Abstract
Metabolic bone disease of prematurity is a condition characterized by reduction in bone mineral content (osteopenia). It is a problem faced by very low birth weight (VLBW) infants because of lack of fetal mineralization during the last trimester. Our aim was to assess serum alkaline phosphatase (ALP) level as an early biomarker for osteopenia in premature infants and to estimate an optimal cutoff value of serum ALP at which osteopenia is detected radiologically in premature newborns.
This prospective study was conducted on a cohort of 120 newborn infants of both sex of ≤34 weeks’ gestational age and <1500 g birth weight. Two blood samples, from each infant on at least 2 consecutive weeks, were reported for calcium, phosphorus, and ALP. Evidence of osteopenia was evaluated radiologically by performing wrist/knee x-ray.
Sixteen infants (13.3%) had evidence of osteopenia in x-ray, whereas 104 infants (86.7%) were nonosteopenic and all the osteopenic infants were <1000-g birth weight. Birth weight and gestational age were significantly inversely related to serum ALP levels. Both samples showed statistically significantly higher mean ALP level in osteopenic than nonosteopenics (P < 0.001, and P < 0.001 respectively). There was no constant value of serum ALP related to radiologic evidence of osteopenia. However, the optimal cutoff value of serum ALP at which osteopenia is detected is 500 IU/L with 100% sensitivity and 80.77% specificity.
High levels of ALP can be considered a reliable biomarker to predict the status of bone mineralization and the need for radiological evaluation in premature infants particularly those <1000-g birth weight and <32 weeks’ gestation.
Keywords: alkaline phosphatase, osteopenia, prematurity
1. Introduction
There is a significant rate of preterm birth worldwide and literature reports show that in 2010, 1 of 10 children was born premature.[1] Metabolic bone disease (MBD) of prematurity is a comparatively frequent illness in preterm infants born before completing 32 weeks’ gestation.[2] It happens in 20% to 30% of preterm infants <1500-g birth weight and in 50% to 60% of preterm infants with birth weight <1000 g.[3] The prevalence is 40% in breastfed premature infants, in comparison to 16% of those fed with a formula specific for preterm infants and supplemented with calcium and phosphorus.[4,5]
Osteopenia of prematurity is characterized by diminished bone mineral content, which occurs chiefly because of diminished mineral stores in premature infants that may be exaggerated by increased mineral requirements in the neonatal period. Normally, Calcium (Ca) and phosphorus (P) are maximally gained by the fetus during the last trimester of pregnancy, so preterm infants are born with significantly reduced mineral stores compared to term infants.[5] Premature very low birth weight (VLBW) infants miss out on the fetal acquisition of nutrients in the last trimester and have slow weight gain postnatally.[6] Moreover, supplementation of minerals in preterm infants at the levels required to equalize the transplacental accretion in the last trimester has turned out to be challenging, especially in infants who do not tolerate enteral feeding and require prolonged total parenteral nutrition (TPN).[7] Treatment with corticosteroids, methylxanthines and diuretics also seem to share in the development of MBD of prematurity.[8]
The MBD usually occurs between the tenth and sixteenth weeks of life, but it may stay still until severe demineralisation (a reduction of bone mineral density [BMD] of 20% to 40%) takes place. The clinical picture is diverse, ranging from a totally occult condition to a full blown picture of overt rickets, with multiple fractures, when marked demineralization occurs.[9]
Evidence indicates that osteopenia may be linked to diminished linear growth potential even after correction of radiographic and biochemical alterations.[10,11]
Serum alkaline phosphatase (ALP) activity, serum P, and serum Ca have been previously used for screening of osteopenia in preterm infants. Increased ALP and reduced serum P have been shown to be related to increased risk of MBD of prematurity.[12,13] Correlations between the existence of skeletal osteopenia and low birth weight, decreased gestational age, diminished enteral feeds, and high serum ALP levels in infants <1500-g birth weight have been reported in literature.[14] Nevertheless, the usefulness of ALP and serum P as screening tests is still challenging.[15] Currently, there are no standard recommendations on screening for MBD and/or rickets in premature infants.[16] ALP activity elevation is common in extreme low birth weight (ELBW) infants and birth weight is significantly inversely related to both ALP levels and radiological rickets.[17]
In this study, we aimed at detection of MBD in VLBW infants radiologically and to assess serum ALP level as a biomarker for osteopenia in premature infants after exclusion of other conditions that cause elevation of serum ALP. We thought also to estimate an optimal cutoff value of serum ALP at which osteopenia is detected in premature infants.
2. Patients and methods
The present prospective study was conducted on a cohort of 120 newborn infants of both sexes in the neonatal intensive care units (NICU), Children Hospital (Abu Elreesch), and NICU of Obstetrics and Gynecology Hospital, Faculty of Medicine, Cairo University, along the period from March 2010 to December 2011. The study was approved by the scientific research committee of the Pediatric Department, Faculty of Medicine, Cairo University, and an informed consent was obtained from the parents of all infants involved in the study. The consent was implied upon admission, after counseling the parent(s) to the possible investigations and management that most of preterm infants were liable to. The consent included an agreement of x-ray performance.
The current study included preterm newborn infants with gestational age <34 weeks and birth weight <1500 g. We excluded infants admitted at postnatal age of >30 days, died, discharged, or being transferred to another institution before staying, at least, 8 weeks in the NICU. Also, we excluded patients with any skeletal deformities or cholestasis where both may affect the ALP level.
All enrolled infants were subjected to full medical history, anthropometric measurements, and assessment of gestational age using the criteria of the new Ballard score system.[18] Complications such as sepsis, broncopulmonary dysplasia, necrotizing enterocolitis (NEC), or gastrointestinal perforation were determined and recorded.
2.1. Laboratory investigations
During their hospital stay, and according to the policy of our unit, we measured serum Ca, P, and ALP in all VLBW infants weekly until the infants received full enteral feeds and had shown a pattern that the ALP was no longer increasing. So, at least 2 blood samples starting at the age of 8 weeks postnatally are reported for each infant on at least 2 consecutive weeks and centrifuged for 15 minutes and then examined for levels of calcium, phosphorous, and ALP. Serum albumin and bilirubin were evaluated to exclude hepatic dysfunction, as it may affect serum ALP levels. Laboratory evaluation also included complete blood count, and C-reactive protein (CRP).
2.2. Radiological assessment
Wrist or knee radiographs were performed for detection of osteopenia for the enrolled infants and were reviewed by a pediatric radiologist who was not aware of the laboratory values of these patients. Patients were classified either as normal (nonosteopenic group), or as having osteopenia if there is poor mineral density; rachitic changes in the form of widening, cupping, and fraying of the epiphyseal ends; and/or fractures[19] (osteopenic group).
2.3. Statistical analysis
Quantitative data were presented as mean and standard deviation (SD) values. Student t test was used for comparisons between osteopenic and normal infants. Qualitative data were presented as frequencies and percentages. χ2 test was used for studying the comparisons and associations between different qualitative variables. Pearson correlation coefficient was used to determine significant correlations between ALP level and different quantitative variables. The significance level was set at P ≤ 0.05. Statistical analysis was performed with IBM SPSS® Statistics Version 20 for Windows (IBM Corporation).
3. Results
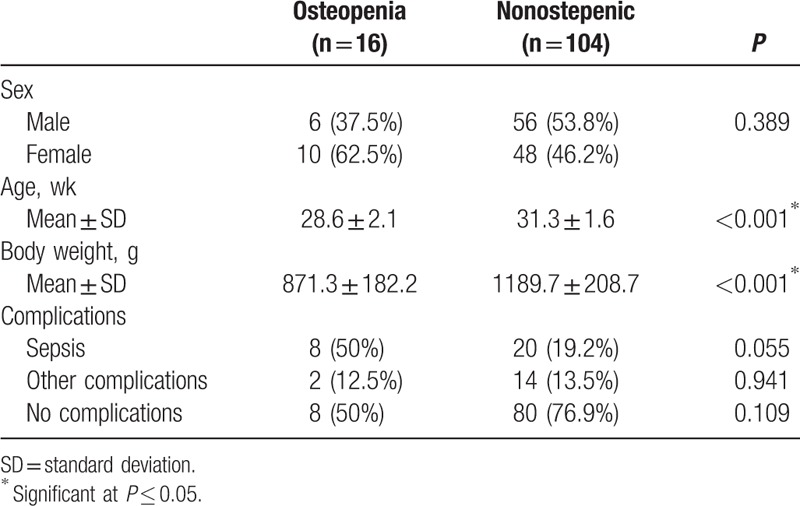
A total of 120 preterm newborn infants, with gestational age <34 weeks and birth weight <1500 g, were included in the study. Sixteen infants (13.3%) had evidence of osteopenia in x-ray, whereas 104 infants (86.7%) showed no osteopenia in their radiological study. Osteopenic infants showed statistically significant lower mean gestational age and mean body weight than normal infants and there was no statistically significant difference between complications in the 2 groups (Table 1).
Table 1.
Descriptive statistics of the studied infants.
By asking the mothers of the osteopenic infants about their compliance to calcium medicinal supplementation during gestation, we found that 75% (n = 12) of them were not receiving supplemental calcium regularly during gestation.
The hematological profile of the studied infants is detailed in Table 2. The first sample showed that osteopenic infants had statistically significantly higher mean total leucocytic count (TLC) and hemoglobin (Hgb) levels than nonosteopenic infants (P = 0.044, P = 0.007, respectively). The second sample, taken 1 week later, showed that there was no statistically significant difference between mean TLC or Hgb in the 2 groups. As regards the percent increase in TLC, osteopenic infants showed statistically significantly lower mean percent increase in TLC than nonosteopenic infants. Osteopenic infants showed higher mean percent decrease in Hgb than nonosteopenic infants (Table 2). CRP showed no statistically significant difference between osteopenic and nonosteopenic infants.
Table 2.
Hematological profile of the studied infants.
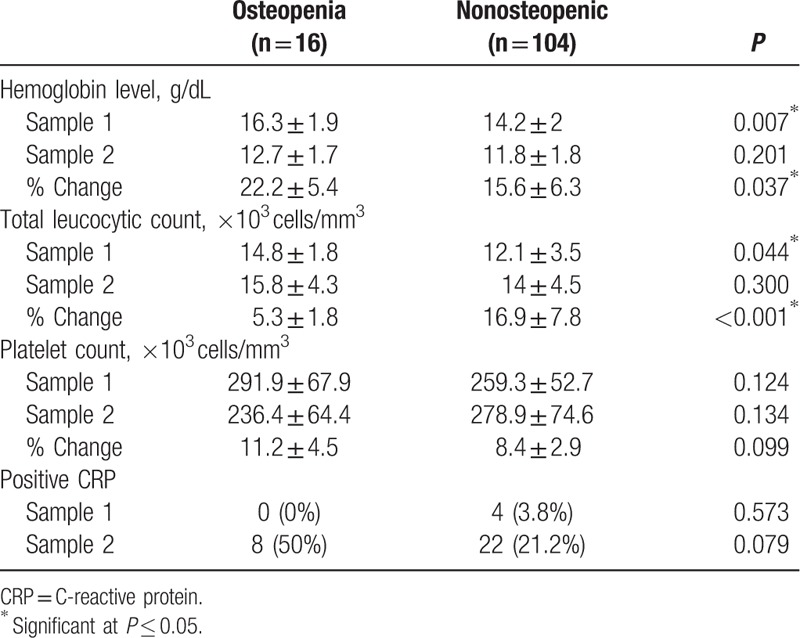
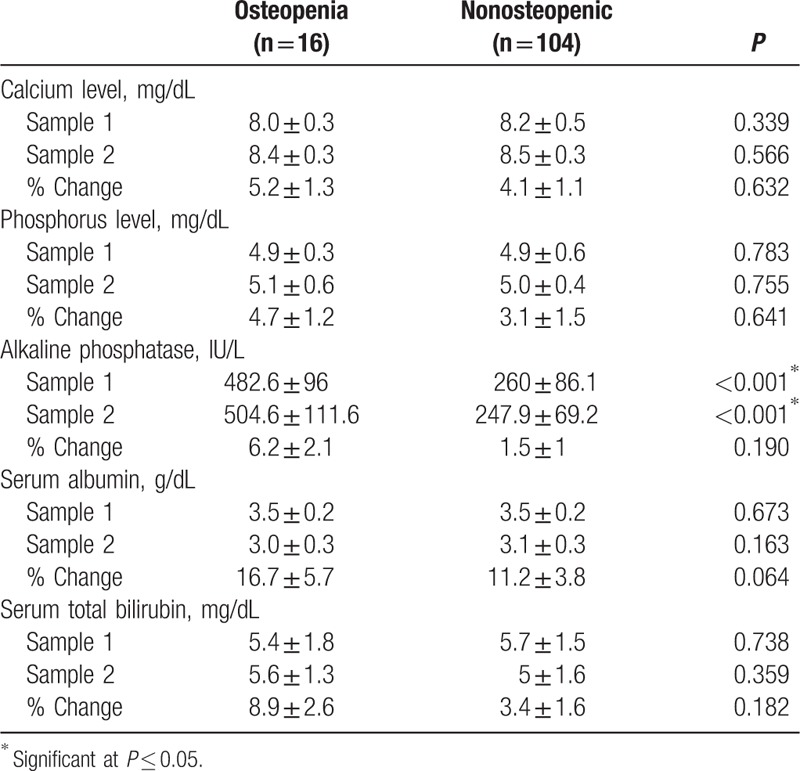
Table 3 shows the blood chemistry results of the studied infants. In both samples, osteopenic infants showed statistically significantly higher mean ALP level than nonosteopenics (P ≤ 0.001, and ≤0.001 respectively). As regards the percent change in ALP, there was no statistically significant difference between the 2 groups. Other blood chemistry parameters showed no significant difference between the 2 groups (Table 3).
Table 3.
Blood chemistry of the studied infants.
Some osteopenic findings as detected radiologically in our patients are shown in Figure 1.
Figure 1.
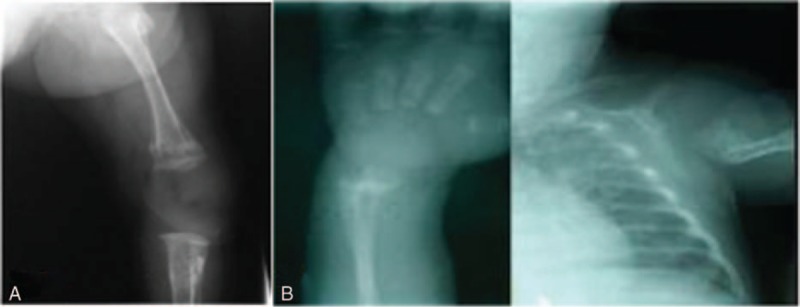
(A) Male infant, 30 weeks of gestational age, 1000-g birth weight with evidence of osteopenia in knee x-ray (reduced bone density and widening of epiphyseal ends). (B) Female infant, 30 weeks of gestational age, 1000-g birth weight with evidence of osteopenia in wrist x-ray reduced bone density with widening and cupping of epiphyseal ends).
3.1. Correlation between ALP and different variables
ALP was inversely correlated to both gestational age and birth weight in the 2 taken samples separated by 1 week apart. There was a statistically significant positive (direct) correlation between the ALP level and Hgb level also in both samples (Table 4).
Table 4.
Correlation between alkaline phosphatase and different variables.
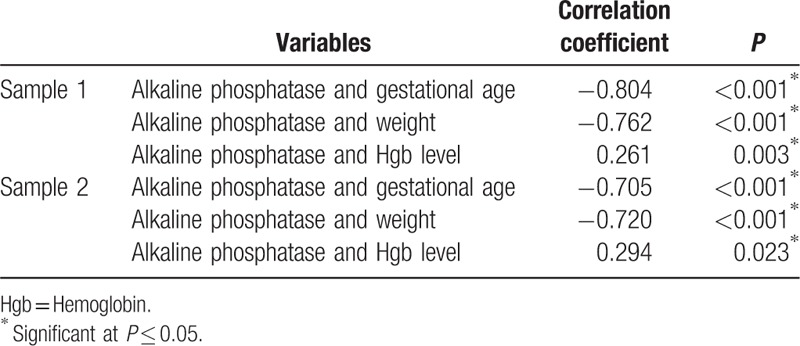
Receiver-operating characteristic curve analysis of ALP values for the diagnosis of osteopenia in the present study showed that the optimal cutoff point was determined at the ALP level of 500 IU/L (Table 5, Fig. 2). At this level, sensitivity was found to be 100%, whereas specificity is 80.77%. The area under the curve is 0.946 denoting very high diagnostic accuracy of ALP in detecting osteopenia.
Table 5.
ROC curve analysis for alkaline phosphatase.
Figure 2.
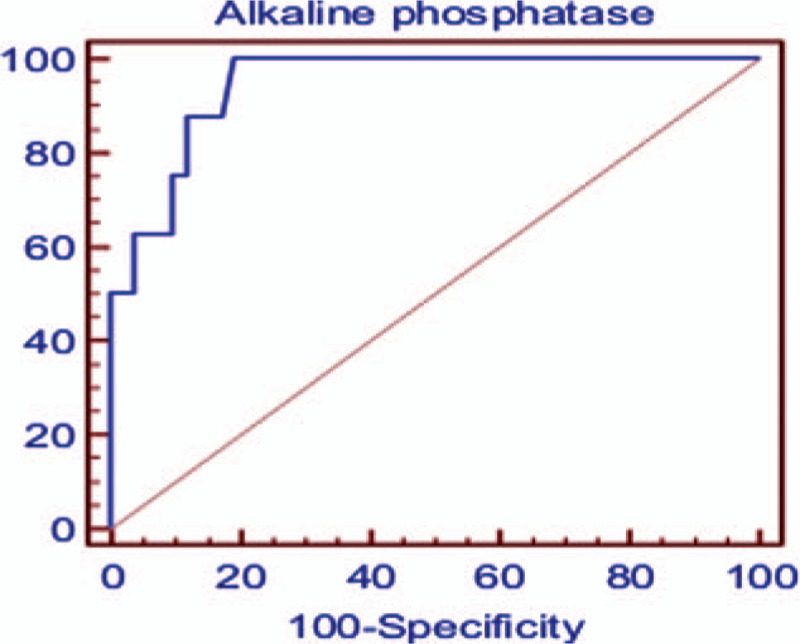
Receiver-operating characteristic curve analysis for alkaline phosphatase in detecting osteopenia.
4. Discussion
In this work, we studied 120 preterm infants ≤34 weeks of gestational age and <1500 g birth weight by x-ray wrist/knee and assessment of serum ALP level as an indicator for osteopenia in premature infants. We also searched for an optimum cutoff value of ALP at which osteopenia of prematurity can be detected.
Based on radiological assessment, only 16 infants (13.3%) with a birth weight of <1000 g had evidence of osteopenia. This was in agreement with Figueras-Aloy et al[20] who reported MBD of prematurity in 17% of their studied preterm neonates (≤31 weeks’ gestation and birth weight ≤1500 g). Similarly, in a study done by Hung et al,[21] 18 preterm infants (≤34 weeks’ gestation) comprising 39.1% of the study population showed osteopenia in radiographic examination. More recently, Viswanathan and et al reported that 30.9% of extremely low birth weight infants they studied developed radiological evidence of MBD of which 33.8% developed spontaneous fractures.[22]
As regards to birth weight and gestational age, they have a significant inverse correlation to both serum ALP level and radiographic evidence of osteopenia in our preterm infants (P < 0.005). Similar to other researches,[17,23–24] our study confirms the fact that the risk of osteopenia increases with decreased birth weight and gestational age. All the osteopenic infant groups were preterm (<32 weeks’ gestation) neonates with extremely low birth weight (<1000 g). Such results denote the high incidence of osteopenia in VLBW and ELBW infants.
We investigated the compliance of mothers of infants with osteopenia to calcium supplementation as a confounding maternal factor that might contribute to the risk of osteopenia. Almost three-fourths of osteopenic infant mothers did not receive calcium supplementation during their pregnancy. This was in agreement with Koo et al[25] who found that low maternal dietary intake of calcium during gestation is associated with increased risk of osteopenia in prematurity and a supplement of calcium to women with a low dietary calcium intake resulted in better bone mineral content of the total body in the infants born at term. This was in contrast with Jarjou et al[26] who concluded that calcium supplementation of pregnant women had no significant correlation with bone mineral status in the first year of life. This difference can be explained by the fact that they did not limit their study to mothers of premature babies.
We could not find a significant relation between the incidence of MBD in preterm infants and the associated complications such as sepsis, air leak syndromes, and NEC. Compared with these results, Eliakim et al[27] reported that neonatal sepsis in premature infants was not correlated with biochemical prove of diminished bone turnover. Nevertheless, Cakir et al[28] reported positive correlation between NEC and increased bone resorption in premature infants, which might be related to the reduced glucagon like peptide-2 levels, an intestinal hormone that is chiefly secreted from the distal small intestine.
We also found no significant difference between the 2 groups of studied preterm infants in terms of serum calcium and phosphorus. This was in agreement with So and Ng, who reported normal calcium level in osteopenic infants which, as they explained, was maintained by parathormone effect that stimulates calcium reabsorption.[8] Similarly, Hung et al reported similar levels of calcium concentration in osteopenic premature infants (≤34 weeks’ gestation) compared to those with no evidence of osteopenia.[21] On the contrary, regarding serum phosphorus level, Hitrova et al[29] concluded that hypophosphatemia in 2 weeks of age (P < 1.6 mmol/L) followed by a gradual rise of phosphorus levels and normalization at eight weeks of postnatal age could be considered as one of the biochemical markers of osteopenia of prematurity. Recently, authors reported that preterm infants with low serum inorganic phosphate (<2 mmol/L) are at risk of osteopenia, and levels <1.8 mmol/L have been strongly related to the presence of radiographically evident rickets. They suggested that the use of serum phosphate levels in combination with ALP levels can increase the sensitivity of the screening and identification of infants at risk of MBD.[30]
Serum ALP is known as a marker of bone turnover. High levels indicate increased bone cellular activity and when reaching levels >700 to 750 IU/L, they are linked to osteopenia, which is still not clinically apparent.[21,31] In our study, serial measurements of serum alkaline phosphatase levels on at least 2 consecutive weeks revealed significantly higher values in osteopenic infants compared with nonosteopenic infants (P < 0.001 in both samples). Also, the analysis of ALP values for the diagnosis of osteopenia showed that there is no constant value at which the evidence of osteopenia was detected. However, the optimal cutoff point with ALP level of 500 IU/L revealed 100% sensitivity but 80.77% specificity. This was in agreement with authors who reported that in the absence of liver disease, serum ALP levels >4 times normal adult levels have been considered as a marker of bone disease and serum ALP levels >600 IU/L have a diagnostic sensitivity of 100% and specificity of 70%.[11] Mitchel et al[17] in one review of 100 extremely low birth weight infants reported that ALP levels of >600 IU/L were commonly detected. However, no single value of ALP could be determined as predictive of the bone changes. Moreover, they recommended a radiograph of the wrist and/or knee to evaluate for rickets in premature infants when multiple measurements of ALP are >800 IU/L (2 values measured at least 1 week apart). Hung et al[21] found that ALP levels >700 IU/L, at 3 weeks of age, resulted in a sensitivity and specificity of 73% in predicting MBD in premature infants. Nevertheless, Backström et al reported that a combination of serum total ALP activity >900 IU/L and serum inorganic phosphate concentrations <1.8 mmol/L yielded a sensitivity of 100% and a specificity of 70%. They also suggested this method for screening of low BMD in preterms.[32]
To the best of our knowledge, this is the first Egyptian study that utilized serial serum ALP measures correlated with radiological changes for early detection of MBDs of preterm infants. The significant findings in this study add to the existing literature, supporting the role of serial ALP levels as a biomarker for osteopenia of prematurity. Nonetheless, important study limitations should be addressed. One of the study restrictions was its relatively small sample size. However, guided by the time frame of our study and the availability of our target study population, the sample size can be considered convenient. Another limitation was taking only 2 time points of ALP that were 7 days apart. More frequent sampling could show more cases with higher ALP values. One more limitation is the use of the wrist or knee radiographs for the detection of osteopenia. To be detected by x-ray, at least 20% of demineralization need to occur.[33] However, x-ray has the advantage over dual-energy x-ray absorptiometry, which is more sensitive to detect minor changes of bone mineral content,[34] by being clinically feasible and applicable.
5. Conclusion
In conclusion, high levels of ALP can be considered a reliable biomarker to predict the status of bone mineralization and the need for radiological evaluation in premature infants particularly those <1000-g birth weight or <32 weeks’ gestation. The optimal cutoff value of serum ALP, at which osteopenia is detected, is 500 IU/L with 100% sensitivity and 80.77% specifity.
6. Implications
MBD of prematurity has been recognized as a condition that deserves more global health attention. Taken into account the very high risk of osteopenia among ELBW infants, we recommend consideration of early mineral supplementation, particularly in those with ALP values of >500 IU/L in at least 2 consecutive weeks.
Footnotes
Abbreviations: ALP = alkaline phosphatase, BMD = bone mineral density, Ca = calcium, CRP = C-reactive protein, ELBW = extreme low birth weight, Hgb = hemoglobin, MBD = metabolic bone disease, NEC = necrotizing enterocolitis, NICU = neonatal intensive care unit, P = phosphorus, ROC curve = receiver-operating characteristic curve, SD = standard deviation, SPSS = Statistical Package for the Social Science, TLC = total leucocyte count, TPN = total parenteral nutrition, VLBW = very low birth weight.
This research received no specific grant from any funding agency in the public, commercial, and not-for-profit sectors.
The authors have declared no conflict of interest.
References
- 1.Blencowe H, Cousens S, Oestergaard M, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012; 379:2162–2172. [DOI] [PubMed] [Google Scholar]
- 2.Pieltain C, de Halleux V, Senterre T, et al. Prematurity and bone health. World Rev Nutr Diet 2013; 106:181–188. [DOI] [PubMed] [Google Scholar]
- 3.Backström MC, Kuusela AL, Mäki R. Metabolic bone disease of prematurity. Ann Med 1996; 28:275–282. [DOI] [PubMed] [Google Scholar]
- 4.Takada M, Shimada M, Hosono S. Trace elements and mineral requirements for very low birth weight infants in rickets of prematurity. Early Hum Dev 1992; 29:333–338. [DOI] [PubMed] [Google Scholar]
- 5.Abrams AS. In utero physiology role in nutrient delivery and fetal development for calcium, phosphorus and vitamin D. Am J Clin Nutr 2007; 85:604S–607S. [DOI] [PubMed] [Google Scholar]
- 6.Valentine CJ, Fernandez S, Rogers LK, et al. Early amino-acid administration improves preterm infant weight. J Perinatol 2009; 29:428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koo WW. Parenteral nutrition-related bone disease. J Parenteral Enteral Nutr 1992; 16:386–394. [DOI] [PubMed] [Google Scholar]
- 8.So KW, Ng PC. Treatment and prevention of neonatal osteopenia. Curr Paediatr 2005; 15:106–113. [Google Scholar]
- 9.Bozzetti V, Tagliabue P. Metabolic Bone Disease in preterm newborn: an update on nutritional issue. Ital J pediatr 2009; 35:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fewtrell MS, Cole TJ, Bishop NJ, et al. Neonatal factors predicting childhood height in preterm infants: evidence for a persisting effect of early metabolic bone disease? J Pediatr 2000; 137:668–673. [DOI] [PubMed] [Google Scholar]
- 11.Lucas A, Brooke OG, Baker BA, et al. High alkaline phosphatase activity and growth in preterm neonates. Arch Dis Child 1989; 64:902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aiken CG, Sherwood RA, Lenney W. Role of plasma phosphate measurements in detecting rickets of prematurity and in monitoring treatment. Ann Clin Biochem 1993; 30:469–475. [DOI] [PubMed] [Google Scholar]
- 13.Kovar I, Mayne P, Barltrop D. Plasma alkaline phosphatase activity: a screening test for rickets in preterm neonates. Lancet 1982; 1:308–310. [DOI] [PubMed] [Google Scholar]
- 14.Koo WW, Gupta JM, Nayanar VV, et al. Skeletal changes in preterm infants. Arch Dis Child 1982; 57:447–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faerk J, Peitersen B, Petersen S, et al. Bone mineralisation in premature infants cannot be predicted from serum alkaline phosphatase or serum phosphate. Arch Dis Child Fetal Neonatal Ed 2002; 87:133–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison CM, Johnson K, McKechnie E. Osteopenia of prematurity: a national review of practice. Acta Paediatr 2008; 4:407–413. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell MS, Rogers PS, Hicks DP, et al. High frequencies of elevated alkaline phosphatase activity and rickets exist in extremely low birth weight infants despite current nutritional support. BMC Pediatr 2009; 9:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ballard JL, Khoury JC, Wedig K, et al. New Ballard Score, expanded to include extremely premature infants. J Pediatr 1991; 119:417–423. [DOI] [PubMed] [Google Scholar]
- 19.Vachharajani AJ, Mathur AM, Rao R. Metabolic bone disease of prematurity. NeoReviews 2009; 10:e402–e411. [Google Scholar]
- 20.Figueras-Aloy J, Alvarez-Domínguez E, Pérez-Fernández JM, et al. Metabolic bone disease and bone mineral density in very preterm infants. J Pediatr 2014; 164:499–504. [DOI] [PubMed] [Google Scholar]
- 21.Hung YL, Chen PC, Jeng SF, et al. Serial measurements of serum alkaline phosphatase for early prediction of osteopaenia in preterm infants. J Paediatr Child Health 2011; 47:134–139. [DOI] [PubMed] [Google Scholar]
- 22.Viswanathan S, Khasawneh W, McNelis K, et al. Metabolic bone disease: a continued challenge in extremely low birth weight infants. J Parenter Enteral Nutr 2014; 38:982–990. [DOI] [PubMed] [Google Scholar]
- 23.Koo WW, Sherman R, Succop P, et al. Fractures and rickets in very low birth weight infants: conservative management and outcome. J Pediatr Orthop 1989; 9:326–330. [PubMed] [Google Scholar]
- 24.Gül YM, Heves K, Elif Ö, et al. Metabolic bone disease of prematurity: report of four cases. J Clin Res Pediatr Endocrinol 2014; 6:111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koo WW, Walters JC, Esterlitz J, et al. Maternal calcium supplementation and fetal bone mineralization. Obstet Gynecol 1999; 94:577–582. [DOI] [PubMed] [Google Scholar]
- 26.Jarjou LM, Prentice A, Sawo Y, et al. Randomized, placebo-controlled Ca supplementation study of pregnant Gambian women: effects on breast-milk Ca concentration and infant birth weight, growth and bone mineral accretion in the first year of life. Am J Clin Nutr 2006; 83:657–666. [DOI] [PubMed] [Google Scholar]
- 27.Eliakim A, Shiff Y, Nemet D, et al. The effect of neonatal sepsis on bone turnover in very-low birth weight premature infants. J Pediatr Endocrinol Metab 2003; 16:413–418. [DOI] [PubMed] [Google Scholar]
- 28.Cakir M, Mungan I, Karahan C, et al. Necrotizing enterocolitis increases the bone resorption in premature infants. Early Hum Dev 2006; 82:405–409. [DOI] [PubMed] [Google Scholar]
- 29.Hitrova S, Slancheva B, Popivanova A, et al. Osteopenia of prematurity-- prophylaxis, diagnostics and treatment. Akush Ginekol (Sofiia) 2012; 51:24–30. [PubMed] [Google Scholar]
- 30.Harrison CM, Gibson AT. Osteopenia in preterm infants. Arch Dis Child Fetal Neonatal Ed 2013; 98:F272–F275. [DOI] [PubMed] [Google Scholar]
- 31.Tsakalidis C, Dokos C, Tragiannidis A, et al. Gestational age, body weight and bone metabolism markers in premature infants: a single institution experience of Northern Greece. Acta Paediatrica 2010; 99:99.19764924 [Google Scholar]
- 32.Backström MC, Kouri T, Kuusela AL, et al. Bone isoenzyme of serum alkaline phosphatase and serum inorganic phosphate in metabolic bone disease of prematurity. Acta Paediatr 2000; 89:867–873. [PubMed] [Google Scholar]
- 33.Done SL. Fetal and neonatal bone health: update on bone growth and manifestations in health and disease. Pediatr Radiol 2012; 42 Suppl 1:S158–S176. [DOI] [PubMed] [Google Scholar]
- 34.Brunton JA, Bayley HS, Atkinson SA. Validation and application of dual-energy x-ray absorptiometry to measure bone mass and body composition in small infants. Am J Clin Nutr 1993; 58:839–845. [DOI] [PubMed] [Google Scholar]


