Abstract
Idiopathic membranous nephropathy (IMN) is known to be associated with antibodies acting on the M-type phospholipase A2 receptor (PLA2R) of the podocyte. However, the mechanism underlying de novo membranous nephropathy (dn MN) posttransplantation remains unclear. In this study, we aimed to elucidate the mechanism underlying dn MN.
We selected 8 cases with dn MN and compared them to 20 IMN cases. Fifteen cases of stable grafts were selected as controls.
Several differences between the dn MN group and the IMN group were detected. IgG4 showed negligible positive staining in patients with dn MN, while it was predominant in the IMN group (1/8 vs 20/20, P < 0.001). Serum anti-PLA2R antibodies and anti-PLA2R antibodies of the podocyte were very few in the dn MN patients; however, these antibodies were detected in most of the IMN patients (serum anti-PLA2R antibodies: 1/8 vs 16/20, P = 0.002, anti-PLA2R antibodies of the podocyte: 0/8 vs 17/20, P < 0.001). The dn MN patients also showed higher ratio of interstitial inflammation, peritubular capillaritis, and peritubular capillary C4d deposition. Importantly, human leukocyte antigens (HLA)-DR expression was detected on the podocytes in most of the dn MN patients, but none of the IMN patients and stable graft patients showed HLA-DR expression.
These data suggested that the PLA2R pathway, which is known to play a role in IMN, was not involved in the mechanism underlying dn MN. On the contrary, dn MN might be associated with the alloimmune response directed against the podocyte.
Keywords: membranous nephropathy, M-type phospholipase A2 receptor, podocyte, renal transplantation
1. Introduction
Recurrent glomerulonephritis can influence renal allograft survival, which was found to be the 3rd most common cause of graft loss in recent reports.[1] Membranous nephropathy (MN) is one of the most common forms of glomerulonephritis found in renal transplant recipients, and recurrent MN occurs as frequently as 42% to 44%, as reported in previous literatures.[2–4] Recent studies revealed that M-type phospholipase A2 receptor (PLA2R) was expressed on the podocyte in idiopathic membranous nephropathy (IMN), which suggested that PLA2R played a role in IMN; subsequently, the PLA2R pathway was verified to be responsible for the pathogenesis of IMN.[5–7] Recent studies disclosed that recurrent MN, which is a type of posttransplant MN, might be associated with the PLA2R antibody in the recipients.[8–10] In addition to recurrent MN, many patients develop de novo membranous nephropathy (dn MN), which is a type of posttransplant MN, but is different from recurrent MN. Currently, the etiology of dn MN is controversial.[11–13]
The discovery of the anti-PLA2R antibody resulted in a great step forward in the elucidation of the etiology of MN, especially when PLA2R was identified to play a key role in IMN.[5,6] Recent studies revealed that the PLA2R pathway in podocytes was related to IMN. Anti-PLA2R antibodies could be detected in the serum of IMN patients, and the titer of the PLA2R antibody was strongly associated with the activity and prognosis of IMN.[5–8] These studies proved that the PLA2R pathway in podocytes plays a crucial role in the pathogenesis of IMN. Unlike IMN, secondary MN could be caused by several diseases such as systemic lupus erythematosus, HBV infection, and carcinoma.[14] The secondary MN in such cases was caused by autoantibodies, virus-mediated antigen-antibodies reaction, and carcinoma antigen-mediated immune reactions.[15]
Larsen and Walker[16] showed that recurrent MN was strongly correlated with PLA2R positive staining on the podocyte of renal allografts. However, PLA2R staining was barely observed in dn MN,[16] which drove us to ask if there might be different pathways causing MN posttransplantation. Human leukocyte antigen (HLA)-DR is an MHC class II cell surface receptor encoded by the HLA. The complex of HLA-DR and its ligand constitute a ligand for T-cell receptors. HLA was original defined as a cell surface antigen that mediates graft-versus-host disease, which causes the rejection of tissue transplants in HLA-mismatched donors. HLA-DR staining was has been found to be positive on the endothelium and renal tubular epithelial cell in acute or chronic rejection.[17–20] HLA-DR has also been detected in cellular and humoral rejection.[21,22] More interestingly, HLA-DR is also known to be expressed on the podocytes in some types of primary glomerulonephritis such as focal segmental glomerulosclerosis, minimal change disease, and mesangial proliferative glomerular nephropathy, but is not expressed on the podocytes in IMN patients.[23–25]
Therefore, we hypothesized that dn MN was related to the alloimmunization response to the podocyte of the renal allograft, which was different from the traditional antibody-mediated rejection. Accordingly, we compared the morphological features, PLA2R expression, and HLA-DR staining on the podocyte of IMN and dn MN patients to elucidate the pathways underlying these 2 conditions.
2. Patients and methods
2.1. Patients
Between May 2006 and June 2014, 8 patients were diagnosed with posttransplant dn MN at the Jinling Hospital, Nanjing University School of Medicine, Nanjing, China. We applied the following inclusion criteria for selecting the patients for our study: light microscopy, immunofluorescence, and electron microscopy confirmed MN; allograft biopsy at the time of transplantation excluded donor glomerulonephritis including MN; the recipient showed no evidence of MN before transplantation; and HBV antigens or HCV antibodies were negative, as determined by immunohistochemical staining; electron microscopy showed no granular electron-dense deposits associated with HBV or HCV. The primary kidney diseases, as determined by biopsy, included IgA nephropathy (IgAN; n = 3), minimal change disease (n = 1), diabetic nephropathy (n = 1), and mesangial proliferative glomerular nephropathy (n = 1). Two patients who did not receive renal biopsy only had hematuria, and the sera (pretransplantation) of 2 patients were negative for the PLA2R antibody; therefore, we considered that the primary kidney diseases of 2 patients were excluded with IMN. During the same period, 20 patients with biopsy-confirmed IMN were selected after age- and gender-matching. Fifteen patients with stable grafts were selected as the control group.
This study was approved by the ethics committee or institutional review board of the Jinling Hospital (approved ethics number: 2014NZGKJ-057).
2.2. Histological examination
Renal tissues were stained with hematoxylin-eosin, periodic acid-Schiff, periodic acid-silver methenamine, and Masson for light microscopic examination. The evaluation of the histological injuries was performed according to the Banff 2013 criteria.[26]
2.3. Immunohistochemistry and immunofluorescence (IF) staining
Cryosections of the kidney biopsies were tested for immunoglobulins and complement deposition by using direct IF. The IgG subclasses were evaluated by indirect IF with monoclonal antibodies directed to IgG1, IgG2, IgG3, and IgG4 in the all patients. A mouse antihuman IgG 1–4 monoclonal antibody (Sigma–Aldrich) was used as the primary antibody. PLA2R expression was assessed in the glomerular deposits by confocal microscopy in the paraffin-embedded biopsy samples obtained from each patient using rabbit affinity purified specific anti-PLA2R antibodies (Atlas antibodies) followed by the use of goat Alexa 488-conjugated antirabbit Fab IgG (Molecular Probes). Circulating antibodies against PLA2R were assessed using the commercially available indirect IF test (Euroimmun). The biopsy samples were also stained for HLA-DR (Dako) and Wilms’ tumor-1 (WT-1) (Santa Cruz), which is termed as a podocyte specific protein.
The clinical and pathological characteristics of all the patients were studied, and specific immunohistochemistry and IF staining results were analyzed for each case.
2.4. Statistical analyses
Values were represented as mean ± standard error (SE). Statistical analysis was performed using the Student t test or nonparametric Mann–Whitney U test for comparison of continuous variables between 2 groups. All the analyses were performed using the SPSS 16.0 package. A P value <0.05 was considered statistically significant.
3. Results
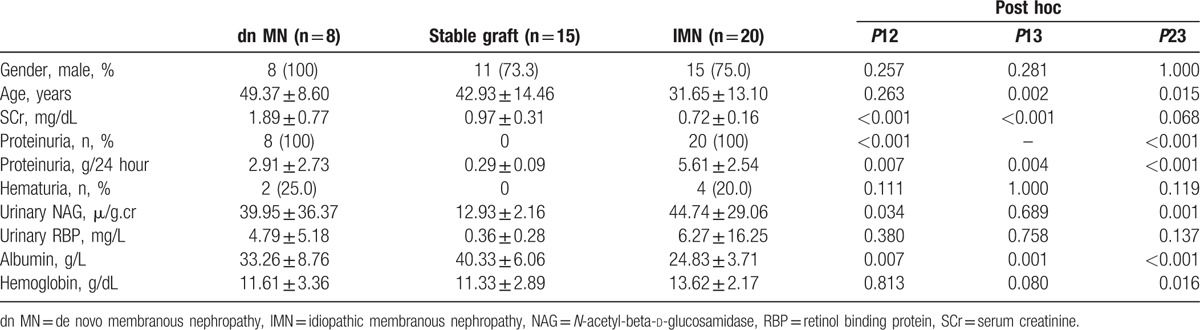
3.1. Clinical profiles
The period between the transplantation and the renal biopsy in patients diagnosed with posttransplant dn MN ranged from 7 to 87 months (41.75 ± 26.28 months). The clinical profiles of the 3 groups are summarized in Table 1. The mean ages of the transplant patients (in the dn MN group and stable graft group) were more than that of the patients in the IMN group. The dn MN patients had the highest level of serum creatinine at the time of biopsy. Compared to the IMN patients, the dn MN patients showed less proteinuria (2.84 ± 2.79 g/24 hour vs 5.61 ± 2.54 g/24 hour, P = 0.018) and a higher level of serum albumin (33.26 ± 8.76 g/L vs 24.83 ± 3.71 g/L, P = 0.030).
Table 1.
Clinical profiles of dn MN and IMN patients.
3.2. Histological characteristics
The histological features of the 3 groups are shown in Table 2. Compared with the patients in the IMN group, the patients in the dn MN group had a marginally higher ratio of glomerulitis (37.5% vs 5.0%, P = 0.058) and a significantly higher ratio of tubulitis (50.0% vs 0, P = 0.003). However, the incidences of these lesions were similar between the dn MN group and the stable graft group. It is notable that the dn MN group had a high prevalence of interstitial infiltrate and peritubular capillaritis; according to the Banff 2013 diagnostic criteria, 87.5% of the patients in the dn MN group had interstitial infiltrate and 62.5% had peritubular capillaritis, both of which were significantly higher than that observed in the other 2 groups (Table 2). With regard to chronic lesions, the patients in the dn MN group had a higher ratio of interstitial fibrosis than the patients in the IMN group (87.5% vs 40.0%, P = 0.038), but the incidences of tubular atrophy were similar between these 2 groups (62.5% vs 30.0%, P = 0.200). The pathology images obtained from the dn MN and IMN patients are shown in Fig. 1A and B, respectively.
Table 2.
Histological characteristics of dn MN and IMN patients.
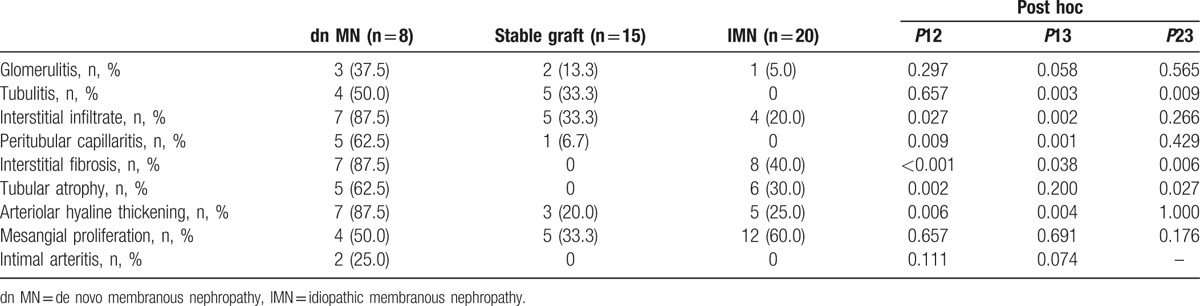
Figure 1.
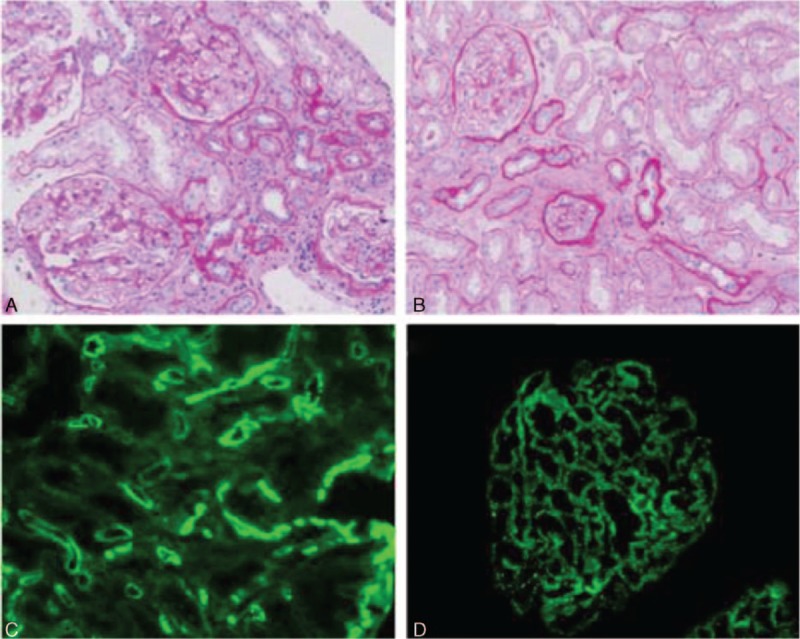
Characteristics of dn MN and IMN patients posttransplantation, as observed by light microscopy and immunofluorescence. Inflammatory cell infiltrations were observed more frequently in the dn MN patients (A) than in the IMN patients (B). (C) C4d deposition in the peritubular capillary walls of the dn MN patients. (D) Deposition of IgG4 in the glomerular capillary walls of the IMN patients. Original magnification: A and B, ×200; C and D, ×400. dn MN = de novo membranous nephropathy, IMN = idiopathic membranous nephropathy.
3.3. IF staining
IF staining was performed to examine the presence of immune complex deposits and IgG subtypes. It is important to note that peritubular capillary C4d deposition was detected in 5 of the 8 dn MN patients (62.5%), which was not observed in the other 2 groups (P = 0.002 compared with the stable graft group; P = 0.001 compared with the IMN group). Compared with the patients in the stable graft group, the patients in the dn MN group had a higher ratio of IgG and C3 deposition (100% vs 6.7%, P < 0.001 for IgG deposition, 75.0% vs 0%, P < 0.001 for C3 deposition). There were no significant differences in the levels of IgG, IgA, and IgM, and in C3 and C1q deposition between the patients in the dn MN group and those in the IMN group (Table 3). The results of IF staining are shown in Table 3.
Table 3.
Immunofluorescence findings of dn MN and IMN patients.
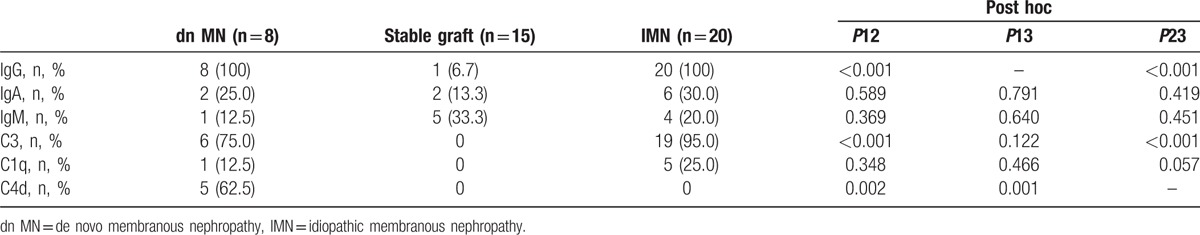
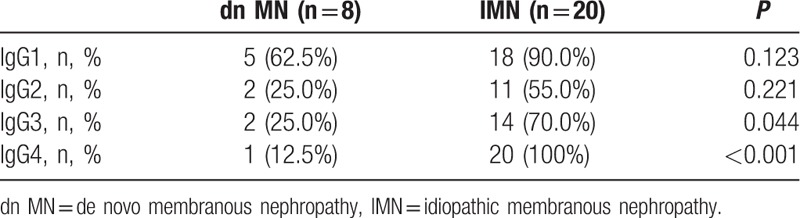
With regard to the IgG subtypes, a predominance of IgG1 deposition was apparent in the patients in the dn MN group (62.5%), and a predominance of IgG4 deposition was observed in the IMN group (100%). Only 1 patient in the dn MN group had IgG4 deposition, the ratio of which was much lower than that in the IMN group (12.5% vs 100%, P < 0.001). The results of the examination of the IgG subtypes are shown in Table 4. Representative images of C4d and IgG4 staining are shown in Fig. 1C and D, respectively.
Table 4.
IgG subtypes of dn MN and IMN patients.
3.4. PLA2R and HLA-DR staining
In order to study the potential etiological pathways in dn MN, PLA2R and HLA-DR staining was performed on the serial sections used for histological analyses.
First, we used the immunofluorescence assay to assess the glomerular deposition of PLA2R antibodies. None of the patients in the dn MN group showed detectable glomerular PLA2R antibodies, which was predominant in the patients of the IMN group (0% vs 85.0%, P < 0.001). Representative images of PLA2R IF staining are shown in Fig. 2. Circulating PLA2R antibodies were also studied in the dn MN and IMN groups. Anti-PLA2R antibodies were detected in 16/20 (80.0%) patients in the IMN group, but were not observed in any of the patients in the dn MN group (P < 0.001).
Figure 2.
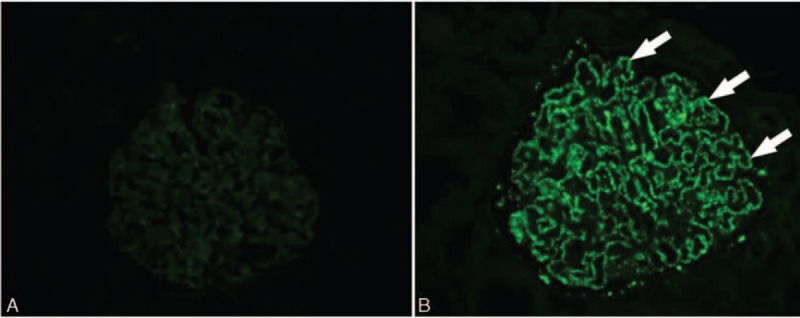
Immunofluorescence staining of PLA2R in the dn MN and IMN patients posttransplantation. (A) PLA2R showed negative staining (white arrows) on the podocytes in the dn MN group. (B) PLA2R showed positive staining in the glomerulus in the IMN group. Original magnification: ×400. dn MN = de novo membranous nephropathy, IMN = idiopathic membranous nephropathy, PLA2R = M-type phospholipase A2 receptor.
Second, HLA-DR antigens were assessed by immunohistochemical assay. WT-1 staining was used to identify podocytes. In the dn MN group, 75% of the patients had HLA-DR antigens expressed on the podocytes, but no HLA-DR expression could be observed in the podocytes of patients in the IMN group (75.0% vs 0%, P < 0.001). Representative images from HLA-DR and WT-1 staining are shown in Fig. 3.
Figure 3.
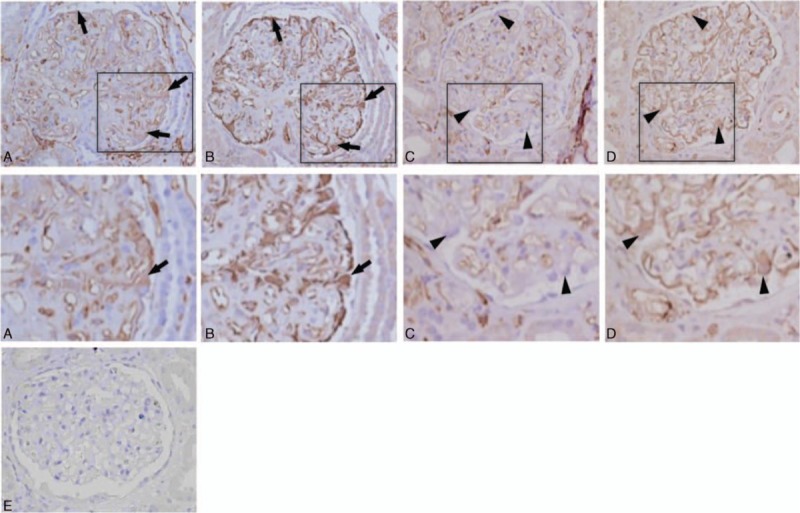
Immunohistochemical staining for HLA-DR and WT-1 in the serial sections. (A) HLA-DR showed positive staining (black arrows) on the podocytes in the dn MN group. (B) WT-1 staining of the same glomerulus from A indicates the position of the podocytes (black arrows). (C) HLA-DR showed less staining on the podocytes in the IMN group than in the dn MN group. Black arrowheads indicate HLA-DR-negative podocytes. (D) WT-1 staining of the same glomerulus from A indicates the position of the podocytes (black arrowheads). A′, B′, C′, and D′ are magnified images of A, B, C, and D, respectively. (E) Negative control staining without primary antibody. Original magnification: ×400. dn MN = de novo membranous nephropathy, HLA = human leukocyte antigen, IMN = idiopathic membranous nephropathy, WT-1 = Wilms’ tumor-1.
4. Discussion
MN that is found in renal allografts can be of 2 different types, recurrent MN and dn MN, according to the primary kidney disease pretransplant. Recurrent MN has been found to be related to the PLA2R pathway, which has been discussed in previous studies.[5,7,8,16] The titer of the PLA2R antibody in the serum could be a marker that can help predict recurrent risk and assess disease activity posttransplantation.[6] On the other hand, the mechanism of dn MN that occurs after renal transplantation is largely unknown. In this study, we aimed to identify the molecules and pathways involved in the pathogenesis of dn MN. We found that HLA-DR, and not PLA2R, is expressed on the podocytes in kidney allografts in dn MN.
The PLA2R antibody can barely be detected in dn MN patients after renal transplantation.[8,16] Therefore, Ponticelli and Glassock[13] hypothesized that hidden podocyte antigens might be exposed in the inflammatory environment of the allograft, which then presents the antigen to immune competent cells, thus taking part in glomerular injury. This hypothesis suggested that some de novo antigens might express and play key roles in dn MN posttransplantation.
In the current study, PLA2R staining was found to be negative on the podocytes of posttransplant dn MN patients, while HLA-DR staining was positive in the glomeruli colocated with the podocytes. It is well known that, under normal conditions, HLA-DR expression could be detected on glomerular endothelial cells and on some type of lymphocytes.[17–20] A previous study in the 1990s suggested that HLA-DR was not expressed on human podocytes,[27] but some recent studies demonstrated that HLA-DR expression could be detected on podocytes under certain conditions of primary glomerular diseases such as focal segmental glomerulosclerosis.[24,25] Our previous study showed that HLA-DR expression on the tubular epithelium was related to acute rejection.[23] However, HLA-DR expression on the podocyte in renal allografts has barely been reported before. In our current work, we detected HLA-DR expression on podocytes from dn MN patients; however, the podocytes of IMN patients did not show HLA-DR expression. Based on our data, we suggest that the expression of HLA-DR on podocytes might be a de novo pathogenic mechanism underlying dn MN.
The 2nd major finding of this study was the histological damage of rejection, especially humoral rejection, frequently found in the dn MN patients. Five of the 8 dn MN patients also showed C4d deposition on the peritubular capillaries, which was a marker of humoral rejection.[12] The glomerulitis, interstitial infiltrate, peritubular capillaritis, interstitial fibrosis, arteriolar hyaline thickening, and tubulitis were more severe and common in the dn MN patients than in the IMN patients. Humoral rejection lesions were barely detected in the recurrent IMN patients. Taken together, these results suggest that the HLA-DR expression on the podocytes might be related to alloimmunization on the podocytes. Under normal conditions, HLA is not exposed in the podocytes. However, under stimulation in the pathological state, these antigens will be exposed in the podocytes. If the recipient can produce antibodies against antigens presented on the podocytes, the podocytes might be damaged, leading to dn MN. Major histocompatibility complex class I-related chain A (MICA) on the endothelium was considered as the target antigen in antibody-mediated rejection during recent studies. Our data show that the percent of HLA antibody was 6/8, whether MICA antigen can express on the podocyte and whether MICA antigen was related to the target antigen should be investigated in further study. However, this hypothesis should be verified in the future. This possible alloimmunization on the podocytes remains to be further clarified in many aspects such as the antigen types and the possible molecular pathways.
Antigens from the podocytes are the target in the pathogenesis of MN. Unfortunately, the PLA2R antibody in the serum can be tested only in 70% to 80% of IMN cases. A recent study showed that THSD7A might be another antigen presented on podocytes in IMN.[28] Therefore, the specific antigen presented in dn MN patients should be confirmed in the future, which will help us understand the pathogenesis of this disease.
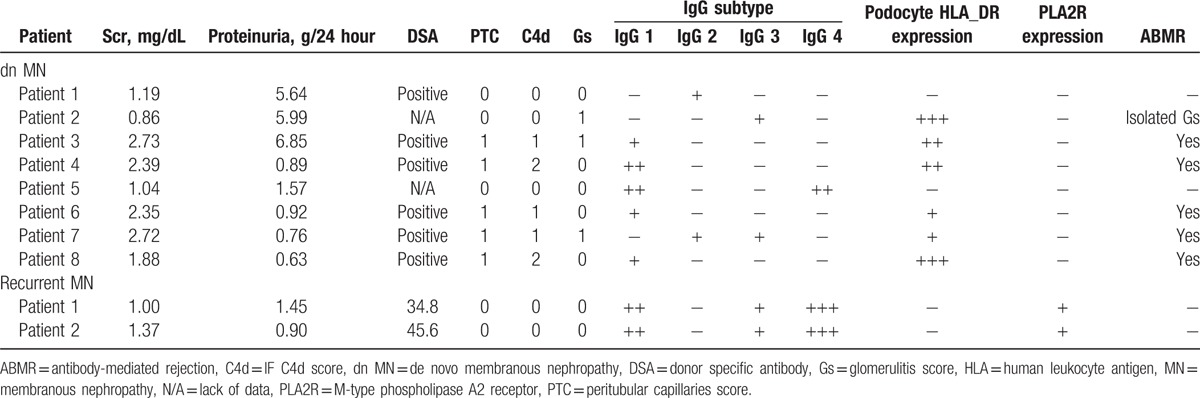
Moreover, the IgG subtypes were very different between the dn MN group and the IMN group in our study. IgG1 was mainly found in the dn MN group, while IgG4 was found in the IMN group. Two patients were diagnosed with recurrent MN based on our data. Their PLA2R expression and PLA2R antibody detection levels were similar to the IMN patients (Table 5).
Table 5.
dn MN and recurrent MN patients clinic-pathological features.
In summary, to the best of our knowledge, this is the 1st report on the fact that HLA-DR, and not PLA2R, is expressed on podocytes in dn MN posttransplantation. This expression might be related to the allogeneic immune damage of podocytes and consequently the pathogenesis of dn MN. Further studies on this topic should focus on determining the antigen and equivalent antibody presented on the podocytes for membranous lesions in dn MN.
Footnotes
Abbreviations: dn MN = de novo membranous nephropathy, HLA = human leukocyte antigen, IMN = idiopathic membranous nephropathy, MICA = major histocompatibility complex class I-related chain A, MN = membranous nephropathy, PLA2R = M-type phospholipase A2 receptor.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Maisonneuve P, Agodoa L, Gellert R, et al. Distribution of primary renal diseases leading to end-stage renal failure in the United States, Europe, and Australia/New Zealand: results from an international comparative study. Am J Kidney Dis 2000; 35:157–165. [DOI] [PubMed] [Google Scholar]
- 2.Dabade TS, Grande JP, Norby SM, et al. Recurrent idiopathic membranous nephropathy after kidney transplantation: a surveillance biopsy study. Am J Transplant 2008; 8:1318–1322. [DOI] [PubMed] [Google Scholar]
- 3.El-Zoghby ZM, Grande JP, Fraile MG, et al. Recurrent idiopathic membranous nephropathy: early diagnosis by protocol biopsies and treatment with anti-CD20 monoclonal antibodies. Am J Transplant 2009; 9:2800–2807. [DOI] [PubMed] [Google Scholar]
- 4.Sprangers B, Lefkowitz GI, Cohen SD, et al. Beneficial effect of rituximab in the treatment of recurrent idiopathic membranous nephropathy after kidney transplantation. Clin J Am Soc Nephrol 2010; 5:790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck LH, Jr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 2009; 361:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin W, Beck LH, Jr, Zeng C, et al. Anti-phospholipase A2 receptor antibody in membranous nephropathy. J Am Soc Nephrol 2011; 22:1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck LH, Jr, Salant DJ. Membranous nephropathy: recent travels and new roads ahead. Kidney Int 2010; 77:765–770. [DOI] [PubMed] [Google Scholar]
- 8.Debiec H, Martin L, Jouanneau C, et al. Autoantibodies specific for the phospholipase A2 receptor in recurrent and De Novo membranous nephropathy. Am J Transplant 2011; 11:2144–2152. [DOI] [PubMed] [Google Scholar]
- 9.Stahl R, Hoxha E, Fechner K. PLA2R autoantibodies and recurrent membranous nephropathy after transplantation. N Engl J Med 2010; 363:496–498. [DOI] [PubMed] [Google Scholar]
- 10.Debiec H, Ronco P. PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N Engl J Med 2011; 364:689–690. [DOI] [PubMed] [Google Scholar]
- 11.Honda K, Horita S, Toki D, et al. De novo membranous nephropathy and antibody-mediated rejection in transplanted kidney. Clin Transplant 2011; 25:191–200. [DOI] [PubMed] [Google Scholar]
- 12.Lim BJ, Kim MS, Kim YS, et al. C4d deposition and multilayering of peritubular capillary basement membrane in posttransplantation membranous nephropathy indicate its association with antibody-mediated injury. Transplant Proc 2012; 44:619–620. [DOI] [PubMed] [Google Scholar]
- 13.Ponticelli C, Glassock RJ. De novo membranous nephropathy (MN) in kidney allografts. A peculiar form of alloimmune disease? Transpl Int 2012; 25:1205–1210. [DOI] [PubMed] [Google Scholar]
- 14.Rihova Z, Honsova E, Merta M, et al. Secondary membranous nephropathy – one center experience. Ren Fail 2005; 27:397–402. [PubMed] [Google Scholar]
- 15.Glassock RJ. Human idiopathic membranous nephropathy – a mystery solved? N Engl J Med 2009; 361:81–83. [DOI] [PubMed] [Google Scholar]
- 16.Larsen CP, Walker PD. Phospholipase A2 receptor (PLA2R) staining is useful in the determination of de novo versus recurrent membranous glomerulopathy. Transplantation 2013; 95:1259–1262. [DOI] [PubMed] [Google Scholar]
- 17.Hall BM, Bishop GA, Duggin GG, et al. Increased expression of HLA-DR antigens on renal tubular cells in renal transplants: relevance to the rejection response. Lancet 1984; 2:247–251. [DOI] [PubMed] [Google Scholar]
- 18.Mohanakumar T, Rhodes C, Mendez-Picon G, et al. Renal allograft rejection associated with presensitization to HLA-DR antigens. Transplantation 1981; 31:93–95. [DOI] [PubMed] [Google Scholar]
- 19.Henny FC, Weening JJ, Baldwin WM, et al. Expression of HLA-DR antigens on peripheral blood T lymphocytes and renal graft tubular epithelial cells in association with rejection. Transplantation 1986; 42:479–483. [DOI] [PubMed] [Google Scholar]
- 20.Russ GR, Pascoe V, d’Apice AJ, et al. Expression of HLA-DR, -DQ, and -DP antigens on renal tubular cells during rejection episodes. Transplant Proc 1986; 18:293–297. [PubMed] [Google Scholar]
- 21.Bishop GA, Hall BM, Waugh J, et al. Diagnosis of renal allograft rejection by analysis of fine-needle aspiration biopsy specimens with immunostains and simple cytology. Lancet 1986; 2:645–650. [DOI] [PubMed] [Google Scholar]
- 22.Strom TB, Garovoy MR. Clinical and experimental aspects of renal allograft rejection. Am J Kidney Dis 1981; 1:5–14. [DOI] [PubMed] [Google Scholar]
- 23.Wen J, Zhang M, Chen J, et al. HLA-DR overexpression in tubules of renal allografts during early and late renal allograft injuries. Exp Clin Transplant 2013; 11:499–506. [DOI] [PubMed] [Google Scholar]
- 24.Bariéty J, Bruneval P, Hill G, et al. Posttransplantation relapse of FSGS is characterized by glomerular epithelial cell transdifferentiation. J Am Soc Nephrol 2001; 12:261–274. [DOI] [PubMed] [Google Scholar]
- 25.Gluhovschi C, Gluhovschi G, Potencz E, et al. What is the significance of HLA-DR antigen expression in the extraglomerular mesangium in glomerulonephritis? Hum Immunol 2012; 73:1098–1101. [DOI] [PubMed] [Google Scholar]
- 26.Haas M, Sis B, Rcusen LC, et al. Banff meeting report writing committee: Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 2014; 14:272–283. [DOI] [PubMed] [Google Scholar]
- 27.Baudeau C, Delarue F, Hé CJ, et al. Induction of MHC class II molecules HLA-DR, -DP and -DQ and ICAM 1 in human podocytes by gamma-interferon. Exp Nephrol 1994; 2:306–312. [PubMed] [Google Scholar]
- 28.Tomas NM, Beck LH, Jr, Meyer-Schwesinger C, et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 2014; 371:2277–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]


