Abstract
Cross-reactivity of pneumococcal capsular polysaccharides is a key element for formulating pneumococcal vaccines and evaluating vaccine efficacy. This study examined whether 23-valent pneumococcal polysaccharide vaccine (PPSV23), which only contains 6B, can elicit cross-functional immune responses against recently discovered serotypes (6C and 6D), as well as against 6A, in 2 adult age groups.
Young adults (25–51 years; N = 28) and elderly subjects (over 65 years; N = 60) were immunized with PPSV23. Functional antibody responses were determined in pre- and postimmune sera via multiplexed opsonophagocytic killing assay against serotypes 6A/B/C/D.
At postimmunization, the geometric mean opsonic indices (OIs) for 6B and nonvaccine serotypes (6A, 6C, and 6D) significantly increased in both age groups. The geometric fold increases of OIs for 6B/A/C/D significantly differed (18.2, 24.8, 3.1, and 7.1, respectively). Proportions of subjects with 4-fold increases in OIs for 6B/A/C/D were 73%, 70%, 31%, and 49%, respectively. Correlations of fold increases in OIs were highest between 6B and 6A, followed by 6B and 6D, then by 6B and 6C. Comparisons of young adults and the elderly revealed that most immunogenicity variables were higher in the former group.
Our data demonstrated that 6B in PPSV23 induced cross-functional immune responses against serotypes 6A, 6C, and 6D, according to the degree of similarity in their capsular polysaccharide structures. In addition, we found significant age-related differences in PPSV23-induced cross-reactivity.
Keywords: 23-valent pneumococcal capsular polysaccharide vaccine, antibodies, bacterial, cross-protection, opsonin proteins, phagocytosis
1. Introduction
Streptococcus pneumoniae is an important disease agent with high morbidity and mortality among older adults worldwide. To date, nearly 100 serotypes have been identified through the differing antigenic properties of capsular polysaccharides, a major virulence factor of pneumococci.[1,2] To provide protection against the majority of pneumococcal infections, a 14-valent capsular polysaccharide vaccine was developed in 1977 using serotype 6A (representing serogroup 6).[3] After more epidemiologic information became available, the formulation was changed to 23-valent pneumococcal polysaccharide vaccine (PPSV23), which uses serotype 6B; 6B is as prominent as 6A, induces considerable cross-reactive immunogenicity to 6A, and is also more structurally stable.[4]
Within the past few decades, 2 novel serogroup 6 members—6C and 6D—have been identified[5–7] that differ serologically, genetically, and biochemically from 6A and 6B.[7,8] Of the new serotypes, 6C was initially considered uncommon among both invasive and colonizing isolates,[9] but its prevalence has increased in some countries after the introduction of 7-valent pneumococcal conjugate vaccine (PCV7).[8,10–12] Serotype 6D was primarily isolated from nasopharynx and remains fairly rare.[13,14] However, some isolates have been reported from invasive diseases, and multidrug-resistant strains exist.[15,16]
Both PPSV23 and 13-valent pneumococcal conjugate vaccine (PCV13) are currently used for the prevention of pneumococcal diseases in adults, but the former's wider serotype coverage and lower cost makes it a more attractive option for routine immunization in the elderly. Therefore, considerable interest exists regarding whether PPSV23 can elicit cross-functional immune responses against 6C and 6D, as it does against 6A. To date, only 1 study has addressed this issue and found that PPSV23 failed to induce sufficient levels of functional antibodies to 6C in the elderly.[17] No similar study is available for 6D. Furthermore, no prior work has evaluated the effect of age on PPSV23's ability to elicit cross-reactivity. Thus, in this study, we assessed PPSV23-induced cross-functional immune responses to 6A, 6C, and 6D in adults of various age groups.
2. Methods
2.1. Subjects
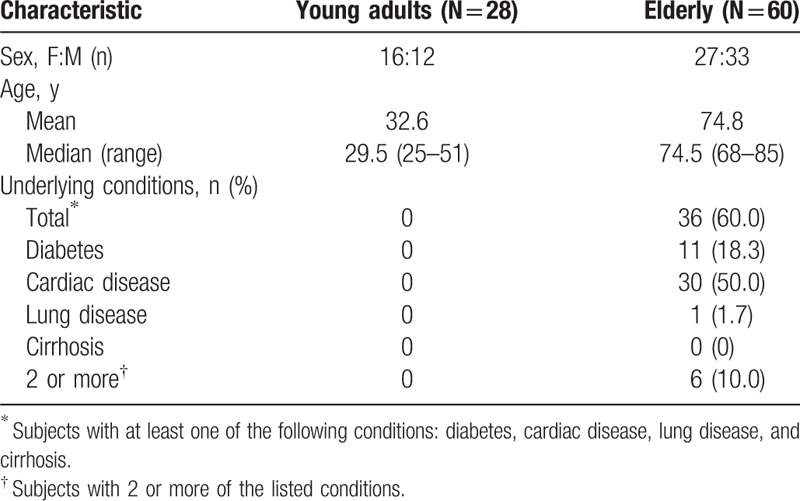
Demographic characteristics of the study subjects are summarized in Table 1. The study enrolled 88 subjects split into 2 groups: healthy, 25- to 51-year-old young adults and elderly individuals over 65 years. Exclusion criteria were immune-compromising conditions such as HIV infection, leukemia, generalized malignancy, chronic renal failure, primary or acquired immunodeficiency, diseases requiring immunosuppressive-drug treatments (including systemic corticosteroids or radiation therapy), functional or anatomic asplenia, cerebrospinal fluid leaks or cochlear implants, a history of vaccine hypersensitivity, any coagulation disorder, and a history of antibiotic use within a week. We were unable to exclude elderly subjects with preexisting but stable health conditions (e.g., well controlled diabetes mellitus, cardiovascular disease, pulmonary disease, and liver cirrhosis) because these diseases are prevalent at high percentages in this population. None of the subjects had received PPSV23 or PCV13 before this experiment. Immunization history for other vaccines (e.g., influenza) was not collected. Both sexes were well represented in each group, and sex ratios did not differ significantly between groups.
Table 1.
Demographic characteristics of the participants.
2.2. Study design
The young adults were immunized with a single dose of PPSV23 (Prodiax-23, Merck & Co. Inc., Whitehouse Station, NJ) from March 2011 to June 2011. The elderly were immunized with a single dose of PPSV23 (Pneumo23, Sanofi-Pasteur, Lyon, France) from August 2013 to January 2014. Vaccine was administered via intramuscular injection in the deltoid muscle using 25-G needles. Blood samples were collected before and approximately 4 weeks after vaccination. Sera were stored at −70 °C until tested.
During both study periods, the vaccines were available in the Republic of Korea. Both brands of PPSV23 contain 25 μg each of the 23 pneumococcal capsular polysaccharides (1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, and 33F) per 0.5-mL dose.
2.3. Multiplexed opsonophagocytic assay for immunogenicity assessment
Following previously described methods,[18] multiplexed opsonophagocytic assay was performed for 4 serotypes (6B, 6A, 6C, and 6D). First, HL-60 cells were differentiated into granulocytes through a 4- to 5-day culture in RPMI 1640 (Welgene, Daegu, South Korea) with 10% fetal bovine serum and 0.8% dimethylformamide. After differentiation, HL-60 cells were diluted to 107 cells/mL in Hanks’ buffer, containing 0.1% gelatin and 5% fetal bovine serum. All serum samples were diluted in the same buffer.
Each pneumococcal serotype used in this experiment possessed derived or naturally acquired resistance to 1 of 4 antibiotics (optochin, streptomycin, spectinomycin, and trimethoprim) and susceptibility to the other 3. Their antibiotic resistance and sensitivity were monitored periodically because these properties are key to obtaining serotype-specific results in our assay.
Equal volumes of the 4 bacterial suspensions were then pooled. Serum samples were tested at an initial dilution of 1:2. Serially 3-fold diluted serum (20 μL) was mixed with 10 μL of pneumococcal suspension containing 2000 CFU in each well of a 96-well microtiter plate. After 30 minutes of shaking incubation at room temperature, 40 μL of HL-60 cell suspension (4 × 105 cells per well) and 10 μL of baby rabbit complement (Pel-Freez, Brown Deer, WI) were added to each well. Plates were incubated again in a tissue culture incubator (37 °C, 5% CO2) with shaking for 45 minutes.
An aliquot of the final reaction mixture (10 μL) was spotted onto 4 Todd-Hewitt agar yeast extract plates. After the fluid was absorbed into the agar, each plate was overlaid with molten Todd-Hewitt agar (0.75%) containing yeast extract, 1 of the 4 antibiotics, and 100 mg/L of 2,3,5-triphenyltetrazolium chloride. The inclusion of 1 antibiotic in the overlay agar ensured that among the 4 pneumococcal serotypes, only target bacteria resistant to that antibiotic were able to grow. After overnight incubation in a candle jar at 37 °C, the bacterial colonies on the agar plates were counted using National Institute of Standards and Technology, US's Integrated Colony Enumerator. The opsonic indices (OIs) were determined with linear interpolation and defined as the serum dilution that killed 50% of total bacteria.
2.4. Statistical analysis
Geometric mean opsonic indices (GMIs) were calculated for each pneumococcal serotype in both groups, and 2-sided 95% confidence intervals were determined. A Friedman test with post hoc Wilcoxon signed-rank tests was used to look for significant differences between the serotypes. We assessed immune response to PPSV23 using seropositive rates and the proportion of subjects with at least a 4-fold increase in OI. Currently, no standard threshold for seropositive rates in adults exists. However, in children, an OI of 8 is the confirmed protective standard for invasive pneumococcal infection, whereas a previous study had used 64 as a threshold in adults.[19] Therefore, we applied both thresholds for seropositive rates in this study. Percentage of ≥4-fold OI increases was calculated to evaluate seroresponse, as this measure is especially reliable in subjects with positive preimmunization OI. For related proportions, Cochran Q test and McNemar test were used. Adjustments for multiple comparisons were made with the Bonferroni method. The percentage of participants that achieved a particular OI for each serotype was represented using reverse cumulative distribution curves (RCDC). Overall significance was set at P < 0.05. All analyses were performed in SPSS (version 23.0; IBM Corp., New York, NY).
2.5. Ethical considerations
The study protocol was reviewed and approved by the Institutional Review Board of Ewha Womans University Mokdong Hospital (ECT 11-13-43 and ECT 13-24B-21). The study was conducted in accordance with clinical best practices (national regulations and ICH E6) and the principles of the Helsinki Declaration. Written informed consent was obtained from all participants following a detailed explanation of the study.
3. Results
Following vaccination, all subjects showed significant increases in the GMI for 6B, as well as for 6A, 6C, and 6D (Table 2). Young adults exhibited significantly higher postimmunization GMI for 6A, 6C, and 6D compared with the elderly.
Table 2.
GMIs, geometric mean fold increases, and proportion of subjects with 4-fold or greater increase in OI before and after immunization with PPSV23.
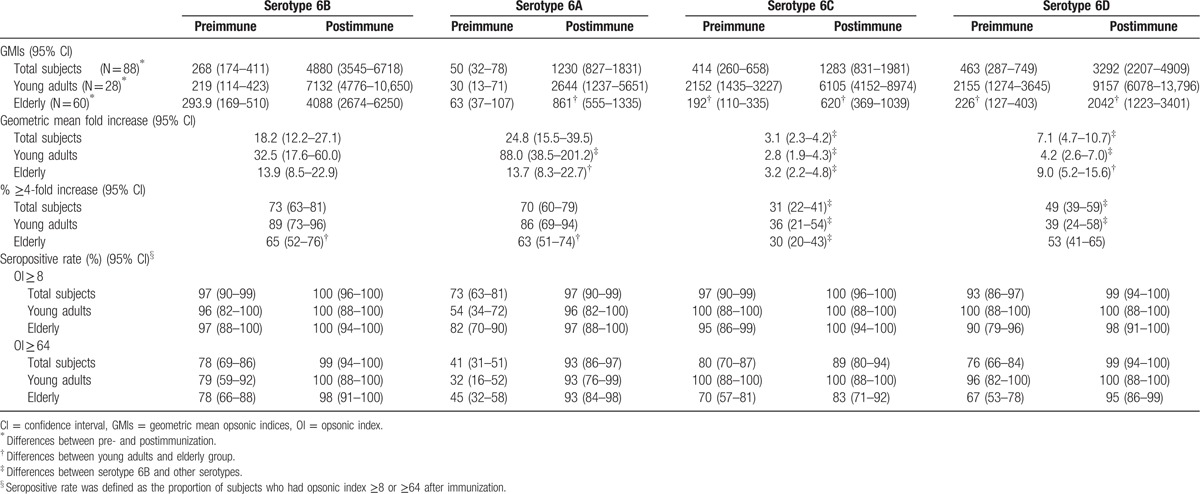
Geometric mean fold increase (GMFI; ratio of post- to preimmunization OI) significantly differed among the 4 serotypes overall and within each age group (Table 2). When considering all subjects, the GMFI for 6A was higher than for 6B, but not significantly so (P = 0.82), whereas GMFIs for 6C and 6D were significantly lower. When subjects were divided by age, the GMFI in young adults were significantly higher for 6A than for 6B, but significantly lower for 6C and 6D. In contrast, the GMFIs of the elderly group did not significantly differ for the most part, except between 6B and 6C. When comparing between age groups, the elderly exhibited significantly lower GMFI for 6A and significantly higher GMFI for 6D compared with the young adults.
The percentages of ≥4-fold increases to OI induced by 6B versus 6A did not significantly differ when considering all subjects (Table 2). However, OIs for 6C and 6D were significantly lower than for 6B. Young adults exhibited similar patterns, but among the elderly, only 6C resulted in significantly lower percentages of OI increases compared with the other serotypes. When comparing the young adults with the elderly, significantly lower percentages of subjects in the latter age group exhibited ≥4-fold OI increases for serotypes 6A and 6B.
After immunization, nearly every individual had an OI ≥ 8 and most had an OI ≥ 64 for all 4 serotypes. The proportion of subjects with OIs ≥ 64 was lowest in 6C (Table 2). Young and old adults did not majorly differ in the proportion of individuals with OI ≥ 8. However, at the higher threshold of OI ≥ 64, the elderly group exhibited lower seropositive rates for 6C than did the young adults. In addition, the mean fold increase between 6B and the other 3 serotypes were linearly correlated (Fig. 1), with the strongest relationship between serotypes 6B and 6A (r2 = 0.76), followed by 6B and 6D. However, the correlation between 6B and 6C was relatively weak. Finally, the RCDC plots for each serotype-specific OI shifted to the right and exhibited larger area under curve (AUC) compared with prevaccination OIs in both groups for all 4 serotypes (Fig. 2). However, postimmunization plots for serotypes 6C and 6D in the elderly were further left than even the preimmunization plots in young adults. The pre- and postimmunization changes in AUC were also much greater for young adults than for the elderly in 6A and 6B, but similar age group differences were not visible in 6C and 6D.
Figure 1.
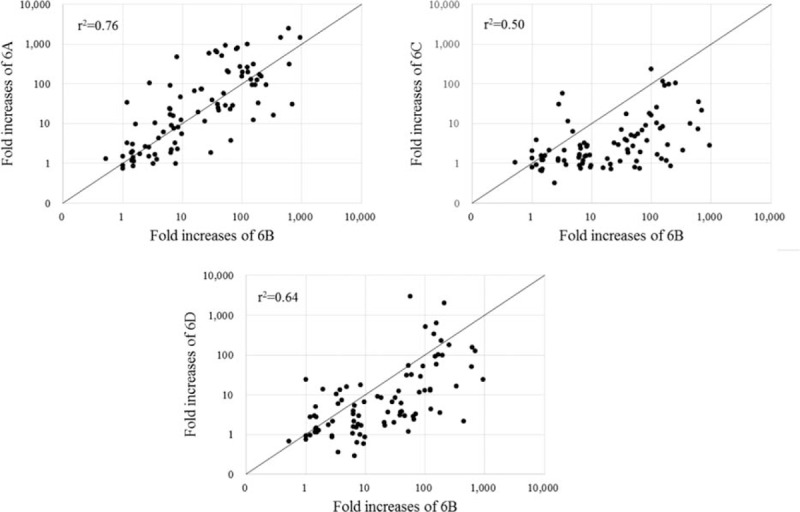
Correlation of fold increases in opsonic indices between 6B and other serotypes in serogroup 6 after immunization with pneumococcal polysaccharide vaccine.
Figure 2.
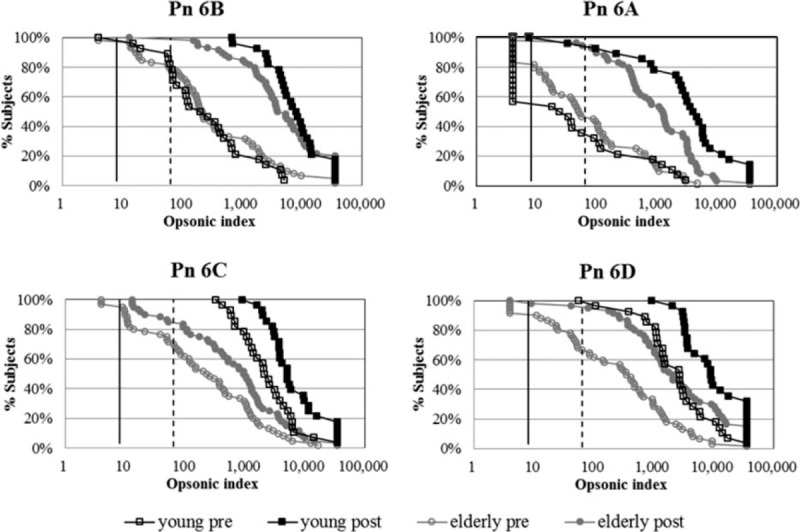
Reverse cumulative distribution curves for opsonic indices of each group. Vertical solid line—opsonic index = 8; vertical dashed line—opsonic index = 64; open circle—preimmunization; closed circle—postimmunization; black line—young adult group; gray line—elderly group.
4. Discussion
In this study, we found that serotype 6B from PPSV23 elicits cross-functional immune responses against serotypes 6A, 6C, and 6D, but to a different extent among young adults compared with the elderly.
Overall, cross-reactivity against 6A after PPSV23 immunization, as measured by OI increases, indicated that 6A and 6B did not significantly differ in their induced immune response. These results are unsurprising due to the structural similarity between 6A and 6B, meaning cross-functional antibodies against 6A can be induced by the capsular polysaccharide antigen of 6B. Our results are thus consistent with previous studies[20–23] that support the use of 6B in PPSV23 to replace 6A in PPSV14.[20]
In contrast, cross-functional immune responses after PPSV23 immunization were significantly lower against 6C than against 6A. This outcome is attributable to the greater structural differences between 6C and the other 2 serotypes: 6C differs from both 6B and 6A in the rhamnose–ribitol linkage of the capsular polysaccharide repeating unit. Moreover, hydroxyl groups of the glucose subunits constituting the capsular polysaccharide vary in location between 6C and 6B.[6]
A previous study examining PPSV23-induced immune responses in adults supports our finding that the 6B-containing vaccine elicits significant increases in cross-opsonic antibodies to 6A, but not to 6C.[17] Although the formulation of PCV7 differs from PPSV23, cross-reactivity induced by 6B in PCV7 has been studied in children since the serotype's discovery.[17,24,25] These works hint at the structural dissimilarity between 6A and 6C by showing high levels of 6A but not 6C functional activity after PCV7 immunization. Moreover, an analysis of pneumococcal serotypes in the United States after PCV7 introduction demonstrated that 6A declined, while 6C did not; thus, low cross-reactivity was reflected in the actual occurrence of invasive pneumococcal diseases.[17]
Our results also revealed that PPSV23-induced cross-functional immune responses were significantly lower against 6D than against 6A. In addition, more cross-functional antibodies were induced against 6D than against 6C. Relatively few studies are available on cross-reactivity against 6D due to the serotype's novel status; in fact, our study is the first to examine cross-functional immune response against 6D induced by 6B in PPSV23. Our results nonetheless find some corroboration with previous work; 1 study conducted on children in 2013 showed that 6B in PCV7 induced 6D cross-functional antibodies at levels similar to 6A antibodies.[24]
While we observed variation in cross-functional immunogenicity among serogroup 6 serotypes, most subjects exhibited high rates of OI ≥ 8 or OI ≥ 64 for all serotypes postvaccination. In a recent study examining pneumococcal serotype prevalence post-PPSV23 introduction, 6A, 6C, and 6D were among the 10 most commonly found serotypes in adults aged over 65 years, with 6D exhibiting the highest frequency.[26] In light of these findings, it is difficult to explain the high seropositive rates we observed in our elderly subjects. Currently, no known protective OI threshold exists for adults and the elderly. The only standard is an OI of 8, confirmed as the protective OI standard for invasive pneumococcal infection among children. Therefore, more research should be conducted to investigate whether the standards for pneumococcal infection used here (i.e., OIs of 8 and 64) are appropriate for adults, including older populations.[27,28] We note that a previous work had used the seropositive rate standard of 64 for some serotypes.[19] In addition, because the required OI for protective immunity against serogroup 6 could be higher in the elderly than against other serotypes or for other age groups, the actual seropositive rates might actually be even lower than what we have reported. Therefore, further analysis is required once we have established more appropriate protective OI standards, reflective of data from epidemiological studies on serogroup 6 in the elderly.
We observed significant age-related differences in cross-reactive immune responses induced by serotype 6B from PPSV23. First, postimmunization GMI and OI increases for 6A were all significantly higher among young adults than among the elderly. The results for 6C were less dramatic, with postimmunization GMI being higher in young adults, but no age group difference for OI increases. In contrast, cross-reactive immune response for 6D was higher in the elderly than in young adults. Previous research has shown that PPSV23-induced immune response is lower among the elderly than among younger adults, due to the induction of antibodies with low avidity and reduced Immunoglobul in M production.[29–31] Nevertheless, in the present study, we observed no age-related difference in vaccine responses against serotypes 6C and 6D. In summary, cross-reactive immune responses induced by 6B in PPSV23 were lower for all serotypes among the elderly compared with young adults, while cross-reactive immune response against 6D was higher than the response against 6C in both groups.
In closing, we note several limitations of the present study. First, we only obtained participant immunization history for the pneumococcal vaccine. We thus have no direct knowledge of other vaccines (e.g., for influenza) that may affect our results. However, most elderly patients in this study probably received influenza vaccine, because both it and pneumococcal vaccine are included in the national immunization program of Korea. We recognize that a history of influenza strongly contributes to the development of severe pneumococcal infection, especially pneumococcal pneumonia.[32,33] Therefore, prior influenza immunization could potentially affect the efficacy of the pneumococcal vaccine. However, we believe this lack does not greatly affect the conclusions we could draw from our results, because we focused on the immunogenicity of pneumococcal vaccine, rather than its effectiveness. Previous research has demonstrated that earlier or concomitant influenza immunization does not affect pneumococcal vaccine immunogenicity.[34,35]
Other limitations included small sample size, especially for the young adults, and the different experimental timing for each age group. Although the study was designed to be prospective, the time lag in data collection between cohorts occurred due to funding limitations. Separately funded projects targeting each age group also resulted in the use of PPSV23 from different manufacturers, but the vaccines were identical beyond their supplier. Nonetheless, it remains possible, though unlikely, that the distinct vaccine source for each population may have biased our results. Moreover, this study is based on the immune response to PPSV23, and PCV13 has been recommended for the elderly population, instead.[36,37] Therefore, we propose further studies to test other vaccine formulations in different population or ethnic groups.
To conclude, serotype 6B in PPSV23 induced differing cross-functional immune responses against 6A, 6C, and 6D, as expected given their differences in capsular polysaccharide structures. Furthermore, we demonstrated that young adults and the elderly differed in PPSV23-induced immune response and cross-reactivity. Although further clarification of protective OIs is recommended for all studied serotypes in adults, this study should prove to be pivotal for research in cross-functional immune response to pneumococcal polysaccharide vaccine.
Acknowledgments
We thank Dr Moon Nahm (Birmingham, AL) for providing the target pneumococcal strains used in the multiplexed opsonophagocytic assay (MOPA). University of Alabama at Birmingham (UAB) owns intellectual property rights on the reagents used for the MOPA studies. The authors would like to thank Kyung Ae Kong (Ewha Medical Center and Ewha Medical Research Institute, Ewha Womans University School of Medicine, Seoul, South Korea) for her statistical advice, as well as Soo Young Lim and Je Eun Cha (Center for Vaccine Evaluation and Study, Medical Research Institute, Ewha Womans University School of Medicine) for laboratory support.
Footnotes
Abbreviations: AUC = area under curve, GMFI = geometric mean fold increase, GMIs = geometric mean opsonic indices, OIs = opsonic indices, PCV13 = 13-valent pneumococcal conjugate vaccine, PCV7 = 7-valent pneumococcal conjugate vaccine, PPSV23 = 23-valent pneumococcal polysaccharide vaccine, RCDC = reverse cumulative distribution curves.
HWK and SL have contributed equally to the article.
Funding/support: This study was supported by an intramural research promotion grant awarded to Kyung-Hyo Kim from Ewha Womans University School of Medicine (RP-grant 2014), the Korea Center for Disease Control and Prevention (2013-E32005-00), and the Ministry of Food and Drug Safety (11172MFDS360 and 15172MFDS164).
The authors have no conflicts of interest to disclose.
References
- 1.Park IH, Geno KA, Yu J, et al. Genetic, biochemical, and serological characterization of a new pneumococcal serotype, 6H, and generation of a pneumococcal strain producing three different capsular repeat units. Clin Vaccine Immunol 2015; 22:313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calix JJ, Porambo RJ, Brady AM, et al. Biochemical, genetic, and serological characterization of two capsule subtypes among Streptococcus pneumoniae Serotype 20 strains: discovery of a new pneumococcal serotype. J Biol Chem 2012; 287:27885–27894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bureau of Biologics. Release guidelines for polyvalent pneumococcal vaccine. Bethesda, MD: Food and Drug Administration; 1982. [Google Scholar]
- 4.Heidelberger M, Rebers PA. Immunochemistry of the pneumococcal types II. V. and VI. I. The relation of type VI to type II and other correlations between chemical constitution and precipitation in antisera to type VI. J Bacteriol 1960; 80:145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park IH, Park S, Hollingshead SK, et al. Genetic basis for the new pneumococcal serotype, 6C. Infect Immun 2007; 75:4482–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park IH, Pritchard DG, Cartee R, et al. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J Clin Microbiol 2007; 45:1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bratcher PE, Kim KH, Kang JH, et al. Identification of natural pneumococcal isolates expressing serotype 6D by genetic, biochemical and serological characterization. Microbiology 2010; 156 (Pt 2):555–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhuo F, Xiao M, Kong F, et al. Prevalence and genetic diversity of pneumococcal serogroup 6 in Australia. Clin Microbiol Infect 2011; 17:1246–1253. [DOI] [PubMed] [Google Scholar]
- 9.Campos LC, Carvalho Mda G, Beall BW, et al. Prevalence of Streptococcus pneumoniae serotype 6C among invasive and carriage isolates in metropolitan Salvador, Brazil, from 1996 to 2007. Diagn Microbiol Infect Dis 2009; 65:112–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nahm MH, Lin J, Finkelstein JA, et al. Increase in the prevalence of the newly discovered pneumococcal serotype 6C in the nasopharynx after introduction of pneumococcal conjugate vaccine. J Infect Dis 2009; 199:320–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho PL, Chiu SS, Chan MY, et al. Changes in nasopharyngeal carriage and serotype distribution of antibiotic-resistant Streptococcus pneumoniae before and after the introduction of 7-valent pneumococcal conjugate vaccine in Hong Kong. Diagn Microbiol Infect Dis 2011; 71:327–334. [DOI] [PubMed] [Google Scholar]
- 12.Ip M, Chau SS, Lai LS, et al. Increased nasopharyngeal carriage of serotypes 6A, 6C, and 6D Streptococcus pneumoniae after introduction of childhood pneumococcal vaccination in Hong Kong. Diagn Microbiol Infect Dis 2013; 76:153–157. [DOI] [PubMed] [Google Scholar]
- 13.Nahm MH, Oliver MB, Siira L, et al. A report of Streptococcus pneumoniae serotype 6D in Europe. J Med Microbiol 2011; 60 (pt 1):46–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao KH, Liu ZJ, Yu JG, et al. Type distribution of serogroup 6 Streptococcus pneumoniae and molecular epidemiology of newly identified serotypes 6C and 6D in China. Diagn Microbiol Infect Dis 2011; 70:291–298. [DOI] [PubMed] [Google Scholar]
- 15.Kuch A, Sadowy E, Skoczynska A, et al. First report of Streptococcus pneumoniae serotype 6D isolates from invasive infections. Vaccine 2010; 28:6406–6407. [DOI] [PubMed] [Google Scholar]
- 16.Ko KS, Baek JY, Song JH. Multidrug-resistant Streptococcus pneumoniae serotype 6D clones in South Korea. J Clin Microbiol 2012; 50:818–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park IH, Moore MR, Treanor JJ, et al. Differential effects of pneumococcal vaccines against serotypes 6A and 6C. J Infect Dis 2008; 198:1818–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burton RL, Nahm MH. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin Vaccine Immunol 2006; 13:1004–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romero-Steiner S, Musher DM, Cetron MS, et al. Reduction in functional antibody activity against Streptococcus pneumoniae in vaccinated elderly individuals highly correlates with decreased IgG antibody avidity. Clin Infect Dis 1999; 29:281–288. [DOI] [PubMed] [Google Scholar]
- 20.Robbins JB, Austrian R, Lee CJ, et al. Considerations for formulating the second-generation pneumococcal capsular polysaccharide vaccine with emphasis on the cross-reactive types within groups. J Infect Dis 1983; 148:1136–1159. [DOI] [PubMed] [Google Scholar]
- 21.Yu X, Gray B, Chang S, et al. Immunity to cross-reactive serotypes induced by pneumococcal conjugate vaccines in infants. J Infect Dis 1999; 180:1569–1576. [DOI] [PubMed] [Google Scholar]
- 22.Kim KH. Functional immunity to cross-reactive serotype 6A induced by serotype 6B in pneumococcal polysaccharide vaccine. Korean J Pediatr 2005; 48:506–511. [Google Scholar]
- 23.Lee H, Nahm MH, Kim KH. The effect of age on the response to the pneumococcal polysaccharide vaccine. BMC Infect Dis 2010; 10:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee H, Cha JH, Nahm MH, et al. The 7-valent pneumococcal conjugate vaccine elicits cross-functional opsonophagocytic killing responses to Streptococcus pneumoniae serotype 6D in children. BMC Infect Dis 2013; 13:474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim KH, Yang JY, Park IH, et al. Cross-reaction of 6b and 19F specific antibodies to serotypes 6A, 6C, and 19A after immunization with 7-valent pneumococcal conjugate vaccine in Korean children aged 12–23 months. Korean J Pediatr Infect Dis 2013; 20:53–62. [Google Scholar]
- 26.Kim MJ, Song JY, Choi WS, et al. A study on the occurrence trend and changes in serotypes causing invasive Streptococcus pneumoniae infection and noninvasive streptococcal pneumonia among Korean elderly over 65: a Korean multi-center observational study (In Korean). Presented at: Annual Meeting of the Korean Society of Infectious Diseases and the Korean Society for Chemotherapy 2014; Jeju, Republic of Korea. Presentation date (Nov 13–14, 2014). [Google Scholar]
- 27.Jodar L, Butler J, Carlone G, et al. Serological criteria for evaluation and licensure of new pneumococcal conjugate vaccine formulations for use in infants. Vaccine 2003; 21:3265–3272. [DOI] [PubMed] [Google Scholar]
- 28.Lee LH, Frasch CE, Falk LA, et al. Correlates of immunity for pneumococcal conjugate vaccines. Vaccine 2003; 21:2190–2196. [DOI] [PubMed] [Google Scholar]
- 29.Usinger WR, Lucas AH. Avidity as a determinant of the protective efficacy of human antibodies to pneumococcal capsular polysaccharides. Infect Immun 1999; 67:2366–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun Y, Hwang Y, Nahm MH. Avidity, potency, and cross-reactivity of monoclonal antibodies to pneumococcal capsular polysaccharide serotype 6B. Infect Immun 2001; 69:336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park S, Nahm MH. Older adults have a low capacity to opsonize pneumococci due to low IgM antibody response to pneumococcal vaccinations. Infect Immun 2011; 79:314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Brien KL, Walters MI, Sellman J, et al. Severe pneumococcal pneumonia in previously healthy children: the role of preceding influenza infection. Clin Infect Dis 2000; 30:784–789. [DOI] [PubMed] [Google Scholar]
- 33.Weinberger DM, Harboe ZB, Viboud C, et al. Serotype-specific effect of influenza on adult invasive pneumococcal pneumonia. J Infect Dis 2013; 208:1274–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song JY, Cheong HJ, Tsai TF, et al. Immunogenicity and safety of concomitant MF59-adjuvanted influenza vaccine and 23-valent pneumococcal polysaccharide vaccine administration in older adults. Vaccine 2015; 33:4647–4652. [DOI] [PubMed] [Google Scholar]
- 35.Schwarz TF, Flamaing J, Rümke HC, et al. A randomized, double-blind trial to evaluate immunogenicity and safety of 13-valent pneumococcal conjugate vaccine given concomitantly with trivalent influenza vaccine in adults aged ≥65 years. Vaccine 2011; 29:5195–5202. [DOI] [PubMed] [Google Scholar]
- 36.Choi WS, Choi JH, Kwon KT, et al. Revised adult immunization guideline recommended by the Korean society of infectious diseases, 2014. Infect Chemother 2015; 47:68–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomczyk S, Bennett NM, Stoecker C, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb Mortal Wkly Rep 2014; 63:822–825. [PMC free article] [PubMed] [Google Scholar]


