Abstract
Virchow–Robin spaces (VRS) are ubiquitous and commonly observed as the resolution of magnetic resonance imaging (MRI) continues to improve. The function of VRS and the etiology of their dilation is still a subject of research. Diagnosing dilated VRS (dVRS) can be challenging because they may appear similar to other pathologies such as cystic neoplasms, infectious cysts, and even arteriovenous malformations (AVMs) on certain MRI pulse sequences. We reported a unique case of brainstem dVRS mimicking an AVM. Furthermore, the extensive pontine involvement of our patient's lesion is rarely described in neurosurgical literature. Understanding the imaging characteristics of dVRS is critical to accurately diagnose these lesions and avoid unnecessary tests and procedures.
KEYWORDS: Arteriovenous malformations, perivascular spaces, Virchow–Robin spaces
INTRODUCTION
Virchow–Robin spaces (VRS) are pia-lined, interstitial fluid-filled structures that surround blood vessels as they enter the brain parenchyma.[1,2,3] VRS are continuous with the subpial space and not the subarachnoid space.[1,2,3] Although some authors consider VRS to be lymphatic spaces of the central nervous system with immunological properties, the clinical significance of VRS remains under investigation.[1] We report a patient with dilated VRS (dVRS) which was initially diagnosed as an arteriovenous malformation (AVM) on magnetic resonance imaging (MRI).
CASE REPORT
A 40-year-old female presented to neurosurgery clinic with a 2-week history of headaches and positional vertigo. Her neurological examination was nonfocal. A brainstem lesion was identified on a limited outside hospital MRI (diffusion-weighted imaging and fluid-attenuated inversion recovery [FLAIR] sequences). The lesion consisted of multiple oval-shaped hypointensities that were concerning for flow voids, with surrounding parenchymal signal abnormality involving the right half of the inferior midbrain and pons [Figure 1a and b]. A cerebral angiogram was performed since the diagnosis of vascular malformation was suspected based on the presumption of flow voids. There was no angiographic evidence of an underlying vascular malformation [Figure 1c]. A repeat brain MRI revealed multiple, mesencephalopontine ovoid lesions that were isointense to cerebrospinal fluid (CSF) on T1- and T2-weighted sequences and did not contrast enhance nor restrict diffusion [Figure 1d–e]. There was no signal dropout on susceptibility-weighted imaging to suggest hemorrhage or calcification [Figure 1f]. The lesion was diagnosed as dVRS of the midbrain and pons and not believed to be the etiology of the patient's symptoms.
Figure 1.
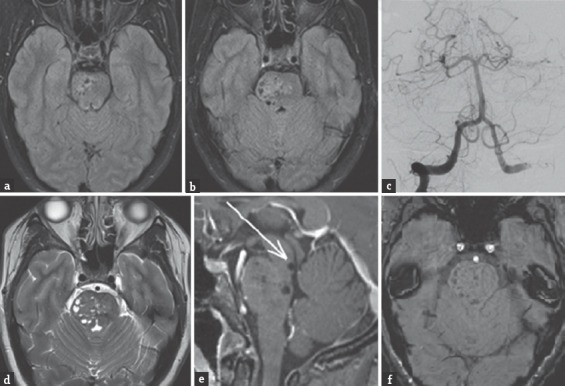
(a and b) Axial fluid-attenuated inversion recovery (FLAIR) MRI performed at an outside hospital reveals multiple, oval-shaped hypointensities located within the right midbrain and pons. (c) Right vertebral artery injection diagnostic cerebral angiogram (Waters view) shows no cerebrovascular abnormalities. (d) Axial T2-weighted MRI with multiple cystic isointense (compared to cerebrospinal fluid) areas in midbrain and pons predominantly on right side. (e) Sagittal T1-weighted MRI with gadolinium contrast shows no associated enhancement. Note the close proximity of the patientfs dilated Virchow-Robin spaces (dVRS) to the cerebral aqueduct. This increases her risk for developing obstructive hydrocephalus if the dVRS continue to expand. (f) Axial susceptibility-weighted imaging (SWI) performed at our institution reveals no evidence of signal dropout to suggest hemorrhage or calcification
The patient was treated with nonsteroidal anti-inflammatory medication, which ameliorated her headaches. A Dix-Hallpike maneuver suggested a diagnosis of benign positional paroxysmal vertigo, which persisted despite treatment with the Epley maneuver.
DISCUSSION
Small, non-dVRS (<2 mm) are ubiquitous.[2,4] Groeschel et al. found VRS in the supratentorial white matter and basal ganglia near the anterior commissure in 100% of 125 healthy subjects using high resolution three-dimensional, fast low angle shot MRI.[1] In the same series, the prevalence of dVRS was found to be 1.6%.[1] dVRS can exert local mass effect, resulting in obstructive hydrocephalus and neurological decline.[3,4] VRS become dVRS when they expand beyond 2 mm,[2] but the mechanism for VRS enlargement is currently unknown. However, current explanations include (1) ex vacuo enlargement after atrophy of surrounding brain tissue, (2) perivascular myelin loss, (3) coiling of an aging artery (i.e., “adventitial loosening”), (4) changes in arterial wall permeability, (5) obstruction of lymphatic drainage, and (6) increased arteriolar tortuosity.[1]
dVRS may be misinterpreted as other pathology, most commonly cystic tumors; therefore, an accurate understanding of the imaging characteristics of dVRS is imperative to making the correct diagnosis.[1,2,5] Despite appearing similar to CSF on all pulse sequences, dVRS have a significantly lower mean signal intensity. This is consistent with dVRS containing interstitial fluid rather than CSF.[2] dVRS lack contrast enhancement, do not restrict diffusion, and have no susceptibility artifact due to calcifications or hemosiderin.[3] MR spectroscopy can further differentiate dVRS from other cystic lesions. Infectious cysts and abscesses exhibit abnormal combinations of lactate, acetate, succinate, and amino acids in the absence of normal brain metabolites. Cystic neoplasms have reduced N-acetylaspartate and increased choline levels. MR spectroscopy of dVRS reveal no major abnormalities, although there have been reports of increased lactate levels.[4] If imaging fails to confirm the diagnosis, surgical biopsy can be used to definitively determine the presence of a suspected dVRS.[5]
In our case, the patient's right-sided mesencephalopontine dVRS appeared similar to AVM flow voids on axial FLAIR MRI. After cerebral angiography revealed no vascular abnormalities, additional MRI pulse sequences were necessary to make the correct diagnosis. A significant proportion of our patient's dVRS were found caudal to the midbrain, which is rare and virtually unacknowledged in the literature except for one patient reported by Salzman et al.[3] The proximity of our patient's dVRS to the cerebral aqueduct increases her risk for developing obstructive hydrocephalus [Figure 1e]. We have recommended follow-up imaging to our patient to monitor for further dilation of her perivascular spaces and evidence of progressive mass effect or obstructive hydrocephalus.
The extensive pontine involvement of our patient's dVRS is not only unusual but completely unaccounted for by the current dVRS classification system. Kwee and Kwee categorized dVRS by location: Type I dVRS are found in the basal ganglia along the lenticulostriate arteries, Type II dVRS are in the cortex along penetrating medullary arteries, and Type III dVRS are in the midbrain surrounding penetrating branches of the collicular and accessory collicular arteries.[2] We propose expanding the category of Type III dVRS to include dVRS anywhere along the brainstem and not limited to the midbrain.
CONCLUSION
VRS are ubiquitous and commonly observed as the resolution of MRI continues to improve. The function of VRS and the etiology of their dilation is still a subject of research. Diagnosing dVRS can be challenging because they may appear similar to other pathology such as cystic neoplasms, infectious cysts, and even AVMs (on certain MRI pulse sequences).[5] We reported a unique case of brainstem dVRS mimicking an AVM. Furthermore, the extensive pontine involvement of our patient's lesion is rarely described in neurosurgical literature. Understanding the imaging characteristics of dVRS is critical to accurately diagnose these lesions and avoid unnecessary tests and procedures.
Highlights
Small Virchow–Robin spaces (VRS) less than 2 mm are a ubiquitous finding
Dilated VRS (dVRS) can be easily mistaken for other pathology
dVRS below the midbrain are rare
In our case, dVRS appeared similar to arteriovenous malformation flow voids on MRI
Accurate understanding of the imaging characteristics of VRS is key to making the diagnosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Groeschel S, Chong WK, Surtees R, Hanefeld F. Virchow-Robin spaces on magnetic resonance images: Normative data, their dilatation, and a review of the literature. Neuroradiology. 2006;48:745–54. doi: 10.1007/s00234-006-0112-1. [DOI] [PubMed] [Google Scholar]
- 2.Kwee RM, Kwee TC. Virchow-Robin spaces at MR imaging. Radiographics. 2007;27:1071–86. doi: 10.1148/rg.274065722. [DOI] [PubMed] [Google Scholar]
- 3.Salzman KL, Osborn AG, House P, Jinkins JR, Ditchfield A, Cooper JA, et al. Giant tumefactive perivascular spaces. AJNR Am J Neuroradiol. 2005;26:298–305. [PMC free article] [PubMed] [Google Scholar]
- 4.Papayannis CE, Saidon P, Rugilo CA, Hess D, Rodriguez G, Sica RE, et al. Expanding Virchow Robin spaces in the midbrain causing hydrocephalus. AJNR Am J Neuroradiol. 2003;24:1399–403. [PMC free article] [PubMed] [Google Scholar]
- 5.Jhawar SS, Garewal SS, Bhargava P, Nittala PP. Dilated Virchow Robin spaces mimicking cystic neoplasm of cingulated gyrus. Neurol India. 2012;60:136–7. doi: 10.4103/0028-3886.93595. [DOI] [PubMed] [Google Scholar]


