Abstract
An association may exist between obstructive sleep apnea (OSA) and depression. However, results regarding this association are inconsistent, and the direction of the association between OSA and depression remains unknown. Therefore, we used the Taiwan National Health Insurance Research Database to investigate the bidirectional association between OSA and depression.
A total of 6427 OSA patients and 32,135 age and sex-matched control subjects were enrolled to analyze the risk of depression among patients with OSA, where 27,073 patients with depression and 135,365 control subjects were enrolled to address the risk of OSA among patients with depression. All subjects were followed to identify their outcomes of interest from January 1, 1997 to December 31, 2012.
Cox proportional-hazards models, after adjusting for potential confounders, demonstrated that patients with OSA had an increased risk (adjusted hazard ratio 2.48, 95% confidence interval 2.20–2.79) of developing depression, whereas those with depression were associated with an increased risk of future OSA (adjusted hazard ratio 2.30, 95% confidence interval 2.11–2.50).
Our results suggested that a strong bidirectional relationship exists between OSA and depression, with each disease influencing the development of the other. Health providers are recommended to ensure the early detection and management of depression among patients with OSA and vice versa.
Keywords: bidirectional association, depression, obstructive sleep apnea
1. Introduction
Obstructive sleep apnea (OSA), one of the most clinically important forms of sleep-related breathing disorders, is characterized by repetitive, partial, or complete collapse of the upper airway during sleep, causing impaired gaseous exchange and sleep disturbance. The prevalence of OSA has increased dramatically during the past 2 decades.[1] On the contrary, depression is one of the most common psychiatric illnesses, and is expected to be one of the top leading causes of global morbidity.[2] Some studies have demonstrated significantly higher rates of depression among OSA patients.[3,4] Both OSA and depression are associated with increased morbidity and mortality, and decreased quality of life. Surely, both of them will pose enormous challenges in the decades to come.[5–8] Therefore, the association between OSA and depression deserves careful examination.
Obstructive sleep apnea and depression have overlapping symptoms,[9] such as fatigue, daytime sleepiness, and poor concentration. In addition, they share various biological mechanisms and risk factors suggesting a potential bidirectional association between them.[10] Studies on the link between OSA and depression are limited and inconsistent, with some demonstrating a positive association between OSA and depression,[11,12] whereas others show no association at all.[13–15] However, the existing literature is flawed due to methodological limitations, such as cross-sectional designs, small sample sizes, and use of self-reported symptoms rather than diagnosis by a certificated physician. Longitudinal follow-up studies have found that patients with OSA had an increased risk of developing subsequent depression,[16,17] but less attention has been given to the investigation of whether the opposite direction also occurs. Moreover, until now, there have been no studies that have investigated the bidirectional relationship between OSA and depression from individuals within the same population.
Therefore, the aim of this study was to investigate the bidirectional temporal relationship between OSA and depression using a large administrative database from the Taiwan National Health Insurance (NHI) program. We hypothesized that patients with OSA would have an increased risk of subsequent depression and vice versa.
2. Materials and methods
2.1. Data source
The NHI program in Taiwan was launched in 1995. This mandatory health insurance program covers up to 98% of the Taiwanese population. The National Health Insurance Research Database (NHIRD) was extracted from the claims data from the NHI program for research purposes. This anonymous database contains comprehensive information on insured patients, such as demographic data, dates of clinical visits, and disease diagnoses. The diagnostic codes used were based on the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM). The Longitudinal Health Insurance Database (LHID)2005, a subset of the NHIRD, contains claims data for 1 million randomly sampled beneficiaries who were insured in 2005. This dataset had been confirmed as having no significant difference in age, sex, or healthcare cost from its whole population, which is composed of all beneficiaries under the NHI program. The requirement for informed consent was waived as all claims data in the NHIRD were anonymized and de-identified at all times. This study was reviewed by the Institutional Review Board of Mackay Memorial Hospital (No. 15MMHIS222e).
2.2. Study population
Patients diagnosed with OSA (ICD-9-CM codes 780.51, 780.53, and 780.57) between 1997 and 2009 were selected as the OSA cohort. Patients younger than 30 years of age or older than 80 years of age were excluded. The age exclusion criteria were selected because of the following reasons. First, OSA is a common disease among adults. The prevalence of OSA increases steadily during the middle age and reaches a plateau at about the age of 65 years.[18,19] Taking these epidemiological characteristics into account, we included patients older than 30 years. Second, it takes time after OSA onset to develop a subsequent depression, and patients older than 80 years of age might have less chance for this to occur. Hence, patients older than 80 years were excluded. In addition to the age exclusion criteria, OSA patients diagnosed with depression (ICD-9-CM codes 296.2x, 296.3x, 300.4, and 311) by a psychiatrist before the diagnosis of OSA were also excluded. The initial date of OSA diagnosis was set as the index date. We selected beneficiaries with no OSA diagnosis from the LHID2005 as the control candidate group, and the cases were then matched 1:5 by age and sex to individuals from this group. Cases were only matched to control candidates who were not diagnosed with depression before the index date of the corresponding cases. After matching, the index date was assigned to the matched controls. The cohorts were observed starting from the index date until the diagnosis of depression by a psychiatrist, December 31, 2012, or death, whichever came first.
Patients diagnosed with depression (ICD-9-CM codes 296.2x, 296.3x, 300.4, and 311) by a psychiatrist between 1997 and 2009 were selected as the depression cohort. Patients younger than 30 years of age or older than 80 years of age were excluded.
The age exclusion criteria were selected because of the following reasons. First, prior research has shown that some patients with bipolar disorders have major depression as their initial presentation before mania during the teenage years or early adulthood.[20,21] Second, patients with bipolar disorder have a mean age of onset of 22 years, whereas those with unipolar depression have an average age of onset of about 30 years. We chose patients older than 30 years for the study cases to focus on those with major depression.[22] Finally, because this was considered a bidirectional study for OSA and depression, we applied the same age exclusion criteria for the 2 substudies. In addition to the age exclusion criteria, patients with depression who had been diagnosed with OSA (ICD-9-CM codes 780.51, 780.53, and 780.57) before the diagnosis of depression were also excluded. The initial date of the depression diagnosis was set as the index date. We selected beneficiaries with no depression diagnosis from the LHID2005 as the control candidate group and the cases were then matched 1:5 by age and sex to individuals from this group. Cases were only matched to control candidates who were not diagnosed with OSA before the index date of the corresponding cases. After matching, the index date was assigned to the matched controls. The cohorts were observed starting from the index date until the OSA diagnosis, December 31, 2012, or death, whichever came first.
In addition, medical comorbidities, including diabetes (ICD-9-CM codes 250.x), hypertension (ICD-9-CM codes 401.x–405.x), hyperlipidemia (ICD-9-CM codes 272.x), stroke (ICD-9-CM codes 430.x–438.x), ischemic heart disease (ICD-9-CM codes 410.x–414.x), chronic obstructive pulmonary disease (ICD-9-CM codes 490.x–492.Xx), and obesity (ICD-9-CM codes 278.00–278.02) were investigated as potential confounding factors in both study 1 and study 2. We also examined the level of urbanization (levels 1–5: from the most urbanized region to the most rural region) and insurance salary (<15,840 new Taiwan dollar [NTD], 15,841–25,000 NTD, and >25,001 NTD). We also adopted a disease- screening tool to quickly filter for the presence of comorbidities and the association between OSA and depression.
2.3. Statistical analyses
Demographic data and covariates were expressed as mean ± SD for continuous variables and as proportions for categorical variables. The chi-square test was used for categorical variables and the t test was used for continuous variables. The Kaplan–Meier product-limit method was used to compute cumulative incidence, and differences in estimate were compared using the log-rank test. To examine the relationship between OSA and future risk of incident depressive disorder and vice versa, Cox proportional-hazard regression analysis was used to calculate the crude and adjusted hazards ratios (HRs) with 95% confidence intervals (CIs). Three models were constructed: model 1 estimated the crude association; model 2 was adjusted for age, sex, urbanization level, and insurance salary; and model 3 was based on model 2 and additionally adjusted for medical comorbidities, such as diabetes mellitus, hypertension, hyperlipidemia, stroke, ischemic heart disease, chronic obstructive pulmonary disease, and obesity. Stratified Cox regression analysis (stratified according to sex, age groups [<65 years and ≥65 years]) was performed to examine the modifying effect of sex and age on the bidirectional OSA–depression associations. All tests were 2-tailed, and the level of significance was set at P < 0.05. Analyses were carried out using SAS, version 9.4 (SAS, Cary, NC).
3. Results
3.1. Study 1: OSA and the subsequent risk of depression
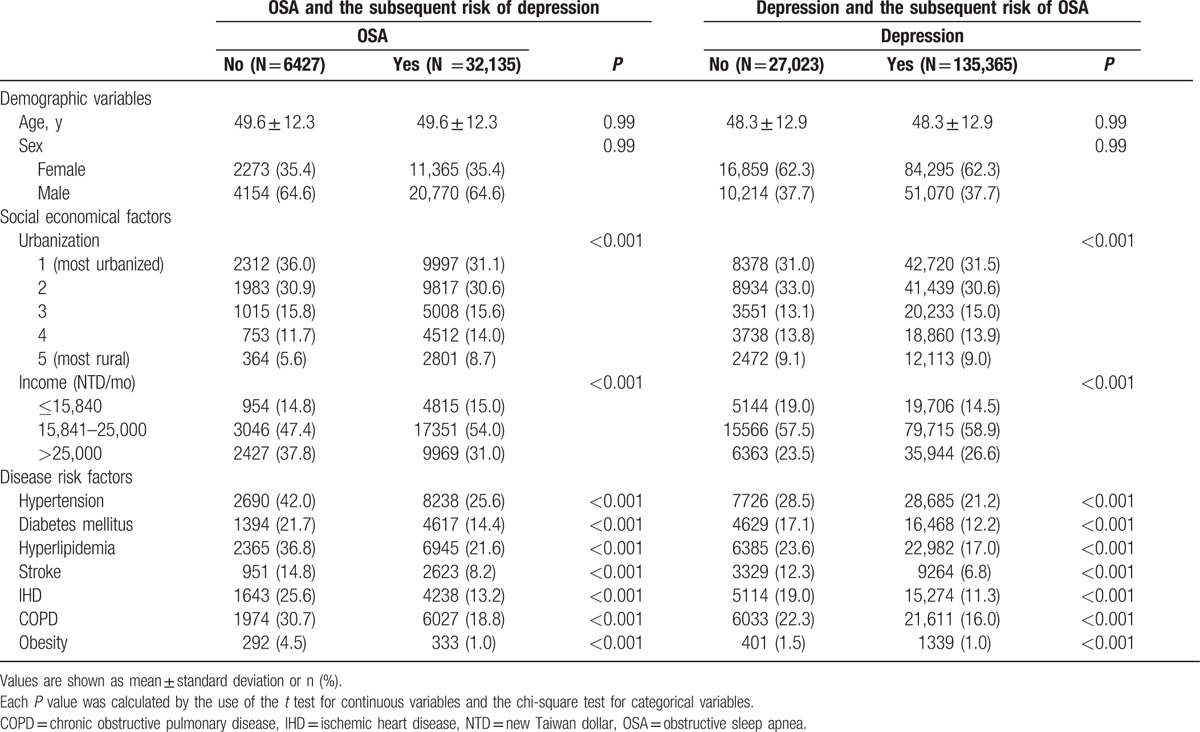
In study 1, we identified 6427 patients with OSA between 1997 and 2009, mean age 49.6 ± 12.3 years, and a male predominance (64.6% vs 35.4%). Compared with those without OSA, patients with OSA were more likely to have hypertension, diabetes mellitus, hyperlipedimia, stroke, ischemic heart disease, and chronic obstructive pulmonary disease, and to be obese, to reside in nonrural areas, and to have higher income (all P < 0.001) (Table 1).
Table 1.
Characteristics of participants with or without obstructive sleep apnea or depression.
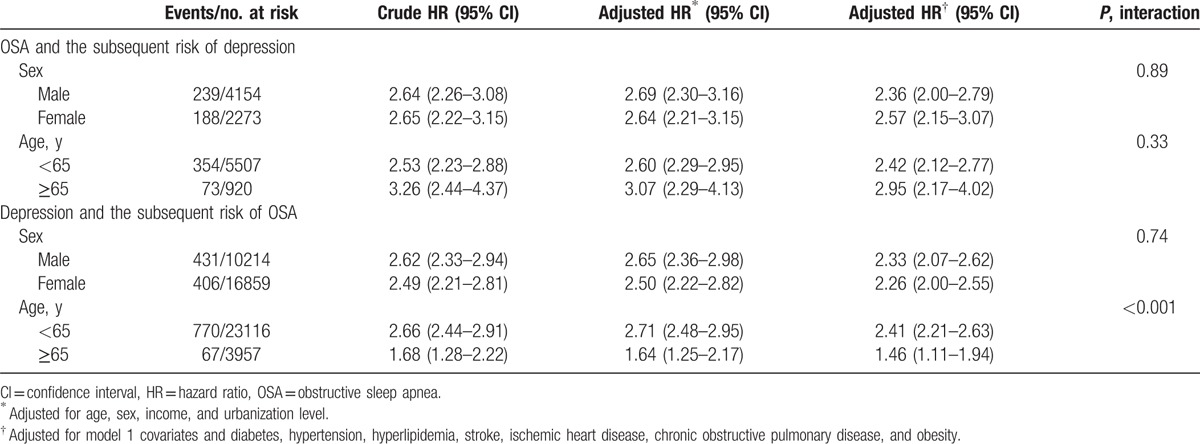
During the follow-up period, there were 427 out of 6427 (6.6%) patients with OSA, and 840 (2.6%) out of 32,145 controls who were diagnosed with depression. In addition, a shorter interval between the index date and OSA diagnosis date was noted (3.1 ± 2.9 vs 4.5 ± 2.9 years; P < 0.001). However, there was no significant difference between the cases and the controls in regards to the average age at the onset of depression. The Kaplan–Meier survival analysis showed that patients with OSA were associated with a higher risk of depression during the follow-up period (log-rank test P < 0.001) (Fig. 1). Compared with the controls, patients with OSA had more than 2 times the risk of a subsequent depression (adjusted for demographic data and medical comorbidities: HR 2.48, 95% CI 2.20–2.79) (Table 2). Subanalyses stratified by sex showed that both males and females had an increased risk for developing depression than the controls (males: HR 2.36, 95% CI 2.00–2.79; females: HR 2.57, 95% CI 2.15–3.07). Furthermore, both the older (≥65 years) group and the younger (<65 years) group showed a significant risk for developing depression at a later time (older group: HR 2.42, 95% CI 2.12–2.77; younger group: HR 2.95, 95% CI 2.17–4.02) (Table 3).
Figure 1.
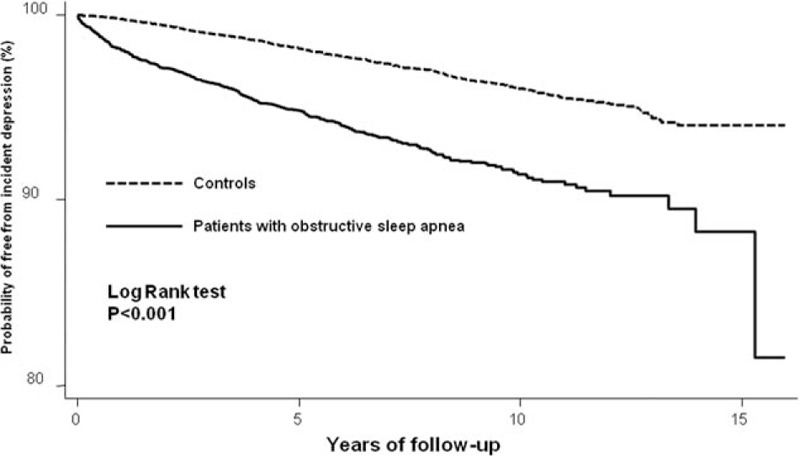
Survival curve of incident depression among patients with or without obstructive sleep apnea.
Table 2.
Bidirectional risk of depression and obstructive sleep apnea.
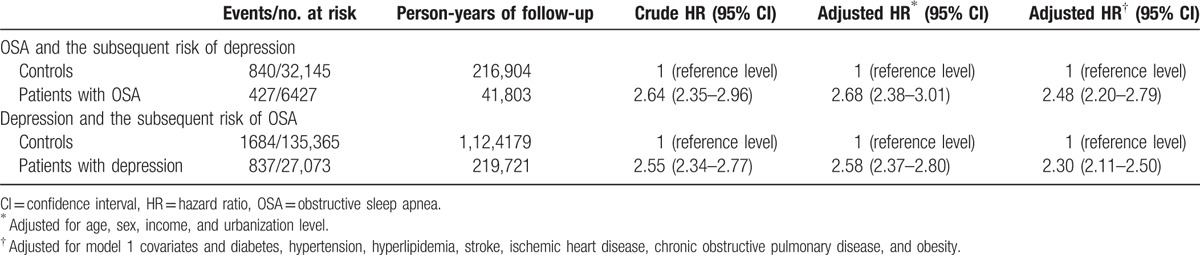
Table 3.
Bidirectional risk of depression and obstructive sleep apnea stratified by age and sex.
3.2. Study 2: Depression and the subsequent risk of OSA
In study 2, we identified 27,073 patients with depression between 1997 and 2009, mean age 48.3 ± 12.9 years, and a female predominance (62.3% vs 37.7%). Patients with depression were more likely hypertension, diabetes mellitus, hyperlipedimia, stroke, ischemic heart disease, and chronic obstructive pulmonary disease comorbidities, and to be obese, to reside in nonrural areas, and to have lower income than the those without depression (all P < 0.001) (Table 1).
During the follow-up period (mean 8.27 years), there were 837 out of 27,073 (3.1%) patients with depression, and 1684 (1.2%) out of 135,365 controls were diagnosed with OSA. Compared with the controls, patients with depression were more likely to be subsequently diagnosed with OSA. Furthermore, we noted a shorter interval between the index date and OSA diagnosis date (4.7 ± 3.6 vs 5.6 ± 3.3 years; P ≤ 0.001), and an earlier age at diagnosis of depressive disorder (52.4 ± 11.2 vs 53.9 ± 11.2 years; P = 0.002) in the case group. The Kaplan–Meier survival analysis showed that patients with depression were associated with a higher risk of OSA during the follow-up period (log-rank test P < 0.001) (Fig. 2). Patients with depression had more than 2 times the risk of developing OSA (adjusted for demographic data and medical comorbidities: HR 2.30, 95% CI 2.11–2.50) (Table 2). Stratified by sex, both male and female with depression were prone to subsequent OSA compared with those without depression (P value for interaction 0.74). Furthermore, both the older (≥65 years) group and the younger (<65 years) group showed a significant risk for developing OSA later (older group: HR 1.46, 95% CI 1.11–1.94; younger group: HR 2.41, 95% CI 2.21–2.63). Compared with their counterparts without depression, patients with depression aged <65 years had more than 2 times the risk of developing OSA and patients with depression aged ≥65 years had less than 2 times the risk of OSA (P value for interaction <0.001) (Table 3).
Figure 2.
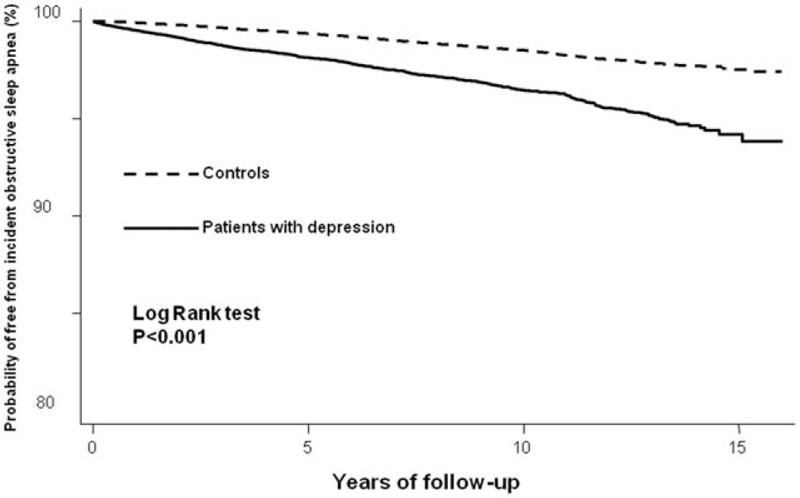
Survival curve of incident obstructive sleep apnea among patients with or without depression.
In this bidirectional study, we revealed the strong association between OSA and depression. Among patients with OSA, the risk for subsequent depression increased more than 2-fold, and vice versa.
4. Discussion
The present study is the first to examine the bidirectional temporal relationship between OSA and depression in the same population. The results demonstrate that patients having OSA increases the risk of subsequent physician-diagnosed depression, and conversely, baseline depression was associated with an increased risk of incident OSA at follow-up. All the associations were independent of sociodemographic factors and medical comorbidities.
Obstructive sleep apnea and depression commonly coexist. Previous studies have supported that OSA was associated with an increased risk of depression later in life. In a 4-year follow-up study, Peppard et al found an association between the severity of OSA and subsequent risk of depression. Patients with mild OSA had a 2-fold increased risk in developing depression later. The risk for development of depression increased by 1.6-fold in patients with minimal OSA, by 2.0-fold in patients with mild OSA, and by 2.6-fold in those with moderate or severe OSA.[17] However, the diagnosis of depressive symptoms has relied on self-administered questionnaires rather than by a clinical diagnosis. Similarly, a large population-based study that assessed depression-free survival in patients with OSA in comparison with patients without OSA found a 2.3-fold higher risk of developing depression in patients with OSA during follow-up.[16] However, the follow-up time from OSA to depression was just 1 year, raising the possibility of reversal causality or misdiagnosis.
Our study again confirmed the results previously described, such as the fact that OSA might be a risk factor for future depression and that the effect sizes were markedly similar. None of these studies examined whether a current depression diagnosis was a risk factor for having OSA later in life or not. A novel finding of our study is that depression was an equally strong risk factor for incident OSA. We found that the direction of the link is not predominately from OSA to depression, but, in fact, is equally significant from depression to future OSA. Thus, our results suggested that the association between OSA and depression might be bidirectional.
Although our study provided evidence to support a “reciprocal link” between OSA and depression, the actual pathophysiological mechanisms involved in the link between these 2 diseases remain unknown. OSA and depression have similar risk factors and common pathophysiological mechanisms, which possibly predispose an individual to the simultaneous development of both diseases.
One possible common link is inflammation. Patients with OSA also have shown to have a dysfunctional immune response and abnormal activation of the inflammatory response system, along with increased release of several pro-inflammatory cytokines. Substantial evidence indicates that patients with OSA have increased circulating concentrations of inflammatory markers such as C-reactive protein, interleukin-6, circulating adhesion molecules, and tumor necrosis factor-α.[23] Previous evidence has also indicated that these pro-inflammatory cytokines may mediate the response to various threats to the homeostatic balance, and might play a significant role in the initiation and perpetuation of depression.[24] On the contrary, a similar immune response, with increased pro-inflammatory cytokines, appears in patients with depression.[25] Elevated inflammatory cytokines might cause neural injury and alter homeostatic and circadian processes underlying the sleep–wake cycle, which may increase the risk of OSA and exaggerate OSA symptoms.[26]
Another possible common link between OSA and depression is neurotransmitter dysfunction. Abnormalities in the neurotransmission of serotonin have been implicated as a potential cause of major depression.[27] Serotonin also can directly affect the upper airway dilator muscles and therefore reduce the upper airway size,[28] which may increase the risk of OSA and exaggerate OSA symptoms. Structural brain abnormalities in patients with OSA occur in sites that are also affected in patients with depression. Neuroimaging studies[29] have revealed significant gray matter reduction in brain regions that regulate mood and respiratory function, including the hippocampus, anterior cingulate, amygdala, and frontal cortex, in both OSA and depression with overlapping results.[30,31] This raises the possibility that depression may contribute to the development of the brain structural abnormalities found in patients with OSA, or that the neural alterations accompanying OSA fosters the development of depression.
Additionally, poor sleep quality and frequent arousals associated with OSA could have a deleterious impact on both emotions and mood, and result in an increased risk of depression.[32] Benzodiazepines and certain antianxiety medication, commonly used to treat depression in patients, might decrease the muscle tone of the upper airway dilator muscle, blunt the response to hypoxia, and increase the risk of OSA.[33]
Our results are consistent with previous epidemiological studies demonstrating that those affected by OSA are predominantly male and those affected by depression are predominantly female.[34,35] Some studies have suggested that there is a sex difference in regards to the relationship between OSA and depression.[36] However, our study found that the risk of subsequent depression among patients with OSA or subsequent risk of OSA among patients with depression was comparably increased in both men and women. In addition, we found that both patients with depression aged <65 or ≥65 years had a higher risk of developing depression in comparison with their counterparts without depression. But, patients with depression aged <65 years had a higher risk of developing subsequent OSA than those with depression aged ≥65 years. This might be explained by the higher prevalence of other age-related comorbidities, such as stroke, heart failure, and cancer, in older adults, which may preclude them from seeking medical services for OSA. Longitudinal studies have also indicated a plateau, or perhaps even a decline, in OSA prevalence in the older population.[18] Future studies are warranted to investigate the effects of sex and age on the relationship between OSA and depression.
Our study has some potential limitations. First, our study was limited by the data from the insurance claims database; this might have led to an underestimation of the incidence of OSA and depression because only those who actively sought healthcare services were included in our study. Second, the diagnosis of sleep apnea and depression were based on the ICD-9-CM coding, and there are concerns regarding the accuracy of medical coding in the claims data. However, coding of OSA from LHID2015 has been found to have good validity in previous studies.[37] To ensure the accuracy of the diagnostic codes for depression, only patients diagnosed with depression by certified psychiatrists were recorded. Nevertheless, coding errors could not be completely ruled out. Sources of error may include misdiagnosis or miscoding by the physicians and hospital billing staff. However, the error might be equal between patients with OSA and depression, and would not be expected to bias the associations.
Third, information about the severity of OSA and major treatment modalities such as the apnea–hypopnea index and continuous positive airway pressure treatment were not available in the claims database. However, the inclusion of both patients with mild OSA and OSA patients receiving treatment might result in underestimation of the risk of depression in our study. In addition, the medications used to treat depression were not investigated in our study because such an analysis would be very complicated in a study with such a long follow-up period. Thus, further research is necessary to clarify the influence of these factors on the bidirectional association between OSA and depression.
Fourth, the claims data did not provide information on lifestyle, dietary habits, social networks, and educational level, which are important risk factors for OSA and depression. Finally, residual unmeasured confounding factors may have been present, as in any observational study.
Notwithstanding these limitations, our study is the first to investigate the bidirectional relationship between OSA and depression in the same population. The population-based sample used in this study, which is highly representative, reduced the possibility of selection bias. We followed a nationwide cohort without any loss in participants, making a nonresponse bias unexpected in our study. The large sample sizes used in our study ensured sufficient statistical power to investigate the link between OSA and depression. Lastly, this retrospective 15-year cohort study allowed us to examine the bidirectional relationship between OSA and depression that may have had a long latency period.
4.1. Clinical implications
From a clinical perspective, it would be valuable to monitor the sleep quality and sleep architecture in depressed patients, and also mood status in patients with OSA. The reciprocal association between OSA and depression found in this study stresses the importance of early detection of each disease and the development of prevention and treatment strategies that can reduce the risk of both diseases.
5. Conclusions
Our study supported a bidirectional association between OSA and depression. Future studies are needed to confirm our findings in different populations and to investigate the potential mechanism(s) contributing this association. Given the serious burden that these 2 diseases have on the healthcare system, more studies are needed to evaluate whether early screening and collaborative care for patients with OSA or depression could reduce the burden of both diseases.
Footnotes
Abbreviations: CI = confidence interval, HR = hazard ratio, ICD-9-CM = International Classification of Diseases, 9th Revision, Clinical Modification, LHID = Longitudinal Health Insurance Database, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, NTD = New Taiwan dollar, OSA = obstructive sleep apnea.
The authors report no conflicts of interest.
References
- 1.Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med 2013; 10:e1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharafkhaneh A, Giray N, Richardson P, et al. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep 2005; 28:1405–1411. [DOI] [PubMed] [Google Scholar]
- 4.Ejaz SM, Khawaja IS, Bhatia S, et al. Obstructive sleep apnea and depression: a review. Innov Clin Neurosci 2011; 8:17–25. [PMC free article] [PubMed] [Google Scholar]
- 5.Xie W, Zheng F, Song X. Obstructive sleep apnea and serious adverse outcomes in patients with cardiovascular or cerebrovascular disease: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2014; 93:e336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Q, Kling JM. Depression and the risk of myocardial infarction and coronary death: a meta-analysis of prospective cohort studies. Medicine (Baltimore) 2016; 95:e2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cong L, Dou P, Chen D, et al. Depression and associated factors in the elderly cadres in Fuzhou, China: a community-based study. Int J Gerontol 2015; 9:29–33. [Google Scholar]
- 8.Molnar MZ, Mucsi I, Novak M, et al. Association of incident obstructive sleep apnoea with outcomes in a large cohort of US veterans. Thorax 2015; 70:888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sforza E, de Saint Hilaire Z, Pelissolo A, et al. Personality, anxiety and mood traits in patients with sleep-related breathing disorders: effect of reduced daytime alertness. Sleep Med 2002; 3:139–145. [DOI] [PubMed] [Google Scholar]
- 10.Harris M, Glozier N, Ratnavadivel R, et al. Obstructive sleep apnea and depression. Sleep Med Rev 2009; 13:437–444. [DOI] [PubMed] [Google Scholar]
- 11.Smith R, Ronald J, Delaive K, et al. What are obstructive sleep apnea patients being treated for prior to this diagnosis? Chest 2002; 121:164–172. [DOI] [PubMed] [Google Scholar]
- 12.Aloia MS, Arnedt JT, Smith L, et al. Examining the construct of depression in obstructive sleep apnea syndrome. Sleep Med 2005; 6:115–121. [DOI] [PubMed] [Google Scholar]
- 13.Ishman SL, Cavey RM, Mettel TL, et al. Depression, sleepiness, and disease severity in patients with obstructive sleep apnea. Laryngoscope 2010; 120:2331–2335. [DOI] [PubMed] [Google Scholar]
- 14.Macey PM, Woo MA, Kumar R, et al. Relationship between obstructive sleep apnea severity and sleep, depression and anxiety symptoms in newly-diagnosed patients. PLoS One 2010; 5:e10211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rezaeitalab F, Moharrari F, Saberi S, et al. The correlation of anxiety and depression with obstructive sleep apnea syndrome. J Res Med Sci 2014; 19:205–210. [PMC free article] [PubMed] [Google Scholar]
- 16.Chen YH, Keller JK, Kang JH, et al. Obstructive sleep apnea and the subsequent risk of depressive disorder: a population-based follow-up study. J Clin Sleep Med 2013; 9:417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peppard PE, Szklo-Coxe M, Hla KM, et al. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med 2006; 166:1709–1715. [DOI] [PubMed] [Google Scholar]
- 18.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002; 165:1217–1239. [DOI] [PubMed] [Google Scholar]
- 19.Garvey JF, Pengo MF, Drakatos P, et al. Epidemiological aspects of obstructive sleep apnea. J Thorac Dis 2015; 7:920–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu CC, Hsu YC, Chang KH, et al. Depression and the risk of peptic ulcer disease: a nationwide population-based study. Medicine 2015; 94:e2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfennig A, Ritter PS, Hofler M, et al. Symptom characteristics of depressive episodes prior to the onset of mania or hypomania. Acta Psychiatr Scand 2016; 133:196–204. [DOI] [PubMed] [Google Scholar]
- 22.Hirschfeld RM. Differential diagnosis of bipolar disorder and major depressive disorder. J Affec Disord 2014; 169:S12–S16. [DOI] [PubMed] [Google Scholar]
- 23.Teramoto S, Yamamoto H, Ouchi Y. Increased C-reactive protein and increased plasma interleukin-6 may synergistically affect the progression of coronary atherosclerosis in obstructive sleep apnea syndrome. Circulation 2003; 107:E40–E140. [DOI] [PubMed] [Google Scholar]
- 24.Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry 2010; 67:446–457. [DOI] [PubMed] [Google Scholar]
- 25.Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun 2007; 21:374–383. [DOI] [PubMed] [Google Scholar]
- 26.Vgontzas AN, Zoumakis E, Lin HM, et al. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-alpha antagonist. J Clin Endocrinol Metab 2004; 89:4409–4413. [DOI] [PubMed] [Google Scholar]
- 27.Jans LA, Riedel WJ, Markus CR, et al. Serotonergic vulnerability and depression: assumptions, experimental evidence and implications. Mol Psychiatry 2007; 12:522–543. [DOI] [PubMed] [Google Scholar]
- 28.Fenik P, Veasey SC. Pharmacological characterization of serotonergic receptor activity in the hypoglossal nucleus. Am J Respir Crit Care Med 2003; 167:563–569. [DOI] [PubMed] [Google Scholar]
- 29.Cross RL, Kumar R, Macey PM, et al. Neural alterations and depressive symptoms in obstructive sleep apnea patients. Sleep 2008; 31:1103–1109. [PMC free article] [PubMed] [Google Scholar]
- 30.Evans KC, Banzett RB, Adams L, et al. BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. J Neurophysiol 2002; 88:1500–1511. [DOI] [PubMed] [Google Scholar]
- 31.Peiffer C, Poline JB, Thivard L, et al. Neural substrates for the perception of acutely induced dyspnea. Am J Respir Crit Care Med 2001; 163:951–957. [DOI] [PubMed] [Google Scholar]
- 32.Bardwell WA, Norman D, Ancoli-Israel S, et al. Effects of 2-week nocturnal oxygen supplementation and continuous positive airway pressure treatment on psychological symptoms in patients with obstructive sleep apnea: a randomized placebo-controlled study. Behav Sleep Med 2007; 5:21–38. [DOI] [PubMed] [Google Scholar]
- 33.Guilleminault C. Benzodiazepines, breathing, and sleep. Am J Med 1990; 88:25S–28S. [DOI] [PubMed] [Google Scholar]
- 34.Young T, Evans L, Finn L, et al. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997; 20:705–706. [DOI] [PubMed] [Google Scholar]
- 35.Martin LA, Neighbors HW, Griffith DM. The experience of symptoms of depression in men vs women: analysis of the National Comorbidity Survey Replication. JAMA Psychiatry 2013; 70:1100–1106. [DOI] [PubMed] [Google Scholar]
- 36.Enright PL, Newman AB, Wahl PW, et al. Prevalence and correlates of snoring and observed apneas in 5,201 older adults. Sleep 1996; 19:531–538. [DOI] [PubMed] [Google Scholar]
- 37.Lee YC, Hung SY, Wang HK, et al. Sleep apnea and the risk of chronic kidney disease: a nationwide population-based cohort study. Sleep 2015; 38:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]


