Abstract
The recently discovered long noncoding RNAs have the potential to regulate many biological processes, which are aberrantly expressed in many tumor types. Our previous study showed that the long noncoding RNA-growth arrest-specific transcript 5 (GAS5) was decreased in lung cancer tissue, which contributed to the proliferation and apoptosis of nonsmall cell lung cancer (NSCLC). GAS5 was also associated with the prognosis of lung cancer patients. These results suggest that GAS5 may represent a novel prognostic indicator and a target for gene therapy in NSCLC. However, the expression and diagnosis significance of GAS5 in the plasma of NSCLC patients was unknown. The plasma samples were more readily available than the tissue samples in clinical, so we designed the study to investigate the diagnosis value of GAS5 in blood samples. In our study, 90 patients with NSCLC and 33 healthy controls were included. Blood samples were collected before surgery and therapy. We extracted the free RNA in the plasma and analyzed the expression of GAS5 with quantitative reverse transcription PCR. Suitable statistics methods were used to compare the plasma GAS5 levels of preoperative and postoperative plasma samples between the NSCLC patients and healthy controls. Receiver-operating characteristic curve analysis was used to evaluate the diagnostic sensitivity and specificity of plasma GAS5 in NSCLC. The results showed that GAS5 was detectable and stable in the plasma of NSCLC patients. Furthermore, the plasma levels of GAS5 were significantly down-regulated in NSCLC patients compared with healthy controls (P = 0.000). Moreover, GAS5 levels increased markedly on the seventh day after surgery compared with preoperative GAS5 levels in NSCLC patients (P = 0.003). GAS5 expression levels could be used to distinguish NSCLC patients from control patients with an area under the curve of 0.832 (P < 0.0001; sensitivity, 82.2%; specificity, 72.7%). The combination of the GAS5 and carcinoembryonic antigen could produce an area of 0.909 under the receiver-operating characteristic curve in distinguishing NSCLC patients from control subjects (95% confidence interval 0.857–0.962, P = 0.000). We have demonstrated that GAS5 expression was decreased in NSCLC Plasma. Plasma samples were more accessible than tissue samples in clinical; therefore, GAS5 could be an ideal biomarker for the diagnosis of NSCLC.
Keywords: biomarker, diagnostic, GAS5, long noncoding RNA, nonsmall cell lung cancer, plasma
1. Introduction
Lung cancer is the most commonly diagnosed cancer, and also the leading major cause of cancer death in the world.[1] Nonsmall cell lung cancer (NSCLC) accounts for approximately 80% of all lung cancer, which is organized into 2 types—adenocarcinoma (ADC) and squamous cell carcinoma (SCC). It has been predicted that NSCLC could remain a major health problem for at least the next 50 years.[2] Although advancements in diagnosis and treatment have promoted the survival of patients, the 5-year survival rate is no higher than 15% after the initial diagnosis, and most of the patients had no obvious clinical symptoms until disease progression.[3] In recent years, the early diagnosis of lung cancer has been based on fiber endoscopy of the bronchus, organizing cytology, computed tomography (CT), and other imaging techniques. The low sensitivity of sputum cytology is a problem for early detection of NSCLC.[4] Although CT can detect the noninvasive early pulmonary nodules, its false-positive rate is high, which would lead to unnecessary surgeries.[5] The most widely used classical serum biomarkers for lung cancer screening are carcinoembryonic antigen (CEA), cytokeratin-19 fragment (CA199), neuron-specific enolase (NSE), cancerantigen-125 (CA125), and cyfra21-1.[6] Based on the studies, the sensitivity and specificity of these markers range between 50% and 90%; however, the low sensitivity and specificity of these markers have limited their clinical diagnostic value.[7] Thus, to develop noninvasive techniques for the diagnosis of early-stage NSCLC is clinically important. Plasma samples are easily acquired in a relatively noninvasive manner compared with biopsy or surgery.
Long noncoding RNAs (lncRNAs), which are defined as transcribed RNA molecules greater than 200 nucleotides in length and that are not translated into a protein, could regulate gene expression by diverse mechanisms.[8] Many subsequent studies have shown that lncRNAs regulate many biological processes, such as transcription, translation, cellular differentiation, gene expression regulation, cell cycle regulation, and chromatin modification.[9–11] lncRNAs are frequently regulated by epigenetic mechanisms and nuclear transport, which are implicated in transcriptional regulation of gene expression in biological processes.[12] Increasing evidence has shown that lncRNAs are abnormally expressed in human cancers, suggesting that lncRNAs may act as tumor suppressors or oncogenes. For example, tumor-suppressor lncRNAs are generally expressed at low levels.[13,14] In recent years, lncRNAs have led to the development of a new field for molecular diagnosis of cancer. It has been reported that, in human plasma, lncRNAs exist in a stable form that can be isolated under various harsh conditions, including multiple freeze–thaw cycles, and incubation of plasma at room temperature or at 45°C.[15] More importantly, blood-based tests are low-risk and noninvasive, and can be explored for potential biomarkers as well. Accumulating reports suggest that circulating lncRNAs are present in blood and have the potential as biomarkers for a variety of tumors, including NSCLC.[16–20] Moreover, several lncRNAs, such as MALAT1, was identified to be associated with the progression of cancer. MALAT1 was demonstrated to be significantly associated with metastasis in NSCLC patients. The high expression of MALAT1 was highly predictive of a poor prognosis in early-stage NSCLC.[21] These findings strongly suggest a decisive role of lncRNAs in the molecular etiology of lung cancer. These studies compelled us to investigate the lncRNAs in the blood samples as biomarker of cancer.
Growth arrest-specific transcript 5 (GAS5) was originally isolated from mouse NIH3T3cells using subtractive hybridization.[22] As a small nucleolus RNA (snoRNA), GAS5 is an encoded at locus 1q25 and is approximately 630 nt in length, which has 12 exons and 10 box C/D snoRNAs within its introns, and a member of the 5′-terminaloligopyrimidine (5′TOP) gene family.[23] The GAS5 transcript is increased during growth arrest induced by either serum starvation or treatment with translation inhibitors.[24,25] Accumulating evidence emerged showing GAS5 is down-regulated in many other cancers and functions as a tumor suppressor.[12,26,27] Interestingly, our previous studies showed that GAS5 levels were significantly decreased in NSCLC tissues and were related to tumor size and TNM stage. The overexpression of GAS5 transcript induced growth arrest and apoptosis in human lung cancer cell lines. Meanwhile, the knockdown of GAS5 promoted tumor cell growth.[28] These results indicated that GAS5 might also be a potential biomarker of NSCLC.
Therefore, we set out to investigate whether GAS5 could be detected in plasma and used as non-invasive biomarkers for NSCLC.
2. Materials and methods
2.1. Clinical specimens
A total of 90 patients with NSCLC (age 61.47 ± 10.51 years) and 33 healthy people (age 42.73 ± 14.72 years) were recruited from Jinling Hospital (Nanjing, China) from March to November 2014. In these NSCLC patients, 11 postoperative pairing plasma samples were also collected in the seventh day after surgery. Another 79 cancer patients had not been treated by surgery, chemotherapy, or radiation therapy before blood collection. Thirty-three control samples were collected from healthy donors without cancer. The study protocol was approved by the Institutional Review Board of Nanjing University. All of the participants signed an informed consent form. Tumor staging was performed according to the TNM classification of malignant tumors. All cases were diagnosed via pathological analysis by 2 experienced pathologists. The clinical and pathological data of the NSCLC patients listed in the study are displayed in Table 1.
Table 1.
Descriptive statistics of patient characteristics and cancer-free controls.
2.2. Blood sample
All samples were processed within 2 hours after collection as follows: approximately 2 mL of venous blood was collected from each participant. The whole blood samples were separated into plasma cell-free nucleic acids in ethylenediaminetetraacetic acid tubes by centrifugation at 1200g for 10 minutes at 4°C to spin down blood cells, followed by centrifugation at 12000g for 10 minutes at 4°C to completely remove cellular components or cell debris. The supernatant plasma was then carefully collected, and the samples were stored at −80°C until further analyses.
2.3. RNA isolation
The total RNA was isolated from supernatant plasma using a mirVanaParis Kit (Ambion 1556), according to the manufacturers’ protocol. Briefly, 400 μL plasma was used to extract total RNA. Each sample was eluted in 35 μL of RNase-free water or preheated elution buffer by using Eppendorf Concentrator Plus 5301 (Eppendorf, Germany). The absorbance at 260/280 (RNA/DNA) and 260/230 (RNA/Protein) was assessed by NanoDropTM 1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). All purified RNA samples were stored at −80°C until use.
2.4. Quantitative reverse-transcriptase polymerase chain reaction analyses
The isolated RNA was reverse-transcribed into cDNA using a reverse transcription kit (Takara, Dalian, China). Retrieved RNA concentration was further calculated and normalized with RNase-free water during cDNA synthesis. GAS5 lncRNA was quantified 3 times by quantitative reverse transcription PCR (qRT-PCR) using SYBR Premix Ex Taq (Takara, Dalian, China), according to the manufacturer's instructions. For the qRT-PCR reaction, 2 μL cDNA was used as template. To ensure the accuracy of the amplifications reactions, we included a nontemplate.
The gene-specific primers were as follows: GAPDH sense: 5′-GTCAACGGATTTGGTCTGTATT-3′, GAPDH reverse: 5′-AGTCTTCTGGGTGGCAGTGAT-3′, GAS5 sense: 5′-CTTGCCTGGACCAGCTTAAT-3′, GAS5 reverse: 5′-CAAGCCGACTCTCCATACCT-3′.
Samples were analyzed in duplicate. All the reactions were carried out on an ABI7500 real-time PCR system (Applied Bio systems, Foster City, CA) according to the manufacturer's instructions. The PCR amplification was performed as follows: an initial denaturation at 95°C for 30 seconds, followed by 45 cycles at 95°C for 5 seconds and 60°C for 34 seconds. The relative quantification of GAS5 expression was calculated using the ΔCt method, and ΔCt = Ct (GAS5) − Ct (GAPDH), GAPDH expression is used as the standard. Lower ΔCt values indicate higher expression of GAS5. The value of CEA was detected by radioimmunoassay method in the Department of Clinical laboratory in Jinling Hospital (cut-off value is 9.7 ng/mL). The index tests and reference standard were blind in the course of detection. No adverse events appeared from performing the index tests. All the results were expressed as the means ± SD of 3 independent experiments.
2.5. Statistical analysis
The GAS5 expression levels were compared using the Mann–Whitney U test, and the Wilcoxon t test was used to compare the paired plasma samples obtained from preoperative and postoperative patients. The Spearman correlation analysis was performed to compare the GAS5 expressions in NSCLC clinical stage. The association between GAS5 expression and clinicopathological characteristics was analyzed using the Mann–Whitney U test. Receiver-operating characteristic (ROC) curves were established to evaluate the diagnostic value of plasma GAS5 for differentiating tumors from controls. All of the statistical calculations were performed using the SPSS software (version17.0) and GraphPad Prism 5.0 (GraphPad Software Inc., CA) was used to generate graphs. All of the P values <0.05 were considered to be statistically significant.
3. Results
3.1. GAS5 expression level was stable subjected to freeze–thaw cycles multiple times
The stability of lncRNAs in plasma should be determined because this is an important requirement for utility as tumor biomarker. We next sought to examine the stability of plasma GAS5 samples under harsh conditions, including incubation at room temperature for 0, 6, 12, and 24 hours, repeated freeze–thaw cycles, and incubation at −80°C. At room temperature, the levels of GAS5 were not significantly altered from 0 to 6 hours, but then slightly decreased at 12 hours after compared with at 6 hours (Fig. 1A). Moreover, when the plasma storage time was protracted, GAS5 showed no significant changes (Fig. 1B). GAS5 expression levels remained stable when the plasma was subjected to freeze–thaw cycles multiple times (Fig. 1C). These results provided a foundation for evaluating plasma GAS5 as potential tumor biomarkers.
Figure 1.
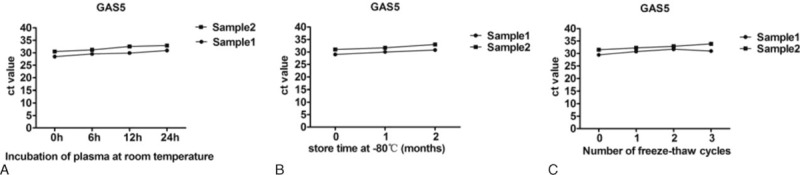
The stability of GAS5 plasma in harsh environments. Plasma GAS5 began to show signs of mild instability. A, Incubation of plasma at room temperature; B, the store time at −80°C; C, the freeze–thawing processes. CT values represent the GAS5 levels. CT = computed tomography, GAS5 = growth arrest-specific transcript 5.
3.2. The plasma GAS5 was decreased in NSCLC patients compared with healthy controls
We first analyzed whether GAS5 levels could serve as circulating biomarkers, by comparing the plasma levels between NSCLC patients and normal controls. These samples were also analyzed by real-time PCR. The Mann–Whitney U test showed that GAS5 was significantly down-regulated in NSCLC patients compared with that in controls (P = 0.000; Fig. 2A). These results indicated that plasma GAS5 was decreased in NSCLC patients compared with healthy controls.
Figure 2.
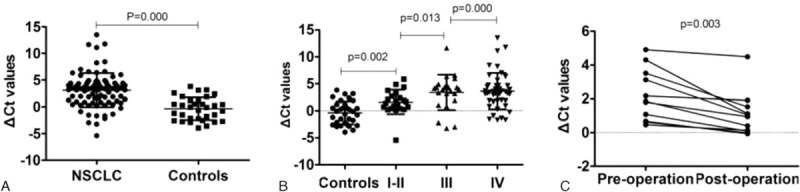
Expression levels of GAS5 in NSCLC patients and healthy controls. A, lncRNA-GAS5 was significantly down-regulated in NSCLC patients (n = 90) compared with healthy controls (n = 33). B, Plasma GAS5 expression levels in NSCLC with various tumor grades. C, Wilcoxon t test analysis of GAS5 expression levels between preoperative and postoperative samples obtained from NSCLC patients. GAS5 = growth arrest-specific transcript 5, NSCLC = nonsmall cell lung cancer.
3.3. The GAS5 expression was strongly associated with NSCLC tumor stage
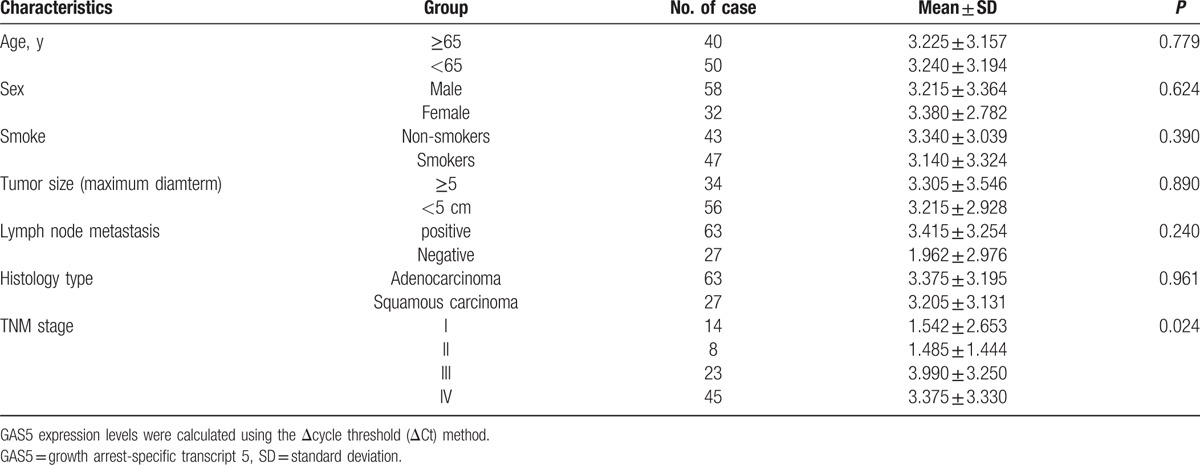
We first examined the correlation between GAS5 expression and the various NSCLC clinical stages. When all NSCLC patients were grouped based on TNM stage, GAS5 expression levels were significantly lower in stage III and IV patients than in stage I or II patients (Fig. 2B). Based on the above findings, we next investigated the associations of GAS5 expression levels between the NSCLC patients with the clinicopathological characteristics. As shown in Table 2, the GAS5 expression was strongly associated with NSCLC tumor stage (P < 0.024). Notably, GAS5 expression levels in the plasma samples from the NSCLC patients were not significantly correlated with age, sex, smoking, tumor size, lymphatic metastasis, or histology type.
Table 2.
Analysis of the relationship between GAS5 level (ΔCt) and clinicopathological factors.
3.4. GAS5 had diagnostic potential significance in the diagnosis of NSCLC
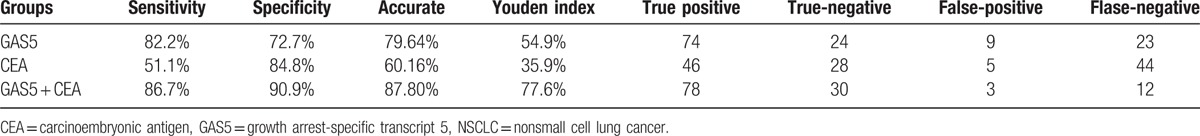
To examine whether GAS5 had diagnostic potential, 123 plasma samples, including 90 from NSCLC patients and 33 from normal controls, were analyzed. No data were missing in the course of detection. A ROC curve was used to evaluate the predictive efficacy of GAS5. The results showed that the value of the area under the ROC curve (AUC) was 0.832 (95% confidence interval [CI] 0.754–0.893), and for the NSCLC and healthy control group (Fig. 3A), the optimal sensitivity and specificity were 82.2% and 72.7%, respectively. Based on the above findings, we further evaluated whether GAS5 expression could distinguish between patients with different stages of NSCLC. Using ROC analyses, stage I to II patients and controls had an AUC value of 0.755 (95% CI 0.620–0.861) (Fig. 3B), stage III patients and controls had an AUC value of 0.850 (95% CI0.729–0.931) (Fig. 3C), stage IV patients and controls had an AUC value of 0.861 (95% CI 0.764–0.929) (Fig. 3D), stage I to II and III patients had an AUC value of 0.723 (95% CI 0.570–0.846) (Fig. 3E), and stage III and IV patients had an AUC value of 0.538 (95% CI 0.413–0.660) (Fig. 3F). These results suggest GAS5 shows predominately diagnostic accuracy. Because the plasma level of CEA is commonly used for screening of NSCLC.[29] Therefore, we compared the sensitivity and specificity of the GAS5 expression with the plasma level of CEA, and found that CEA had an AUC value of 0.700 (95% CI 0.611–0.779) (Fig. 4A). In addition, the optimal sensitivity and specificity were 43.3% and 100%, respectively, indicating, compared with CEA, GAS5 is a more sensitive and specific biomarker for NSCLC. In the next step, we further explored whether the combination of GAS5 and CEA significantly improved the diagnostic efficiency (AUC 0.909, 95% CI 0.857–0.962, P = 0.000; Fig. 4B). The sensitivity, specificity, accuracy of CEA, GAS5, and the combination (CEA + GAS5) for distinguishing NSCLC from healthy controls were summarized in Table 3. The results showed the combination group had higher sensitivity and specificity. Together, these results indicated that plasma GAS5 had potential significance with respect to the sensitivity and specificity in the diagnosis of NSCLC.
Figure 3.
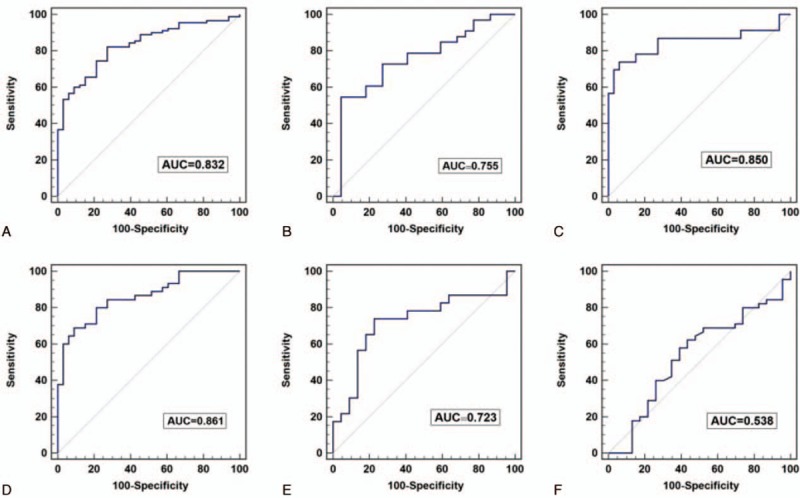
The ROC curve analysis of the differences between NSCLC patients of various tumor grades from healthy controls. A, Comparison of NSCLC patients with the control group. B, Comparison of tumor stage I to II patients versus controls. C, Comparison of tumor stage III patients versus controls. D, Comparison of tumor stage IV patients versus controls. E, Comparison of stage I to II with stage III patients. F, Comparison of stage III patients with stage IV patients. NSCLC = nonsmall cell lung cancer, ROC = receiver-operating characteristic curve.
Figure 4.
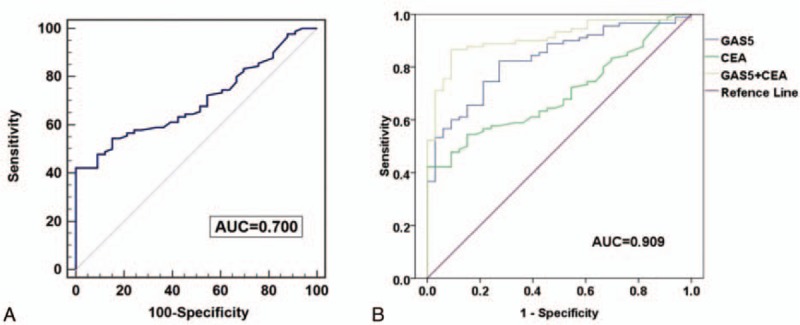
The ROC curve analysis of GAS5 in NSCLC cases and healthy controls. A, The results of the ROC curve analysis of the CEA assay. B, The analysis of the predictive effect of CEA and GAS5. CEA = carcinoembryonic antigen, GAS5 = growth arrest-specific transcript 5, NSCLC = nonsmall cell lung cancer, ROC = receiver-operating characteristic curve.
Table 3.
Performance of CEA, GAS5, and both CEA and GAS5, in the differential diagnosis NSCLC from healthy controls.
3.5. GAS5 increased after operation in NSCLC
We next ask whether the plasma expression level of GAS5 could be used to monitor tumor dynamics. We compared the GAS5 expression levels between preoperative and postoperative samples obtained from NSCLC patients. In 11cases, the GAS5 expression levels of the postoperative samples were significantly increased compared with those of the preoperative samples (P = 0.003, Wilcoxon t test; Fig. 2C), indicating GAS5 increased after operation in NSCLC.
4. Discussion
Noncoding RNAs are classified as small or long ncRNAs (lncRNAs) based on their size and function. Numerous recent studies have shown that miRNAs are rapidly released from tissues into the circulation in many pathological conditions. Studies have shown that circulating miRNAs are detectable as stable molecules in blood, urine, and sputum of cancer patients, and might be potential new biomarkers for cancer. For example, serum miR-21 could be a potential novel biomarker for the diagnosis of NSCLC, and miR-200c plays a significant role in the prognosis of patients with NSCLC.[30]
There are distinct expression signatures of miRNAs in human plasma or serum with various cancers. However, the expression of lncRNA in plasma or serum still needs further investigation. So far, numerous studies have shown that lncRNAs play an important role in tumor occurrence, invasion, and metastasis.[31] Currently, an increasing number of lncRNAs have been identified to be correlated with cancers. For example, LINC01133 was up-regulated in lung squamous cell cancer (LSCC). LSCC patients with higher expression level of LINC01133 had shorter survival time.[32] Recent studies have confirmed that the specificity and sensitivity of the lncRNAPOU3F3 are superior to the current specific biomarkers of serum squamous cell carcinoma antigen (SCCA). POU3F3 could serve as a potential biomarker for diagnosis of esophageal squamous cell carcinoma.[33] Together, these observations provide evidence for the potential roles of lncRNAs as tumor biomarkers. To date, no study has quantified the transcript levels of lncRNA GAS5 in blood plasma. The purpose of this study was to identify and validate circulating GAS5 in human plasma for use as diagnostic biomarkers in NSCLC. We here first investigated the diagnostic value of GAS5 in plasma distinguishing NSCLC patients from healthy controls. It should be noticed that as Trizol extraction may result in a lower production, this procedure might be more cumbersome to perform in the laboratory. Instead, we used a mirVana PARIS kit, which was the optimal column-based kit, for extracting the RNAs. A recent study showed that circulating lncRNAs were released from tumor cells; thus, when the tumor was resected, it reverted to a normal level status regarding lncRNAs.[18] Our study detected markedly increased levels of GAS5 after the surgical removal of the primary tumors. Therefore, our results support this explanation. Moreover, we confirmed that circulating GAS5 lncRNA was relatively stable under several harsh conditions. This finding was consistent with prostate cancer and gastric cancer in patients.[15,18]
Since the identification of CEA in 1965, various studies have demonstrated its role as a follow-up in NSCLC patients.[34] Approximately 70% of NSCLC patients have CEA that is overexpressed in advanced stages.[35] CEA is useful for monitoring disease, and detecting recurrence and metastasis, and prognostics.[36–38] However, the use of CEA as a prognostic and predictive marker in lung cancer patients has been widely controversial. The specificity is higher and sensitivity is lower than other biomarkers in diagnosis of NSCLC patients.[37] Thus, novel biomarkers urgently need to be explored; particularly, the use of noninvasive detection instead of invasive detection, which can improve the diagnosis of NSCLC, should be pursued. In our study, the combined use of GAS5 and CEA AUC values could provide a significantly more accurate differential diagnosis between NSCLC patients and healthy controls compared with use of either GAS5 or CEA alone. The experimental data showed that the joint diagnosis is effective for NSCLC. Furthermore, the AUC values obtained for GAS5 and CEA were 0.832 (95% CI 0.754–0.893) and 0.700 (95% CI 0.611–0.779), respectively. These results indicated the combination of GAS5 and CEA has better diagnosis accuracy.
Circulating RNAs could be detected and are surprisingly stable in the serum and plasma of cancer patients, despite the large amount of RNases circulating in the blood there. This implies that the RNAs might be protected from degradation by being packaged into microparticles.[39] At present, the exact biological roles of circulating lncRNAs in cancer patients remain unclear and require further investigation. Our data show a strong association between the expression of GAS5 in NSCLC and healthy controls, indicating that GAS5 expression may be exploited as a promising noninvasive biomarker of NSCLC.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81302032, No. 81401903, No. 81572937, No. 81572273) and the Natural Science Foundation of Jiangsu province (No. BK2011658, No. BK20140736). We apologize to all researchers whose relevant contributions were not cited due to space limitations.
Footnotes
Abbreviations: ADC = adenocarcinoma, CEA = carcinoembryonic antigen, CT = computed tomography, GAPDH = glyceraldehyde-3-phosphate dehydrogenase, GAS5 = growth arrest-specific transcript 5, lncRNAs = long noncoding RNAs, MALAT1 = metastasis-associated lung adenocarcinoma transcript 1, NSCLC = nonsmall cell lung cancer, ROC = receiver-operating characteristic curve, qRT-PCR = quantitative reverse transcription PCR, SCC = squamous cell carcinoma, snoRNA = small nucleolus RNA.
WL and TL contributed equally to the work.
The authors report no conflicts of interest.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011; 61:69–90. [DOI] [PubMed] [Google Scholar]
- 2.Foss KM, Sima C, Ugolini D, et al. Weiss GJ. miR-1254 and miR-574-5p: serum-based microRNA biomarkers for early-stage non-small cell lung cancer. J Thorac Oncol 2011; 6:482–488. [DOI] [PubMed] [Google Scholar]
- 3.Rosell R, Karachaliou N. Lung cancer: Maintenance therapy and precision medicine in NSCLC. Nat Rev Clin Oncol 2013; 10:549–550. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch FR, Franklin WA, Gazdar AF, et al. Early detection of lung cancer: clinical perspectives of recent advances in biology and radiology. Clin Cancer Res 2001; 7:5–22. [PubMed] [Google Scholar]
- 5.Shen J, Todd NW, Zhang H, et al. Plasma microRNAs as potential biomarkers for non-small-cell lung cancer. Lab Invest 2011; 91:579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tarro G, Perna A, Esposito C. Early diagnosis of lung cancer by detection of tumor liberated protein. J Cell Physiol 2005; 203:1–5. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Hu Z, Wang W, et al. Identification of ten serum microRNAs from a genome-wide serum microRNA expression profile as novel noninvasive biomarkers for nonsmall cell lung cancer diagnosis. Int J Cancer 2012; 130:1620–1628. [DOI] [PubMed] [Google Scholar]
- 8.Spizzo R, Almeida MI, Colombatti A, et al. Long non-coding RNAs and cancer: a new frontier of translational research? Oncogene 2012; 31:4577–4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li G, Zhang H, Wan X, et al. Long noncoding RNA plays a key role in metastasis and prognosis of hepatocellular carcinoma. Biomed Res Int 2014; 2014:780521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilusz JE, Sunwoo H, Spector DL. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev 2009; 23:1494–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoon JH, Abdelmohsen K, Gorospe M. Posttranscriptional gene regulation by long noncoding RNA. J Mol Biol 2013; 425:3723–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pickard MR, Mourtada-Maarabouni M, Williams GT. Long non-coding RNA GAS5 regulates apoptosis in prostate cancer cell lines. Biochim Biophys Acta 2013; 1832:1613–1623. [DOI] [PubMed] [Google Scholar]
- 13.Renganathan A, Kresoja-Rakic J, Echeverry N, et al. GAS5 long non-coding RNA in malignant pleural mesothelioma. Mol Cancer 2014; 13:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutschner T, Hammerle M, Eissmann M, et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res 2013; 73:1180–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arita T, Ichikawa D, Konishi H, et al. Circulating long non-coding RNAs in plasma of patients with gastric cancer. Anticancer Res 2013; 33:3185–3193. [PubMed] [Google Scholar]
- 16.Shao Y, Ye M, Jiang X, et al. Gastric juice long noncoding RNA used as a tumor marker for screening gastric cancer. Cancer 2014. [DOI] [PubMed] [Google Scholar]
- 17.Isin M, Ozgur E, Cetin G, et al. Investigation of circulating lncRNAs in B-cell neoplasms. Clin Chim Acta 2014; 431:255–259. [DOI] [PubMed] [Google Scholar]
- 18.Ren S, Wang F, Shen J, et al. Long non-coding RNA metastasis associated in lung adenocarcinoma transcript 1 derived miniRNA as a novel plasma-based biomarker for diagnosing prostate cancer. Eur J Cancer 2013; 49:2949–2959. [DOI] [PubMed] [Google Scholar]
- 19.Xie H, Ma H, Zhou D. Plasma HULC as a promising novel biomarker for the detection of hepatocellular carcinoma. Biomed Res Int 2013; 2013:136106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber DG, Johnen G, Casjens S, et al. Evaluation of long noncoding RNA MALAT1 as a candidate blood-based biomarker for the diagnosis of non-small cell lung cancer. BMC Res Notes 2013; 6:518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji P, Diederichs S, Wang W, et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene 2003; 22:8031–8041. [DOI] [PubMed] [Google Scholar]
- 22.Schneider C, King RM, Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell 1988; 54:787–793. [DOI] [PubMed] [Google Scholar]
- 23.Smith CM, Steitz JA. Classification of gas5 as a multi-small-nucleolar-RNA (snoRNA) host gene and a member of the 5′-terminal oligopyrimidine gene family reveals common features of snoRNA host genes. Mol Cell Biol 1998; 18:6897–6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coccia EM, Cicala C, Charlesworth A, et al. Regulation and expression of a growth arrest-specific gene (gas5) during growth, differentiation, and development. Mol Cell Biol 1992; 12:3514–3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleming JV, Hay SM, Harries DN, et al. Effects of nutrient deprivation and differentiation on the expression of growth-arrest genes (gas and gadd) in F9 embryonal carcinoma cells. Biochem J 1998; 330 (Pt 1):573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mourtada-Maarabouni M, Pickard MR, Hedge VL, et al. GAS5, a non-protein-coding RNA, controls apoptosis and is downregulated in breast cancer. Oncogene 2009; 28:195–208. [DOI] [PubMed] [Google Scholar]
- 27.Sun M, Jin FY, Xia R, et al. Decreased expression of long noncoding RNA GAS5 indicates a poor prognosis and promotes cell proliferation in gastric cancer. BMC Cancer 2014; 14:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi X, Sun M, Liu H, et al. A critical role for the long non-coding RNA GAS5 in proliferation and apoptosis in non-small-cell lung cancer’. Mol Carcinog 2015; 54 Suppl 1:E1–E12. [DOI] [PubMed] [Google Scholar]
- 29.Chen X, Zhou F, Li X, et al. Folate receptor-positive circulating tumor cell detected by LT-PCR-based method as a diagnostic biomarker for non-small-cell lung cancer. J Thorac Oncol 2015; 10:1163–1171. [DOI] [PubMed] [Google Scholar]
- 30.Liu XG, Zhu WY, Huang YY, et al. High expression of serum miR-21 and tumor miR-200c associated with poor prognosis in patients with lung cancer. Med Oncol 2012; 29:618–626. [DOI] [PubMed] [Google Scholar]
- 31.Fan L, Hu Z. Progress of long non-coding rnas in non-small cell lung cancer. Zhongguo Fei Ai Za Zhi 2016; 19:108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, Zhu N, Chen X. A novel long noncoding RNA Linc01133 is upregulated in lung squamous cell cancer and predicts survival. Tumour Biol 2015; 36:7465–7471. [DOI] [PubMed] [Google Scholar]
- 33.Tong YS, Wang XW, Zhou XL, et al. Identification of the long non-coding RNA POU3F3 in plasma as a novel biomarker for diagnosis of esophageal squamous cell carcinoma. Mol Cancer 2015; 14:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moro D, Villemain D, Vuillez JP, et al. CEA, CYFRA21-1 and SCC in non-small cell lung cancer. Lung Cancer 1995; 13:169–176. [DOI] [PubMed] [Google Scholar]
- 35.Robbins PF, Eggensperger D, Qi CF, et al. Definition of the expression of the human carcinoembryonic antigen and non-specific cross-reacting antigen in human breast and lung carcinomas. Int J Cancer 1993; 53:892–897. [DOI] [PubMed] [Google Scholar]
- 36.Lee DS, Kim SJ, Kang JH, et al. Serum carcinoembryonic antigen levels and the risk of whole-body metastatic potential in advanced non-small cell lung cancer. J Cancer 2014; 5:663–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer 2012; 76:138–143. [DOI] [PubMed] [Google Scholar]
- 38.Matsuoka K, Sumitomo S, Nakashima N, et al. Prognostic value of carcinoembryonic antigen and CYFRA21-1 in patients with pathological stage I non-small cell lung cancer. Eur J Cardiothorac Surg 2007; 32:435–439. [DOI] [PubMed] [Google Scholar]
- 39.Orozco AF, Lewis DE. Flow cytometric analysis of circulating microparticles in plasma. Cytometry A 2010; 77:502–514. [DOI] [PMC free article] [PubMed] [Google Scholar]


