Abstract
Anti-N-methyl-d-aspartate receptor (NMDAR) encephalitis is one of the most common autoimmune encephalitis that presents with a wide variety of movement disorders. The purpose of our study is to review the manifestations and duration of movement disorders in different ages with NMDAR encephalitis.
A retrospective cohort of 28 patients (20 females and 8 males) with positive cerebrospinal fluid (CSF) anti-NMDAR antibody in a 5-year period from major hospitals in Taiwan was enrolled. They were categorized into 3 age groups: 7 patients were ≤10 years, 14 patients were 10 to 18 years, and 7 patients were >18 years.
Total 28 patients (20 females and 8 males) with age ranging from 8 months to 38 years were enrolled. Nearly all patients (n = 27/28, 96%) presented with at least 2 types of disorders, including orofacial–lingual dyskinesia (OFLD; n = 20), catatonia (n = 19), tremor (n = 11), bradykinesia (n = 11), dystonia (n = 11), choreoathethosis (n = 9), and ballism (n = 3). Only 1 patient below 10 years presented with isolated periodic choreoathethosis without other movement disorders. OFLD was common in all age groups. Choreoathetosis was most common in patients aged ≤10 years, while catatonia was most common in patients aged >10 years (P = 0.001 and 0.020, respectively). Bradykinesia was also more common in patients aged >10 years (P = 0.020). The clinical presentations of movement disorders were not significantly different in the age of 10 to 18 years and those >18 years. Neither patient ≤10 years old nor male patients had associated tumors. All patients’ movement disorders were improved after treatment, while female patients with tumors had worse short-term outcome (P = 0.014). Compared with other disorders, choreoathetosis persisted significantly longer in patients ≤10 years (P = 0.038), while OFLD and catatonia last longer in patients >10 years (P = 0.047 and 0.002, respectively).
Our study shows that hyperkinetic movements such as choreoathetosis are more common and last longer in younger age groups, whereas hypokinetic movements such as catatonia and bradykinesia are more common and last longer in older age groups. Female patients with ovarian tumors had worse short-term outcome.
Keywords: anti-N-methyl-d-aspartate receptor, autoimmune, encephalitis, movement disorders, treatment
1. Introduction
Anti-N-methyl-d-aspartate receptor (NMDAR) encephalitis is one of the most common autoimmune encephalitis. It is also a treatable and reversible disease and has received much attention in the past 10 years.[1,2] It predominantly affects young women with or without ovarian teratoma.[3] The clinical manifestations of anti-NMDAR encephalitis have a multistage course[4,5]: the prodromal phase includes nonspecific symptoms of headache, fever, vomiting, and general malaise, followed by a neuropsychiatric phase presenting with unusual manifestations, agitation, delirium, or acute psychotic changes. If the disease persists without proper diagnosis or treatment, the patients can deteriorate rapidly with language impairment; memory or cognitive impairment; and decreased responsiveness accompanied by hyper or hypokinetic movement disorders such as catatonia, dystonia, and chorea. Patients may also have seizures, which can be difficult to treat and refractory to anti-epileptic medications unless the underlying disease has been appropriately treated. Some patients may develop autonomic and breathing instability or even death if there is delayed treatment or treatment failure.[5–8]
The major clinical manifestations between adults and toddlers suffering from anti-NMDAR encephalitis are different.[9,10] Psychiatric symptomatology and memory impairment is more prominent in adults, whereas abnormal behavior, movement disorders, and seizure are more frequently observed in children.[4,8,11,12] Movement disorders are a prominent feature of autoimmune encephalitis, particularly in anti-NMDAR encephalitis.[13,14] For patients with anti-NMDAR encephalitis, they may also present with a wide variety and combination of movement disorders.[4,12,13] In Dale's series, patients with anti-NMDAR encephalitis are more likely to present with stereotyped and preserved movements that are less commonly observed in other encephalitis, whereas tremor and akinesia were more dominant in other encephalitis such as basal ganglion encephalitis[13]; however, there are limited data in the literature discussing the movement disorders of anti-NMDAR encephalitis in different age groups, the duration of these disorders, and the treatment response.[4] Therefore, the purpose of our study is to investigate the differences in clinical manifestations and duration of the movement disorders between different age groups after treatment with the aim of aiding early diagnosis and treatment of this challenging disease.
2. Methods
2.1. Patients
A retrospective cohort of 28 patients with positive cerebrospinal fluid (CSF) anti-NMDAR antibody in a 5-year period from major hospitals in Taiwan was enrolled for analysis. They were categorized into 3 age groups: ≤10 years, 10 to 18 years, and >18 years according to previous experience and study that maturation of dopamine receptor levels may be possibly related to different movement disorders. The demographic data and manifestations of movement disorders were collected and analyzed. The movement disorder phenomenology is discussed according to the recognized terminology, which includes tremor, choreoathetosis, catatonia, dystonia, ballism, and orofacial–lingual dyskinesia (OFLD), and was evaluated by pediatric neurologists. A modified Rankin scale (mRS) was used to evaluate the disease severity and neurological outcome before and 6 months after treatment. The institutional review board of National Taiwan University Hospital had approved the retrospective study.
2.2. Statistical Analysis
The frequency of movement disorders, duration of treatment response, and outcomes were collected and compared between the groups. The Fisher exact tests for categorical variables and Mann–Whitney tests were used to compare groups. P < 0.05 was considered statistically significant. All statistical analyses were performed using Stata 13 (StataCorp. 2013. Stata Statistical Software: Release 13; StataCorp LP, College Station, TX).
3. Results
3.1. Demographic data
A total of 28 patients (20 females and 8 males) with ages ranging from 8 months to 38 years (mean age 15.4 ± 8.8 years, median 15 years) were enrolled. Among these patients, 7 were <10 years old (mean age 4.0 ± 2.8 years, median 3.1 years), 14 patients were within the 10 to 18 years range (mean age 14.6 ± 1.8 years, median 14.5 years), and 7 patients were >18 years old (mean age 26.7 ± 6.4 years, median 23). There were no associated tumors in patient aged ≤10 years and male patients. Only female patients aged >10 years had associated ovarian tumors, including 4 patients (29%) in 10 to 18 years range and 3 (43%) in those aged >18 years (Table 1).
Table 1.
Characteristics of anti-NMDAR encephalitis patients: demographic data, movement disorders manifestation and treatment protocols.
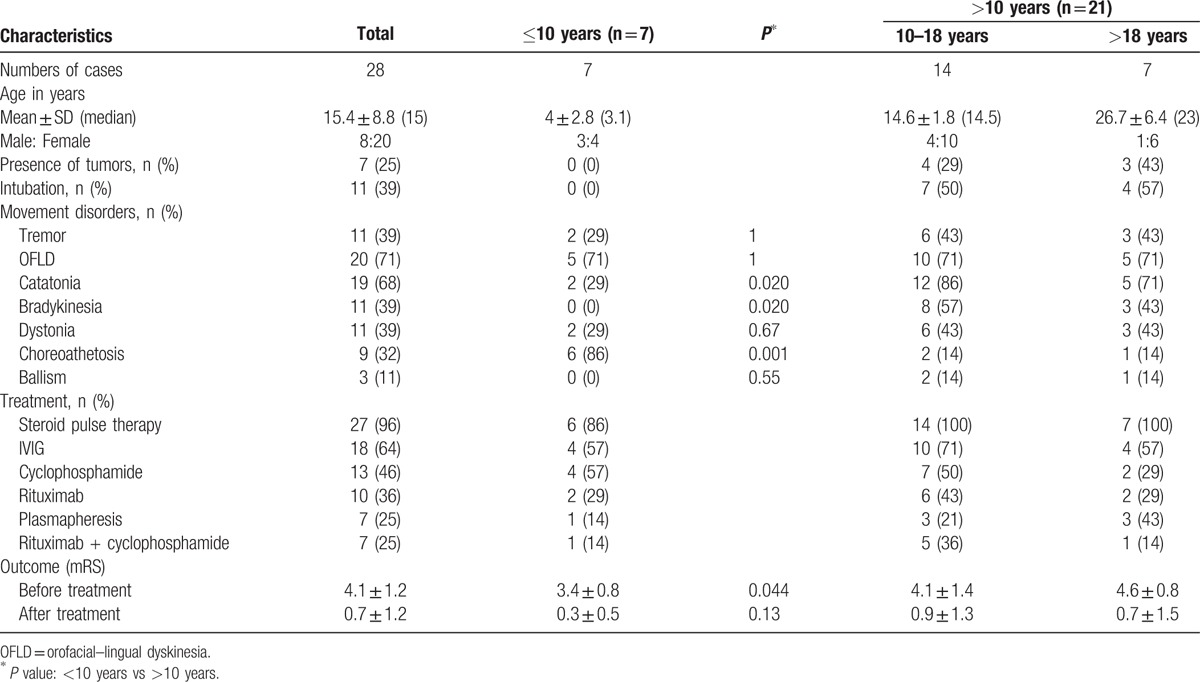
3.2. Features of movement disorders
A summary of the movement disorders in different age groups is summarized in Table 1 and Fig. 1. Nearly all patients (n = 27/28, 96%) in our study presented with at least 2 types of movement disorders, including OFLD (n = 20, 71%), catatonia (n = 19, 68%), tremor (n = 11, 39%), bradykinesia (n = 11, 39%), dystonia (n = 11, 39%), choreoathethosis (n = 9, 32%), and ballism (n = 3, 11%). Only 1 female patient who was 6 years old presented with isolated periodic choreoathethosis without other movement disorders. The most common manifestations of movement disorders in children aged <10 years were choreoathetosis (86%) and OFLD (71%), followed by tremor (29%), dystonia (29%), and catatonia (29%). Catatonia (86%) was the most common feature in children of age 10 to 18 years, followed by OFLD (71%), bradykinsesia (57%), tremor (43%), dystonia (43%), choreoathetosis (14%), and ballism (14%). In contrast, OFLD (71%) and catatonia (71%) were more common in those aged >18 years, followed by tremor (43%), bradykinesia (43%), dystonia (43%), choreoathetosis (14%), and ballism (14%). Only patients aged >10 years had manifestations of ballism and bradykinesia. The manifestations of movement disorders did not reveal a statistically significant difference between the 10 to 18 years group and the group aged >18 years. We attempted to investigate whether there was any statistically significant difference between patients aged ≤10 years and patients aged >10 years, and found that the hyperkinetic movements of choreoathetosis (n = 6/7, 86%) was significantly more common in patients aged ≤10 years as compared with those aged >10 years (P = 0.001). In contrast, the hypokinetic movements of catatonia and bradykinesia were significantly more common in patients aged >10 years as compared with those aged ≤10 years (P = 0.020; Table 1). OFLD, a striking feature with stereotypical pouting and chewing oral movements as well as a repetitive grimacing face, was commonly observed in all age groups (71%) without statistically significant differences (Table 1).
Figure 1.
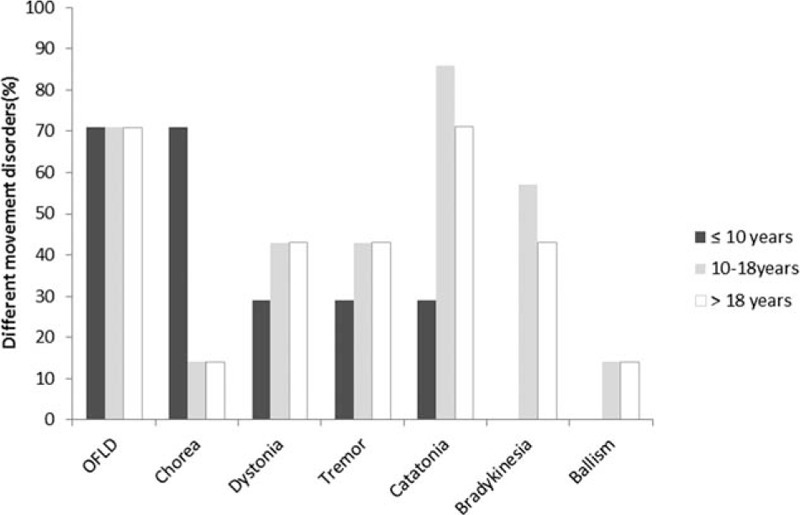
Percentage of different movement disorders in different age groups. OFLD = orofacial–lingual dyskinesia.
3.3. Treatment and outcomes
A total of 11 out of 21 (52%) patients aged >10 years required intubation and ventilator support due to autonomic dysfunction and severe dystonia before treatment. They included 7 (50%) patients in the 10 to 18 years group and 4 (57%) patients in the >18 years group. No patients aged <10 years needed ventilator support during hospitalization. Among patients requiring respiratory support, most are female (n = 8/11, 73%) and 6 of them also had associated tumors. Therefore, 6 out of 7 patients with associated tumors required intubation (n = 6/7, 86%).
Nearly all patients (n = 27, 96%) had received 5 days of steroid pulse therapy as the first-line treatment. Of these, more than half of the patients (n = 18, 64%) still required intravenous immunoglobulin treatment after steroid therapy. Only 7 patients (25%) received plasmapheresis during the entire clinical course and only 1 was aged ≤10 years. Among second-line therapies, cyclophosphamide (n = 13, 46%) was more commonly used than rituximab (n = 10, 36%). Approximately 25% (n = 7) of patients received both cyclophosphamide and rituximab, and all of these patients were female. A total of 6 out of these 7 patients were aged >10 years and only 1 was aged ≤10 years. There was no mortality in our study. Approximately 25% (n = 7/28) of female patients had associated ovarian tumors, and all had received tumor resection after diagnosis of the tumor. One patient had symptom relapse twice with tumor recurrence during the follow-up period and received bilateral oophorectomy due to disease progression. In those with tumors, nearly all (n = 6/7, 86%) had to receive at least 1 of the second-line therapies, and 3 patients (n = 3/7, 43%) had to use 2 second-line therapies.
We then used mRS to evaluate the disease severity before and 6 months after treatment. Disease severity was reported mild for mRS of 0 to 1, moderate for 2 to 3, and severe for 4 to 5. The mean mRS before treatment was 4.1 ± 1.2, which decreased to 0.7 ± 1.2 after treatment. The mean mRS before treatment in patients aged <10 years was significantly lower than that in those aged >10 years (3.4 ± 0.8 vs 4.3 ± 0.8, P = 0.044). There was no significant difference in mRS at 6 months after treatment for patients aged >10 years and those aged <10 years (P = 0.13).
In female patients with tumors (n = 7), only 1 patient's initial disease severity was moderate (mRS = 3) and the remaining 6 patients were all severe (mRS = 5); however, after treatment, including tumor removal, 3 patients had symptoms that completely resolved with an mRS of 0, 1 had minimal symptoms with an mRS of 1, 1 had moderate symptoms with an mRS of 3, and the remaining 2 patients had severe sequelae with an mRS of 4. In patients aged >10 years without tumors (n = 14), 1 patient's initial symptoms were mild, 3 were moderate, and 10 were severe before treatment. Six months after treatment, 9 had an mRS of 0, 4 had an mRS of 1, and 1 had an mRS of 2. Therefore, in our series, the short-term outcome was better for those without tumors than for those with tumors (P = 0.014).
In patients aged <10 years, 4 patients’ initial mRS was 4, 2 patients’ mRS was 3, and the remaining 1 was 1 before treatment. A total of 6 months after treatment, only 2 patients had minimal symptoms with mRS of 1 and the other 5 were 0.
3.4. Duration of different movement disorders
The duration of movement disorders in anti-NMDAR encephalitis was different in different age groups (Table 2). Compared with patients aged <10 years, OFLD and catatonia last significantly longer in patients aged >10 years (0.68 ± 0.47 vs 1.49 ± 1.60, 0.11 ± 0.20 vs 1.96 ± 2.40; P = 0.047 and 0.002, respectively). Only 2 cases aged <10 years had catatonia, and both last for <2 weeks. In contrast, the duration of choreoathetosis is significantly longer in patients aged <10 years compared with that in those aged >10 years (1.93 ± 1.72 vs 0.20 ± 0.60, P = 0.038). Only patients aged >10 years had bradykinesia and ballism (2.27 ± 1.92 and 0.67 ± 0.24 months, respectively). There was no statistically significant difference in the duration of tremor in both groups. Although there was also no statistically significant difference in the duration of dystonia, there is a trend for dystonia lasting longer in patients aged >10 years.
Table 2.
Duration of different movement disorders in months at different age groups.
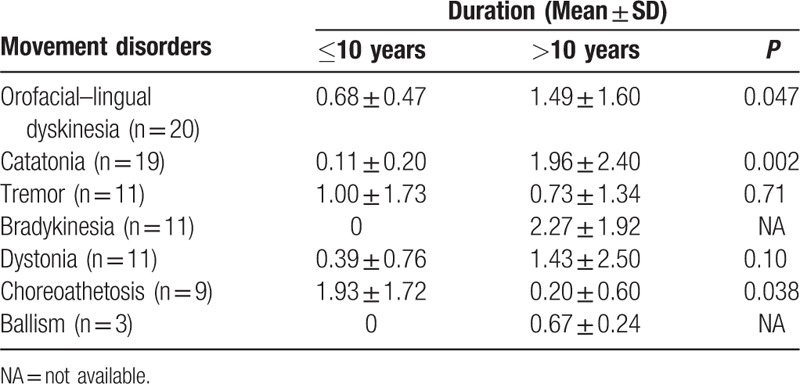
Of these movement disorders, bradykinesia and catatonia last significantly longer than ballism and choreoathetosis in patients aged >10 years; however, in patients aged <10 years, choreoathetosis lasts significantly longer than other movement disorders (1.93 ± 1.72 months, P < 0.05).
4. Discussion
Movement disorders have been reported in anti-NMDAR encephalitis in both adults and children, with prevalence rates of 86% and 84%, respectively.[4,5,11,12,14] According to a Spanish cohort study, all children with anti-NMDAR encephalitis had involuntary movements, and these movements were present in 30% of patients at the time of presentation.[11] Our study illustrated that all patients with anti-NMDAR encephalitis had a broad array of movement disorders, and nearly 70% of patients presented with at least 2 different features such as OFLD, tremor, catatonia, dystonia, bradykinesia, ballism, and choreoathetosis.[4] The most common movement disorder in our study was OFLD. Approximately 71% (n = 20/28) of the patients presented with this manifestations and had a similar proportion in children, adolescents, and adults. This feature was also the most common feature in previous studies with 85% in adults and 45% reported in children.[12,13]
OFLD has previously been established as a paraneoplastic immune-mediated neurological disorder, mostly occurring in patients with ovarian tumors, and may also occur without a detectable tumor.[15,16] In our study, only 25% (n = 7/28) of female patients aged >10 years had associated ovarian tumors. Although there was no associated tumor in patients ≤10 years old, OFLD was still the most prominent feature in this age group (71%, n = 5/7). Previous hypothesis of paraneoplastic immune-mediated mechanisms for OFLD reminds us that patients with this feature need to be followed up seriously for possibility of associated tumor. Various neurochemical hypotheses have been proposed for the development of OFLD, including hypersensitivity of the dopaminergic system, the instability between dopamine and cholinergic systems, impairments of striatonigral GABAergic neurons, and excitotoxicity.[17] According to recent reports, the anti-NMDAR antibodies may reversibly suppress the gathering of the NMDA receptors on cell membrane. Therefore, the inhibition of the NMDA receptors on presynaptic GABAergic interneurons by antibodies may decrease the release of GABA. This may cause the loss of inhibition of postsynaptic glutamatergic transmission and massive release of glutamate in the prefrontal/subcortical areas. Therefore, loss of fronto-striatal inhibition, or loss of cortico-limbic control over the hypothalamus and brainstem, may play a role.[13]
Our study also showed that approximately 86% (n = 6/7) of patients aged ≤10 years had choreoathetosis, but only 3 patients (14%, n = 3/21) aged >10 years had choreoathetosis. Eight of them presented with persistent choreoathetosis, whereas 1 patient, who was 6 years old, presented with isolated symptoms of periodic athetosis without other associated movement disorders; the athetosis lasted for only 2 weeks. Therefore, choreoathetosis may also present periodically in anti-NMDAR encephalitis. In contrast, there were no patients aged ≤10 years presenting with bradykinesia and only 2 patients presenting with catatonia, whereas in patients aged >10 years, catatonia and bradykinesia were the most prominent movement disorders. Neurological diseases affecting the basal ganglia, wherein the expression of NR1/NR2 receptors and D1,D2 receptors is relatively high, may cause failure to facilitate desired movements causing bradykinesia, or failure to inhibit undesired movements causing hyperkinetic movements such as chorea.[11,18–21] Specific types of movement disorders may represent dysfunction of particular localized regions of the brain.[20,22,23] Chorea, particularly dominant in children with anti-NMDAR encephalitis, typically occurs in patients with injury to the subthalamic nucleus, but it can also develop in patients with widespread cerebral injury.[4,24] In contrast, bradykinesia most likely occurred with injury to the substantia nigra or striatum, which may result in presynaptic or postsynaptic failure of dopaminergic transmission.[18,25] Therefore, dysfunction in basal ganglion-cortical loops due to anti-NMDAR antibodies might lead to different movement disorders in different patients. Furthermore, the densities of D1 and D2 receptors may decrease with increasing age and may be distributed heterogeneously within the brain; the developmental changes of dopaminergic receptors may possibly lead to the different presentations of movement disorders in different age groups.[21,26,27] Catatonia, one of the specific features in anti-NMDAR encephalitis, increases with age from childhood to adolescence to adulthood and is caused by impairment of the cortical GABA-ergic system.[28] Furthermore, the reduction of the D1 and D2 receptors with increasing age potentially causes catatonia.[29] Although the exact pathogenic mechanisms for different movement disorders remain unknown, we speculate that it might be related to different levels of the receptor distribution, variation of receptor sensitivity, dysfunction of different regions of basal ganglion at different ages, or an age-dependent reduction in dopamine receptors.[26,27]
Nearly all of the patients had good treatment response to immunotherapy with either the single first-line therapy or with a combination of both the first and second-line treatments. Although different movement disorders took variable times to recover, there were no statistically significant differences between the age groups studied. In our study, we observed that some older patients (>10 years) had more severe movement disorders and needed to be treated more aggressively than patients in younger age groups; however, all patients improved after second-line immunotherapy with both cyclophosphamide and rituximab. Only 1 patient relapsed during the follow-up. Rituximab reacts with B cells leading to B cell depletion, which then downregulates the antigen-presenting cells and cytokine-producing cells participating in the immune response.[30] Cyclophosphamide can modulate the immune system by reacting with both B and T cells. Therefore, in severe and refractory cases, second-line therapy with both rituximab and cyclophosphamide may provide higher efficacy than monotherapy with either cyclophosphamide or rituximab alone.[31,32]
Previous studies have shown that female patients with ovarian tumors had better outcomes and good responses to the immunotherapy after tumor removal[5,9]; however, our study found that female patients with ovarian tumors did not actually have favorable short-term outcomes or good responses to treatment compared with other without tumors. They had severe initial presentations and had more chances to receive second-line therapy than male patients. Furthermore, the only relapsed patient in our study was a single female patient with a teratoma, and she still had moderate residual symptoms with an mRS of 3 after tumor removal and bilateral oophorectomy.
Ovarian tumors are more common in female patients aged >10 years with an incidence of 58%, 31%, and 15%, for patients aged >18, <18, and <14 years, respectively, but is less common in patients aged <10 and in male patients.[3,5,33] Although the exact mechanism remains unknown, we speculate that the hormonal effect in reproductive females secondary to the estrogenic signaling in the development of ovarian teratoma might have some correlation with it.[34] With the possible hormonal effects, in anti-NMDAR encephalitis, paraneoplastic tumor seemed to be more prominent in the reproductive-female age groups, and became less observed in the nonreproductive groups and male patients.[35–37] However, because our case number is small, it is difficult to conclude whether the hormone has a possible effect upon gender difference in NMDAR encephalitis.
5. Conclusion
Anti-NMDAR encephalitis is a treatable and reversible disease if diagnosed early, and the treatment response largely depends on the timing of the treatment plan.[2,15,36,37] Although OFLD was prevalent in both age groups, it lasts longer in patients aged >10 years. In contrast, patients aged ≤10 years tended to have choreoathetosis, while patients aged >10 years tended to have catatonia and bradykinesia. Our study also provided more information about the duration of different movement disorders, thereby helping clinicians gain confidence in the management of the disease.
Acknowledgment
The authors would like to thank all the physicians in Taiwan anti-NMDAR encephalitis study group, who helped us in collecting the clinical data.
Footnotes
Abbreviations: CSF = cerebrospinal fluid, mRS = modified Rankin scale, NMDAR = N-methyl-d-aspartate receptor, OFLD = orofacial–lingual dyskinesia.
B-C D, W-C W, and K-L L contributed equally to the paper.
The authors disclose no conflicts of interest.
References
- 1.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013; 12:157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wandinger KP, Saschenbrecker S, Stoecker W, et al. Anti-NMDA-receptor encephalitis: a severe, multistage, treatable disorder presenting with psychosis. J Neuroimmunol 2011; 231:86–91. [DOI] [PubMed] [Google Scholar]
- 3.Hsu MH, Huang CC, Hung PL, et al. Paraneoplastic neurological disorders in children with benign ovarian tumors. Brain Develop 2014; 36:248–253. [DOI] [PubMed] [Google Scholar]
- 4.Baizabal-Carvallo JF, Stocco A, Muscal E, et al. The spectrum of movement disorders in children with anti-NMDA receptor encephalitis. Mov Disord 2013; 28:543–547. [DOI] [PubMed] [Google Scholar]
- 5.Dalmau J, Lancaster E, Martinez-Hernandez E, et al. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 2011; 10:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas A, Rauschkolb P, Gresa-Arribas N, et al. Anti-N-methyl-D-aspartate receptor encephalitis: a patient with refractory illness after 25 months of intensive immunotherapy. JAMA Neurol 2013; 70:1566–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barry H, Byrne S, Barrett E, et al. Anti-N-methyl-d-aspartate receptor encephalitis: review of clinical presentation, diagnosis and treatment. BJPsych Bull 2015; 39:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldberg EM, Titulaer M, de Blank PM, et al. Anti-N-methyl-D-aspartate receptor-mediated encephalitis in infants and toddlers: case report and review of the literature. Pediatr Neurol 2014; 50:181–184. [DOI] [PubMed] [Google Scholar]
- 9.Armangue T, Titulaer MJ, Malaga I, et al. Pediatric anti-N-methyl-D-aspartate receptor encephalitis-clinical analysis and novel findings in a series of 20 patients. J Pediatr 2013; 162:850–856.e852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raha S, Gadgil P, Sankhla C, et al. Nonparaneoplastic anti-N-methyl-d-aspartate receptor encephalitis: a case series of four children. Pediatr Neurol 2012; 46:246–249. [DOI] [PubMed] [Google Scholar]
- 11.Hacohen Y, Dlamini N, Hedderly T, et al. N-methyl-D-aspartate receptor antibody-associated movement disorder without encephalopathy. Develop Med Child Neurol 2014; 56:190–193. [DOI] [PubMed] [Google Scholar]
- 12.Florance NR, Davis RL, Lam C, et al. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol 2009; 66:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohammad SS, Fung VS, Grattan-Smith P, et al. Movement disorders in children with anti-NMDAR encephalitis and other autoimmune encephalopathies. Mov Disord 2014; 29:1539–1542. [DOI] [PubMed] [Google Scholar]
- 14.Baizabal-Carvallo JF, Jankovic J. Movement disorders in autoimmune diseases. Mov Disord 2012; 27:935–946. [DOI] [PubMed] [Google Scholar]
- 15.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008; 7:1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poloni C, Korff CM, Ricotti V, et al. Severe childhood encephalopathy with dyskinesia and prolonged cognitive disturbances: evidence for anti-N-methyl-D-aspartate receptor encephalitis. Develop Med Child Neurol 2010; 52:e78–e82. [DOI] [PubMed] [Google Scholar]
- 17.Kulkarni SK, Naidu PS. Pathophysiology and drug therapy of tardive dyskinesia: current concepts and future perspectives. Drugs Today (Barcelona, Spain: 1998) 2003; 39:19–49. [DOI] [PubMed] [Google Scholar]
- 18.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci 1989; 12:366–375. [DOI] [PubMed] [Google Scholar]
- 19.Mink JW. The basal ganglia and involuntary movements: impaired inhibition of competing motor patterns. Arch Neurol 2003; 60:1365–1368. [DOI] [PubMed] [Google Scholar]
- 20.Berardelli A. Symptomatic or secondary basal ganglia diseases and tardive dyskinesias. Curr Opin Neurol 1995; 8:320–322. [PubMed] [Google Scholar]
- 21.Palacios JM, Camps M, Cortés R. Obeso JA, Horowski R, Marsden CD, et al. Mapping dopamine receptors in the human brain. Continuous Dopaminergic Stimulation in Parkinson's Disease. Vol 27. Vienna: Springer; 1988. 227–235. [DOI] [PubMed] [Google Scholar]
- 22.Herrero MT, Barcia C, Navarro JM. Functional anatomy of thalamus and basal ganglia. Childs Nerv Syst 2002; 18:386–404. [DOI] [PubMed] [Google Scholar]
- 23.Sanger TD. Pathophysiology of pediatric movement disorders. J Child Neurol 2003; 18 Suppl 1:S9–S24. [DOI] [PubMed] [Google Scholar]
- 24.Salvucci A, Devine IM, Hammond D, et al. Pediatric anti-NMDA (N-methyl D-aspartate) receptor encephalitis. Pediatr Neurol 2014; 50:507–510. [DOI] [PubMed] [Google Scholar]
- 25.Hallett M. THe neurophysiology of dystonia. Arch Neurol 1998; 55:601–603. [DOI] [PubMed] [Google Scholar]
- 26.Jucaite A, Forssberg H, Karlsson P, et al. Age-related reduction in dopamine D1 receptors in the human brain: from late childhood to adulthood, a positron emission tomography study. Neuroscience 2010; 167:104–110. [DOI] [PubMed] [Google Scholar]
- 27.Rinne JO, Lönnberg P, Marjamäki P. Age-dependent decline in human brain dopamine D1 and D2 receptors. Brain Res 1990; 508:349–352. [DOI] [PubMed] [Google Scholar]
- 28.Northoff G. Catatonia and neuroleptic malignant syndrome: psychopathology and pathophysiology. J Neural Transm 2002; 109:1453–1467. [DOI] [PubMed] [Google Scholar]
- 29.Carroll BT. The universal field hypothesis of catatonia and neuroleptic malignant syndrome. CNS Spect 2000; 5:26–33. [DOI] [PubMed] [Google Scholar]
- 30.Binstadt BA, Caldas AMC, Turvey SE, et al. Rituximab therapy for multisystem autoimmune diseases in pediatric patients. J Pediatr 2003; 143:598–604. [DOI] [PubMed] [Google Scholar]
- 31.Nosadini M, Mohammad SS, Ramanathan S, et al. Immune therapy in autoimmune encephalitis: a systematic review. Expert Rev Neurotherap 2015; 15:1391–1419. [DOI] [PubMed] [Google Scholar]
- 32.Wang H. Efficacies of treatments for anti-NMDA receptor encephalitis. Front Biosci (Landmark edition) 2016; 21:651–663. [DOI] [PubMed] [Google Scholar]
- 33.Sartori S, Nosadini M, Cesaroni E, et al. Paediatric anti-N-methyl-D-aspartate receptor encephalitis: the first Italian multicenter case series. Eur J Paediatr Neurol 2015; 19:453–463. [DOI] [PubMed] [Google Scholar]
- 34.Hung YC, Chang WC, Chen LM, et al. Non-genomic estrogen/estrogen receptor alpha promotes cellular malignancy of immature ovarian teratoma in vitro. J Cell Physiol 2014; 229:752–761. [DOI] [PubMed] [Google Scholar]
- 35.Lazar-Molnar E, Tebo AE. Autoimmune NMDA receptor encephalitis. Clin Chim Acta 2015; 438:90–97. [DOI] [PubMed] [Google Scholar]
- 36.Kurian M, Fluss J, Korff C. Anti-NMDA receptor encephalitis: the importance of early diagnosis and aggressive immunotherapy in tumor negative pediatric patients. Eur J Paediatr Neurol 2012; 16:764–765. [DOI] [PubMed] [Google Scholar]
- 37.Mann AP, Grebenciucova E, Lukas RV. Anti-N-methyl-D-aspartate-receptor encephalitis: diagnosis, optimal management, and challenges. Therap Clin Risk Manag 2014; 10:517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]


