Abstract
Occupational lung diseases are well recognized risk factors for tuberculosis (TB). However, little research investigated the effect of TB on the clinical course and outcome of occupational lung diseases.
We conducted a 13-year observational study of a nationwide cohort to evaluate the risk and prognosis of TB among patients with occupational lung diseases in Taiwan.
By using the Taiwan National Health Insurance database, occupational lung diseases cohort was identified according to diagnosis codes from 1998 to 2008 and prospectively monitored until the end of 2010, loss to follow-up, or death. Newly diagnosed TB, comorbidities, and demographic characteristics were evaluated as prognostic variables in the survival analysis of patients with occupational lung diseases using Cox proportional hazard regression models.
A total of 12,787 study participants were enrolled with an average of 9.69 years of follow-up. Among them, 586 (4.58%) had newly diagnosed TB and 3180 (24.87%) died during follow-up. The incidence of TB was 473 per 100,000 person-years, and the risk of TB infection significantly increased over time. The independent risk factors for mortality included male gender (hazard ratio [HR]: 2.23, 95% confidence interval [CI]: 1.91–2.60), age (HR: 1.05, 95% CI: 1.05–1.06), TB (HR: 1.17, 95% CI: 1.01–1.37), congestive heart failure (HR: 1.44, 95% CI: 1.17–1.79), cerebrovascular disease (HR: 1.34, 95% CI: 1.15–1.57), chronic obstructive pulmonary disease (HR: 1.44, 95% CI: 1.33–1.56), and asthma (HR: 1.27, 95% CI: 1.15–1.40). In addition, patients with TB infections had worse outcomes in the survival analysis than those without TB (log-rank test P = 0.02).
Despite the low prevalence of occupational lung diseases in Taiwan, patients with those diseases had a higher TB incidence than the general population did (473 vs 55 per 100,000 person-years). Furthermore, even with effective antimicrobial chemotherapy, TB infection was a prognostic factor leading to poor outcomes in the patients with occupational lung diseases. We recommend intensive medical surveillance of TB in these high-risk patients for better control of TB and improvement of occupational health in Taiwan.
Keywords: nationwide, occupational lung diseases, prognosis, tuberculosis
1. Introduction
Coal workers’ pneumoconiosis, asbestosis, and silicosis are included in the spectrum of occupational lung diseases caused by inhalation of coal dust, asbestos, or crystalline silica. Environmental exposure to these toxic particles has a cumulative effect, and long durations ultimately lead to irreversible fibrotic changes in the lungs and a decline in pulmonary function.[1] All of these environmental exposures contribute to functional disability and impaired quality of life in these patients. Although modern industrial technology has been developed to minimize occupational exposure, data from the National Institute for Occupational Safety and Health Program revealed an ongoing increase in both the prevalence and severity of pneumoconiosis among US coal workers.[2] No proven curative treatment for these occupational lung diseases exists, and strong evidence has demonstrated that higher mortality is found in these patients compared with the general male population.[3–5]
Occupational lung diseases, especially silicosis, are well established risk factors for the development of active tuberculosis (TB).[6–8] Moreover, other pulmonary disorders including chronic obstructive pulmonary disease (COPD)[9,10] and lung cancer[11–13] are also associated with these occupational lung diseases. Both COPD and lung cancer manifest as irreversible disease courses and further result in poor prognoses among these patients. However, with effective antimicrobial chemotherapy, TB becomes a curable disease but still has a significant impact on pulmonary dysfunction because of structural damage and changes in local immunity during the mycobacterial infection. Little research has emphasized the correlation between the complications of TB and the mortality of occupation lung diseases, and these studies are limited by out-of-date or different epidemiological characteristics of the region.[14,15] The aim of the present study was to evaluate the role of TB in the outcomes of patients with occupational lung diseases in Taiwan, using a nationwide population.
2. Methods
2.1. Data sources
National Health Insurance (NHI) is a mandatory universal health insurance program in Taiwan, providing comprehensive medical care to more than 95% of Taiwanese residents since 1996. The NHI databases, released by the National Health Research Institute (NHRI), contain the patients’ identification numbers, gender, date of birth, dates of ambulatory visits, hospital admissions and discharge data, medications, diagnoses, and procedures. Diagnoses and procedures were coded using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) format. The NHRI encrypted all information to protect the anonymity of the patients while allowing specific patients to be selected for study and follow-up. Our study used the Catastrophic Illness dataset of the NHI data, which provides comprehensive utilization and enrollment information for all patients with severe illnesses in Taiwan. Occupational lung diseases are recognized as catastrophic illnesses in Taiwan, and they are exempted from copayment under the NHI program. Six types of cancers including lung, liver, colon, cervical, breast, and nasopharynx were not included in our database because the database was initially designed for other purposes.
The confidentiality of the dataset abides by the data regulations of the Bureau of NHI. The study was approved by the institutional review board of National Chung Kang University Hospital (B-EX-104-019). According to the NHRI regulations, only citizens of Taiwan (Republic of China) who fulfill the requirements for conducting research projects are eligible to apply for use of the NHRI database. Therefore, the data availability is restricted by Taiwanese law. The contact email address to which requests for the data is as follows: nhird@nhri.org.tw.
2.2. Study design and population
A prospective, nationwide cohort study was conducted from January 1, 1998 to December 31, 2008 based on ambulatory care and inpatient discharge records. Patients with the diagnosis of compatible ICD-9-CM codes (500, 501, 502, 503, and 505) were enrolled as the occupational lung diseases cohort. The certification of occupational lung diseases as catastrophic illnesses in Taiwan requires a complete occupational history, classic radiological findings in the lungs, optional pulmonary function testing, and a full review by occupational medicine specialists or pulmonologists. In addition, we excluded patients younger than 20 years, with a history of TB and human immunodeficiency virus (HIV) infection before the diagnosis of occupational lung diseases. All enrollees were monitored until December 31, 2010, loss to follow-up (canceled health insurance before December 31, 2010), or death.
2.3. Definition of prognostic variables
A new TB diagnosis was defined as compatible ICD-9-CM codes (010–018) plus concurrent prescriptions of 1 (including fixed-dose combination drugs of isoniazid/rifampin/pyrazinamide/ethambutol or isoniazid/rifampin) or at least 2 (including isoniazid, rifampin, pyrazinamide, or ethambutol) anti-TB drugs for more than 60 days. The Taiwan Center for Disease Control set up a reporting system for TB control and surveillance. All cases of suspicious TB were registered, and patients were finally notified once a TB diagnosis was confirmed by physicians through bacterial, radiological, or clinical evidence of TB. All notified TB patients were treated with anti-TB drugs for at least 6 months. By way of directly linking the ICD-9-CM data with the registry of drug prescriptions pooled in the databases, we identified TB patients with definite notifications confirmed by physicians. TB involvement was categorized into intrathoracic and extrathoracic involvement based on the ICD-9-CM coding (010–012 for intrathoracic involvement and 013–018 for extrathoracic involvement). Comorbidities, based on the claims data, included diabetes mellitus (ICD-9-CM code 250), dyslipidemia (ICD-9-CM code 272), hypertension (ICD-9-CM code 401–405), ischemic cardiovascular disease (ICD-9-CM code 410–414), cardiac arrhythmia (ICD-9-CM code 427), congestive heart failure (ICD-9-CM code 428), cerebrovascular disease (ICD-9-CM code 430–438), COPD (ICD-9-CM code 490–492, 496), asthma (ICD-9-CM code 493), bronchiectasis (ICD-9-CM code 494), liver cirrhosis (ICD-9-CM code 571.2, 517.5, 517.6), end-stage renal disease (ICD-9-CM code 585), and connective tissue diseases (ICD-9-CM code 710, 714). Comorbidities were recorded when the selected ICD-9-CM codes were present in the claims data of enrollees for an average of more than 3 times per year during the observational period.
2.4. Statistical analysis
Data for continuous and categorical variables are presented as means ± standard deviations or number (%). Baseline differences between groups with and without TB were analyzed using the chi-square test or Fisher exact test for comparison of nominal variables. Two-tailed Student t test was used to analyze the differences between continuous variables. Univariate and multivariate Cox proportional hazard regressions were utilized to identify prognostic factors for mortality. Risk factors with P values <0.05 in the univariate Cox analysis were further entered into the multivariate analysis, and the result was represented as the hazard ratio (HR) with 95% CIs. Kaplan–Meier survival analysis was used for outcome evaluation, which was compared using the log-rank test. A P value <0.05 was considered statistically significant. Data extraction and statistical analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC).
3. Results
3.1. Baseline characteristics of study population
The flowchart for patient selection is illustrated in Fig. 1. From January 1, 1998 to December 31, 2008, we enrolled 12,787 patients as the study cohort after excluding patients with unknown gender and age less than 20 years, antecedent TB and HIV infections before the diagnosis of occupational lung diseases. The average observational period was 9.69 ± 2.94 years. Among the enrollees with occupational lung diseases, 586 (4.58%) were newly diagnosed TB, and 3180 (24.87%) had died by the end of the observational period (December 31, 2010). The incidence of TB was 473 per 100,000 person-years.
Figure 1.
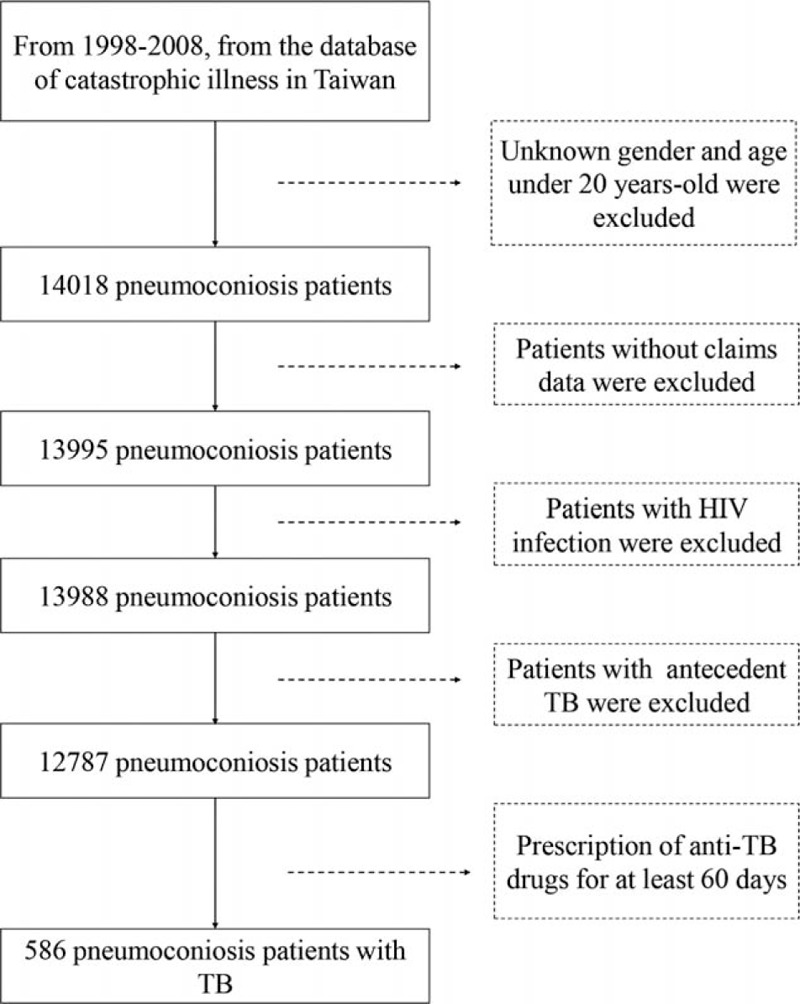
Flow diagram for the enrollment of study participants from 1998 to 2008. After excluding patients with human immunodeficiency virus infection, antecedent tuberculosis (TB), and those less than 20 years of age, 586 patients infected with TB were identified in the study cohort (n = 12,787).
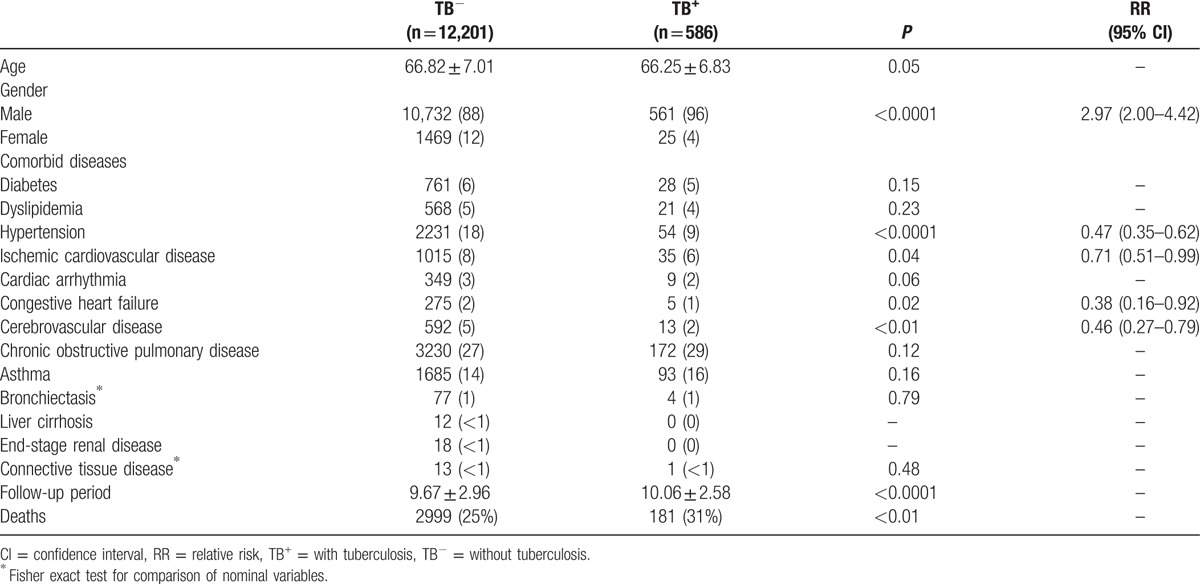
Table 1 shows that males tended to have TB, and comorbid diseases including hypertension, ischemic cardiovascular disease, congestive heart failure, and cerebrovascular disease occurred with greater frequency among patients without TB. Patients with TB were monitored longer and had higher mortality.
Table 1.
Characteristics of study cohort with and without TB.
3.2. Duration and locations of TB diagnosis
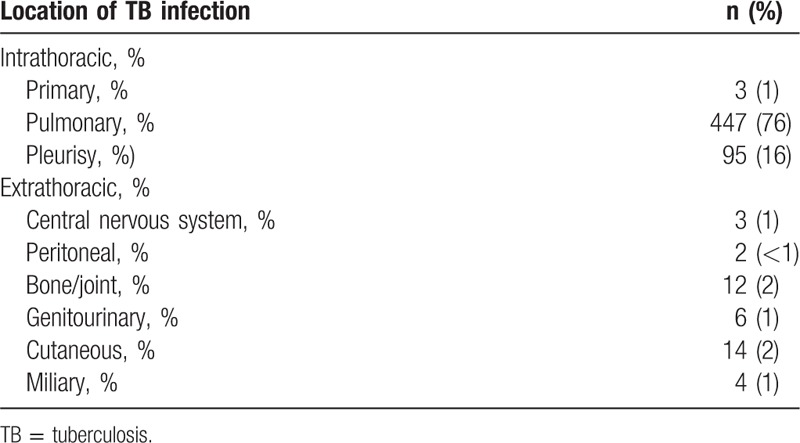
The average follow-up period between study enrollment and the date of TB diagnosis was 5.18 ± 3.37 years (minimum: 0.12 years, maximum: 12.38 years). The average age at TB diagnosis of the 586 patients was 71.42 ± 7.42 years. As demonstrated in Fig. 2 and Table 2, the incidence of TB among patients with occupational lung diseases increased significantly over time. Table 3 shows that intrathoracic TB comprised almost 93% of all cases, and pulmonary TB was the predominant type (76%).
Figure 2.
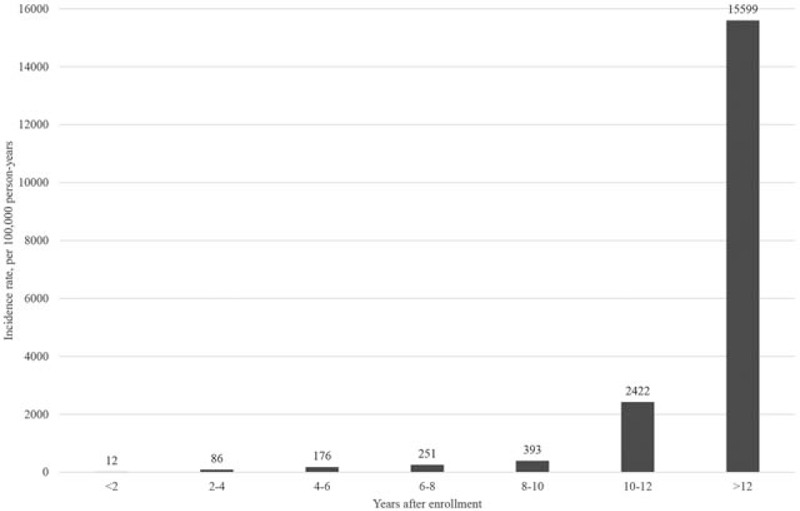
Incidence of tuberculosis (TB) among patients with occupational lung diseases. The TB incidence increased over time during the study period and peaked late in the stages of occupational lung diseases.
Table 2.
Time to TB diagnosis in years during follow-up.
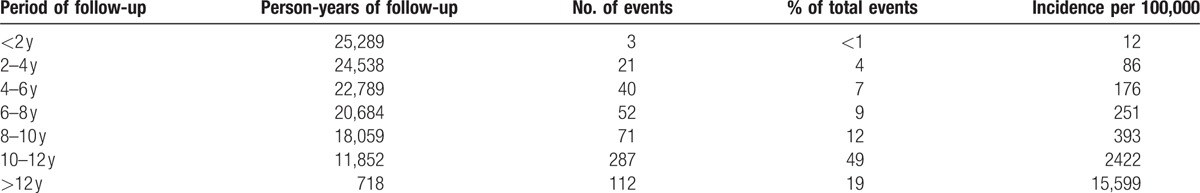
Table 3.
Locations of TB involvement.
3.3. Survival analysis among patients with occupational lung diseases
Table 4 demonstrates gender, age, and different comorbidities between surviving and deceased patients. Univariate Cox proportional hazard regression analysis identified 11 risk variables (all P < 0.05). Further multivariate regression analysis showed that the following 7 variables were independent risk factors for mortality: male gender, old age, TB, congestive heart failure, cerebrovascular disease, COPD, and asthma. In addition, Fig. 3 reveals significantly worse survival in patients with TB compared to those without TB.
Table 4.
Overall survival analysis among patients with occupational lung diseases.
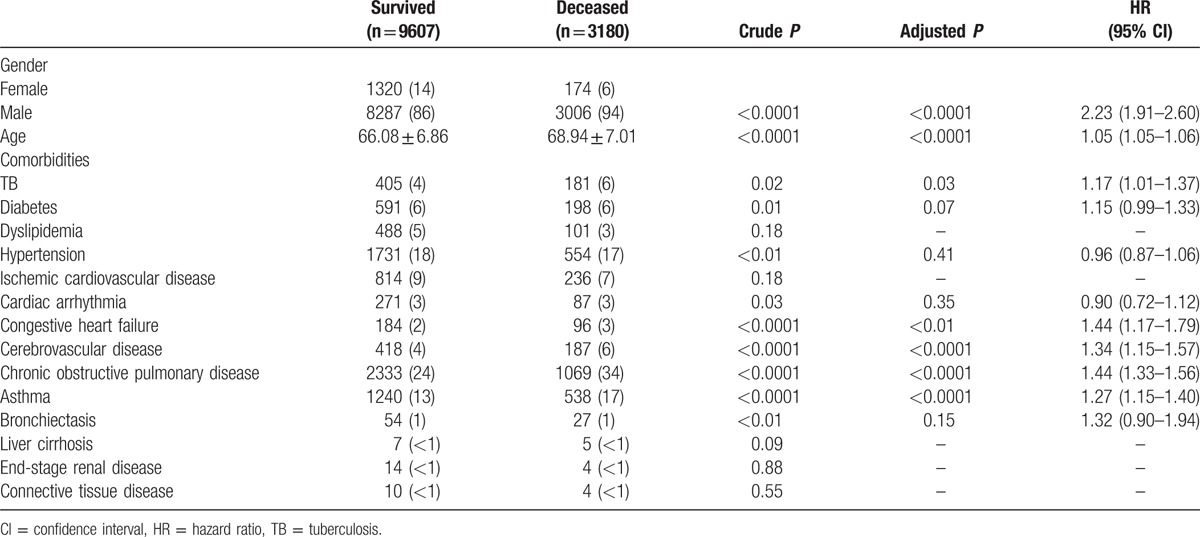
Figure 3.
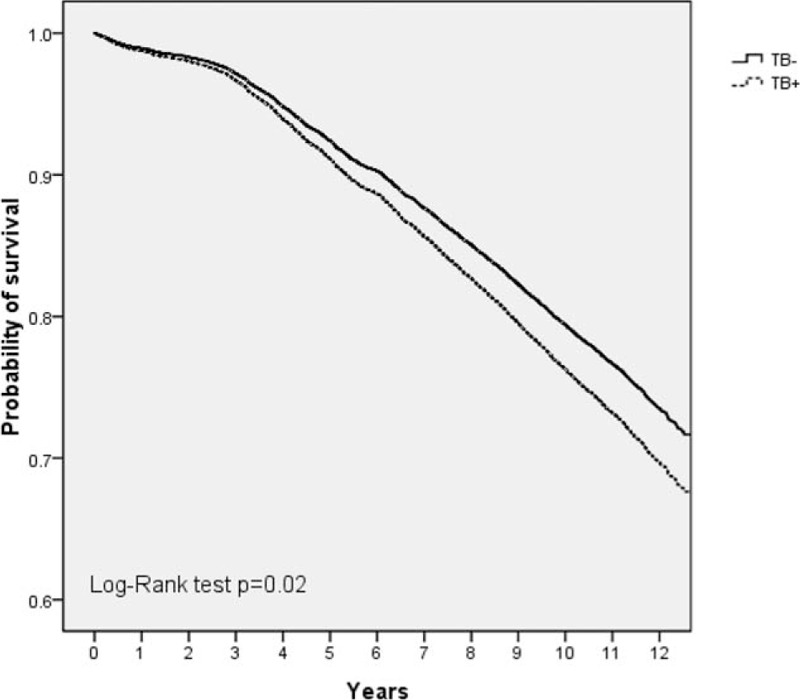
Overall survival rates for occupational lung disease patients with and without tuberculosis (TB). During the study period, patients with TB infections had significantly worse survival than did patients without TB infections.
4. Discussion
From 1998 through 2010, the cumulative incidence and incidence of TB among patients with occupational lung diseases were 4.58% and 473 per 100,000 person-years, respectively. Although there is a small percentage of the workforce working in mining among the industries in Taiwan (less than 1% of the total labor force),[16] the incidence of combined occupational lung diseases and TB was not much less than that in other countries with higher prevalences of occupational lung diseases.[17,18] A systematic analysis of 2001 to 2011 studies revealed that the pooled rate of coal workers’ pneumoconiosis patients combined with TB was 10.82%.[17] Another research investigated from 2008 to 2013 reported a proportion of 6.6% among cases with combined pneumoconiosis and TB.[18] Both these studies were conducted in China, where the pooled prevalence of coal workers’ pneumoconiosis was 6.02% from 2001 to 2011[17] and much higher than in Taiwan. In our study results, we found that TB developed on an average of 5.18 years after the diagnosis of an occupational lung disease. Besides, the incidence of TB in these patients was higher than in the general Taiwanese population after 2 years of follow-up (86 per 100,000 person-years compared with 55 per 100,000 person-years in 2012 in Taiwan).[19] In addition to male gender, old age, congestive heart failure, cerebrovascular disease, COPD, and asthma, TB was also an independent prognostic factor in predicting mortality in patients with occupational lung diseases.
Compared with other countries,[17] the prevalence of occupational lung diseases in Taiwan is quite low (<1% of Taiwan's total population, 0.8% in the United Kingdom from 1998 to 2000, and 3.2% in USA during the 2000s). Nonetheless, Taiwanese public health officials are concerned about chronic lung injury due to occupationally inhaled particulate toxins.[16] Furthermore, data from the Department of Labor Insurance Statistics show that pneumoconiosis contributed to the majority of hospitalizations lasting over 4 days, permanent disability, and deaths from occupational disease in Taiwan from 1999 to 2003.[16] Ongoing inflammatory effects from toxic particles retained in airways, even after exposure ceases, result in irreversible biological and functional abnormalities. These abnormalities cause a broad spectrum of respiratory complications and a large burden of lung diseases. Identification of risk factors for morbidity and mortality in occupational lung diseases benefits not only the academic research community and governmental policy-makers but also the employees of companies involved in occupationally hazardous work.
Epidemiological[6–8] and experimental studies[20,21] have shown an association between changes induced by these toxic particles and increased susceptibility to mycobacterial infection. Consistent with these results, we demonstrated that the risk of TB infection in these patients was higher than in the general population of Taiwan (473 vs 55 per 100,000 person-years, relative risk: 8.6) and continued to increase during the whole clinical course of occupational lung diseases (Fig. 2). From a review article,[22] the risk of developing pulmonary TB was reported to be 2.8 to 39 times higher for patients with silicosis than for healthy controls, depending on different epidemiologic areas (2.8 times in South Africa,[23] 3 times in Spain,[24] and 39.5 times in the United States).[25] Besides, some evidence could be supported for the dramatically increased risk of TB in the late stage of diseases. First, a literature review showed that little research has been done on the incidence of TB in cases of silicosis of various durations, except for one conducted by Cowie[23] in 1990s. They concluded that the TB incidence increased in direct proportion to the duration and extent of silicosis in their 7-year follow-up study, which was in concordance with our findings in a similar trend. Our study result could be partially explained by latency and the progressive and chronic course of both diseases. Second, host factors including aging and local immunity within lungs may both facilitate the risk of TB infection in proportion to the observation period. Third, we speculate that the dramatically increased risk of active TB in the late stage of occupational lung diseases in our cohort could be due to inadequate screening or alertness of clinicians. In a silicosis cohort study conducted in Hong Kong,[26] a high prevalence of latent TB infection was reported based on interferon-γ release assays or tuberculin skin testing (66.2% and 65.9%, respectively). Whether or not there is an association between the prevalence of latent TB infection in Taiwan and the related risk of subsequent progression to active TB is not well understood. Thus, comprehensive identification of the characteristic risk factors and an individualized understanding of the risk trends over time in this subgroup would help improve TB control in Taiwan. We emphasize the importance of routine TB checkups in the active or latent stages, with increasing frequency in the late stages of occupational lung diseases.
The dose–response of silica dust in the decline of pulmonary function and the increased risk of mortality in coal workers were well established.[4,27] Previous researches[14,15] have emphasized on the relationship between the survival of patients with occupational lung diseases and TB morbidity, but the results are limited to the efficacy of medical treatment in different eras, changes in occupational safety and infectious disease control, and various epidemiological features of both the occupational diseases and TB. A survival analysis collected from a pneumoconiosis cohort in China was conducted in the 1990s by Yi and Zhang,[14] and the results indicate that TB was a risk factor for premature death (HR: 2.0, P < 0.01). However, the study results may not be representative of the current times because the study was designed in the 1990s. Effective prevention and control of both diseases in the United States decreased silicosis-respiratory TB deaths by 99.5% during the study period from 1968 to 2006, and this decline paralleled decreases in both silicosis and TB deaths.[15] Nonetheless, despite progress toward elimination of occupational lung diseases and TB in Taiwan, our findings showed that TB still posed a significant threat to these patients’ overall survival (HR: 1.17, P = 0.03). To our knowledge, higher treatment failure and relapse rates are reported in patients with silicosis who are receiving anti-TB therapy.[28] Moreover, TB itself is also a strong facilitator in the progression of pneumoconiosis severity.[14] Furthermore, a retrospective cohort study of South American gold miners also showed excessive lung function decline among those treated for pulmonary TB.[29] All of these complications caused by TB could have a negative impact on the overall mortality of these patients, as indirectly demonstrated with our study results. Furthermore, a recent work that investigated 3202 male silicotic workers in Hong Kong during 1981 to 2006 also concluded a significantly excess risk of death for pulmonary TB (standardized mortality ratio = 6.57, 95% CI: 5.01–8.61).[30]
The present study has some inherent strengths and limitations. It is the first nationwide-based assessment of TB in Taiwanese workers with occupational lung diseases and covers an extended longitudinal period from 1998 to 2010. However, observations were based on diagnostic codes and prescription histories. We were unable to identify changes in pulmonary function or radiological differences that could have potentially affected the severity or activity of occupational lung diseases of each subject, and thus, influenced their overall outcome. Moreover, because most of our study subjects were recruited in the retired age, the definitive number of years they spent in the workforce was not available. Thus, the relationship of exposure-response with survival could not be clarified. Nonetheless, we still found that the risk of TB infection increased dramatically over time, even in the late stages of occupational lung diseases. We suggest that TB screening should be done in routine health checkups to improve the occupational safety and workers’ health outcomes in Taiwan.
Occupational health issues have caught the public's attention in Taiwan. The Programme to Reduce Exposures by Surveillance Systems was introduced in 1993 and has been in full operation since 1995 in Taiwan to improve industrial hygiene.[31] Nonetheless, a substantial gap in our understanding of the morbidity and mortality associated with occupational diseases in Taiwan exists. Since Taiwan has an intermediate TB incidence, our results emphasize the importance of TB research in an occupational health setting. Strengthening a multilateral collaboration of occupational health and infectious disease control with an efficient surveillance system provides benefits to promote workers’ welfare.
Acknowledgments
This study is based, in part, on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health and managed by National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of Bureau of National Health Insurance, Department of Health, or National Health Research Institutes. We also sincerely thank Yi-Ting Chen, from the Department of Public Health, College of Medicine, National Cheng Kung University, for NHI database extraction and statistical help.
Footnotes
Abbreviations: CI = confidence interval, COPD = chronic obstructive pulmonary disease, HIV = human immunodeficiency virus, HR = hazard ratio, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, NHI = National Health Insurance, NHRI = National Health Research Institute, TB = tuberculosis.
C-LH and P-LS have contributed equally to the article.
Funding: This study was funded by NCKUH-10503022 grant from the Clinical Research Fund of National Cheng Kung University Medical Center, Tainan, Taiwan.
The authors have no conflicts of interest to disclose.
References
- 1.Castranova V, Vallyathan V. Silicosis and coal workers’ pneumoconiosis. Environ Health Perspect 2000; 108 Suppl 4:675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petsonk EL, Rose C, Cohen R. Coal mine dust lung disease. New lessons from old exposure. Am J Respir Crit Care Med 2013; 187:1178–1185. [DOI] [PubMed] [Google Scholar]
- 3.Starzyński Z, Marek K, Kujawska A, et al. Mortality among different occupational groups of workers with pneumoconiosis: results from a register-based cohort study. Am J Ind Med 1996; 30:718–725. [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Liu Y, Wang H, et al. Long-term exposure to silica dust and risk of total and cause-specific mortality in Chinese workers: a cohort study. PLoS Med 2012; 9:e1001206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tse LA, Chen MH, Au RK, et al. Pulmonary tuberculosis and lung cancer mortality in a historical cohort of workers with asbestosis. Public Health 2012; 126:1013–1016. [DOI] [PubMed] [Google Scholar]
- 6.Hnizdo E, Murray J. Risk of pulmonary tuberculosis relative to silicosis and exposure to silica dust in South African gold miners. Occup Environ Med 1998; 55:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rees D, Murray J. Silica, silicosis and tuberculosis. Int J Tuberc Lung Dis 2007; 11:474–484. [PubMed] [Google Scholar]
- 8.Kim YM, Kim M, Kim SK, et al. Mycobacterial infections in coal workers’ pneumoconiosis patients in South Korea. Scand J Infect Dis 2009; 41:656–662. [DOI] [PubMed] [Google Scholar]
- 9.Rushton L. Chronic obstructive pulmonary disease and occupational exposure to silica. Rev Environ Health 2007; 22:255–272. [DOI] [PubMed] [Google Scholar]
- 10.Rosenman KD, Reilly MJ, Gardiner J. Results of spirometry among individuals in a silicosis registry. J Occup Environ Med 2010; 52:1173–1178. [DOI] [PubMed] [Google Scholar]
- 11.Pelucchi C, Pira E, Piolatto G, et al. Occupational silica exposure and lung cancer risk: a review of epidemiological studies 1996–2005. Ann Oncol 2006; 17:1039–1050. [DOI] [PubMed] [Google Scholar]
- 12.Lacasse Y, Martin S, Gagné D, et al. Dose–response meta-analysis of silica and lung cancer. Cancer Causes Control 2009; 20:925–933. [DOI] [PubMed] [Google Scholar]
- 13.Hung YP, Teng CJ, Liu CJ, et al. Cancer risk among patients with coal workers’ pneumoconiosis in Taiwan: a nationwide population-based study. Int J Cancer 2014; 134:2910–2916. [DOI] [PubMed] [Google Scholar]
- 14.Yi Q, Zhang Z. The survival analyses of 2738 patients with simple pneumoconiosis. Occup Environ Med 1996; 53:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasrullah M, Mazurek JM, Wood JM, et al. Silicosis mortality with respiratory tuberculosis in the United States, 1968–2006. Am J Epidemiol 2011; 174:839–848. [DOI] [PubMed] [Google Scholar]
- 16.Shih TS, Chang HY, Yeh WY, et al. Occupational health research in Taiwan. Ind Health 2004; 42:124–134. [DOI] [PubMed] [Google Scholar]
- 17.Mo J, Wang L, Au W, et al. Prevalence of coal workers’ pneumoconiosis in China: a systematic analysis of 2001–2011 studies. Int J Hyg Environ Health 2014; 217:46–51. [DOI] [PubMed] [Google Scholar]
- 18.Xia Y, Liu J, Shi T, et al. Prevalence of pneumoconiosis in Hubei, China from 2008 to 2013. Int J Environ Res Public Health 2014; 11:8612–8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang SL, Lo HY, Shi WY, et al. Tuberculosis Control Report. Taiwan, ROC: Centers for Disease Control, Department of Health; 2012. [Google Scholar]
- 20.Allison AC, Hart PD. Potentiation by silica of the growth of Mycobacterium tuberculosis in macrophage cultures. Br J Exp Pathol 1968; 49:465–476. [PMC free article] [PubMed] [Google Scholar]
- 21.Gross P, Westrick ML, McNerney JM. Experimental tuberculosilicosis. A comparison of the effects produced by some of its various pathogenic compounds. Am Rev Respir Dis 1961; 83:510–527. [DOI] [PubMed] [Google Scholar]
- 22.Barboza CE, Winter DH, Seiscento M, et al. Tuberculosis and silicosis: epidemiology, diagnosis and chemoprophylaxis. J Bras Pneumol 2008; 34:959–966. [DOI] [PubMed] [Google Scholar]
- 23.Cowie RL. The epidemiology of tuberculosis in gold miners with silicosis. Am J Respir Crit Care Med 1994; 150:1460–1462. [DOI] [PubMed] [Google Scholar]
- 24.Mosquera JA, Rodrigo L, Gonzálvez F. The evolution of pulmonary tuberculosis in coal miners in Asturias, northern Spain. An attempt to reduce the rate over a 15-year period, 1971–1985. Eur J Epidemiol 1994; 10:291–297. [DOI] [PubMed] [Google Scholar]
- 25.Calvert GM, Rice FL, Boiano JM, et al. Occupational silica exposure and risk of various diseases: an analysis using death certificates from 27 states of the United States. Occup Environ Med 2003; 60:122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leung CC, Yam WC, Yew WW, et al. T-Spot.TB outperforms tuberculin skin test in predicting tuberculosis disease. Am J Respir Crit Care Med 2010; 182:834–840. [DOI] [PubMed] [Google Scholar]
- 27.Ehrlich RI, Myers JE, te Water Naude JM, et al. Lung function loss in relation to silica dust exposure in South African gold miners. Occup Environ Med 2011; 68:96–101. [DOI] [PubMed] [Google Scholar]
- 28.[No authors listed]. A controlled clinical comparison of 6 and 8 months of antituberculosis chemotherapy in the treatment of patients with silicotuberculosis in Hong Kong. Hong Kong Chest Service/Tuberculosis Research Centre, Madras/British Medical Research Council. Am Rev Respir Dis 1991; 143:262–267. [DOI] [PubMed] [Google Scholar]
- 29.Ross J, Ehrlich RI, Hnizdo E, et al. Excess lung function decline in gold miners following pulmonary tuberculosis. Thorax 2010; 65:1010–1015. [DOI] [PubMed] [Google Scholar]
- 30.Tse LA, Yu IT, Qiu H, et al. Joint effects of smoking and silicosis on diseases to the lungs. PLoS One 2014; 9:e104494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu TN, Liou SH, Shen CY, et al. Occupational disease surveillance in Taiwan. Lancet 1996; 348:827. [DOI] [PubMed] [Google Scholar]


