Abstract
This study aims to describe the unique characteristics of absorption fever in patients with a hematoma after percutaneous renal biopsy (PRB) and distinguish it from secondary infection of hematoma.
We retrospectively studied 2639 percutaneous renal biopsies of native kidneys. We compared the clinical characteristics between 2 groups: complication group (gross hematuria and/or perirenal hematoma) and no complication group. The axillary temperature of patients with a hematoma who presented with fever was measured at 06:00, 10:00, 14:00, and 18:00. The onset and duration of fever and the highest body temperature were recorded. Thereafter, we described the time distribution of absorption fever and obtained the curve of fever pattern.
Of 2639 patients, PRB complications were observed in 154 (5.8%) patients. Perirenal hematoma was the most common complication, which occurred in 118 (4.5%) of biopsies, including 74 small hematoma cases (thickness ≤3 cm) and 44 large hematoma cases (thickness >3 cm). Major complications were observed in only 6 (0.2%) cases resulting from a large hematoma. Of 118 patients with a perirenal hematoma, absorption fever was observed in 48 cases. Furthermore, large hematomas had a 5.23-fold higher risk for absorption fever than the small ones.
Blood pressure, renal insufficiency, and prothrombin time could be risk factors for complications. Fever is common in patients with hematoma because of renal biopsy and is usually noninfectious. Evaluation of patients with post-biopsy fever is necessary to identify any obvious infection sources. If no focus is identified, empiric antibiotic therapy should not be initiated nor should prophylactic antibiotics be extended for prolonged durations. Absorption fevers will resolve in time without specific therapeutic interventions.
Keywords: absorption fever, complication, hematoma, renal biopsy
1. Introduction
Percutaneous renal biopsy (PRB) introduced by Iversen and Bran[1] has become a key diagnostic technique in the study of kidney disease. However, its invasive nature results in potential complications. Perirenal hematoma is the most common complication, which has been studied in several articles.[2–7] In clinical practice, we observed that absorption fever is a common clinical sign in patients with a hematoma after PRB. Absorption fever can aggravate the suffering and psychological pressure of patients, cause antibiotic abuse, and result in prolonged bed rest. Furthermore, we also found that absorption fever related to hematoma after PRB is unique compared with absorption fever after surgical operations[8]; however, few articles describe this phenomenon. Thus, in this study, we reported 118 cases of biopsy-related perirenal hematoma from January 2004 to October 2013 in Tongji Hospital and described the unique features of the associated absorption fever.
2. Materials and methods
We retrospectively reviewed nearly all cases of PRB of native kidneys in our institution from January 2004 to October 2013. Information regarding biopsy-related complications was lacking in 252 reports; thus, these cases were excluded. A total of 2639 cases were included in our analysis. A complete laboratory examination before the biopsy was performed in all patients, including routine blood count, urine analysis, functional renal evaluation, coagulation parameters, and renal ultrasound. In general, PRB was performed in patients with a normal prothrombin time (PT) and activated partial thromboplastin time (APTT). Aspirin and other nonsteroidal anti-inflammatory drugs and anticoagulants were discontinued 1 week before the biopsy. Patients with hypertension received antihypertensive agents, and biopsy was performed only if blood pressure was under controlled (<160/90 mmHg). Moreover, PRB was contraindicated in patients with contracted kidneys (length of kidney <9.5 cm) or solitary kidneys. Informed consent was obtained from all patients. This study was approved by the Ethical Committee of Tongji Hospital, Tongji Medical College, Hubei, China.
All biopsies, guided by real-time ultrasonography, were performed by experienced nephrologists. After biopsy, the patients were advised for bed rest for at least 24 h. Postbiopsy urine samples were inspected visually by the physician for gross hematuria, and persistent gross hematuria was considered with a duration >24 h. Vital signs and symptoms of complications, such as intense abdominal pain (pain rating of 4–10), hypotension, and fever (temperature >37.3°C), were closely monitored. Blood count and renal ultrasound were only performed for clinical symptoms (pain, persistent gross hematuria, hypotension, fever).
We defined bleeding complications as gross hematuria and/or perirenal hematoma. Baseline values, including age, sex, pre-PRB blood pressure (<140/90 mmHg), hemoglobin (Hb) (male, 130–175 g/L; female, 115–150 g/L), PT (11–13 s), APTT (31–43 s), and serum creatinine (Scr) (male, 59–104 μmol/L; female, 45–84 μmol/L), between patients with and without complications were compared. Hematomas were classified according to their size, as previously described[2]: small (thickness ≤3 cm) and large (thickness >3 cm), and post-PRB blood pressure, hemoglobin, and temperature of the patients were monitored. We measured the axillary temperature at 06:00, 10:00, 14:00, and 18:00 in patients with fever owing to perirenal hematoma and recorded the onset and duration of fever and the highest body temperature. We distinguished infection from absorption fever based on the patients’ symptoms and signs and results of laboratory examinations, imaging tests, and pathogen tests. Patients who presented with cough, purulent sputum, pulmonary crackles, increased white blood cell count (>10 × 109 cells/L) and neutrophil ratio (>75%), radiographic changes, and sputum culture indicating evidence of pathogenic organism by Gram-staining were diagnosed as having pulmonary infection. Those who presented with persistent severe lumbago/abdominal pain (>7 pain rating) and remittent fever, increased white blood cell count (>10 × 109 cells/L) and neutrophils ratio (>75%), and ultrasonography-guided aspiration indicating evidence of pathogenic organism were diagnosed as having secondary infection of hematoma. After cases of infection were excluded, we described the time distribution of absorption fever, compared the incidence and characteristics of absorption fever in small and large hematomas, and obtained the curve of fever pattern.
Statistical analysis was performed using the SPSS 17 package (SPSS Inc, Chicago, IL). Values were expressed as mean ± SD and percentages. Kolmogorov–Smirnov test revealed that the samples were normally distributed. The optimal cutoff point to dichotomize each variable was determined. Some articles have reported that the risk of postbiopsy bleeding was higher in women, younger, and older subjects, and in patients with hypertension, renal insufficiency, low pre-PRB hemoglobin, and higher PT or APTT.[2,6–8,15–21] In this study, we used following reference variables: male, 18- to 59 years’ old, systolic blood pressure (SBP) <140 mmHg, Scr ≤177 μmol/L, pre-Hb ≥110 g/L, and PT <12 s, APTT <37 s. To test for potential differences in complication rate, we used the χ2 test or Fisher exact test when the assumption underlying statistical tests was satisfied. Subsequently, unadjusted relative risk analyses were performed and reported as odds ratio with 95% confidence interval. P values <0.05 were considered significant.
3. Results
3.1. Baseline demographic, clinical, and laboratory data
The mean age of the 2639 patients who underwent PRB of native kidneys was 33 ± 12 years (range = 11–74 years; 7.4% were <18 years, 12.0% were ≥50 years, 2.4% were ≥60 years). A total of 1213 (46.0%) were males and 1426 (54.0%) females. The pre-PRB SBP was ≥ mmHg in 20.8% of cases. The pre-PRB hemoglobin was 127 ± 21 g/L and the pre-PRB hemoglobin was <110 g/L in 475 cases (18.0%). The platelet count was <100 × 109 cells/L in 158 cases (6.0%). The Scr was 86.6 ± 76.8 μmol/L, >177 μmol/L, 442 μmol/L, 707 μmol/L in 127 (4.8%), 24 (0.9%), and 13 (0.5%) cases, respectively. PT and APTT were in the normal range.
Clinical and laboratory data of patients with bleeding complications and perirenal hematomas are summarized in Tables 1 and 2. Bleeding complications, perirenal hematoma, and gross hematuria occurred in 154 (5.8%), 118 (4.5%), and 52 (2.0%) cases, respectively. Small hematoma was observed in 74 patients, and large hematoma in 44 patients. Major complications (those necessitating transfusion of blood products, invasive procedures, and nephrectomy, or that could result in death) were observed in only 6 (0.2%) cases, all of which required blood transfusion because of hypotension. Angiography was needed in 3 patients, and 2 of them were treated by embolization. No nephrectomy or death was reported in this study.
Table 1.
Clinical data of patients with and without post-PRB bleeding complications.
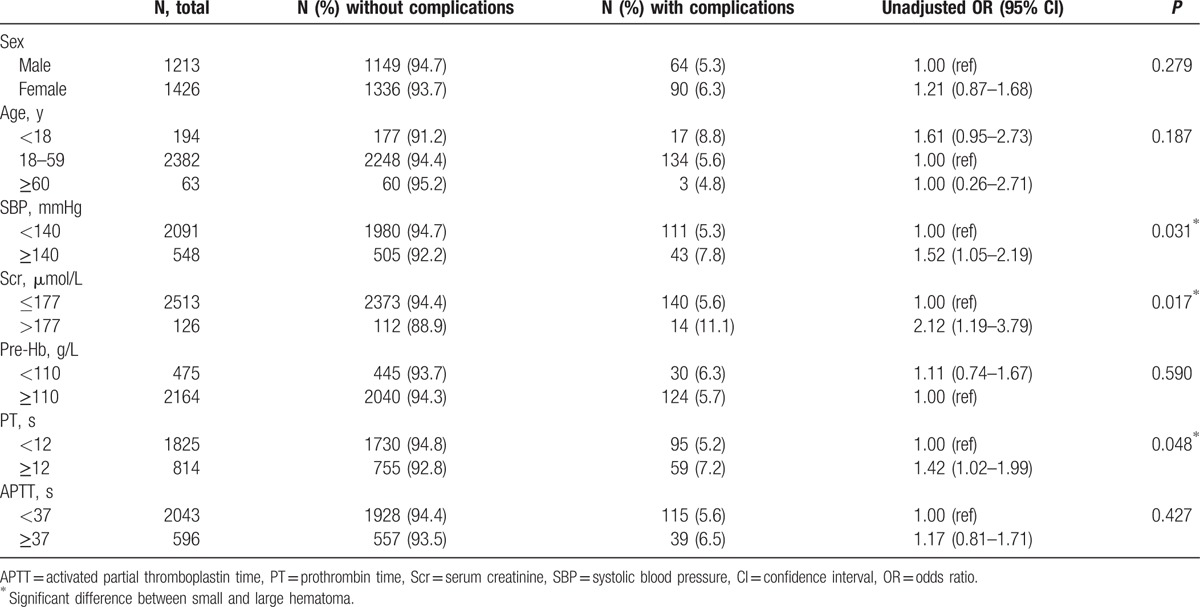
Table 2.
Clinical data of patients with small and large hematomas.
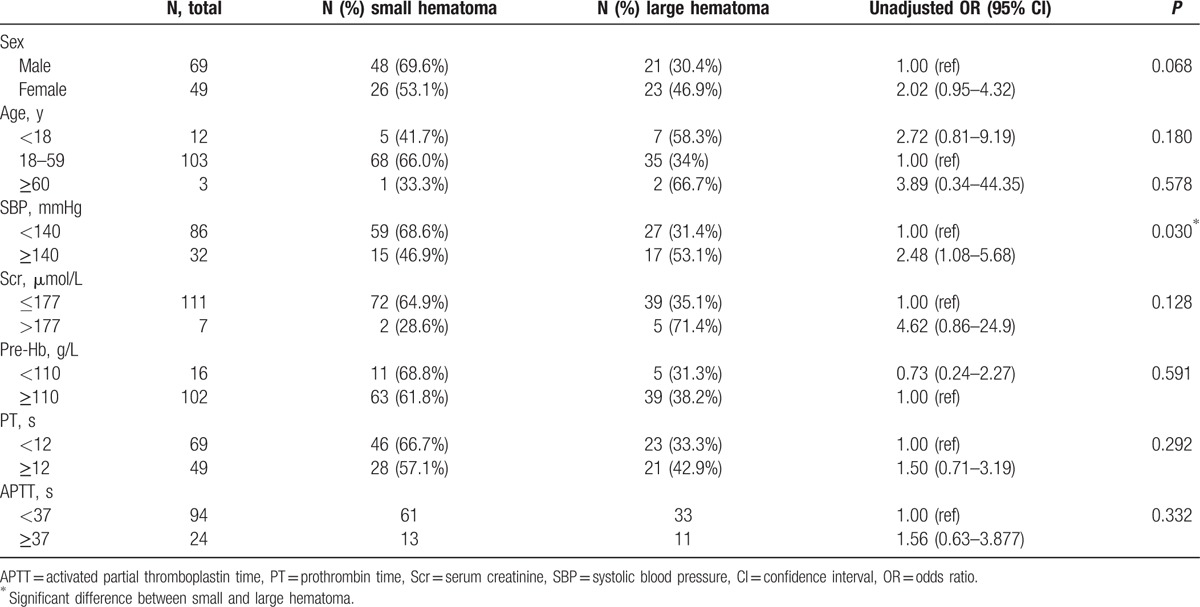
To test for potential differences in complication rate, we used the χ2 test. The assumption underlying statistical tests was satisfied. No significant differences in sex, age, pre-PRB hemoglobin, and APTT were observed between patients with and without complications. However, patients with complications had higher SBP, Scr, and PT before PRB (Table 1). Furthermore, we found that patients with a large hematoma had higher SBP than those with a small hematoma (Table 2).
3.2. Absorption fever owing to perirenal hematoma after PRB
Of 118 patients who developed a perirenal hematoma, fever was observed in 57 cases, of which 9 were because of infection (pulmonary infection, 3; secondary infection of hematoma, 6). Thus, absorption fever was noted in the remaining 48 cases, accounting for 40.7% of all patients with perirenal hematoma.
Absorption fever characteristics are as follows: fever onset 4.51 ± 1.63 (1–7) days after PRB with a duration of 4.09 ± 2.47 (1–10) days and body temperature of 38.26°C ± 0.39°C (37.5°C –39.0°C). The time distribution of absorption fever is shown in Figure 1.
Figure 1.
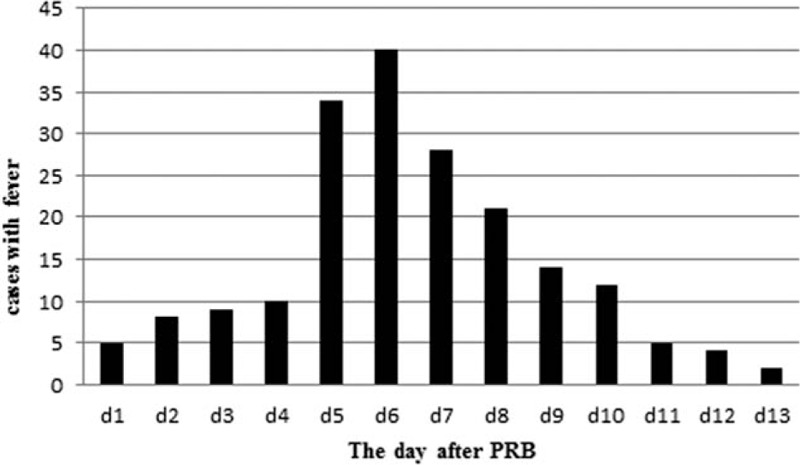
Time distribution of absorption fever. The cases of fever reach its peak on the sixth postbiopsy day.
After the exclusion of the 9 cases with infection, the remaining 109 cases with a hematoma were divided into 2 groups: small (thickness ≤3 cm, 70 cases) and large (thickness >3 cm, 39 cases). Twenty-one (30%) patients with small hematomas and 27 (69.2%) cases with large hematomas developed absorption fever. Patients with a large hematoma were 5.25 times more likely to develop absorption fever compared with those with a small hematoma (P < 0.001) (Table 3). Furthermore, we compared the absorption fever characteristics between small and large hematomas (Table 4). No differences were observed in fever onset and duration and the highest body temperature between the 2 groups.
Table 3.
Frequency of absorption fever in small and large hematomas.

Table 4.
Comparison of absorption fever between small and large hematomas.

We recorded the axillary temperature of patients with absorption fever at 06:00, 10:00, 14:00, and 18:00 every day, according to the temperature chart. Thereafter, we confirmed the unilateral 95% ceiling of temperature for each timing and connected them to obtain the curve of fever pattern (Fig. 2).
Figure 2.
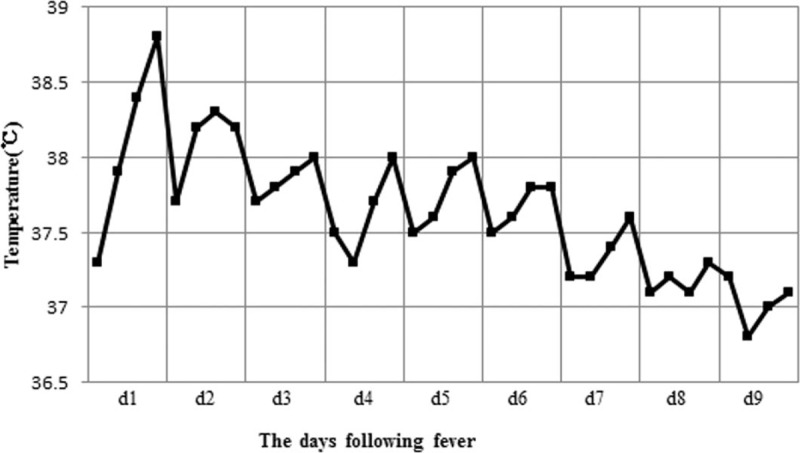
The curve of absorption fever pattern. Body temperature peaks on the first day of fever and gradually falls each day. Fever generally lasts <1 week.
4. Discussion
4.1. Low incidence of postrenal biopsy bleeding complications
With the use of automated spring loaded biopsy device guided by real-time ultrasonography, PRB has become safe and reliable. In previous studies, perirenal hematoma incidence ranged from 11% to 91%,[3–7] gross hematuria occurred in 4.5% to 20% of biopsies,[7,9–12] and major complications were observed in 0.36% to 6.6% of patients.[7,9,11,13–16] In our study of 2639 patients, the incidence of bleeding complications was lower than that previously reported. Perirenal hematoma, gross hematuria, and major complications occurred in 118 (4.5%), 52 (2.0%), and 6 (0.2%) cases, respectively. We must highlight that no nephrectomy or death was reported in this study. The low complication rate can be attributed to the biopsies performed by experienced nephrologists (each of them performed >100 biopsies annually) with the guidance of real-time ultrasonography operated by the skilled radiologists, which ensured good coordination. However, we followed a strict patient selection protocol for PRB, carefully evaluated the risk of complications, and excluded high-risk patients. The pre-PRB blood pressure of most patients was controlled (160/90 mmHg). Only 6.0% of cases had a PLT <100 × 109 cells/L. The PT and APTT were in the normal range in nearly all patients. Furthermore, we ensured that all patients discontinued anti-platelet drugs, anticoagulants, or other medicines that may affect blood coagulation at least 1 week before the biopsy. Contraindications for biopsy included contracted or solitary kidneys.
4.2. Potential risk factors of PRB-related bleeding
Recently, a few articles have analyzed the risk factors of complications, such as age, female, hypertension, renal insufficiency, low pre-PRB hemoglobin, coagulation disorders.[2,6,7,9,16–22] In this study, the following variables were found to be significantly associated with bleeding complications: SBP ≥140 mmHg, Scr >177 μmol/L, and PT ≥12 s (Table 1). Moreover, SBP was significantly higher in patients with a large hematoma than in those with a small hematoma, thereby supporting that hypertension is a risk factor for PRB-related bleeding.
4.3. Absorption fever due to PRB-related hematoma
In clinical practice, we observed that patients with a PRB-related hematoma were predisposed to a fever (about 41% of all hematomas), which is similar to the absorption fever after surgical operations. Fever is common within the first 48 h after any major operation, which is often noninfectious in origin, is typically related to surgical tissue trauma, and resolves within 3 days without any specific therapeutic interventions.[8,23] In this study, we found that absorption fever because of hematoma occurred almost 4 to 6 days after biopsy, and the duration was longer (mean, 4.09 ± 2.47 days). The above-mentioned difference may be because of hemorrhage. Furthermore, we found that the large hematoma had 5.23-fold higher risk for an absorption fever than the small ones. However, the size of hematoma had no influence on the onset and duration of fever and the highest body temperature.
Absorption fever can increase metabolism, increase oxygen and energy consumption, quicken the loss of moisture, aggravate the suffering and psychological pressure of patients, and extend hospital stay.[24] Moreover, absorption fever is often confused with secondary infection of hematoma, causing antibiotic abuse. According to the time distribution of absorption fever shown in Figure 1, the cases of fever reached its peak on the sixth postbiopsy day, and measurements can be taken in advance, by increasing the temperature measurement frequency. Figure 2 shows the curve of fever pattern with the following features: body temperature is higher in the afternoon than in the morning and always reaches the highest at dusk, the temperature usually peaks on the first day of fever (<39°C) and gradually falls each day, fever generally lasts <1 week, and the everyday temperature fluctuates within 1°C. A differential diagnosis from secondary infection of hematoma is necessary when the temperature rises up to 39°C, duration of fever is >1 week, temperature fluctuates widely or the temperature peak elevates every day. The prognosis of absorption fever, which can be alleviated with antipyretics or other symptomatic relief and supportive treatment, is good. The characteristics of a hematoma-related absorption fever described previously provide the basis for medical interventions. Mastery on the regularity of absorption fever prevents a series of meaningless examinations for differential diagnosis and an excessive antibiotic treatment, which in turn conserves medical resources and reduces the economical burden of patients, and enables clinical physicians to identify an abnormal fever pattern and discover the case of infection timely.
5. Conclusion
The rate of major complications in our center was 0.2%. Blood pressure, renal insufficiency, and PT could be risk factors for complications. Fever is common in patients with hematoma because of renal biopsy and is usually noninfectious. Patients with postbiopsy fevers should be evaluated to identify any obvious infection sources. If no focus is identified, empiric antibiotic therapy should not be initiated nor should prophylactic antibiotics be extended for prolonged durations. Absorption fevers will resolve in time without specific therapeutic interventions.
Acknowledgments
None.
Footnotes
Abbreviations: APTT = activated partial thromboplastin time, BUN = blood urea nitrogen, DBP = diastolic blood pressure, Hb = hemoglobin, NSAIDs = non-steroidal anti-inflammatory drugs, PLT = blood platelet, PRB = percutaneous renal biopsy, PT = prothrombin time, SBP = systolic blood pressure, Scr = serum creatinine.
None of the authors have any conflicts of interest.
References
- 1.Iversen P, Brun C. Aspiration biopsy of the kidney. Am J Med 1951; 11:324–330. [DOI] [PubMed] [Google Scholar]
- 2.Jackson GG, Poirier KP, Grieble HG. Concept of pyelonephritis: experience with renal biopsies and long-term clinical observations. Ann Intern Med 1957; 47:1165–1183. [DOI] [PubMed] [Google Scholar]
- 3.Rosenbaum R, Hoffsten PE, Stanley RJ, et al. Use of computerized tomography to diagnose complications of percutaneous renal biopsy. Kidney Int 1978; 14:87–92. [DOI] [PubMed] [Google Scholar]
- 4.Ralls PW, Barakos JA, Kaptein EM, et al. Renal biopsy-related hemorrhage: frequency and comparison of CT and sonography. J Comput Assist Tomogr 1987; 11:1031–1034. [DOI] [PubMed] [Google Scholar]
- 5.Christensen J, Lindequist S, Knudsen DU, et al. Ultrasound-guided renal biopsy with biopsy gun technique-efficacy and complications. Acta Radiol 1995; 36:276–279. [PubMed] [Google Scholar]
- 6.Manno C, Strippoli GF, Arnesano L, et al. Predictors of bleeding complications in percutaneous ultrasound-guided renal biopsy. Kidney Int 2004; 66:1570–1577. [DOI] [PubMed] [Google Scholar]
- 7.Eiro M, Katoh T, Watanabe T. Risk factors for bleeding complications in percutaneous renal biopsy. Clin Exp Nephrol 2005; 9:40–45. [DOI] [PubMed] [Google Scholar]
- 8.Garibaldi RA, Brodine S, Matsumiya S, et al. Evidence for the non-infectious etiology of early postoperative fever. Infect Control 1985; 6:273–277. [DOI] [PubMed] [Google Scholar]
- 9.Korbet SM, Volpini KC, Whittier WL. Percutaneous renal biopsy of native kidneys: a single-center experience of 1,055 biopsies. Am J Nephrol 2014; 39:153–162. [DOI] [PubMed] [Google Scholar]
- 10.Stratta P, Canavese C, Marenqo M, et al. Risk management of renal biopsy: 1387 cases over 30 years in a single centre. Eur J Clin Invest 2007; 37:954–963. [DOI] [PubMed] [Google Scholar]
- 11.Whittier WL, Korbert SM. Renal biopsy: update. Curr Opin Nephrol Hypertens 2004; 13:661–665. [DOI] [PubMed] [Google Scholar]
- 12.Rianthavorn P, Kerr SJ, Chiengthong K. Safety of paediatric percutaneous native kidney biopsy and factors predicting bleeding complications. Nephrology (Carlont) 2014; 19:143–148. [DOI] [PubMed] [Google Scholar]
- 13.Preda A, Van Dijk LC, Van Oostaijen JA, et al. Complication rate and diagnostic yield of 515 consecutive ultrasound-guided biopsies of renal allografts and native kidneys using a 14-gauge biopsy gun. Eur Radiol 2003; 13:527–530. [DOI] [PubMed] [Google Scholar]
- 14.Mackinnon B, Fraser E, Simpson K, et al. Is it necessary to stop antiplatelet agents before a native renal biopsy? Nephrol Dial Transplant 2008; 23:3566–3570. [DOI] [PubMed] [Google Scholar]
- 15.Soares SM, Fervenza FC, Lager DJ, et al. Bleeding complications after transcutaneous kidney biopsy in patients with systemic amyloidosis: single-center experience in 101 patients. Am J Kidney Dis 2008; 52:1079–1083. [DOI] [PubMed] [Google Scholar]
- 16.Whittier WL, Korbet SM. Timing of complications in percutaneous renal biopsy. J Am Soc Nephrol 2004; 15:142–147. [DOI] [PubMed] [Google Scholar]
- 17.Tøndel C, Vikse BE, Bostad L, et al. Satety and complications of percutaneous kidney biopsies in 715 children and 8573 adults in Norway 1988–2010. Clin J Am Soc Nephrol 2012; 7:1591–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corapi KM, Chen JL, Balk EM, et al. Bleeding complications of native kidney biopsy: a systematic review and meta-analysis. Am J Kidney Dis 2012; 60:62–73. [DOI] [PubMed] [Google Scholar]
- 19.Shidham GB, Siddiqi N, Beres JA, et al. Clinical risk factors associated with bleeding after native kidney biopsy. Nephrology (Carlton) 2005; 10:305–310. [DOI] [PubMed] [Google Scholar]
- 20.Torres Muñoz A, Valdez-Ortiz R, González-Parra C, et al. Percutaneous renal biopsy of native kidneys: efficiency, safety and risk factor associated with major complications. Arch Med Sci 2011; 7:823–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pendón-Ruiz de Mier MV, Espinosa-Hernández M, Rodelo-Haad C, et al. Prospective study of the complications associated with percutaneous renal biopsy of native kidneys experience in a centre. Nefrologia 2014; 34:383–387. [DOI] [PubMed] [Google Scholar]
- 22.Ishikawa E, Nomura S, Hamaguchi T, et al. Ultrasonography as a predictor of overt bleeding after renal biopsy. Clin Exp Nephrol 2009; 13:325–331. [DOI] [PubMed] [Google Scholar]
- 23.Rhee C, Sax PE. Evaluation of fever and infections in cardiac surgery patients. Semin Cardiothorac Vasc Anesth 2015; 19:143–153. [DOI] [PubMed] [Google Scholar]
- 24.Christabel A, Sharma R, Manikandhan R, et al. Fever after maxillofacial surgery: a critical review. J Maxillofac Oral Surg 2015; 14:154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]


