Abstract
Context:
No evidence-based recommendation exists regarding how far clinicians should insert a rectal thermistor to obtain the most valid estimate of core temperature. Knowing the validity of temperatures at different rectal depths has implications for exertional heat-stroke (EHS) management.
Objective:
To determine whether rectal temperature (Trec) taken at 4 cm, 10 cm, or 15 cm from the anal sphincter provides the most valid estimate of core temperature (as determined by esophageal temperature [Teso]) during similar stressors an athlete with EHS may experience.
Design:
Cross-sectional study.
Setting:
Laboratory.
Patients or Other Participants:
Seventeen individuals (14 men, 3 women: age = 23 ± 2 years, mass = 79.7 ± 12.4 kg, height = 177.8 ± 9.8 cm, body fat = 9.4% ± 4.1%, body surface area = 1.97 ± 0.19 m2).
Intervention(s):
Rectal temperatures taken at 4 cm, 10 cm, and 15 cm from the anal sphincter were compared with Teso during a 10-minute rest period; exercise until the participant's Teso reached 39.5°C; cold-water immersion (∼10°C) until all temperatures were ≤38°C; and a 30-minute postimmersion recovery period. The Teso and Trec were compared every minute during rest and recovery. Because exercise and cooling times varied, we compared temperatures at 10% intervals of total exercise and cooling durations for these periods.
Main Outcome Measure(s):
The Teso and Trec were used to calculate bias (ie, the difference in temperatures between sites).
Results:
Rectal depth affected bias (F2,24 = 6.8, P = .008). Bias at 4 cm (0.85°C ± 0.78°C) was higher than at 15 cm (0.65°C ± 0.68°C, P < .05) but not higher than at 10 cm (0.75°C ± 0.76°C, P > .05). Bias varied over time (F2,34 = 79.5, P < .001). Bias during rest (0.42°C ± 0.27°C), exercise (0.23°C ± 0.53°C), and recovery (0.65°C ± 0.35°C) was less than during cooling (1.72°C ± 0.65°C, P < .05). Bias during exercise was less than during postimmersion recovery (0.65°C ± 0.35°C, P < .05).
Conclusions:
When EHS is suspected, clinicians should insert the flexible rectal thermistor to 15 cm (6 in) because it is the most valid depth. The low level of bias during exercise suggests Trec is valid for diagnosing hyperthermia. Rectal temperature is a better indicator of pelvic organ temperature during cold-water immersion than is Teso.
Key Words: esophagus, exertional heat stroke, hyperthermia
Key Points
The rectal depth to which the thermistor is inserted affects measurement of rectal temperature.
Clinicians should insert flexible rectal thermistors 15 cm (6 in) into the rectum.
If an inflexible thermistor is being used, the clinician should follow the manufacturer's recommendation regarding insertion depth.
Exertional heat stroke (EHS) is one of the leading causes of sudden death during physical activity.1 Exertional heat stroke is diagnosed when body temperature exceeds 40.5°C and the athlete displays signs or symptoms of central nervous system dysfunction.2,3 It is essential to obtain an accurate and valid measure of body core temperature (Tcore) when EHS is suspected because the signs and symptoms can vary considerably among patients and mimic those of other serious conditions.2 By accurately diagnosing EHS and monitoring Tcore, clinicians can implement proper treatment protocols, such as cold-water immersion (CWI), and return-to-play criteria.
The Tcore, by definition, is the temperature of the hypothalamus. Given the difficulty of directly measuring hypothalamic temperature, other sites have been used to estimate Tcore, including the axilla, mouth, esophagus, intestines, rectum, ear canal, forehead, and pulmonary artery.4,5 Although measurement of pulmonary artery temperature is considered the criterion standard for estimating Tcore,6 it is prohibitively invasive and impractical to use in clinical situations. Many scientists prefer measuring esophageal temperature (Teso) to estimate Tcore because it is close to the heart and major arteries supplying blood to the hypothalamus and has a rapid response to acute temperature changes and high correlation with pulmonary artery6 and aortic temperatures.7 However, Teso is also invasive and impractical to use in the field. Thus, clinicians measure rectal temperature (Trec) in EHS situations because it is a valid estimate of Tcore in exercising, hyperthermic humans4 and practical in emergency situations.5
The National Athletic Trainers' Association2,3 and American College of Sports Medicine8 recommend measuring Trec if EHS is suspected. However, no evidence-based recommendation exists regarding how far into the rectum clinicians should insert a thermometer to obtain the most valid estimate of Tcore in simulated EHS scenarios. Some athletic trainers advised inserting a rectal thermometer 2.54 cm (1 in)9 to 10 cm (3.9 in)10 in EHS scenarios, but no evidence was provided to support these recommendations. In the scientific literature, Trec has been measured at depths ranging from 4 to 27 cm (1.6 to 10.6 in), with most experimenters using a depth around 10 cm (3.9 in).11,12 Because Trec can vary as much as 0.84°C at various depths in the rectum,11−13 rectal depth may affect diagnosis, and thereby treatment, of exertional heat illnesses. Understanding which rectal depth provides the most valid estimate of Tcore under various external stressors is vital for appropriate EHS management.
The purpose of our study was to compare Trec at 4 cm (T4cm), 10 cm (T10cm), and 15 cm (T15cm) from the anal sphincter to Teso. Our goal was to identify the rectal depth with the least bias (ie, least difference from Teso) during 4 experimental periods meant to simulate the stressors an athlete might encounter in an EHS situation (eg, rest, exercise to a state of hyperthermia, CWI, postimmersion recovery). Based on the deeper location, we hypothesized that bias would be lowest at T15cm. We also hypothesized that the greatest bias for all rectal depths would occur during CWI because Teso responds rapidly to acute changes in temperature, whereas Trec responds much more slowly.7,14
METHODS
Participants
We recruited a convenience sample of 19 healthy, recreationally active, unacclimatized individuals. Two individuals discontinued testing due to the difficulty of the exercise protocol; 17 participants completed the study (Table 1). Individuals were excluded from participating if they self-reported (1) an injury or illness that impaired their ability to exercise; (2) any neurologic, respiratory, gastrointestinal, esophageal, or cardiovascular disease; (3) taking any medication that might affect fluid balance or temperature regulation; (4) sedentary lifestyle (defined as exercising less than 30 minutes, 3 times per week)15; (5) history of heat-related illness in the 6 months before data collection; (6) current pregnancy; or (7) cold allergy. Females must also have been at least 10 days postmenstruation to participate. All procedures were approved by Central Michigan University's institutional review board, and recruits provided written consent before participation.
Table 1. .
Participant Demographics and Descriptive Information (Mean ± SD; N = 17)
| Characteristic |
Men (n = 14) |
Women (n = 3) |
| Age, y | 23 ± 2 | 22 ± 2 |
| Height, cm | 181.3 ± 6.2 | 161.3 ± 5.5 |
| Body mass index | 25 ± 3 | 25 ± 3 |
| Sum of skinfolds, mma | 30 ± 9 | 55 ± 17 |
| Body density | 1.1 ± 0.0 | 1.1 ± 0.0 |
| Body fat, % | 8 ± 3 | 15 ± 4 |
| Body surface area, m2 | 2.0 ± 0.1 | 1.7 ± 0.2 |
| Pre-exercise body mass, kg | 82.9 ± 10.3 | 64.7 ± 11.6 |
| Postexercise body mass, kg | 81.5 ± 10.2 | 64.0 ± 11.8 |
| Pre-exercise urine specific gravity | 1.004 ± 0.003 | 1.001 ± 0.000 |
| Sweat rate, L/hb | 1.6 ± 0.4 | 0.9 ± 0.2 |
| Hypohydration, %c | 1.8 ± 0.7 | 1.1 ± 0.4 |
Measured at the chest, abdomen, and thigh for males and at the triceps brachii, abdomen, and thigh for females.
Calculated by taking the difference between body mass measures and dividing by total exercise time.
Calculated by subtracting postexercise body mass from pre-exercise body mass, dividing by pre-exercise body mass, and multiplying by 100.
Procedures
Participants reported for 1 day of testing between 8:00 am and 3:00 pm. They were instructed to abstain from exercise, stimulants (eg, caffeine), and depressants (eg, alcohol) for at least 24 hours before testing. They were also directed to drink water regularly throughout the day preceding testing to ensure their urine was clear or light yellow and to fast for 2 hours before the start of testing. Compliance was self-reported before testing.
Participants voided their bladders completely, and we measured urine specific gravity (SUR-Ne refractometer; Atago USA Inc, Bellevue, WA) to assess hydration status. If urine specific gravity indicated participants were hypohydrated (ie, >1.020),16 they were rescheduled. If euhydrated, participants were weighed nude on a scale (Defender #5000; Ohaus Corp, Parsippany, NJ). They dressed in undergarments (including sports bras for females), shorts, socks, and T-shirts. We measured skinfolds (Baseline skinfold caliper #12-1110; Fabricated Enterprises, Inc, White Plains, NY) at the chest, abdomen, and thigh (men) and at the triceps brachii, abdomen, and thigh (women) in triplicate per Pollack et al.17 Skinfolds were averaged at each site and summed to estimate body density18 and percentage of body fat.19 Body surface area was estimated using the Dubois and Dubois equation.20
Participants donned a heart-rate monitor (Polar Electro, Inc, Lake Success, NY). Then we inserted a pediatric esophageal thermistor 42 cm into the participant's esophagus via the nasal passage while the participant sipped water. This distance ensured that the tip of the thermistor was below the tracheal bifurcation and near the level of the left ventricle.21 We used Teso as our criterion standard for 4 reasons: (1) it is sensitive to acute changes in Tcore and ambient temperature conditions14,22,23; (2) it is valid as compared with pulmonary artery temperature6 and para-aortic temperature7; (3) the esophagus is close in proximity to the major blood vessels supplying blood to the hypothalamus; and (4) the esophagus has a deep body location. The esophageal thermistor was taped to the participant's cheek, looped behind the left ear, and secured to the upper back.
Participants self-inserted a custom-made rectal thermistor 15 cm into the rectum (Physitemp Instruments, Inc, Clifton, NJ). The thermistor was 2.4 m (8 ft) long and consisted of 3 type T thermocouples permanently affixed within a single protective nonstick casing so that Trec at 4 cm (1.5 in), 10 cm (3.9 in), and 15 cm (5.9 in) from the anal sphincter could be measured simultaneously. The rectal thermistor also had a 0.95-cm (0.37-in)–diameter ball permanently affixed at 15.1 cm from the tip to help ensure it did not exit the rectum during testing. Participants were instructed to insert the thermistor until they could no longer feel the ball when they palpated the anal sphincter. To further prevent movement of the rectal thermistor during testing, it was secured to the lower back.
Participants entered an environmental chamber (40.3°C ± 0.5°C, 27% ± 5% relative humidity; Kestrel Heat Stress Tracker #4400; Nielsen-Kellerman, Boothwyn, PA) and stood on a treadmill for 10 minutes to acclimate to the heat.24 After this rest period, they performed an incremental exercise protocol consisting of walking for 3 minutes at 4.83 km/h (3 mph) and then running at 90% of their age-predicted maximum heart rate for 2 minutes (0% incline). After each 5-minute bout, participants stopped the treadmill and rested for 30 seconds. During this time, participants palpated their anus to confirm that the 0.95-cm–diameter ball on the probe remained just inside the anus. Rectal temperature was then recorded. After this 30-second rest period, participants resumed walking at 4.83 km/h for the remainder of the 3-minute walking period. This walking-running-rest protocol continued until Teso reached 39.5°C. We monitored Teso continuously to determine when participants reached 39.5°C.
On reaching a Teso of 39.5°C, participants stopped the treadmill, checked the depth of the rectal thermistor, and had their Trec recorded. They stepped off the treadmill, removed only their shoes, and entered a 1135.6-L capacity, noncirculating water tub (160.7 cm [length] × 175.3 cm [width] × 63.5 cm [height]; model 4247; Rubbermaid, Atlanta, GA). Participants immersed themselves up to the neck for the duration of cooling. We started a standard stopwatch the moment each participant's foot touched the water and stopped it when he or she exited the bath so we could calculate cooling rates for each temperature site. Cooling rates for each site were calculated by determining the difference in body temperatures at each site from postexercise to post-CWI and dividing it by the amount of time necessary to reduce each temperature site to 38°C. Participants remained in the water bath until all temperatures were ≤38°C. A 401 thermistor (Advanced Industrial Systems Inc, Prospect, KY) was secured at 21 cm from the bottom of the water bath. Initial water-bath temperature was 9.9°C ± 0.2°C. The water bath was kept in the environmental chamber to minimize transfer time and to simulate the ambient conditions an athlete might experience while being cooled at an outdoor athletic event in the heat. The water bath was stirred every 2 minutes.
Once all body temperatures were ≤38°C, participants exited the water bath and sat in the environmental chamber for 30 minutes. After this recovery period, participants exited the environmental chamber, removed the thermistors, towel dried, and were weighed nude a second time and excused. No fluids were given to participants once they entered the environmental chamber.
Instrumentation
A 4600 Precision thermometer with #402 pediatric esophageal thermistor (Advanced Industrial Systems Inc) measured Teso. A 16-channel Iso-Thermex electrothermometer (Columbus Instruments, Columbus, OH) measured Trec at each depth. During pilot testing, we verified each thermometer's calibration against a National Institute of Standards and Technology-certified thermometer under similar conditions (∼40°C, 20% relative humidity) at 3 water-bath temperatures (1°C, 39.5°C, and 50°C). All thermometers were certified to be accurate to within 0.1°C.
The Teso and Trec data were recorded every 30 seconds during the rest, cooling, and recovery periods of the experiment. The data from each whole minute were averaged with the preceding 30-second data point during these periods. During exercise, Teso and Trec were recorded every 1 and 5 minutes, respectively. Rectal temperature was recorded every 5 minutes during exercise because our treadmill emitted electrical radiation that interfered with the rectal-thermistor measurements. When the treadmill was stopped, the rectal thermistors provided valid data. The treadmill did not interfere with the Teso thermometer at any time. We compared Teso and Trec at 10% increments of each person's total exercise and cooling durations because these times differed among participants.
Statistical Analysis
Data were assessed for skewness, kurtosis, and omnibus normality to ensure normal distribution. A 2-way repeated-measures analysis of variance was used to determine if differences in temperatures existed between Teso and Trec at each depth over time. To simplify the statistical analysis of bias, we calculated the average bias for each experimental period and each rectal depth. We then used a 2-way, repeated-measures analysis of variance to examine differences in bias among rectal depths across the 4 experimental periods. Sphericity was assessed using the Mauchly test. Geisser-Greenhouse adjustments to P values and degrees of freedom were made when sphericity was violated. When significant interactions or main effects were demonstrated, we conducted Tukey-Kramer post hoc tests to identify differences at each time point. Significance was demonstrated with P < .05 (version 2007; Number Cruncher Statistical Software, Kaysville, UT).
RESULTS
All data are reported as means and standard deviations. In addition to demographic information, urine specific gravity, sweat rates, and hypohydration levels are reported for descriptive purposes (Table 1). Exercise durations, CWI durations, and cooling rates are also reported for descriptive purposes. Participants exercised for 53.0 ± 16.8 minutes. The time to reduce body temperature to 38°C varied by site (Teso = 1.9 ± 0.49 minutes; T4cm = 9.5 ± 4.8 minutes; T10cm = 9.5 ± 5.8 minutes; T15cm = 7.6 ± 5.1 minutes). Consequently, cooling rates also varied (Teso = 0.79°C·min−1 ± 0.16°C·min−1; T4cm = 0.24°C·min−1 ± 0.13°C·min−1; T10cm = 0.24°C·min−1 ± 0.13°C·min−1; T15cm = 0.28°C·min−1 ± 0.12°C·min−1).
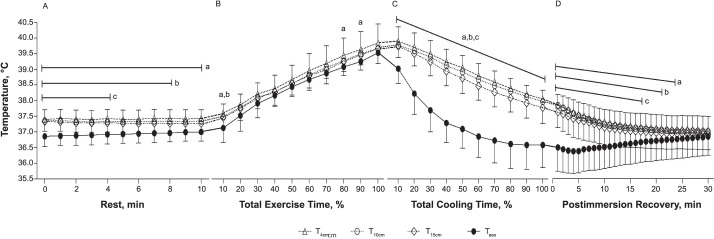
We observed a significant interaction between temperature site and time for body temperatures (F180,2880 = 27.9, P < .001; Figure 1). The T4cm measurement was different from the Teso reading for all 11 measurements during the rest period, 3 measurements during exercise, all 10 cooling measurements, and 23 measurements during postimmersion recovery (47 of 61 measurements; 75% of all measurements). The T10cm measurement differed from the Teso reading for 9 measurements during the rest period, 1 measurement during exercise, all 10 cooling measurements, and 21 measurements during postimmersion recovery (41 of 61 measurements; 67% of all measurements). The T15cm measurement was different from the Teso reading for the first 5 measurements during the rest period, all 10 cooling measurements, and 17 measurements during postimmersion recovery (32 of 61 measurements; 52% of all measurements).
Figure 1. .
Body temperatures measured in the esophagus and rectum during, A, rest, B, exercise, C, cold-water immersion, and D, postimmersion recovery (mean ± standard deviation; n = 17). Abbreviations: T4cm, rectal temperature 4 cm from the anal sphincter; T10cm, rectal temperature 10 cm from the anal sphincter; T15cm, rectal temperature 15 cm from the anal sphincter; Teso, esophageal temperature. a Indicates T4cm was different from Teso within each time point (P < .05). b Indicates T10cm was different from Teso within each time point (P < .05). c Indicates T15cm was different from Teso within each time point (P < .05).
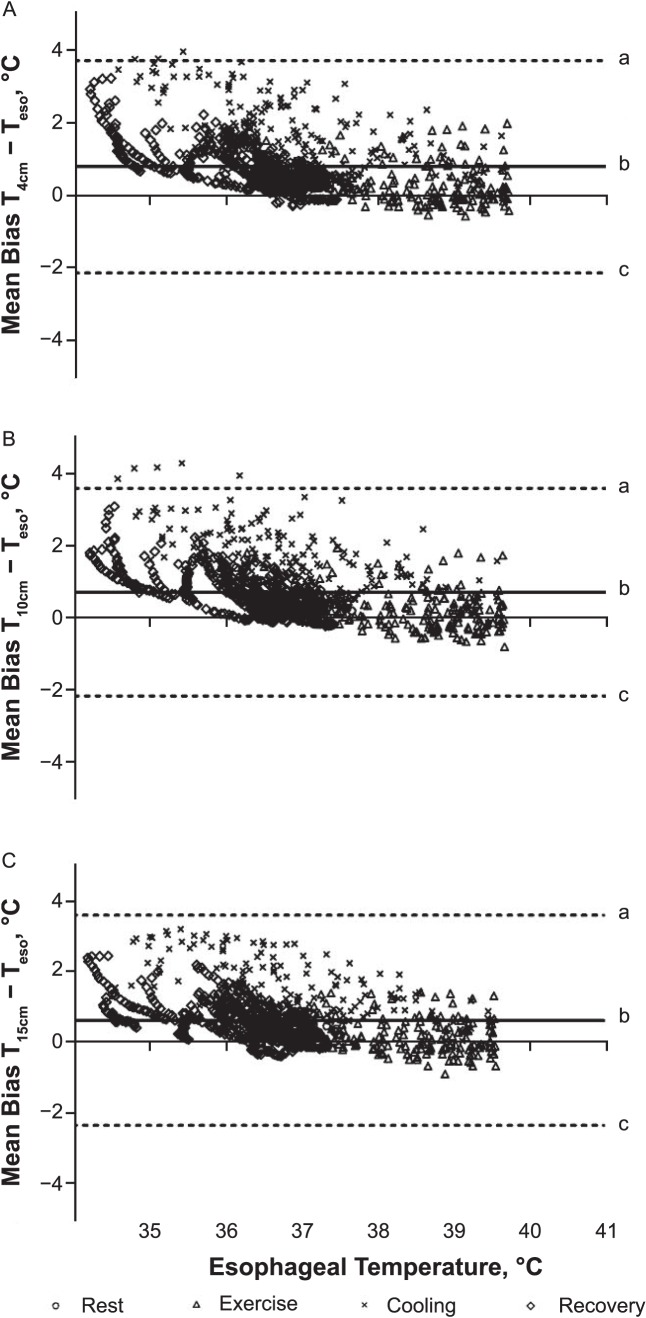
We did not observe an interaction between rectal depth and time for temperature bias (F2,32 = 1.0, P = .37; Table 2). However, we noted differences among rectal depths (F2,24 = 6.8, P = .008). Bias at T15cm was less than at T4cm (P < .05). Bias at T10cm was not different from at T4cm or T15cm (P > .05). Also, bias differed over time (F2,34 = 79.5, P < .001; Table 2). Bias during rest, exercise, and recovery was less than during cooling (P < .05). Bias during exercise was less than during postimmersion recovery (P < .05). We created Bland-Altman plots with 95% limits of agreement to show bias throughout the experiment (Figure 2).
Table 2. .
Bias by Rectal Depth and Experimental Time (Mean ± SD; N = 17)
| Time |
Rectal Temperature, °C, at Distance From Anal Sphincter |
Overall Mean for Timea |
||
| 4 cm |
10 cm |
15 cm |
||
| Rest | 0.49 ± 0.26 | 0.41 ± 0.26 | 0.36 ± 0.29 | 0.42 ± 0.27c |
| Exercise | 0.32 ± 0.57 | 0.19 ± 0.54 | 0.17 ± 0.49 | 0.23 ± 0.53c,d |
| Cooling | 1.86 ± 0.72 | 1.74 ± 0.67 | 1.55 ± 0.56 | 1.72 ± 0.65 |
| Postimmersion recovery | 0.73 ± 0.36 | 0.67 ± 0.36 | 0.55 ± 0.32 | 0.65 ± 0.35c |
| Overall mean for rectal depthb | 0.85 ± 0.78 | 0.75 ± 0.76 | 0.65 ± 0.68e | |
Average of all rectal depths within each time (ie, main effect of time).
Average of all measurements collected during the experiment at each depth (ie, main effect of rectal depth).
Less than cooling period (P < .05).
Less than postimmersion recovery (P < .05).
Less than rectal temperature at 4 cm from the anal sphincter (P < .05).
Figure 2. .
Bland-Altman plots indicate temperature bias between esophageal temperature (Teso) and rectal temperature at, A, 4 cm (T4cm), B, 10 cm (T10cm), and C, 15 cm (T15cm) from the anal sphincter. a Upper limit of agreement. b Mean difference. c Lower limit of agreement.
DISCUSSION
Our most important observation was that rectal depth affected estimates of Tcore. Numerous position statements2,3,8 and experts4,25,26 have advocated for Trec assessment as a vital component of proper EHS diagnosis and management. However, no evidence-based recommendation regarding how far clinicians should insert a rectal thermistor when EHS is suspected has been provided. Although in athletic training textbooks9,10 some clinicians recommended inserting thermometers 2.54 to 10 cm (1 to 4 in), no evidence validating these recommendations in hyperthermic humans was provided. To our knowledge, we are the first to investigate the validity of 3 rectal depths during stressors similar to what an EHS patient may experience in the field. The clinical application of our data is that clinicians should insert flexible thermistors 15 cm (6 in) into the rectum to obtain the most valid estimate of Tcore when diagnosing, treating, and monitoring patients with severe hyperthermia.
Small differences in bias were observed among rectal depths. The T15cm was 0.2°C (0.4°F) and 0.1°C (0.2°F) more accurate in estimating Tcore than were T4cm and T10cm, respectively. Similarly, Nielsen and Nielsen12 observed unsystematic differences in Trec when measured at 12, 17, 22, and 27 cm from the anal sphincter during steady-state exercise. The smallest bias from Teso occurred at the 17-cm depth (0.27°C ± 0.7°C).12 Smaller biases may not have been observed at 22 and 27 cm12 due to the rectum being less than 20 cm (8 in) long.27 Thus, the flexible thermistors may have bent back toward the anal sphincter after encountering the 80° anorectal flexure. Lee et al11 did not measure Teso, and therefore bias, but they did measure Trec at 4, 6, 8, 10, 13, 16, and 19 cm from the anal sphincter at rest and after moderate- to high-intensity exercise. They11 observed the 16-cm depth had the highest and most stable Trec with the longest latency. Moreover, systematic differences in Trec did not occur at depths greater than 10 cm.
We propose 2 reasons for why the deepest rectal depths produced the least bias. First, at deeper depths, the temperature sensors would have been closer to the large blood vessels of the pelvic wall.13 Thus, T15cm may be more consistent with the temperature of the blood in the vessels perfusing the buttocks, upper leg, and external genitalia. The Teso also correlates highly with the temperatures of blood in major arteries (eg, pulmonary artery, aorta).7 Second, at deeper depths, organ mass surrounding the probe would be greater, leading to more stable temperatures and less bias. In contrast, T4cm measures the temperature of the anal cavity, a portion of the rectum surrounded by only the internal and external anal sphincters.27
Consistent with our original hypothesis, the largest bias (∼1.7°C) occurred during CWI, whereas the smallest bias occurred during exercise (∼0.2°C). The small bias during exercise confirms that Trec is a valid estimate of Tcore in exercising, hyperthermic humans.4 Thus, Trec is useful for diagnosing exertional heat illnesses such as EHS. Regarding the large bias during CWI, it is well established that Teso responds rapidly to acute changes in temperature, whereas Trec exhibits slower response times.7,14,22 The Teso cooling rates vary widely depending on the water-bath temperature but can range from 0.06°C·min−1 to 1.04°C·min−1.28−31 In water temperatures between 2°C and 14°C, Teso cooling rates often exceed 0.4°C·min−1.28,29,31 By comparison, Trec cooling rates of hyperthermic humans undergoing CWI range from 0.12°C·min−1 to 0.35°C·min−1.14,29,30,32−35 The faster Teso cooling rates can be explained by less organ mass and density in the thorax compared with the gut14 and rapid incorporation of cooled blood from the periphery into the general circulation.36
The differences in cooling rates between Teso and Trec may have clinical implications. Current recommendations are to use Trec to make clinical judgments on CWI cessation26 and EHS diagnosis.2,3,8 Though Teso may indicate a patient's Tcore has returned to safe levels, considerable heat stress still exists as indicated by the high Trec. In our study, when Teso reached 38°C, T4cm, T10cm, and T15cm were 39.09°C, 39.06°C, and 38.99°C, respectively. Thus, although Teso may be a better indicator of brain and heart temperature,7 Trec provides valuable information about the temperature of internal organs in the gut. For this reason, as well as its ease of use in the field, Trec remains the criterion-standard measurement site for estimating Tcore in clinical situations.
Even though T15cm is the most valid rectal depth, clinicians must consider the type of thermometer available when trying to diagnose and treat patients with EHS. We used flexible, reusable thermistors to measure Trec. These thermistors have long leads and remained inside the rectum during the entire treatment and monitoring processes. If clinicians have inflexible thermometers that cannot or were not designed to be inserted 15 cm into the rectum, we suggest following the manufacturer's insertion recommendations to avoid injuring the rectum or surrounding tissues.
We recognize 3 limitations of our study. First, rectal anatomy can vary considerably among participants,13 and we did not verify the exact position of the rectal thermistor during testing using advanced imaging techniques. However, we took considerable measures to ensure that the thermistors were inserted consistently. For example, a nylon ball was inserted into the rectum to prevent the thermistor from exiting, and participants repeatedly checked for proper insertion depth before Trec measurements were taken. Second, electrical interference from the treadmill prevented us from measuring Trec continuously during exercise. However, Teso was monitored and recorded frequently during exercise to ensure measurements were taken at approximately the same times. Rectal temperature was monitored continuously during all other testing periods. Finally, as in all human experimental studies of significant hyperthermia under laboratory conditions, we did not induce EHS. Thus, our results may not be generalizable to all EHS situations.
In summary, rectal depth affects estimates of Tcore, and bias varies according to the physiological stress placed on the body. Clinicians should insert flexible thermistors 15 cm (6 in) into the rectum for the most valid estimate of Tcore. If clinicians do not have access to flexible thermistors, they should follow the manufacturer's recommendations for insertion depth to avoid damage to the rectum and surrounding tissues.
ACKNOWLEDGMENTS
We thank Greg McGillvary, MA, ATC, and Joseph Fox, MA, ATC, from Central Michigan University's Athletics Department for donating the cooling tub for this study and Central Michigan University's Office of Research and Graduate Studies for funding this project.
REFERENCES
- 1. Mueller FO, Colgate B. . Annual Survey of Football Injury Research: 1931–2011. Chapel Hill, NC: National Center for Catastrophic Sport Injury Research; 2011: 1– 31. [Google Scholar]
- 2. Casa DJ, DeMartini JK, Bergeron MF, et al. . National Athletic Trainers' Association position statement: exertional heat illnesses. J Athl Train. 2015; 50 9: 986– 1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Casa DJ, Guskiewicz KM, Anderson SA, et al. . National Athletic Trainers' Association position statement: preventing sudden death in sports. J Athl Train. 2012; 47 1: 96– 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Casa DJ, Becker SM, Ganio MS, et al. . Validity of devices that assess body temperature during outdoor exercise in the heat. J Athl Train. 2007; 42 3: 333– 342. [PMC free article] [PubMed] [Google Scholar]
- 5. Moran DS, Mendal L. . Core temperature measurement: methods and current insights. Sports Med. 2002; 32 14: 879– 885. [DOI] [PubMed] [Google Scholar]
- 6. Lefrant JY, Muller L, de La Coussaye JE, et al. . Temperature measurement in intensive care patients: comparison of urinary bladder, oesophageal, rectal, axillary, and inguinal methods versus pulmonary artery core method. Intensive Care Med. 2003; 29 3: 414– 418. [DOI] [PubMed] [Google Scholar]
- 7. Cooper KE, Kenyon JR. . A comparison of temperatures measured in the rectum, oesophagus, and on the surface of the aorta during hypothermia in man. Br J Surg. 1957; 44 188: 616– 619. [DOI] [PubMed] [Google Scholar]
- 8. American College of Sports Medicine, LE, Armstrong Casa DJ, et al. . American College of Sports Medicine position stand: exertional heat illness during training and competition. Med Sci Sports Exerc. 2007; 39 3: 556– 572. [DOI] [PubMed] [Google Scholar]
- 9. Prentice WE. . Risk management. : WE, Prentice Arnheim D, . Principles of Athletic Training: A Competency-Based Approach. 14th ed. New York, NY: McGraw-Hill Company, Inc; 2011: 160. [Google Scholar]
- 10. Prentice WE. . Environmental conditions. Principles of Athletic Training: A Competency-Based Approach. 15th ed. New York, NY: McGraw-Hill Company, Inc; 2014: 165. [Google Scholar]
- 11. Lee JY, Wakabayashi H, Wijayanto T, Tochihara Y. . Differences in rectal temperatures measured at depths of 4–19 cm from the anal sphincter during exercise and rest. Eur J Appl Physiol. 2010; 109 1: 73– 80. [DOI] [PubMed] [Google Scholar]
- 12. Nielsen B, Nielsen M. . Body temperature during work at different environmental temperatures. Acta Physiol Scand. 1962; 56 2: 120– 129. [DOI] [PubMed] [Google Scholar]
- 13. Mead J, Bonmarito CL. . Reliability of rectal temperatures as an index of internal body temperature. J Appl Physiol. 1949; 2 2: 97– 109. [DOI] [PubMed] [Google Scholar]
- 14. Gagnon D, Lemire BB, Jay O, Kenny GP. . Aural canal, esophageal, and rectal temperatures during exertional heat stress and the subsequent recovery period. J Athl Train. 2010; 45 2: 157– 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thompson WR, Gordon NF, Pescatello LS. . Preparticipation health screening and risk stratification. ACSM's Guidelines for Exercise Testing and Prescription. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010: 18– 39. [Google Scholar]
- 16. American College of Sports Medicine, Sawka MN, Burke LM, et al. . American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc. 2007; 39 2: 377– 390. [DOI] [PubMed] [Google Scholar]
- 17. Pollack ML, Schmidt DH, Jackson AS. . Measurement of cardio-respiratory fitness and body composition in the clinical setting. Compr Ther. 1980; 6 9: 12– 27. [PubMed] [Google Scholar]
- 18. Jackson AS, Pollack ML. . Generalized equations for predicting body density of men. Br J Nutr. 1978; 40 3: 497– 504. [DOI] [PubMed] [Google Scholar]
- 19. Siri WE. . Body composition from fluid spaces and density: analysis of methods. : Brozek J, Henschel A, . Techniques for Measuring Body Composition. Washington, DC: National Academies Press; 1961: 223– 244. [Google Scholar]
- 20. DuBois D, DuBois EF. . A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916; 17: 863– 871. [Google Scholar]
- 21. Mekjavić IB, Rempel ME. . Determination of esophageal probe insertion length based on standing and sitting height. J Appl Physiol (1985). 1990; 69 1: 376– 379. [DOI] [PubMed] [Google Scholar]
- 22. Teunissen LPJ, de Haan A, de Koning JJ, Daanen HA. . Telemetry pill versus rectal and esophageal temperature during extreme rates of exercise-induced core temperature change. Physiol Meas. 2012; 33 6: 915– 924. [DOI] [PubMed] [Google Scholar]
- 23. Sarkar S, Donn SM, Bhagat I, Dechert RE, Barks JD. . Esophageal and rectal temperatures as estimates of core temperature during therapeutic whole-body hypothermia. J Pediatr. 2013; 162 1: 208– 210. [DOI] [PubMed] [Google Scholar]
- 24. Armstrong LE, Johnson EC, Casa DJ, et al. . The American football uniform: uncompensable heat stress and hyperthermic exhaustion. J Athl Train. 2010; 45 2: 117– 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. DeMartini JK, Casa DJ, Stearns RL, et al. . Effectiveness of cold-water immersion in the treatment of exertional heat stroke at the Falmouth Road Race. Med Sci Sports Exerc. 2015; 47 2: 240– 245. [DOI] [PubMed] [Google Scholar]
- 26. Gagnon D, Lemire BB, Casa DJ, Kenny GP. . Cold-water immersion and the treatment of hyperthermia: using 38.6°C as a safe rectal temperature cooling limit. J Athl Train. 2010; 45 4: 439– 444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. The digestive system. In: Tortora GJ, Derrickson B. eds Principles of Anatomy and Physiology. Hoboken, NJ: John Wiley and Sons, Inc; 2012: 1006. [Google Scholar]
- 28. Proulx CI, Ducharme MB, Kenny GP. . Safe cooling limits from exercise-induced hyperthermia. Eur J Appl Physiol. 2006; 96 4: 434– 445. [DOI] [PubMed] [Google Scholar]
- 29. Lemire B, Gagnon D, Jay O, Dorman L, Ducharme MB, Kenny GP. . Influence of adiposity on cooling efficiency in hyperthermic individuals. Eur J Appl Physiol. 2008; 104 1: 67– 74. [DOI] [PubMed] [Google Scholar]
- 30. Friesen BJ, Carter MR, Poirier MP, Kenny GP. . Water immersion in the treatment of exertional hyperthermia: physical determinants. Med Sci Sports Exerc. 2014; 46 9: 1727– 1735. [DOI] [PubMed] [Google Scholar]
- 31. Taylor NA, Caldwell JN, Van den Heuvel AM, Patterson MJ. . To cool, but not too cool: that is the question—immersion cooling for hyperthermia. Med Sci Sports Exerc. 2008; 40 11: 1962– 1969. [DOI] [PubMed] [Google Scholar]
- 32. Proulx CI, Ducharme MB, Kenny GP. . Effect of water temperature on cooling efficiency during hyperthermia in humans. J Appl Physiol (1985). 2003; 94 4: 1317– 1323. [DOI] [PubMed] [Google Scholar]
- 33. Lemire BB, Gagnon D, Jay O, Kenny GP. . Differences between sexes in rectal cooling rates after exercise-induced hyperthermia. Med Sci Sports Exerc. 2009; 41 8: 1633– 1639. [DOI] [PubMed] [Google Scholar]
- 34. Miller KC, Swartz EE, Long BC. . Cold-water immersion for hyperthermic humans wearing American football uniforms. J Athl Train. 2015; 50 8: 792– 799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miller KC, Long BC, Edwards JE. . Necessity of removing American football uniforms from hyperthermic humans before cold-water immersion. J Athl Train. 2015; 50 12: 1240– 1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pearson J, Ganio MS, Seifert T, Overgaard M, Secher NH, Crandall CG. . Pulmonary artery and intestinal temperatures during heat stress and cooling. Med Sci Sports Exerc. 2012; 44 5: 857– 862. [DOI] [PMC free article] [PubMed] [Google Scholar]