Abstract
The results of neuroimaging studies on migraines have shown that the functions and functional connectivity networks of some brain regions are altered in migraine patients, and different brain structure volumes have also been observed in recent years. However, it is still not known whether the mean thickness of the cortex is different in migraine patients.
A total of 48 migraine without aura (MWoA) patients in interictal phase and 48 healthy controls were enrolled in this study. All subjects received neurological and magnetic resonance imaging (MRI) examinations. Automatic segmentation processing of high-resolution MRI structure images was performed using FreeSurfer software.
The mean cortical thickness of many brain regions in the frontal lobe, temporal lobe, occipital lobe, parietal lobe, and insula in the migraine patient group was significantly decreased compared with that in the healthy control group. The mean cortical thickness of the insula anterior was positively correlated with the duration of the disease course, while the mean cortical thickness of insula superior and insula inferior was negatively correlated with the duration of the disease course.
The results showed that MWoA results from a complex interactive reaction involving many brain regions and many brain network systems together. However, it is still not clear whether the difference in the brain structure of migraine patients is the result or the cause of headache, which is a topic that must be better elucidated. Therefore, longitudinal neuroimaging studies on migraine patients with large samples sizes should be performed using more advanced neuroimaging techniques.
Keywords: brain connectivity, functional network, insula, magnetic resonance imaging, migraine
1. Introduction
Migraine is a debilitating disorder and one of the most common disorders of the nervous system.[1] The prevalence of migraine is approximately 9.3% in China, showing obvious familial aggregation, most of them are migraine without aura (MWoA).[2] Recurrent attacks severely affect the quality of life of patients and can increase the risk of developing cardiovascular and cerebrovascular diseases. The pathogenic mechanism is currently still not clear. Studies have shown that migraine may be a progressive disease; recurrent attacks could increase the risk of subtle lesions and cause functional damage in some brain regions.[3] A variety of imaging techniques are generally used to observe microstructural injury and functional abnormalities in brain regions such as the prefrontal cortex, cingulate gyrus, insula, and thalamus, which are the main regions that participate in the regulation of pain by central nervous system.[4]
With the development of magnetic resonance imaging (MRI) techniques, increasing numbers of new sequences and data processing methods have been applied in neuroimaging studies on migraines. These techniques exhibit a high spatial resolution and are more sensitive to structural changes in the brain compared with other neuroimaging models such as computed tomography and positron emission tomography, thus presenting a more powerful capacity for the analysis of brain function.[5–7] Such studies have mainly focused on the resting state brain function, brain functional connectivity network, and brain structure of migraine patients. Functional MRI studies based on the resting state and task state have shown that the functions of some brain regions and the functional connectivity network of migraine patients are altered, and analyses of voxel-based morphometry have demonstrated that the volumes of some brain structures also exhibit differences. However, it is still not clear whether the differences in the volumes of these brain structures cause changes in thickness in migraine patients. Therefore, the aim of this study was to investigate the differences in cortical mean thickness between patients with MWoA and healthy controls.
FreeSurfer is free software designed for the analysis of morphological measurements through automatic segmentation of brain structure images. It can easily and accurately measure the mean thickness of the cortex in regions of interest in the brain.[8] The aims of this study were to use the reliable automatic MRI tissue segmentation technique to investigate whether the brain cortex of MWoA patients show changes in mean thickness and to investigate the correlation between these changes and clinical variables.
2. Subjects and methods
2.1. Study subjects
2.1.1. Patient group
A total of 49 patients with MWoA confirmed by experts in the clinic of the Department of Neurology of the First Affiliated Hospital of Third Military Medical University, China, between November 2014 and December 2015 were recruited. The inclusion criteria were as follows: satisfaction of the migraine criteria in the International Classification of Headache Disorders, 3rd edition, beta version; a score of more than 50 points on the Headache Impact Test 6 (HIT-6) questionnaire; a score of more than 4 points on the visual analog scale (VAS) of pain intensity; pain greater than grade II in the pain classification system of the World Health Organization (WHO); no history of drug abuse; no abnormal signals in conventional MRI scanning and normal neurological examinations; no neurological or internal diseases other than migraine; and no migraine attack 3 days before scanning or 4 days after scanning. During follow-up, 1 case exhibited right-sided migraine pain 3 days after scanning and was excluded from the study. Finally, 48 cases were enrolled, including 11 men and 37 women, who were all right-handed. Each attack of migraine pain lasted for >5 hours without treatment in all patients, and the shortest disease course duration in the MWoA patient group was 1 year, and the longest was 24 years.
2.1.2. Healthy control group
A total of 48 volunteers recruited from communities were used as the normal control group, all healthy controls are not relatives of migraine patients. These participants were all right-handed and included 37 women and 11 men.
2.1.3. Clinical scale assessment
General information for the included patients, including duration of disease of migraine and medication history, was recorded. The general psychological conditions of all subjects were assessed using the Neuropsychiatric Inventory questionnaire. The 24-item Hamilton Depression Rating Scale, the 14-item Hamilton Anxiety Rating Scale, and the Chinese Classification of Mental Disorders Version 3 were used to assess whether the subjects exhibited depression, anxiety, or bipolar disorder. Subjects without abnormalities were enrolled in this study. The detailed conditions of migraine in the MWoA patient group were assessed using the concise headache questionnaire. In addition, HIT-6, VAS were employed to detect the impact of migraine on the patients’ daily activities and headache severity.
The education level, gender, and age of the subjects did not differ significantly between the 2 groups (P > 0.05). All subjects volunteered to participate in this study. The patients were informed of the experimental methods, aims, and possible risks and discomfort before the study, and the patients volunteered to sign an informed consent form. This study was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of the Third Military Medical University.
2.2. Data collection
Collection of MRI imaging data from all subjects was performed using a 3.0T MRI scanner (Siemens, Germany) with an 8-channel head coil. The patient's head was immobilized, and the patients attempted to remain motionless. First, conventional T1W1 and T2W1 axial images were collected to exclude cranial organic diseases in the subjects. Sagittal high-resolution T1W1 structure images were collected using the 3-dimensional magnetization-prepared rapid acquisition gradient-echo sequence. The scanning parameters were as follows: TR = 1900 ms, TE = 2.52 ms, flip angle = 9°, field of view = 256 × 256 mm2, voxels = 1.0 × 1.0 × 1.0 mm3, and slice thickness = 1 mm. A total of 176 layers were scanned over a period of 4 minutes 26 seconds.
2.3. Data processing
The raw data obtained from MRI scanning were used for format conversion and classification. The high-resolution T2 structure images were introduced into FreeSurfer 5.0 (http://surfer.nmr.mgh.harvard.edu/fswiki/Download) for automatic anatomical segmentation. The following main steps were performed:[9–11] nonbrain tissues such as the skull were removed; optimal linear conversion was performed to calculate the maximum likelihood of the atlas template corresponding to whole-brain tissue images; nonlinear conversion was performed to convert images into the standardized Talairach space; whole-brain gray and white matter segmentation was performed; the signal intensities of the gray and white matter after segmentation were standardized; topological correction of the surface of gray and white matter after segmentation was performed; the boundary of the gray and white matter surface after segmentation was defined; and the mean thickness of a total of 148 brain regions corresponding to 74 regions in each of the left and right hemispheres, set up using the aparc.a2009s template, was calculated. Approximately 16 hours were required for the analysis of each patient.
2.4. Statistical analysis
The subjects were divided into a MWoA patient group and a healthy control group. All data analyses were performed using SPSS 22.0 (http://www.spss.com). The demographic data and clinical parameters of the subjects in these 2 groups were analyzed using descriptive statistics. Analysis of covariance (ANCOVA) was performed for the subjects in these 2 groups using the mean thickness of all brain regions as a dependent variable and gender, age, and the mean thickness of the left or right hemisphere as covariates. The significance level was set to P < 0.05. Pearson analysis was performed to examine the correlation between brain regions that showed differences and the duration of the disease course.
3. Results
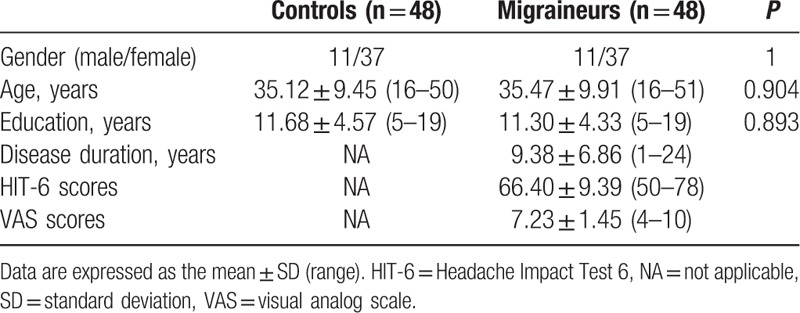
The demographic and general clinical data of all subjects are shown in Table 1. There were 48 subjects in both the MWoA patient and healthy control groups. The gender, age, and education level of the subjects did not differ significantly between these 2 groups (P > 0.05). The Neuropsychiatric Inventory, Hamilton depression rating scale, Hamilton anxiety rating scale, and Chinese Classification of Mental Disorders Version 3 assessment report of all participants were without abnormalities. The shortest disease course duration in the MWoA patient group was 1 year, and the longest was 24 years. HIT-6 scores of patients in the MWoA group ranged from 50 to 78 points, indicating that migraine had significantly affected these patients’ daily lives. Their VAS scores ranged from 4 to 10 points, indicating that these patients suffered from severe migraine and required medical treatment.
Table 1.
Demographic and clinical data.
ANCOVA was performed using the mean thickness of the cortex in the 148 brain regions of the whole brain, calculated with the aparc.a2009s template for automatic segmentation, as a dependent variable and using gender, age, and the mean thickness of the left or right hemisphere as covariates. The statistical analyses are shown in Figure 1 (Warm coloration indicates the brain regions showing an increased cortical mean thickness, and cold coloration indicates the brain regions showing a reduced cortical mean thickness in MWoA patients. The mapping threshold was set at P < 0.05.). Brain regions showing P > 0.05 in the variance homogeneity test and P < 0.05 in ANCOVA were considered to be brain regions with significant differences (Table 2.). These brain regions included the bilateral insula, bilateral cuneus, bilateral cortex surrounding the corpus callosum, left calcarine gyrus, Cingulate (bilateral dorsal anterior cingulate, bilateral posterior cingulate, and bilateral anterior cingulate), Frontal (bilateral orbitofrontal gyrus, bilateral middle frontal gyrus, and right dorsolateral frontal gyrus), Temporal (bilateral middle temporal gyrus, right inferior temporal gyrus), Parietal (bilateral paracentral lobule, bilateral postcentral gyrus, bilateral inferior parietal lobule, and bilateral precentral gyrus), Occipital (bilateral superior occipital gyrus, left middle occipital gyrus). As shown in Figure 2, the statistical significance of the mean thickness of the brain area, MWoA patients were lower than the healthy controls.
Figure 1.
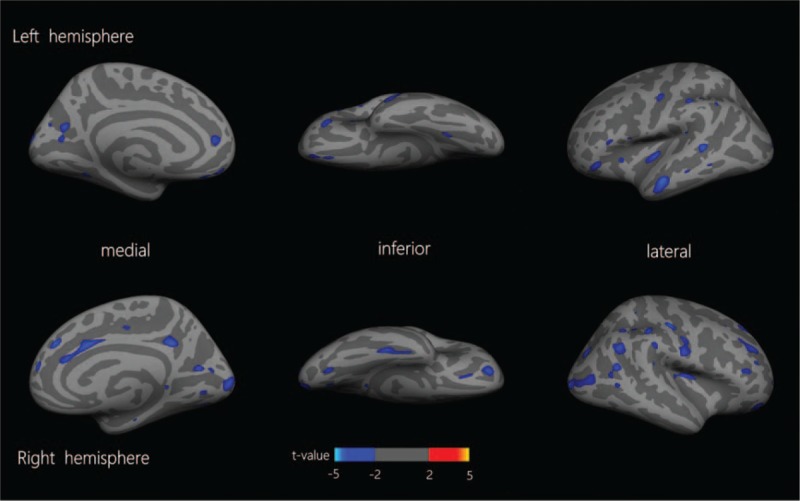
Cortical mean thickness differences between migraine and healthy controls. Warm coloration indicates the brain regions showing an increased cortical mean thickness, and cold coloration indicates the brain regions showing a reduced cortical mean thickness in migraine without aura (MWoA) patients. The mapping threshold was set at P < 0.05.
Table 2.
Significant P values of the mean thickness of cortical areas.
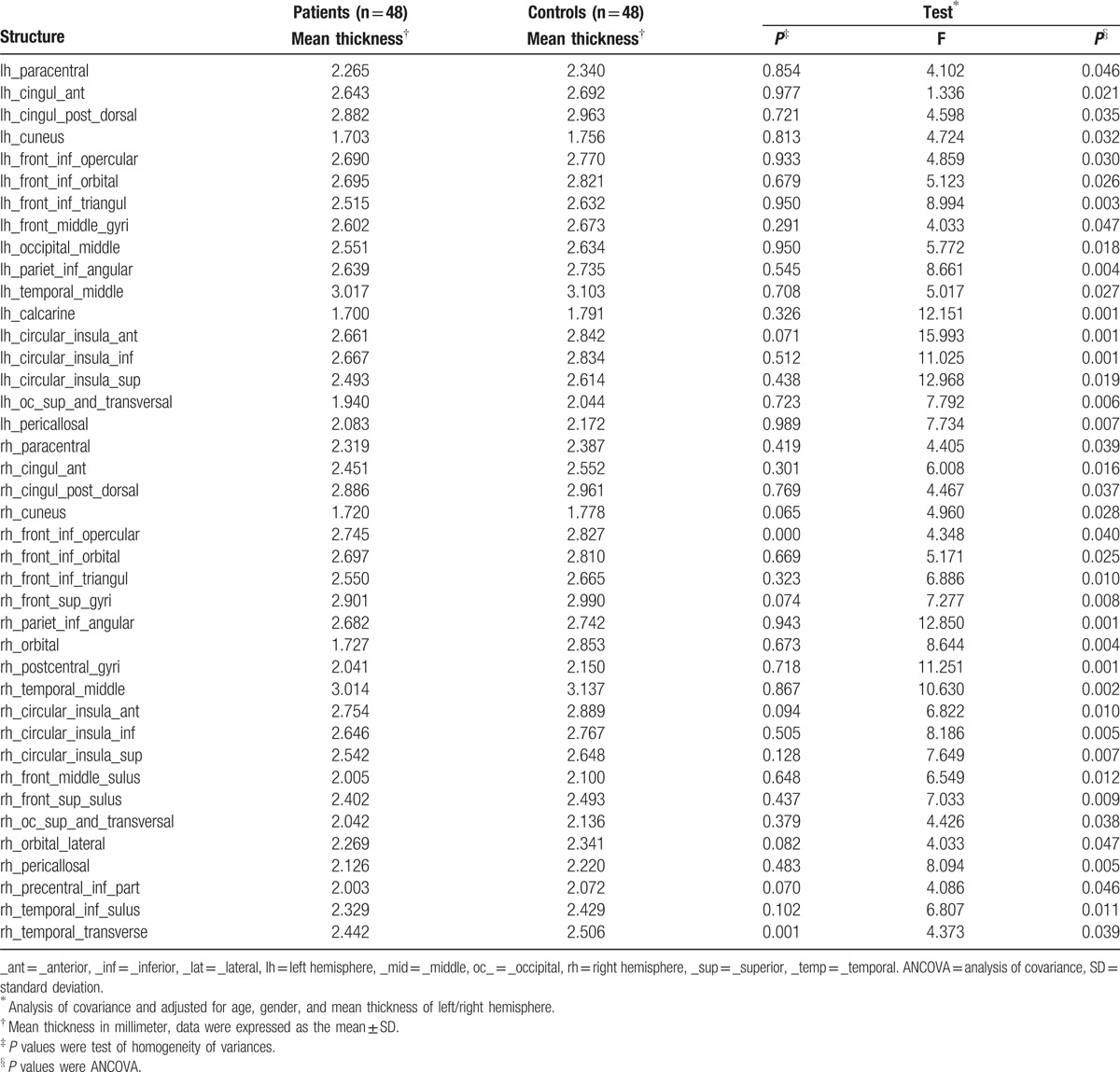
Figure 2.
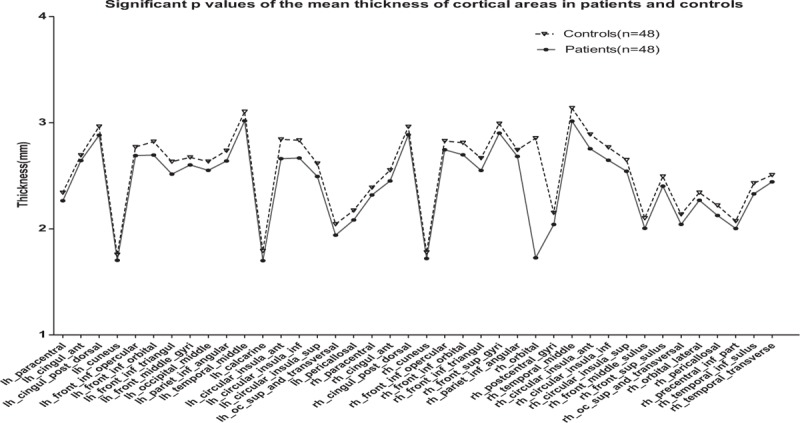
Significant P values of the mean thickness of cortical areas. The statistical significance of the mean thickness of the brain area, migraine without aura (MWoA) patients were lower than the healthy controls.
The brain regions in which the difference in mean thickness exhibited statistical significance were used for comparative analysis between the MWoA patient group and the healthy control group. The results are shown in Figure 3. The results of comparative analysis showed that for brain regions presenting statistically significant differences, the mean cortical thickness in the MWoA patient group was lower than that in the healthy control group.
Figure 3.
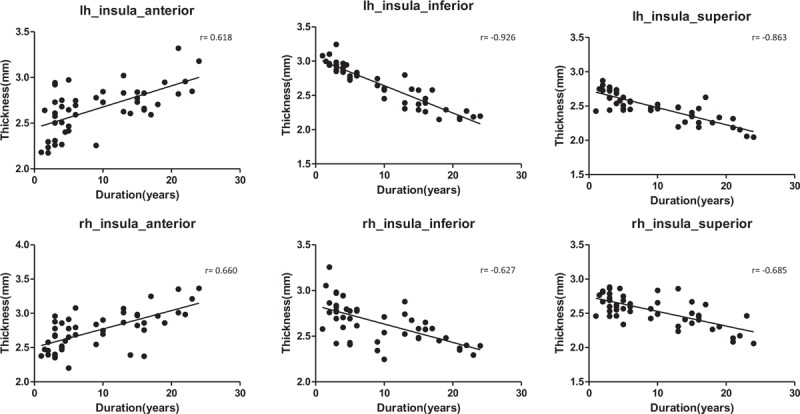
Insula cortex mean thickness of each subregion and the course duration correlation scatterplot. The mean thickness of the cortex of the bilateral insula anterior was positively correlated with the disease course duration, the mean thickness of the cortex of the upper and insula inferior was negatively correlated with the disease course duration.
Pearson correlation analysis was performed on brain regions in which the mean thickness exhibited a significant difference in relation to disease course duration (Fig. 3). The results showed that although the mean thickness of the cortex in the front, upper, and insula inferior in the MWoA patient group was lower than that in the healthy control group, the mean thickness of the cortex in the bilateral insula anterior was positively correlated with the disease course duration (rl = 0.618 and rr = 0.660); the mean thickness of the cortex in the insula superior was negatively correlated with the disease course duration (rl = −0.863 and rr = −0.685); and the mean thickness of the cortex in the insula inferior was negatively correlated with the disease course duration (rl = −0.926 and rr = −0.627).
4. Discussion
This study showed that the mean thickness of the cortex in the bilateral cuneus, posterior cingulate, inferior parietal lobule, inferior frontal gyrus, and middle temporal gyrus was lower in the MWoA patient group than in the healthy control group, the results consistent with Schmitz et al.[12] The cuneus, posterior cingulate, inferior parietal lobule, inferior frontal gyrus, and temporal cortex are important components of the default network.[13] In the resting state, the default network is mainly associated with higher functions of the human brain, such as memory and consciousness. Organized brain function activities occur in the resting state. The cuneus and cingulate are the brain regions with the greatest brain metabolic activity. The cingulate gyrus and cuneus continuously collect surrounding and internal information and allocate this information. The cuneus is involved in high-level cognitive functions related to episodic memory and self-relevant information processing and is associated with conscious short-term memory.[14] The frontal lobe and posterior cingulate gyrus are involved in emotional reactions to pain and the subjective feeling of pain; in addition, they participate in the memory, attention response, and cognition of pain.[15] The inferior parietal lobule, including the supramarginal gyrus and angular gyrus, mainly functions in the pain response and the sensing of temperature and pressure. The mean cortical thickness of these brain regions was found to be decreased in MWoA patients, which might be associated with self-adaptation of the nervous system. Through self-attenuation of the nervous system, emotional reactions to pain and the subjective feeling of pain are reduced as much as possible, and responses to sensory functions such as pain are also actively reduced. Therefore, reduction of the mean cortical thickness of these brain regions in the default network is associated with MWoA. Through self-attenuation, the default network reduces participation in the subjective feeling of pain and the emotional response to pain as much as possible.
The 3 processes of awareness, emotion, and cognition constitute the complete experience of pain. The pain pathway, including both the emotional component and the sensory component, is divided into 2 parallel ascending pathways.[16] The prefrontal lobe and medial hypothalamic nuclei and their projected limbic system constitute the affective pathway of pain, while the lateral hypothalamic nuclei and the corresponding cortical projection area and somatosensory area constitute the sensory pathway of pain. This study showed that the mean cortical thickness of the bilateral angular gyrus, orbital gyrus, dorsolateral superior frontal gyrus, middle frontal gyrus, inferior frontal gyrus, subcallosal gyrus, left calcarine, and right anterior cingulate was lower in the MWoA patient group than in the healthy control group, the results in keeping with Valfrè et al[17] and Datta et al.[18] These brain regions are important components of the limbic system and represent an important link in the constitution of the affective pathway of pain. The anterior cingulate gyrus receives fiber projections from the midline thalamic nuclei and medial nuclear group of the thalamus and exhibits prominent functions in the affective pathway of pain. The results of comparative analyses of subjects with chronic pain and healthy subjects showed that the anterior cingulate gyrus contains a high density of opioid receptor binding sites,[19] indicating that the anterior cingulate gyrus participates in both the formation and regulation of pain. A study by Leknes and Tracey[20] demonstrated that stimulation of regions such as the anterior hypothalamic region and anterior cingulate gyrus in the limbic system can increase the pain threshold. Similarly, the limbic system exhibits the function of inducing sleep activity. The 48 MWoA patients included in this study all required rest while lying down quietly to obtain relief during the migraine attack period, which might be associated with the activation of this function in the limbic system. Therefore, the reduction of the mean cortical thickness in these brain regions in the limbic system was significantly associated with migraine pain. The limbic system is both involved in the medial pain pathway in migraine to generate the sense of pain and participates in the regulation of pain to increase the pain threshold.
The salience network is mainly composed of the orbital frontal lobe, insula, and dorsal anterior cingulate cortex (http://www.psychiatrictimes.com/special-reports/neurobiology-borderline-personality-disorder). The orbital frontal lobe and insular cortex play important roles in the inhibition of noncognition-related tasks and the promotion of higher control tasks, and they exhibit a “switch” function in the conversion between the central executive network and the default network; thus, they display antagonistic functions in the execution of the cognitive tasks of these 2 networks.[21] This study showed that the mean cortical thickness of the bilateral inferior frontal gyrus operculum, middle orbital frontal gyrus, pars triangularis of the inferior frontal gyrus, paracentral lobule, insula anterior, insula superior, insula inferior, and dorsal anterior cingulate was significantly lower in the MWoA patient group than in the healthy control group, the results did not agree with DaSilva et al.[22] The brain regions showing a significant decrease in the mean cortical thickness are the main components of the salience network. The task-state functional MRI analyses conducted in MWoA patients revealed microstructural damage and functional abnormality in the prefrontal cortex, cingulate gyrus, insula, and thalamus. These regions are mainly involved in the regulation of pain by the central nervous system. The paracentral lobe is associated with attention and memory in the cerebral cortex.[23] The present study showed that the reduction of the mean cortical thickness in the bilateral paracentral lobes might result in a reduction of attention. A study by Tian et al[24] showed that dorsal anterior cingulate gyrus was involved in motor and cognition control and was associated with wake and autonomic control functions. According to the external environment, the dorsal anterior cingulate gyrus automatically regulates its own status to adapt to environmental changes. This study showed that the reduction of the mean cortical thickness in the bilateral dorsal anterior cingulate gyrus might be associated with this automatic regulatory control. This study showed that the brain regions exhibiting a reduction of the mean cortical thickness, such as the frontal lobe, inferior parietal lobule, and paracentral lobule, are important brain regions in the pain matrix and might be important brain regions for the production of analgesic effects. The reduction of the mean cortical thickness in these brain regions causes a reduction of analgesic effects, thus inducing migraine attacks. Therefore, we hypothesized that the salience network might play an important role in MWoA. The reduction of the mean cortical thickness in these brain regions in the salience network both regulates the reduction of attention to and memory of the pain response and induces migraine due to the reduction of its analgesic effects.
The insula participates in many physiological processes, including cognition with purpose, consciousness, perception, autonomic regulation, interoceptive awareness, and somatosensory perception. During the process of participation in these complex functions, the insula plays a role in processing and relaying incoming impulses. Functionally, the insula is divided into the anterior insula and the posterior insula.[25] The anterior insula is connected to the middle temporal gyrus, inferior temporal gyrus, and anterior cingulate. It is involved in the regulation of emotion mainly through brain regions in the limbic system.[26] The anterior insula is also an integral part of the salience network.[27] In addition, the anterior insula may be the interactive center of the entire brain network.[28] The posterior insula is closely connected to the premotor region, sensorimotor region, supplementary motor region, and middle/posterior cingulate gyrus and plays an important role in the integration of sensorimotor function.[26] A study by Linnman et al[29] showed that the anterior insula is closely associated with the processing of emotion and cognition, while the posterior insula is even more closely associated with the processing of the differentiation of feelings. Frot et al[30] used laser evoked potentials in the mouse brain to acquire data for the entire insula and found that the primary processing of pain responses occurs in the posterior insula. After coding according to intensity and anatomical location, pain responses are conveyed to the anterior insula. The emotional responses to pain are processed in the anterior insula. The nociceptive input in the anterior insula might be further combined into more complex brain networks, such as the salience network.[31] This study showed that the mean thickness of the cortex in the whole insula was lower in the MWoA patient group than in the healthy control group, which was consistent with previous studies and the results of Maleki et al.[32] These findings indicated that long-term recurrent migraine attacks might alter the structure of this region, thus altering sensory and emotional processing in the insula. This study showed that although the mean cortical thickness of entire bilateral insula was lower in the MWoA patient group than in the healthy control group, the mean thickness of the cortex of the bilateral insula anterior was positively correlated with the disease course duration, while the mean thickness of the cortex of the upper and insula inferior was negatively correlated with the disease course duration, the results in accord with Schmitz et al.[33] According to a study by Sterzer and Kleinschmidt,[34] this phenomenon can be explained as heightened alertness of either stimulus- or task-driven origin, in which the endogenous and exogenous stressors are integrated to challenge homeostasis. With an increase in the disease course duration, the thickness of the rear insula decreases more rapidly, thereby reducing the transmission of nociceptive input to the anterior insula. Conversely, the mean cortical thickness of the anterior insula increases more slowly, thereby increase the process of attention and cognition of pain to maintain the dynamic balance of the entire brain network.
5. Conclusion
The production of pain in MWoA patients involves many interactions involving the pain center of the brain network.[35] Although the present study showed that the mean thickness of the cortex in many brain regions in many brain networks of MWoA patients is decreased and that some brain regions are closely associated with the disease course duration, it is still not clear whether the change in the brain structure of MWoA patients is the result or the cause of headache. To better understand this issue, longitudinal neuroimaging studies on MWoA patients with a large sample size are required.
Acknowledgments
The authors thank National Nature Science Foundation of China (grant no. 81171283 and 81471194) and the Clinical Innovation Foundation of Third Military Medical University (grant no. 20140045) for the support. The authors also thank all the participants for their contribution to this study.
Footnotes
Abbreviations: ANCOVA = analysis of covariance, HIT-6 = Headache Impact Test 6, MRI = magnetic resonance imaging, MWoA = migraine without aura, VAS = visual analog scale.
Z-bY and JP contributed equally to this work.
Ethical standards: The Ethics Committee of the First Affiliated Hospital of the Third Military Medical University (Chongqing, China) approved the current study and all participants gave written informed consent to the participation according to the Declaration of Helsinki.
Funding/support: This study was supported by the National Nature Science Foundation of China (grant no. 81171283 and 81471194) and the Clinical Innovation Foundation of Third Military Medical University (grant no. 20140045).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
References
- 1.Kim JH, Kim S, Suh SI, et al. Interictal metabolic changes in episodic migraine: a voxel-based FDG-PET study. Cephalalgia 2010; 30:53–61. [DOI] [PubMed] [Google Scholar]
- 2.Yu S, Liu R, Zhao G, et al. The prevalence and burden of primary headaches in China: a population-based door-to-door survey. Headache 2012; 52:582–591. [DOI] [PubMed] [Google Scholar]
- 3.Schwedt TJ, Dodick DW. Advanced neuroimaging of migraine. Lancet Neurol 2009; 8:560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiu Y, Noguchi Y, Honda M, et al. Brain processing of the signals ascending through unmyelinated C fibers in humans: an event-related functional magnetic resonance imaging study. Cereb Cortex 2006; 16:1289–1295. [DOI] [PubMed] [Google Scholar]
- 5.Bhaskar S, Saeidi K, Borhani P, et al. Recent progress in migraine pathophysiology: role of cortical spreading depression and magnetic resonance imaging. Eur J Neurosci 2013; 38:3540–3551. [DOI] [PubMed] [Google Scholar]
- 6.Colombo B, Dalla Costa G, Dalla Libera D, et al. From neuroimaging to clinical setting: what have we learned from migraine pain? Neurol Sci 2012; 33 (Suppl. 1):S95–S97. [DOI] [PubMed] [Google Scholar]
- 7.Aydın-Ozemir Z, Baykan B. The face of chronic migraine which has started to be clarified. Arch Neuropsychiatry 2013; 50 (Suppl. 1):21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A 2000; 97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33:341–355. [DOI] [PubMed] [Google Scholar]
- 10.Morey RA, Petty CM, Xu Y, et al. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage 2009; 45:855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Upadhyay J, Maleki N, Potter J, et al. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain 2010; 133:2098–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitz N, Arkink EB, Mulder M, et al. Frontal lobe structure and executive function in migraine patients. Neurosci Lett 2008; 440:92–96. [DOI] [PubMed] [Google Scholar]
- 13.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 2008; 1124:1–38. [DOI] [PubMed] [Google Scholar]
- 14.Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci 2000; 12:1–47. [DOI] [PubMed] [Google Scholar]
- 15.Bluhm RL, Miller J, Lanius RA, et al. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull 2007; 33:1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schnitzler A, Ploner M. Neurophysiology and functional neuroanatomy of pain perception. J Clin Neurophysiol 2000; 17:592–603. [DOI] [PubMed] [Google Scholar]
- 17.Valfrè W, Rainero I, Bergui M, et al. Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache 2008; 48:109–117. [DOI] [PubMed] [Google Scholar]
- 18.Datta R, Detre JA, Aguirre GK, et al. Absence of changes in cortical thickness in patients with migraine. Cephalalgia 2011; 31:1452–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones AK, Qi LY, Fujirawa T, et al. In vivo distribution of opioid receptors in man in relation to the cortical projections of the medial and lateral pain systems measured with positron emission tomography. Neurosci Lett 1991; 126:25–28. [DOI] [PubMed] [Google Scholar]
- 20.Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nat Rev Neurosci 2008; 9:314–320. [DOI] [PubMed] [Google Scholar]
- 21.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007; 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DaSilva AF, Granziera C, Snyder J, et al. Thickening in the somatosensory cortex of patients with migraine. Neurology 2007; 69:1990–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forss N, Merlet I, Vanni S, et al. Activation of human mesial cortex during somatosensory target detection task. Brain Res 1996; 734:229–235. [PubMed] [Google Scholar]
- 24.Tian L, Jiang T, Wang Y, et al. Altered resting-state functional connectivity patterns of anterior cingulate cortex in adolescents with attention deficit hyperactivity disorder. Neurosci Lett 2006; 400:39–43. [DOI] [PubMed] [Google Scholar]
- 25.Borsook D, Veggeberg R, Erpelding N, et al. The insula: a “Hub of activity” in migraine. Neuroscientist 2015; 8:1–21.doi:10.1177/1073858415601369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cauda F, D’Agata F, Sacco K, et al. Functional connectivity of the insula in the resting brain. Neuroimage 2011; 55:8–23. [DOI] [PubMed] [Google Scholar]
- 27.Damoiseaux JS, Rombouts SA, Barkhof F, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A 2006; 103:13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 2010; 214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linnman C, Beucke JC, Jensen KB, et al. Sex similarities and differences in pain-related periaqueductal gray connectivity. Pain 2012; 153:444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frot M, Faillenot I, Mauguière F. Processing of nociceptive input from posterior to anterior insula in humans. Hum Brain Mapp 2014; 35:5486–5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiech K, Lin CS, Brodersen KH, et al. Anterior insula integrates information about salience into perceptual decisions about pain. J Neurosci 2010; 30:16324–16331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maleki N, Becerra L, Brawn J, et al. Concurrent functional and structural cortical alterations in migraine. Cephalalgia 2012; 32:607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitz N, Admiraal-Behloul F, Arkink EB, et al. Attack frequency and disease duration as indicators for brain damage in migraine. Headache 2008; 48:1044–1055. [DOI] [PubMed] [Google Scholar]
- 34.Sterzer P, Kleinschmidt A. Anterior insula activations in perceptual paradigms: often observed but barely understood. Brain Struct Funct 2010; 214:611–622. [DOI] [PubMed] [Google Scholar]
- 35.Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci 2004; 24:10410–10415. [DOI] [PMC free article] [PubMed] [Google Scholar]


