Abstract
The incidence of colorectal cancer (CRC) is increasing in young-age patients, but the clinical history is not established. Authors analyzed the clinical characteristics of young-age onset CRC to support basic information for setting treatment policies.
Between January 2006 to January 2014, 100 CRC patients diagnosed at the age of 10 to 39 were analyzed. The clinicopathologic characteristics were reviewed based on medical records. Survival outcomes including overall survival (OS), disease-free survival (DFS), and progression-free survival (PFS) were analyzed. This study was conducted as a retrospective, observation study.
Among 100 patients, 86 patients were diagnosed as CRC at their thirties. Seventy-nine patients had no familial history of cancer. At initial diagnosis, 59 patients showed the normal CEA level (≤3 ng/mL), and 61 patients were diagnosed as advanced CRC (40% stage III, 21% stage IV). Sixty-four patients had lower location-sigmoid colon, rectosigmoid junction, or rectum. Recurrence rate was 7.9% in stage I to III CRC. Although median OS was not reached, patients with normal CEA level showed better survival outcome (P = 0.013) and patients with perineural invasion showed poorer survival (P = 0.011). The 5-year survival rate of total patient population was estimated as 75%. However, median OS of stage IV patients were 19 months (range 7.9–60.63 months), shorter than historical data of >24 months.
Young-age CRC was most commonly diagnosed at their thirties, with no familial history, normal range of CEA and located below sigmoid colon. In young-age onset stage IV CRC, patients showed inferior OS compared to historical data. Based on our data, different surveillance program other than serum CEA level (e.g., sigmoidoscopy) is needed in young-age patient population.
Keywords: cancer screening, colorectal neoplasm, young-age cancer
1. Introduction
Colorectal cancer (CRC) is third most common cancer in incidence and associated with fourth most common cancer-related death in Korea.[1] The incidence of CRC begin to rise in the age of 40,[1] and >90% of patients are diagnosed as CRC over the age of 55.[2] The overall incidence of CRC is decreasing in total patient population,[3] but the prevalence of young-age onset CRC is increasing worldwide.[4] Among CRC patients, young-aged patients are commonly defined as patients <40 years of age. The incidence of young-age CRC varies between studies and races, but recent studies show 3.5% to 5% incidence rate in Asians and Caucasians.[4,5] The clinical characteristics of young-age CRC is not well validated and controversial about the prognosis, clinical behavior, and histopathology. There are heterogeneous opinions about survival outcomes, clinical characteristics, and pathologic findings of young-age CRC patients.[4–8]
In the present study, we analyzed the clinical characteristics and survival outcomes of CRC patients diagnosed at young age in our center.
2. Materials and methods
2.1. Patients
From January 2006 to January 2014, the medical records of patients diagnosed and treated as colorectal cancer in Seoul St. Mary's hospital were retrospectively reviewed. Among 4894 patients initially diagnosed as CRC, 100 patients were at the age of 13 to 39. The other eligible criteria were as follows: (1) pathologically confirmed as adenocarcinoma, mucinous carcinoma, or signet ring cell carcinoma; (2) patients who regularly followed up in Seoul St. Mary's Hospital. After the pathology was confirmed as CRC, available specimens were analyzed for V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) mutation status. Genomic DNA was extracted from available pathologic specimens for direct sequencing of KRAS exon 2. We analyzed the clinical characteristics, laboratory findings, and survival outcomes through the medical records. Gross and microscopic pathologic findings were reviewed based on operation records and pathology reports. This study was approved by the Institutional Review Board (IRB) of Seoul St. Mary's Hospital, Catholic University of Korea.
2.2. Treatments
In our analysis, initial diagnosis including pathologic confirmation was done within 2 weeks since initial patient visit. Locally advanced CRC patients received surgical treatment within 4 weeks since initial diagnosis. Patients who were diagnosed as stage I or II colon cancer underwent primary surgical resection. Stage III colon cancer patients went through surgical resection followed by adjuvant systemic chemotherapy. Patients diagnosed as stage II or III rectal cancer received preoperative 5-FU-based chemoradiation followed by surgery and systemic adjuvant chemotherapy as standard guideline. After completion of surgery or adjuvant treatment, patients were followed by every 3 months with physical examination, laboratory evaluation including serum CEA, and chest, abdominal computed tomography (CT) for 2 years. After initial 2 years, patients were followed biannually and were followed annually thereafter. Patients who presented with stage IV CRC with resectable metastatic lesion underwent systemic chemotherapy followed by surgical resection or went through surgical resection followed by systemic chemotherapy or adding 5-FU-based chemoradiation, if necessary. Resectability of metastatic lesions was judged by surgeons in multidisciplinary meetings. All these patients were treated with curative intent. Stage IV CRC patients with inoperable metastatic lesions were treated with oxaliplatin or irinotecan-based systemic chemotherapy (FOLFOX, FOLFIRI) with or without cetuximab or bevacizumab according to KRAS mutation status (Fig. 1). Monthly physical examination and laboratory evaluation was performed, with serum CEA and chest, abdominal computed tomography (CT) followed up at regular interval. When cancer recurrences were detected, patients were treated as stage IV CRC. Response evaluation was performed by CT scans every 2 to 3 months, which means every 4 cycles of systemic chemotherapy. Response assessment was measured according to RECIST criteria, ver. 1.0. Among patients who were on chemotherapy holiday, disease status evaluation was performed every 2 to 3 months interval. During the evaluation, serum CEA was followed up simultaneously. Systemic chemotherapy was administered until unaccepted toxicity, disease progression or patients’ refusal.
Figure 1.
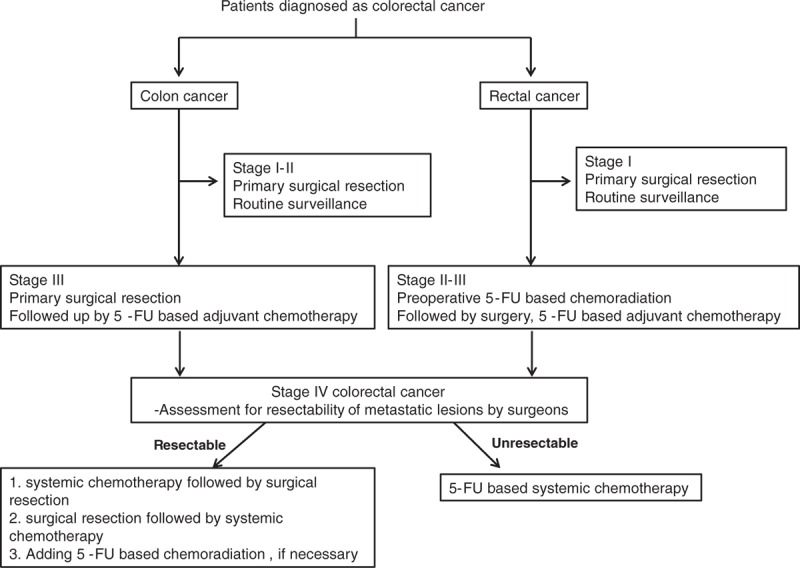
Treatment scheme of patient population.
2.3. Statistical analysis
Overall survival (OS) was calculated from the date of initial diagnosis of CRC to the death of patient or patient's last follow-up date. Disease-free survival (DFS) was calculated from the date of surgical resection to the date of disease recurrence, confirmed by CT scans. Progression-free survival (PFS) was measured from the first administration date of systemic chemotherapy to the date of disease progression, confirmed by CT scans. OS, DFS, and PFS were analyzed using the Kaplan–Meier method. All statistical analyses were performed with SPSS (ver. 18.0).
3. Results
3.1. Patient's characteristics
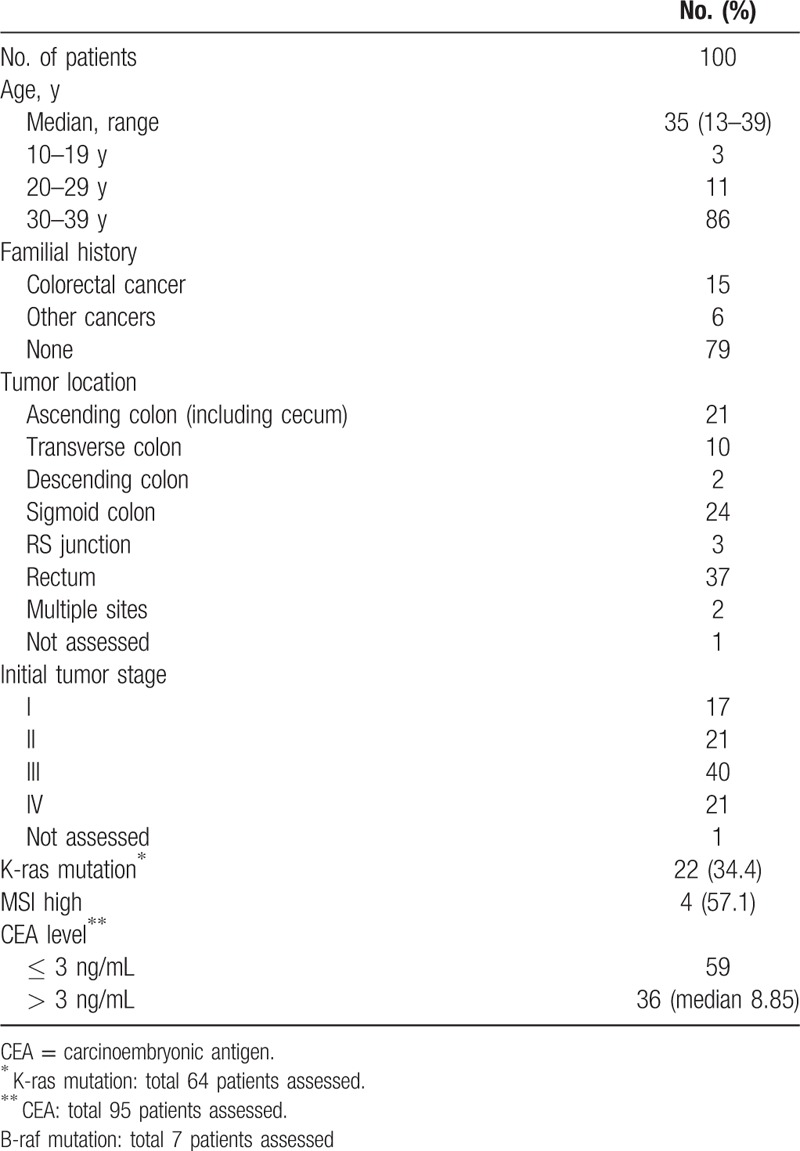
Among 4894 patients diagnosed as CRC in Seoul St. Mary's hospital, 100 patients (2.04%) were diagnosed before 40 years of age. Baseline characteristics of the patients are described in Table 1. The median age was 35 years (range 13–39 years), and 14 patients (14%) were diagnosed before the age of 30. Among 100 patients, 1 patient had history of familial adenomatous polyposis (FAP), and there were no other genetic predisposition of colorectal cancer. There was no past history of inflammatory bowel disease among 100 patients. Seventy-nine patients had no familial history of cancer. The primary site located below descending colon in 64 patients. Of the 64 patients available for KRAS mutation analysis, 22 patients (34.4%) showed mutation for KRAS exon 2. Sixty-one patients (61%) showed advanced stage disease over stage III. Among patients with available serum CEA, 59 patients (59%) showed normal range of serum CEA level (≤3.0 ng/mL). Among total patient population, 9 patients (9%) were diagnosed as mucinous carcinoma and 5 patients (5%) were presented with signet ring cell carcinoma.
Table 1.
Characteristics of patient population.
3.2. Clinical outcomes
The recurrence rate in stage I to stage III CRC was 7.9%. Among these patients, median DFS was 38.4 months (range 0.53 months to 8.5 years). The median OS was not reached yet. Survival outcomes were analyzed based on clinicopathologic factors such as serum CEA level, pathologic subtypes, KRAS mutational status, and microscopic description of surgical specimen. According to the serum CEA level, patients with high serum CEA level showed inferior survival outcomes compared to the normal serum CEA level (P = 0.01, OS not reached; Fig. 2A). Among patients who underwent surgical resection, patients with perineural invasion showed inferior outcome compared to those without perineural invasion (P = 0.01, OS not reached; Fig. 2B). The presence of lymphatic invasion, vascular invasion at surgical specimen, or KRAS mutation status were not associated with survival outcomes (data not shown).
Figure 2.
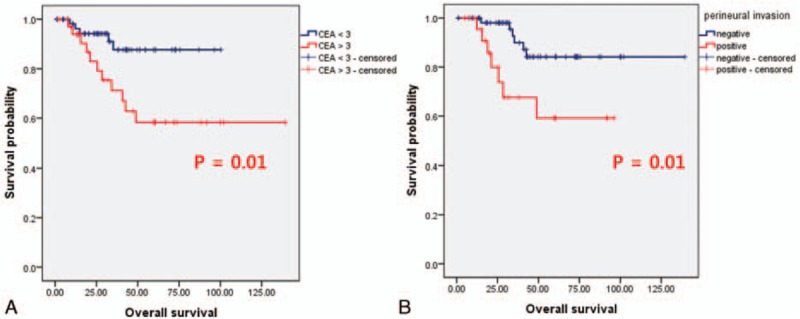
Survival outcomes according to serum CEA concentration (A) and status of perineural invasion (B).
3.3. Characteristics and survival outcomes in stage IV CRC
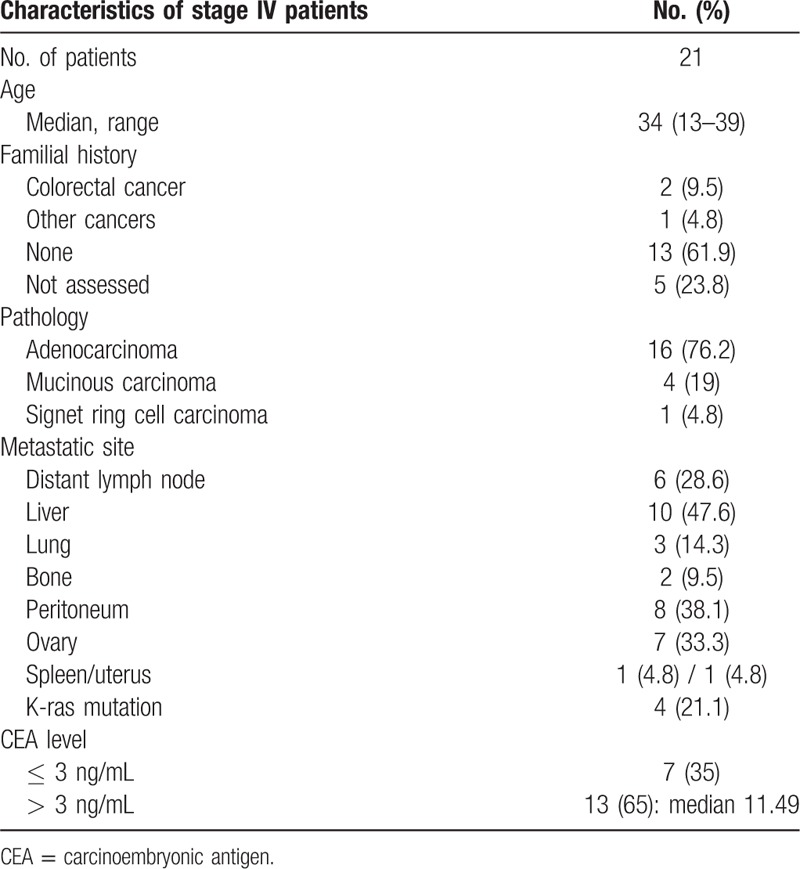
Twenty-one patients were initially presented as stage IV CRC (Table 2), and 2 patients were diagnosed as stage IV at their teens (13 year and 16 year). Among these patients, 13 patients had no familial history (61.9%) and 9 patients had primary tumor below descending colon (42.8%). The proportion of KRAS mutants was relatively lower than total patient population (4 patients, 21.1%). Seven patients (35%) showed a normal range of serum CEA level at initial diagnosis, although patients had systemic metastatic disease.
Table 2.
Characterstics of stage IV young age patients.
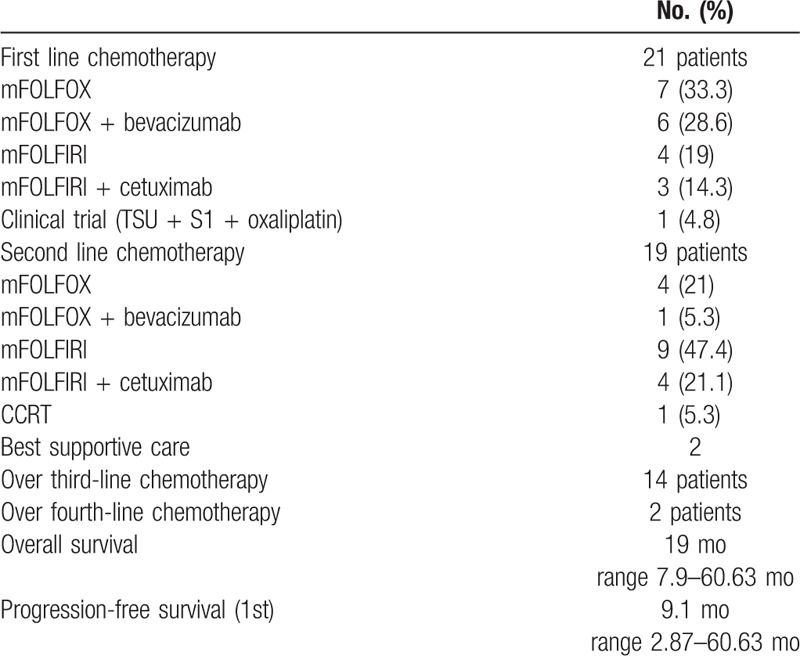
All patients received systemic chemotherapy after diagnosis. Median PFS after first-line chemotherapy was 9.1 months (range 7.9–60.63 months). Oxaliplatin-based chemotherapy was preferred as first-line chemotherapy, and then followed by irinotecan-based chemotherapy as second-line treatment. Nine patients (42.9%) were treated with target agents such as bevacizumab or cetuximab, combined with cytotoxic chemotherapeutic agents. After progression of first-line chemotherapy, 19 patients continued on second-line chemotherapy but 2 patients refused systemic treatment, receiving best supportive care (Table 3). Fourteen patients went on to third-line chemotherapy after failure of second-line chemotherapy. Median OS of stage IV CRC was 19 months (range 7.9–60.63 months, Table 3).
Table 3.
Chemotherapy history and survival outcomes of stage IV patients.
4. Discussion
The prevalence of CRC increases over the age of 50s. Although the overall incidence of CRC is decreasing in western countries and developed countries, the incidence of young-age CRC is arising gradually.[9] The clinical course of young-age CRC is assumed to be different to general patient population. Preceding retrospective studies suggested young-age CRC tended to be diagnosed at advanced stage, with shorter PFS and no difference in OS compared to general population.[10,11] Blanke et al[11] also conducted the study as meta-analysis with Western patient population. However, they defined the young-age patient as below 50 years of age, only including stage IV patients who received systemic chemotherapy. In another previous study, subpopulation analysis was performed based on patients registered at patients registered at Surveillance, Epidemiology, and End Results (SEER) database from 1991 to 1999. Those patients do not reflect recent advances in treatment of metastatic CRC, including active metastasectomy and local control of metastatic lesions. In this study, we conducted an analysis of young-age CRC patients comparing the clinicopathologic outcomes with previous studies.
The incidence of young-age CRC in previous reports is about 8% of all CRC patients,[12] but the incidence of young-age CRC in our institute was 2%, which was lower than expected. The incidence of young-age CRC (age 10–39) in Korea was estimated to 3.1%.[1] Compared to Western, the incidence of young-age CRC in Asia is relatively low.[12,13] Difference of underlying hereditary colorectal disease and inflammatory bowel disease, and the different incidence of young-age CRC between Western and Asian might be a factor for different incidence between Asia and Western.
In our analysis, most patients were presented as advanced stage (III or IV). This late presentation may be due to aggressive cancer behavior in the young-age group. Various analyses suggest young-age onset CRC shows aggressive histopathologic presentation,[14–16] with more lympho-vascular invasion than the historical control group.[10] These features may be associated with more aggressive nature of the cancer compared to old age patient group,[4] leading to advanced stage and rapid progression of the disease. Other than biologic natures, delayed recognition of the primary physician may have contributed to advanced cancer stage of young-age patients.[9]
In view of screening, individuals without familial history or risk factors start screening examination for CRC >50 years of age. Individuals with familial history of CRC, hereditary nonpolyposis colorectal cancer (HNPCC), or polyposis syndromes are known as the high-risk group for CRC.[17] In our data, 79% of the patients did not show familial history for CRC at their first-degree relatives. Negative familial history may lead to delayed evaluation for CRC when patients complain for symptoms suggesting CRC. In our analysis, most patients developed primary cancer at sigmoid colon or below. Considering the prevalent location in young-age CRC, brief screening method such as digital rectal examination or sigmoidoscopy may be useful for early screening in young-age population.
The elevated serum CEA level usually reflects massive tumor burden with advanced cancer stage.[18] Serum CEA is known as a meaningful tumor marker reflecting tumor burden in CRC,[19] but serum CEA showed relatively normal range in advanced CRC in our study. Twenty-eight patients (45.9%) among the 61 patients with stage III or IV CRC showed normal range of serum CEA. In previous literatures, serum concentration CEA tended to be higher in well-differentiated CRC.[20,21] Considering young-age CRC patients show higher frequency of poorly differentiated adenocarcinoma,[8] this difference of differentiation in pathology may have contributed to relatively normal range of serum CEA in our study population.
The incidence of mucinous carcinoma in our data was 9 percent, similar to CRC patients at their average age,[22,23] but the incidence of signet-ring cell carcinoma was higher than general population,[22,24] similar to previous data with young-age patients.[10] This different histology pattern may have contributed in aggressive tumor biology and relatively poor response to systemic chemotherapy.
The OS of total patient population in the present study was not reached yet. The 5-year survival rate was estimated as 75%. This survival outcome is better than that of prior studies with young-age CRC patients and also superior to patients over age of 50.[5,9] This superior survival outcome may be due to improved surgical technique, active adjuvant treatment scheme in locally advanced disease, and development of new agents for advanced disease. In our analysis, patients showed good performance status to tolerate and overcome the toxicity of chemotherapeutic agents. This tolerance to chemotherapy facilitated to the delivery of full-dose intensity of chemotherapeutic agents without alteration of chemotherapy dose and schedule. Aggressive local treatment was combined to systemic chemotherapy, although patient had stage IV CRC. Local treatment such as metastasectomy, radiofrequency ablation (RFA), intensity-modulated radiation therapy (IMRT) was applied, combined or sequential to systemic chemotherapy. This active treatment scheme may have contributed to improved overall survival outcome in total patient population. However, the median OS and PFS in stage IV CRC showed inferior outcome to historical data of above 24 months.[25] Although the standard combination chemotherapy is known to have similar clinical efficacy to young-age patients compared to patients >50 years age,[11] the massive tumor burden with aggressive nature in young-age patients may have contributed to relatively poorer response compared to general CRC patients.
There are some limitations in our study. This study was conducted as retrospective manner, so the results should be interpreted with caution. In the present study, we got the data only from single tertiary center. Patients diagnosed at early stage might be treated in primary clinical practice, and only complicated patients were referred to academic institution. This could lead to selection bias of the patient population. However, based on the referral system in the Korean medical system, most of cancer patient initially diagnosed at local hospital are usually referred to tertiary institution for further treatment. This referral pattern in Korea resulted in homogeneous patient population between major tertiary centers, without regional differences. Based on this referral system, each tertiary center may play a role as a sample group reflecting characteristics of Korean population. Patient population in our analysis served as a representative sample group, and the difference of incidence between our center and Korean patient population does not directly influence the analysis result of our study. This homogenous patient distribution in tertiary center may have declined selection bias in our analysis.
Our study is first report analyzing the characteristics and natural course of young-age CRC in Korea. Although the sample size is relatively small, authors concluded the patient population in our analysis acts as a sample group representing young-age CRC patients in Korea. This study is a pilot study to propose multicenter clinical analysis which comprises total young-age CRC patients in Korea. The outcomes of this analysis will be the basis of further clinical multicenter analysis and will be the milestone for analyzing the characteristics of young-age CRC in Korea.
In summary, young-age CRC patients were diagnosed at more advanced stage without familial history and relatively normal serum CEA level. The primary tumor tended to arise from left-sided colon—especially at sigmoid colon and below. The young patients with stage IV CRC showed inferior survival outcomes to historical data, suggesting the need of early detection of cancer in young-age population. Based on our data, routine surveillance using serum CEA may be inappropriate. Active surveillance is warranted in young-age population when subject complains of symptoms suggesting colorectal cancer in old age population. Aggressive examination comprising colonoscopy or sigmoidoscopy may be needed for cancer surveillance. Furthermore, a prospective, large cohort study of screening young-age CRC patient should be considered to establish screening protocol in the young-age population group.
5. Conclusion
Young-age CRC was most commonly diagnosed at the thirties with no familial history, normal range of CEA, and location below sigmoid colon. The incidence rate of mucinous and signet ring cell carcinoma was higher compared to average-aged CRC patients. Stage IV CRC showed inferior OS compared to historical data. Considering the increasing incidence of CRC in the young-age group, differentiated surveillance program is required for this age group. Active surveillance other than the serum CEA level (e.g., sigmoidoscopy) is warranted.
Footnotes
Abbreviations: CEA = carcinoembryonic antigen, CRC = colorectal cancer, CT = computed tomography, DFS = disease-free survival, HNPCC = hereditary nonpolyposis colorectal cancer, KRAS = V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog, OS = overall survival, PFS = progression-free survival.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Jung K-W, Park S, Won Y-J, et al. Prediction of cancer incidence and mortality in Korea, 2012. Cancer Res Treat 2012; 44:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkin W, Northover J, Cuzick J, et al. Prevention of colorectal cancer by once-only sigmoidoscopy. Lancet 1993; 341:736–740. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63:11–30. [DOI] [PubMed] [Google Scholar]
- 4.Lieu CH, Renfro LA, de Gramont A, et al. Association of age with survival in patients with metastatic colorectal cancer: analysis from the ARCAD clinical trials program. J Clin Oncol 2014; 32:2975–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chew M-H, Koh P-K, Ng K-H, et al. Improved survival in an Asian cohort of young colorectal cancer patients: an analysis of 523 patients from a single institution. Int J Colorectal Dis 2009; 24:1075–1083. [DOI] [PubMed] [Google Scholar]
- 6.Mayo C, Pagtalunan R. Malignancy of colon and rectum in patients under 30 years of age. Surgery 1963; 53:711–718. [PubMed] [Google Scholar]
- 7.Minardi AJ, Jr, Sittig KM, Zibari GB, et al. Colorectal cancer in the young patient. Am Surg 1998; 64:849–853. [PubMed] [Google Scholar]
- 8.Raymond P, Skelton D, Hsu H. Young patients with colorectal cancer: the University of Mississippi Medical Center experience 1970–1990. J Miss State Med Assoc 1991; 32:298. [PubMed] [Google Scholar]
- 9.Ahnen DJ, Wade SW, Jones WF, et al. The increasing incidence of young-onset colorectal cancer: a call to action. Mayo Clin Proc 2014; 89:216–224. [DOI] [PubMed] [Google Scholar]
- 10.O’Connell JB, Maggard MA, Liu JH, et al. Do young colon cancer patients have worse outcomes? World J Surg 2004; 28:558–562. [DOI] [PubMed] [Google Scholar]
- 11.Blanke CD, Bot BM, Thomas DM, et al. Impact of young age on treatment efficacy and safety in advanced colorectal cancer: a pooled analysis of patients from nine first-line phase III chemotherapy trials. J Clin Oncol 2011; 29:2781–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perea J, Alvaro E, Rodriguez Y, et al. Approach to early-onset colorectal cancer: clinicopathological, familial, molecular and immunohistochemical characteristics. World J Gastroenterol 2010; 16:3697–3703.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al. SEER Cancer Statistics Review, 1975–2012, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2012/, based on November 2014 SEER data submission, posted to the SEER web site, April 2015. [Google Scholar]
- 14.Li Q, Cai G, Li D, et al. Better long-term survival in young patients with non-metastatic colorectal cancer after surgery, an analysis of 69,835 patients in SEER database. PloS One 2014; 9:e93756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang J, Huang K, Cheng A, et al. Clinicopathological and molecular biological features of colorectal cancer in patients less than 40 years of age. Br J Surg 2003; 90:205–214. [DOI] [PubMed] [Google Scholar]
- 16.Tricoli JV, Seibel NL, Blair DG, et al. Unique characteristics of adolescent and young adult acute lymphoblastic leukemia, breast cancer, and colon cancer. J Natl Cancer Inst 2011; 103:628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NCCN Clinical Practice Guidelines in Oncology (NCCN guidelines®) Colorectal Cancer Screening version I. 2014. [Google Scholar]
- 18.Bronstein B, Steele G, Ensminger W, et al. The use and limitations of serial plasma carcinoembryonic antigen (CEA) levels as a monitor of changing metastatic liver tumor volume in patients receiving chemotherapy. Cancer 1980; 46:266–272. [DOI] [PubMed] [Google Scholar]
- 19.Yamashita K, Watanabe M. Clinical significance of tumor markers and an emerging perspective on colorectal cancer. Cancer Sci 2009; 100:195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhatnagar J, Tewari H, Bhatnagar M, et al. Comparison of carcinoembryonic antigen in tissue and serum with grade and stage of colon cancer. Anticancer Res 1998; 19:2181–2187. [PubMed] [Google Scholar]
- 21.Goslin R, O’brien M, Steele G, et al. Correlation of plasma CEA and CEA tissue staining in poorly differentiated colorectal cancer. Am J Med 1981; 71:246–253. [DOI] [PubMed] [Google Scholar]
- 22.Nitsche U, Zimmermann A, Späth C, et al. Mucinous and signet-ring cell colorectal cancers differ from classical adenocarcinomas in tumor biology and prognosis. Ann Surg 2013; 258:775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verhulst J, Ferdinande L, Demetter P, et al. Mucinous subtype as prognostic factor in colorectal cancer: a systematic review and meta-analysis. J Clin Pathol 2012; 65:381–388. [DOI] [PubMed] [Google Scholar]
- 24.Gopalan V, Smith RA, Ho Y-H, et al. Signet-ring cell carcinoma of colorectum—current perspectives and molecular biology. Int J Colorectal Dis 2011; 26:127–133. [DOI] [PubMed] [Google Scholar]
- 25.Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 2014; 15:1065–1075. [DOI] [PubMed] [Google Scholar]


