Abstract
The aim of this study was to investigate the relationships between blood–brain barrier (BBB) dysfunction, intrathecal IgG synthesis, and brain glucose consumption as detectable by means of serum/cerebrospinal fluid (CSF) albumin index (Qalb) and IgG index [(CSF IgG/serum IgG) × Serum albumin/CSF albumin)] and 2-deoxy-2-(18F) fluoro-d-glucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) in a selected population affected by Alzheimer disease (AD). The study included 134 newly diagnosed AD patients according to the NINCDS-ADRDA criteria. The mean (±SD) age of the patients was 70 (±6) years; 60 were male and 64 were female. Mini mental State Examination was equal to 18.9 (±7.2). All patients underwent a CSF assay and magnetic resonance before 18F-FDG PET scanning. The relationships were evaluated by means of statistical parametric mapping (SPM8). We found a significant negative correlation between the increase of Qalb and 18F-FDG uptake in the Brodmann Area 42 and 22 that corresponds to the left superior temporal gyrus, with higher Qalb values being related to a reduced glucose consumption in these areas. No significant relationships have been found between brain glucose consumption and IgG index. The results of our study suggest that BBB dysfunction is related to reduction of cortical activity in the left temporal cortex in AD subjects.
Keywords: Alzheimer, blood brain barrier dysfunction, dementia, IgG, PET, SPM
1. Introduction
Alzheimer disease (AD) is a primary neurodegenerative disease pathologically characterized by cortical deposits, the extracellular senile plaques, and the intracellular neurofibrillary tangles. Since its first appearance in the early 90s, the “amyloid hypothesis” has been proposed as a pioneristic explanation of its pathological basis,[1] although recent evidences criticized its validity and suggested the neurofibrillary pathology as the main responsible for cognitive decline of AD.[2] Changes observed in AD brains are mirrored by changes in the content of their constituent proteins in the cerebrospinal fluid (CSF). Assay of the microtubule Tau protein (total and its hyper phosphorylated form) and amyloid peptides (as Aβ42 peptide) in CSF are indeed routinely used as biochemical diagnostic markers of AD and of other neurodegenerative disorders.[3–5]
The blood–brain barrier (BBB) is a highly selective permeability barrier that separates the circulating blood from the brain extracellular fluid in the central nervous system (CNS). Albumin is not synthesized in the CNS but penetrates BBB from the plasma; therefore, the albumin concentration quotient (Qalb, see below) is generally accepted as a method for estimating the function of the BBB.[6,7] Up to 42% of subjects with AD may show a dysfunction in BBB and it has been hypothesized that this could affect the clearance of potentially toxic substances across the BBB.[8–10] Recent evidences showed that Aβ pathology itself could be responsible for the occurrence of an endotheliopathy in AD patients, a condition that was suggested to potentially precipitate the rate of cognitive decline in these patients.[9]
Increased concentrations of free immunoglobulins in CSF indicating an immune response within the CNS are commonly found in neuro-inflammatory diseases as multiple sclerosis. About two-thirds of the patients with this type of disease have increased CSF immunoglobulin (IgG) index (see below), as an indicator of intrathecal class G production and more than 90% of the patients display oligoclonal IgG bands on electrophoretic separation of CSF.[11] Plasma cells from compartimentalized lymphoid tissue (CLT) in CNS are the main source of intratechal-synthesized immunoglobulins.[12] However, despite the solid evidence of the presence of Ig synthesis in multiple sclerosis (MS), the antigens targeted by intratechal immunoglobulins are still largely unknown and many different epitopes have been demonstrated.[13] As a direct consequence, the pathogenic potential of these immunoglobulins is still elusive and a hypothetical role of intratechal synthesis in non-MS neurological diseases cannot be excluded.[14] On the other side, the identification of intratechal-synthesized Ig could represent only the epiphenomenon of the presence of deranged immune cells inside the CNS, and these cells contributing to neuropathology with Ig-independent mechanisms.[15] Contrarily to Qalb, the percentage of AD subjects showing alterations in IgG index is small, with intrathecal IgG synthesis being detectable in ∼4% of subjects with AD.[10] Whether BBB changes could represent a possible marker of vulnerability during AD has not been explored yet. To do this, we investigated the relationship between BBB function markers, IgG index, and 18F-FDG uptake, in a population of clinically diagnosed AD.
The usefulness of 2-deoxy-2-(18F) fluoro-d-glucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) in the diagnosis of AD has been widely investigated showing a good sensitivity, specificity, and diagnostic accuracy in the detection of hypometabolism associated with AD.[16,17]
2. Materials and methods
2.1. Patients
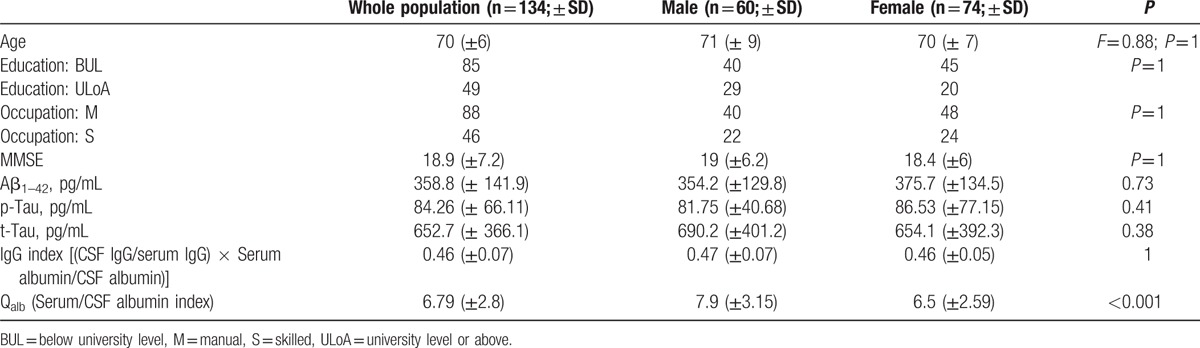
We examined 134 newly diagnosed probable AD patients according to the NINCDS-ADRDA criteria.[18] An overview of the population examined is provided in Table 1.
Table 1.
General overview of the AD population examined, including sociodemographic variables.
All patients underwent a complete clinical investigation, including medical history, neurological examination, mini-mental state examination (MMSE), a complete blood screening (including routine examinations, thyroid hormones, level of B12), neuropsychological examination, a complete neuropsychiatric evaluation.[19] Structural magnetic resonance (MR) was performed within 1 month before 18F-FDG PET/CT brain scan PET/CT in order to exclude any possible brain lesions and to aid in the image analysis (when required, PET and MR data were coregistered in order to exclude any artifact, partial volume effect as in the case of an expanded cerebral sulcus).[20]
Exclusion criteria were the following: patients with isolated deficits and /or unmodified MMSE (≥25/30) on revisit (6, 12, 18 months follow-up), patients with clinically manifest acute stroke in the last 6 months showing an Hachinsky scale >4, and a radiological evidence of subcortical lesions. None of patients revealed pyramidal and/or extrapyramidal signs at the neurological examination. Moreover, patients with diabetes, oncological or HIV histories, or with a history of surgery, radiation, or trauma to the brain were excluded from the study. All AD patients showed a cognitive profile consistent with mild dementia, as assessed by a neuropsychological evaluation including the MMSE and a standardized neuropsychological battery.[19] On the MMSE, AD patients scored a mean of N (± N) and Clinical Dementia Rating (CDR) was N (± N). At the time of the examination, 42 subjects were in treatment for hypertension with angiotensin-converting enzyme (ACE) inhibitor and /or beta blockers and 19 were in treatment for dyslipidemia with statins. We did not consider patients undergoing treatment with drugs that could interfere with 18F-FDG uptake and distribution in the brain as psychotropic drugs and, in particular, as all AD patients were “de-novo,” none of them was treated with cholinesterase inhibitor treatment throughout the study.[21]
The study has been approved by the local ethics committee (Policlinico Tor Vergata) and a written informed consent has been obtained in all cases from the patient themselves in accordance with the Declaration of Helsinki.
2.2. Cognitive evaluation
At the time of enrollment, all recruited patients underwent a neuropsychological battery including the following cognitive domains: general cognitive efficiency: MMSE[22] verbal episodic long-term memory: Rey auditory verbal long term memory (RAVLT) (15-Word List Immediate and 15-min Delayed recall)[23]; visuo-spatial abilities and visuo-spatial episodic long-term memory: Complex Rey's Figure (copy and 10-min Delayed recall)[24] executive functions: phonological word fluency (PWF)[25]; analogic reasoning: Raven's Colored Progressive Matrices (RCPM).[26] For all employed tests, we used the Italian normative data for both score adjustment (gender, age, and education) and to define cut-off scores of normality, determined as the lower limit of the 95% tolerance interval. For each test, normative data are reported in the corresponding references.
2.3. CSF collection and analysis
All the CSF samples were obtained by lumbar puncture (LP) performed at rest, in decubit-position, between 9:00 and 10:00 am, after overnight fasting, using an atraumatic needle. Blood specimens were also obtained at the same time of LP procedure. CSF samples were collected in polypropylene tubes using standard sterile techniques. The first 4 mL CSF sample was used for biochemistry routine analysis including total cell count and lactate levels. A second 4 mL CSF sample was centrifuged to eliminate cells and cellular debris and immediately frozen at -80°C until the analysis to assess t-Tau, p-Tau, and Aβ42 amounts, performed as previously described. Chemistry assays were carried out using commercially available kits following the manufacturer's specifications (Flex reagent cartridge, Dimension Vista System, Siemens Healthcare Diagnostics GmbH, Munich, Germany).[27]
Concentrations of IgG and albumin in serum and CSF were measured by means of a nephelometer BN ProSpec (Siemens Healthcare Diagnostics). The Qalb was calculated by the formula ([ALBUMIN]CSF/[ALBUMIN]SERUM) × 1000. IgG index was calculated by the formula ([IgG]CSF/[Albumin]CSF)/[[IgG]SERUM/[Albumin]SERUM.[28]
2.4. 18F-FDG injection and PET/CT scan
The PET/CT system Discovery VCT (GE Medical Systems, Tennessee) has been used to assess 18F-FDG brain distribution in all patients by means of a 3D-mode standard technique in a 256 × 256 matrix. Reconstruction was performed using the 3-dimensional reconstruction method of ordered-subsets expectation maximization (OSEM) with 20 subsets and with 4 iterations. The system combines a high-speed ultra 16-detector-row (912 detectors per row) CT unit and a PET scanner with 13,440 bismuth germanate crystals in 24 rings (axial full width at half-maximum 1 cm radius, 5.2 mm in 3D mode, axial field of view 157 mm). A low-ampere CT scan of the head for attenuation correction (40 mA; 120 Kv) was performed before PET image acquisition. All the subjects fasted for at least 5 hours before intravenous injection of 18F-FDG; the serum glucose level was up to 95 mg/mL in all of them. All the subjects were injected intravenously with 185 to 210 MegaBequerels of 18F-FDG and hydrated with 500 mL of saline (0.9% sodium chloride). PET/CT acquisition was started 30 minutes after 18F-FDG injection.[27]
3. Statistical analysis
Correlations among brain 18F-FDG uptake, clinical, and CSF data were analyzed using statistical parametric mapping (SPM8, Wellcome Department of Cognitive Neurology, London, UK) implemented in Matlab R2012b (Mathworks, Natick, Massachusetts). MMSE scores, neuropsychological assessment scores, sex, age, and CSF biomarkers were used as covariates in each correlation analysis. 18F-FDG PET data have been subjected to affine and nonlinear spatial normalization into the Montreal Neurological Institute space. The spatially normalized set of images were then smoothed with a 8 mm isotropic Gaussian filter to blur for individual variations in gyral anatomy and to increase the signal-to-noise ratio. Images have been globally normalized to 50 using proportional scaling to remove confounding effects to global cerebral glucose consumption changes, with a masking threshold of 0.8. The resulting statistical parametric maps, SPM[t], have been transformed into normal distribution (SPM[z]) unit. Correction of SPM coordinates to match the Talairach coordinates was achieved by the subroutine implemented by Matthew Brett (http://www.mrc-cbu.cam.ac.uk/Imaging). Brodmann areas (BAs) have been identified at a range from 0 to 3 mm from the corrected Talairach coordinates of the SPM output isocenters, after importing the corrected coordinates, by Talairach client (http://www.talairach.org/index.html). According to Bennett et al,[29] SPM t-maps have been set at P < 0.05, corrected for multiple comparisons with the False Discovery Rate option at voxel level, and at P < 0.01 corrected for multiple comparison at cluster level. Only those clusters containing more than 100 (5 × 5 × 5 voxels, i.e., 11 × 11 × 11 mm) contiguous voxels have been accepted as significant. The voxel-based analyses have been performed using a ‘regression analysis’ design model using sex, age, MMSE, and CSF parameters presented in Table 1 as a covariate. In SPM maps, we searched the brain areas with a significant correlation using a statistical threshold of P = 0.01, family-wise error-corrected for the problem of multiple comparisons, with an extent threshold of 100 voxels.
The cluster obtained by this comparison has been exported and further analyzed after a normalization process. In particular, the mean signal intensities computed of each cluster have been normalized within each subject to the average intensities of the cerebellar volume of interest as defined by Schmahmann et al.[30] This choice was based on the knowledge that the cerebellum is poorly affected by AD pathological processes and on the evidence that, when using cerebellum instead of whole brain counts as the reference region, accuracy in distinguishing AD patients from controls increases.[31] As proposed in another study of Pagani et al,[16] a dataset including cerebellum-normalized 18F-FDG PET values relevant to the examined cluster has been exported. In order to assess that cerebellum-normalized 18F FDG PET values for the cluster examined were of Gaussian distribution, D’Agostino K squared normality test has been applied (where the null hypothesis is that the data are normally distributed). Spearman correlation has been applied in order to investigate the relationships among cerebellum-normalized 18F-FDG PET values, CSF biomarkers (amyloid and Tau, see Table 1), albumin ratio, and IgG index. According to the results of correlation analyses (see below) and population characteristics, patients have been divided into groups according to Qalb values (i.e., <6 and >6, <9 and >9, etc). In order to find an optimal Qalb cut-off value with the highest differences in cortical activity among groups, Mann–Whitney U test has been used in this comparison. A hypothesis was considered valid when P value was less than or equal to 0.05. Then, neuropsychological assessment scores of each patient from each group were evaluated to find differences among groups. No statistically significant differences were observed.
4. Results
Twenty-one out of the 134 subjects examined (15.6%) showed abnormal Qalb values (>9), while 3 subjects (2%) showed an abnormal IgG index (>0.7).
SPM analysis performed in AD patients documented a significant negative correlation between the increase of Qalb and 18F-FDG uptake. In particular, we documented a selective correlation linking CSF albumin levels to the reduced brain glucose consumption occurring in the BA 42 and 22 that corresponds to the left superior temporal gyrus (LSTG, Table 2, Fig. 1), with higher Qalb values being related to a reduced glucose consumption in these areas.
Table 2.
Multiple regression analysis showing the serum/CSF albumin index related areas of decreased 18F FDG brain uptake.
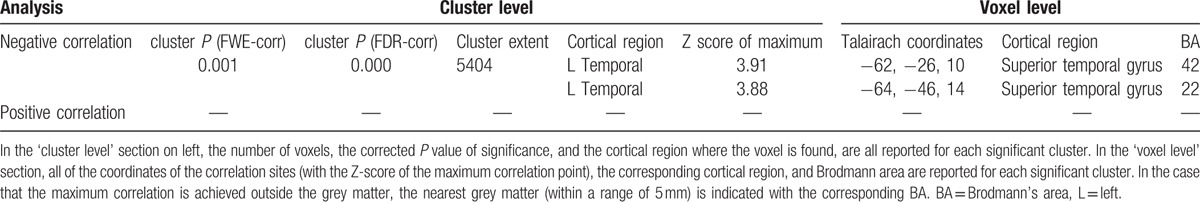
Figure 1.
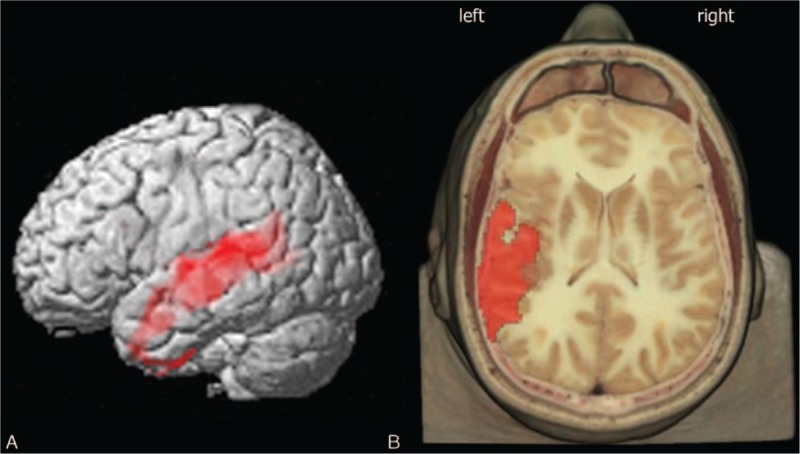
3D rendering of data presented in Table 2 in (A) showing the negative correlation between Qalb and brain glucose consumption in left temporal lobe (left superior temporal gyrus). (B) Axial anatomical brain image superimposition of the brain regions presented in Table 2 (threshold P < 0.01 corrected for multiple comparisons with false discovery rate at the voxel level). Coordinate and regional details are presented in Table 2.
Cerebellum-normalized 18F-FDG PET values for LSTG (BA 42 and 22) resulted equal to 1.05 ± 0.12 (mean ± standard deviation) and were not normally distributed (K2 = 32.2 and P < 0.001). Spearman correlation analysis showed a good correlation between albumin ratio levels and normalized 18F-FDG uptake further showing that low levels of albumin ratio were related to lower levels of 18F-FDG uptake (r = -0.22 and P = 0.009).
The highest difference in cortical 18F-FDG metabolism has been found in subjects with Qalb <6 (n = 61) as compared with those with a Qalb > 6 (n = 73). Cerebellum-normalized 18F-FDG PET values for LSTG resulted equal to 1.08 ± 0.12 and 1.02 ± 0.11, respectively (P = 0.005).
We did not find significant relationships between 18F-FDG uptake and IgG index at any explored statistical threshold in either analysis (positive or negative correlation).
Qalb was not related to Aβ42 (P = 0.81; r = 0.02), t-Tau (P = 0.64, r = 0.04), and p-Tau (P = 0.19, r = -0.11). IgG index was not related to Aβ42 (P = 0.06; r = 0.17), t-Tau (P = 0.70; r = -0.03), and p-Tau (P = 0.97; r = -0.02).
5. Discussion
The main finding of our study is a significant relationship between brain glucose consumption and Qalb in a wide portion of the left temporal lobe (LSTG, left BA 42 and 22). Unexpectedly, the more Qalb increased the worst glucose consumption appeared in that area. The gradient did not correlate with cognitive decline severity as measured with neuropsychological assessment. Mechanisms linked to the neurodegenerative process are likely to be imputed for such increase regional permeability. On one hand, physiological aging could alter the normal immune response of an individual promoting microglial activation and BBB disruption, contributing to neurodegeneration of the brain,[32,33] whereas on the other hand, Aβ42-mediated pathology could be responsible for endothelial dysfunction responsible in turn for increase in BBB permeability.[34,35] Left temporal lobe has been shown to be an important structure in the pathway involved in social cognition processes.[36] Including the superior temporal gyrus, areas more anterior and dorsal within the temporal lobe have been linked to the ability of processing information.[36] Temporal lobes are also considered preferential vulnerable sites in AD patients, whose degeneration is responsible for memory complaining of these patients.[37,38] To the best of our knowledge, very few imaging studies have been carried out to date in order to investigate the relationships between BBB and brain functions. Brain magnetic resonance imaging (MRI) study in a group of mild cognitive impairment (MCI) patients compared with control subjects found that the temporal lobes had a lower vascular space owing to suppose that this area of the brain might represent a constitutional site of vulnerability, responsible for evolutionary aspects of AD.[39] In another study, Starr et al[40] evaluated BBB dysfunction in AD and controls by means of dynamic contrast-enhanced MRI. The authors demonstrated that, in AD subjects, an initial rise in gray matter MRI signal intensity followed by a later increase was detectable thus suggesting a BBB permeability even at the early stages of AD.[40]
Although the hypothesis of a BBB impairment as an evolutionary factor in AD is intriguing, the heterogeneous results reported in literature lead to inconclusive hypothesis.[41] A positron emission tomography (PET) study with [68Ga]EDTA did not find increased BBB permeability in AD[42] and an MRI study performed to specifically examine white matter lesions in 10 demented patients (including 5 with elevated CSF/serum albumin ratios) found no evidence for BBB leakage.[43] In particular, it has been suggested that BBB impairment could affect transport of trophic substances to the CNS and the removal of toxic substances as amyloid[41] responsible for neurodegeneration. Unfortunately, BBB increased permeability was observed only in a small subgroup of AD patients, reducing the possibility to consider BBB disruption as a general mechanisms of neurodegeneration.[44]
The results of our study suggest that in a selected population of AD (∼16%), BBB dysfunction may occur and that this pattern is related to a worse metabolic pattern, as shown in Figs. 1 and 2. Interestingly, the higher difference in brain glucose consumption has been found when a cut-off of 6 in Qalb was used, that is, lower as compared with the standard used in other studies.[8] This latter finding suggests that, in AD subjects, a functional correlate of BBB dysfunction appears for relatively low levels of Qalb. Thus, further studies would be performed to deeply explore the potential impact of BBB dysfunction at the early stages of the disease even in subjects with mild cognitive impairment.
Figure 2.
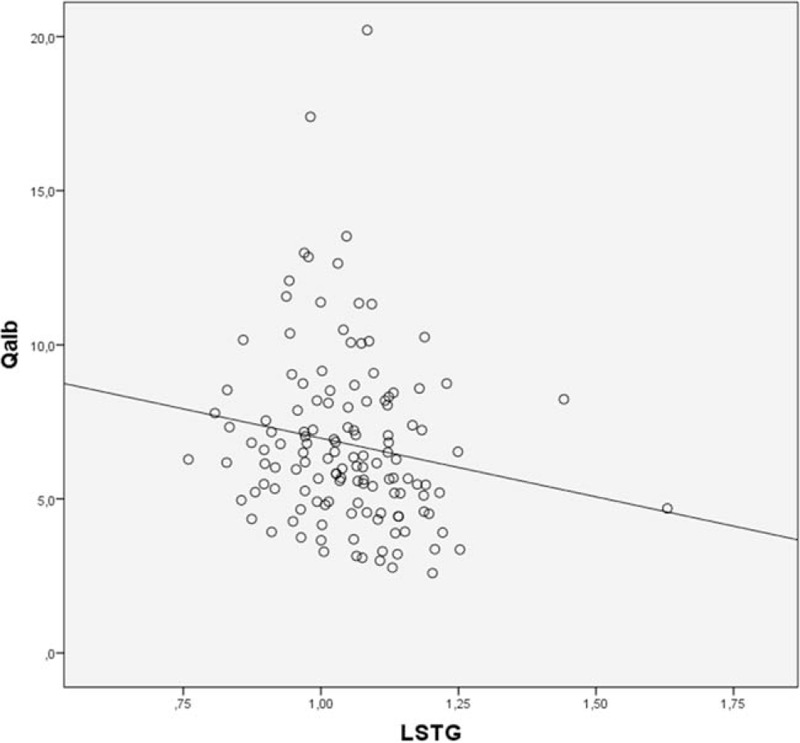
Regression analysis showing the significant relationship between normalized cortical 18F-FDG uptake in left superior temporal gyrus (LSTG) and Qalb.
Few literature evidences demonstrated that intratechal synthesis of Ig could be documented in a small subgroup of AD patients,[45–47] suggesting a potential role in neurodegeneration. In our study, however, we failed to find any significant correlation between glucose brain consumption and CSF/blood IgG index, a solid measure of intratechal Ig synthesis. However, the low prevalence of clinically significant Ig synthesis in our study population did not allow a subanalysis of this distinct category of patients. Globally taken, our data support the hypothesis that the contribution of B-cell dependent CNS inflammation could be considered minimal, at least in the vast majority of AD patients, but no inferences could be made on those with demonstrated intratechal Ig synthesis. On the other side, IgG index alone could be insufficiently sensitive to detect subtle abnormalities, such as intratechal synthesis of disease-specific cytoskeletal proteins as recently demonstrated.[48]
6. Conclusion
BBB impaired permeability in AD patients is restricted to a subset of patients not identifiable by means of neuropsychological, biomarkers, and MRI findings. Whether BBB changes might represent a site of vulnerability and a negative prognostic factor at the stage of our analysis could not be confirmed. Inconclusive and often conflicting results led us to suggest that BBB changes observed during AD might likely represent a paraphysiological phenomenon, a marker of aging process associated with the course of neurodegenerative diseases such as AD.
Footnotes
Abbreviations: 18F-FDG = 2-deoxy-2-(18F) fluoro-d-glucose, AD = Alzheimer disease, BBB = Blood–brain barrier, CSF = Cerebrospinal Fluid, IgG index = [(CSF IgG/serum IgG) × Serum albumin/CSF albumin)], PET/CT = Positron Emission Tomography/Computed Tomography, Qalb = Serum/CSF albumin index, SPM = Statistical Parametric mapping.
The authors have no conflicts of interest to disclose.
References
- 1.Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science 1992; 256:184–185. [DOI] [PubMed] [Google Scholar]
- 2.Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging 1997; 18:351–357. [DOI] [PubMed] [Google Scholar]
- 3.Hansson O, Zetterberg H, Buchhave P, et al. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol 2006; 5:228–234. [DOI] [PubMed] [Google Scholar]
- 4.Dubois B, Epelbaum S, Santos A, et al. Alzheimer disease: from biomarkers to diagnosis. Rev Neurol 2013; 169:744–751. [DOI] [PubMed] [Google Scholar]
- 5.Mattsson N, Zetterberg H. Alzheimer's disease and CSF biomarkers: key challenges for broad clinical applications. Biomark Med 2009; 3:735–737. [DOI] [PubMed] [Google Scholar]
- 6.Tibbling G, Link H, Ohman S. Principles of albumin and IgG analyses in neurological disorders. I. Establishment of reference values. Scand J Clin Lab Invest 1977; 37:385–390. [DOI] [PubMed] [Google Scholar]
- 7.Blennow K, Fredman P, Wallin A, et al. Protein analysis in cerebrospinal fluid. II. Reference values derived from healthy individuals 18–88 years of age. Eur Neurol 1993; 33:129–133. [DOI] [PubMed] [Google Scholar]
- 8.Algotsson A, Winblad B. The integrity of the blood-brain barrier in Alzheimer's disease. Acta Neurol Scand 2007; 115:403–408. [DOI] [PubMed] [Google Scholar]
- 9.Bowman GL, Kaye JA, Moore M, et al. Blood-brain barrier impairment in Alzheimer disease: stability and functional significance. Neurology 2007; 68:1809–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zimmermann R, Beck G, Knispel S, et al. Intrathecal IgG synthesis in patients with alterations in the neurochemical dementia diagnostics. J Alzheimer Dis 2010; 19:1199–1203. [DOI] [PubMed] [Google Scholar]
- 11.Henriksson A, Kam-Hansen S, Link H. IgM, IgA and IgG producing cells in cerebrospinal fluid and peripheral blood in multiple sclerosis. Clin Exp Immunol 1985; 62:176–184. [PMC free article] [PubMed] [Google Scholar]
- 12.Howell OW, Reeves CA, Nicholas R, et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain 2011; 134 (Pt 9):2755–2771. [DOI] [PubMed] [Google Scholar]
- 13.Elliott C, Lindner M, Arthur A, et al. Functional identification of pathogenic autoantibody responses in patients with multiple sclerosis. Brain 2012; 135 (Pt 6):1819–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonnan M. Does disease-irrelevant intrathecal synthesis in multiple sclerosis make sense in the light of tertiary lymphoid organs? Front Neurol 2014; 5:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leszek J, Barreto GE, Gasiorowski K, et al. Inflammatory mechanisms and oxidative stress as key factors responsible for progression of neurodegeneration: role of brain innate immune system. CNS Neurol Disord Drug Targets 2016; 15:329–336. [DOI] [PubMed] [Google Scholar]
- 16.Pagani M, De Carli F, Morbelli S, et al. Volume of interest-based [18F]fluorodeoxyglucose PET discriminates MCI converting to Alzheimer's disease from healthy controls. A European Alzheimer's Disease Consortium (EADC) study. NeuroImage Clin 2015; 7:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morbelli S, Brugnolo A, Bossert I, et al. Visual versus semi-quantitative analysis of 18F-FDG-PET in amnestic MCI: an European Alzheimer's Disease Consortium (EADC) project. J Alzheimer Dis 2015; 44:815–826. [DOI] [PubMed] [Google Scholar]
- 18.Varma AR, Snowden JS, Lloyd JJ, et al. Evaluation of the NINCDS-ADRDA criteria in the differentiation of Alzheimer's disease and frontotemporal dementia. J Neurol Neurosurg Psychiatry 1999; 66:184–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pierantozzi M, Panella M, Palmieri MG, et al. Different TMS patterns of intracortical inhibition in early onset Alzheimer dementia and frontotemporal dementia. Clin Neurophysiol 2004; 115:2410–2418. [DOI] [PubMed] [Google Scholar]
- 20.Schillaci O, Chiaravalloti A, Travascio L, et al. F-FDG PET/MR in herpes simplex virus encephalitis: a case study. Rev Esp Med Nucl Imagen Mol 2014; 2:183–192. [DOI] [PubMed] [Google Scholar]
- 21.Alessandrini M, Pagani M, Napolitano B, et al. Early and phasic cortical metabolic changes in vestibular neuritis onset. PLoS One 2013; 8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198. [DOI] [PubMed] [Google Scholar]
- 23.Schoenberg MR, Dawson KA, Duff K, et al. Test performance and classification statistics for the Rey Auditory Verbal Learning Test in selected clinical samples. Arch Clin Neuropsychol 2006; 21:693–703. [DOI] [PubMed] [Google Scholar]
- 24.Shin MS, Park SY, Park SR, et al. Clinical and empirical applications of the Rey-Osterrieth Complex Figure Test. Nat Protoc 2006; 1:892–899. [DOI] [PubMed] [Google Scholar]
- 25.Henry JD, Crawford JR, Phillips LH. Verbal fluency performance in dementia of the Alzheimer's type: a meta-analysis. Neuropsychologia 2004; 42:1212–1222. [DOI] [PubMed] [Google Scholar]
- 26.Bilker WB, Hansen JA, Brensinger CM, et al. Development of abbreviated nine-item forms of the Raven's standard progressive matrices test. Assessment 2012; 19:354–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiaravalloti A, Martorana A, Koch G, et al. Functional correlates of t-Tau, p-Tau and Abeta(1)(-)(4)(2) amyloid cerebrospinal fluid levels in Alzheimer's disease: a (1)(8)F-FDG PET/CT study. Nucl Med Commun 2015; 36:461–468. [DOI] [PubMed] [Google Scholar]
- 28.Teunissen CE, Tumani H, Bennett JL, et al. Consensus Guidelines for CSF and Blood Biobanking for CNS Biomarker Studies. Mult Scler Int 2011; 2011:246412. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Bennett CM, Wolford GL, Miller MB. The principled control of false positives in neuroimaging. Soc Cogn Affect Neurosci 2009; 4:417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmahmann JD, Doyon J, McDonald D, et al. Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. NeuroImage 1999; 10 (3 Pt 1):233–260. [DOI] [PubMed] [Google Scholar]
- 31.Soonawala D, Amin T, Ebmeier KP, et al. Statistical parametric mapping of (99m)Tc-HMPAO-SPECT images for the diagnosis of Alzheimer's disease: normalizing to cerebellar tracer uptake. NeuroImage 2002; 17:1193–1202. [DOI] [PubMed] [Google Scholar]
- 32.Ryu JK, McLarnon JG. A leaky blood-brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer's disease brain. J Cell Mol Med 2009; 13:2911–2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minogue AM, Jones RS, Kelly RJ, et al. Age-associated dysregulation of microglial activation is coupled with enhanced blood-brain barrier permeability and pathology in APP/PS1 mice. Neurobiol Aging 2014; 35:1442–1452. [DOI] [PubMed] [Google Scholar]
- 34.Kook SY, Hong HS, Moon M, et al. Abeta(1)(-)(4)(2)-RAGE interaction disrupts tight junctions of the blood-brain barrier via Ca(2)(+)-calcineurin signaling. J Neurosci 2012; 32:8845–8854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gheorghiu M, Enciu AM, Popescu BO, et al. Functional and molecular characterization of the effect of amyloid-beta42 on an in vitro epithelial barrier model. J Alzheimer Dis 2014; 38:787–798. [DOI] [PubMed] [Google Scholar]
- 36.Bigler ED, Mortensen S, Neeley ES, et al. Superior temporal gyrus, language function, and autism. Develop Neuropsychol 2007; 31:217–238. [DOI] [PubMed] [Google Scholar]
- 37.Pievani M, Rasser PE, Galluzzi S, et al. Mapping the effect of APOE epsilon4 on gray matter loss in Alzheimer's disease in vivo. NeuroImage 2009; 45:1090–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Filippini N, Rao A, Wetten S, et al. Anatomically-distinct genetic associations of APOE epsilon4 allele load with regional cortical atrophy in Alzheimer's disease. NeuroImage 2009; 44:724–728. [DOI] [PubMed] [Google Scholar]
- 39.Wang H, Golob EJ, Su MY. Vascular volume and blood-brain barrier permeability measured by dynamic contrast enhanced MRI in hippocampus and cerebellum of patients with MCI and normal controls. J Magn Reson Imaging 2006; 24:695–700. [DOI] [PubMed] [Google Scholar]
- 40.Starr JM, Farrall AJ, Armitage P, et al. Blood-brain barrier permeability in Alzheimer's disease: a case-control MRI study. Psychiatry Res 2009; 171:232–241. [DOI] [PubMed] [Google Scholar]
- 41.Erickson MA, Banks WA. Blood-brain barrier dysfunction as a cause and consequence of Alzheimer's disease. J Cereb Blood Flow Metab 2013; 33:1500–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schlageter NL, Carson RE, Rapoport SI. Examination of blood-brain barrier permeability in dementia of the Alzheimer type with [68Ga]EDTA and positron emission tomography. J Cereb Blood Flow Metab 1987; 7:1–8. [DOI] [PubMed] [Google Scholar]
- 43.Bronge L, Wahlund LO. White matter lesions in dementia: an MRI study on blood-brain barrier dysfunction. Dement Geriatr Cogn Disord 2000; 11:263–267. [DOI] [PubMed] [Google Scholar]
- 44.Skoog I, Wallin A, Fredman P, et al. A population study on blood-brain barrier function in 85-year-olds: relation to Alzheimer's disease and vascular dementia. Neurology 1998; 50:966–971. [DOI] [PubMed] [Google Scholar]
- 45.Hampel H, Kotter HU, Padberg F, et al. Oligoclonal bands and blood–cerebrospinal-fluid barrier dysfunction in a subset of patients with Alzheimer disease: comparison with vascular dementia, major depression, and multiple sclerosis. Alzheimer Dis Assoc Disord 1999; 13:9–19. [DOI] [PubMed] [Google Scholar]
- 46.Small GW, Rosenthal M, Tourtellotte WW. Central nervous system IgG synthesis rates in Alzheimer disease: possible differences in early-onset and late-onset subgroups. Alzheimer Dis Assoc Disord 1994; 8:29–37. [DOI] [PubMed] [Google Scholar]
- 47.Blennow K, Wallin A, Fredman P, et al. Intrathecal synthesis of immunoglobulins in patients with Alzheimer's disease. Eur Neuropsychopharmacol 1990; 1:79–81. [DOI] [PubMed] [Google Scholar]
- 48.Bartos A, Fialova L, Svarcova J, et al. Patients with Alzheimer disease have elevated intrathecal synthesis of antibodies against tau protein and heavy neurofilament. J Neuroimmunol 2012; 252:100–105. [DOI] [PubMed] [Google Scholar]


