Abstract
Background:
The ideal measures to prevent postoperative catheter-related bladder discomfort (CRBD) remain unestablished. We conducted the systematic review and meta-analysis to clarify the significance of potential interventions.
Methods:
We followed the Preferred Reporting Items for Systematic review and Meta-Analysis statement guidelines, and searched databases from MEDLINE, EMBASE, and referred Cochrane Library for randomized clinical trials (RCTs) published before December 2014. Reference lists from reviews or related articles were screened and checked for the related RCTs. Data extraction was performed carefully by 2 authors, respectively, and methodological quality was assessed by scoring system. Meta-analysis was applied for studies using the similar strategies or same reagents on the similar participants focused on CRBD. The primary outcome measure was the incidence of postoperative CRBD.
Results:
We identified 8 RCTs with interventions ranging from perioperative managements to pharmacological or multicomponent interventions. Meta-analysis showed ketamine was associated with less incidence of CRBD compared with placebo (pool risk ratio [RR] = −0.75, 95% confidence interval [CI] = 0.17–3.44, P < 0.01) at 0 hour, 1 hour (RR = −0.26, 95%CI = −0.38 to −0.13, P < 0.01), and 2 hours (RR = 0.31, 95%CI = 0.17–0.55, P < 0.01) and 6 hours (RR = 0.23, 95% CI = 0.11–0.49, P < 0.01) after operation. Oxybutynin did not affect the incidence of CRBD (RR = 0.46, 95%CI = 0.20–1.03, P = 0.06). Anticholinergic drugs also lower the incidence of CRBD at 0 hour (RR = 0.52, 95% CI = 0.38–0.71, P < 0.01), 1 hour (RR = 0.66, 95% CI = 0.51–0.86, P < 0.01), 2 hours (RR = 0.62, 95% CI = 0.46–0.84, P < 0.01), and 6 hours (RR = 0.56, 95%CI = 0.38–0.81, P < 0.01) postoperatively. Tramadol and gabapentin were also useful in lower the incidence and severity of CRBD in a RCT with 50 patients.
Conclusion:
The included studies showed great effectiveness in incidence of postoperative CRBD. Meta-analysis supported that ketamine, oxybutynin, and anticholinergic reagents interventions were useful in preventing postoperative catheter-related bladder discomfort.
Keywords: a systematic review and meta-analysis, anticholinergic drugs, catheter-related bladder discomfort, gabapentin, ketamine, oxybutynin, tramadol
1. Introduction
Around 15% to 25% of patients admitted to hospital are indwelled with urinary catheters,[1] while it is a common intervention in operation room. It is widely used to permit the accurate measurement of urinary output in patients undergoing operations. However, urinary catheters sometimes cause catheter-related bladder discomfort (CRBD). CRBD is characterized with the elevated symptoms of urinary frequency and urgency, and a discomfort at the suprapubic region caused by catheter-related bladder irritation.[2,3] The incidence of CRBD has been reported around to be 47%, with 2 independent predictors of being the size of indwelling catheters and patient gender.[4] The occurrence of CRBD is extremely distressing to patients, reduces the quality of recovery, and prolongs hospital stay by exacerbated postoperative discomfort.[5,6]
A number of studies have been conducted to reduce the problems caused by bladder catheterization and identify agents that can be used for prophylactic postoperative treatment.[3] The mechanism of CRBD is similar to that of overactive bladder,[7] which are caused by involuntary contractions of the bladder mediated by muscarinic receptors.[6] Muscarinic receptor antagonists oxybutynin and tolterodine have been used successfully for the management of overactive bladder.[2,8] Agents with antimuscarinic properties, therefore, have been investigated for the prevention of CRBD including butylscopolamine, tolterodine, and oxybutynin. Ketamine is a general anesthetic, which acts rapidly, used for catheter-related discomfort in several conditions.[9]
2. Materials and methods
This systematic review and meta-analysis was conducted following the guidelines of the Preferred Reporting Items for Systematic Review and Meta-Analysis statement.[10,11] Completed studies met all the following criteria were considered eligible for inclusion: randomized clinical trials (RCTs) assessing drugs to prevent CRBD; patients in the studies undergoing general anesthesia; and patients were all adult. Research articles were excluded if they recruited: patients were treated with different size of urinary catheterization; patients undergoing local anesthesia or nerve block; groups including nonsurgical patients (for examples patients in the intensive care unit or ward without surgery); and patients with mental disorders (e.g., dementia, schizophrenia, or depression). The methodological quality score of included studies were conducted and recorded.
2.1. Ethics statement and guidelines
The present systematic review and meta-analysis involved no animal experiments or direct human trials, and neither a special ethics review nor an ethical approval was therefore necessary. The study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.[11]
2.2. Search strategy
We conducted a literature search of Medline, Embase, and The Cochrane Central Database of Controlled Trials electronically from inception to December 2014 for RCTs concerning CRBD. The Medline search strategy retrieved citations containing “catheter-related bladder discomfort” and “general anesthesia.” Medline citations were limited to RCTs using a sensitive strategy[12] that was modified for other databases. Details of the search strategies are available in Fig. 1. We reviewed references lists from included studies and review articles and searched the related articles of identified studies using “Google Scholar.” No language restrictions were applied.
Figure 1.

Risk of bias graph. Reviewing authors’ judgements about each risk of bias item presented as percentages across all included studies.
2.3. Data extraction
Two authors (BH and CL) screened the flow diagram and completed the data extraction. Disagreements were resolved by discussion or applied to the 3rd coauthor for consultation. Authors of the included studies were applied, if the data in the included studies were not converted to the incidence of CRBD. The following characteristics of included studies were collected: primary author, publication year, country of origin, intervention, types of surgery, participant characteristics (gender, age), invention, primary outcome, criteria for and incidence of CRBD. Dichotomous data were converted to incidences for data synthesis.
2.4. Data analysis
The present systematic review and meta-analysis focused on the incidence of CRBD. Placebo or control produces was considered as sham intervention as a study comparing the effects between reagent with placebos. Meta-analysis was performed for 2 or more studies using the similar strategies or same reagents. Analyses were on a model of experiment-to-control basis. Differences between 2 groups were expressed as risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous outcomes. A fixed-effect model was used as there was no significant heterogeneity (P-value of χ2 test no less than 0.10 and I2 not greater than 50%), otherwise, a random-effects model was employed (P-value of χ2 test less than 0.10 and I2 greater than 50%). Publication bias and studies quality were assessed by checking the bias risk in the software of Review Manager, version 5.3 (RevMan, The Cochrane Collaboration, Oxford, UK) and visually inspecting funnel plots performed by the software. A P value of less than 0.05 was considered statistically significant. All statistical analyses were performed using the software of RevMan,
3. Results
The process of literature identification, screening, and selection is summarized by figure diagram. Our primary search yielded 42 articles and 33studies potentially met the inclusion criteria after screening. Eight RCTs[2,5,6,13–17] were left for systematic review and meta-analysis, with 25 studies excluded: 5 studies were retrospective cohort studies; 6 studies patients were treated with epidural anesthesia; 4 studies patients were treated with local anesthesia; 7 studies concerned catheter-related infection; and 3 studies were about catheter product. An overview of the risk of bias was shown in Figs. 1 and 2.
Figure 2.

Risk of bias summary. Reviewing authors’ judgements about each risk of bias item for each included study.
3.1. Studies characteristics
The characteristics of 8 included studies were summarized in Table 1.
Table 1.
The characteristics of included studies.

3.2. Quality scores of included studies
The methodological quality score of included studies were shown in Table 2. The scores ranged from 15[14] to 21.[6] A score of 15 was found in 1 study,[14] 2 studies[2,13] with 16, 4 studies[5,15–17] with 17, and 1 studies[6] got the full score of 21.
Table 2.
Methodological quality score of included studies.

3.3. Quantitative review and meta-analysis
Category 1: Administration of tramadol intraoperatively prevented the incidence of CRBD. Agarwal et al[13] tested the effect of tramadol on prevention of CRBD by comparing tramadol 1.5 mg/kg with normal saline 30 minutes before extubation with 54 patients of 27 in each group were included in the prospective, randomized, double-blind, placebo controlled study. Incidence of CRBD was reduced in tramadol group (n = 27) at 0, 1, 2, 6 hours after extubation compared with normal saline group (n = 27) (P < 0.05). In addition, the severity of CRBD was also reduced by tramadol at all time points (0, 1, 2, 6 hours) (P < 0.05). However, the incidence of postoperative nausea and vomiting together with sedation were higher in tranadol group compared with normal saline group (P < 0.05).
Category 2: Effect of gabapentin on the incidence of CRBD. Agarwal et al[15] conducted an RCT including 108 consecutive adult patients, undergoing elective percutaneous nephrolithotomy. Gabapentin were administrated orally 1 hour before surgery, and 16Fr Foley catheter was applied after induction of anesthesia in the studied group, while placebo were administrated in control group. The result demonstrated that gabapentin reduced the incidence of CRBD to 50% (27 of 54) compared with 80% (43 of 54) observed in the control group.
Category 3: Effect of ketamine on the incidence of CRBD. We identified 2 studies[16,17] with 164 patients compared ketamine with placebo. Meta-analysis using random effect model (χ2 = 64.03, P < 0.01, I2 = 98%) revealed no difference between ketamine and placebo (RR = 0.75, 95%CI = 0.17–3.44, P = 0.72) at 0 hour after operation (Fig. 3). Random effect model (χ2 = 17.18, P < 0.01, I2 = 94%) was also applied in the meta-analysis at 1 hour after operation, and the result demonstrated that ketamine decreased the incidence of CRBD significantly (RR = −0.26, 95%CI = −0.38 to −0.13, P < 0.01) (Fig. 4). Meta-analysis using fixed effect model (χ2 = 1.52, P = 0.22, I2 = 34%) revealing that ketamine reduced the incidence of CRBD (RR = 0.31, 95%CI = 0.17–0.55, P < 0.01) at 2 hours after operation (Fig. 5). Furthermore, meta-analysis using fixed effect model (χ2 = 0.50, P = 0.48, I2 = 0%) demonstrated that ketamine reduced the incidence of CRBD significantly (RR = 0.23, 95%CI = 0.11–0.49, P < 0.01) at 6 hours after operation (Fig. 6).
Figure 3.

The incidence of CRBD was not affected by ketamine at 0 hour after operation. RR = 0.75, 95%CI = 0.17 to 3.44, P = 0.72. CI = confidence interval, CRBD = catheter-related bladder discomfort, RR = risk ratio.
Figure 4.
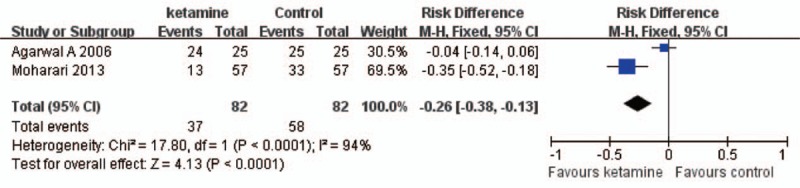
Ketamine decreased the incidence of CRBD at 1 hour after operation. RR = −0.26, 95%CI = −0.38 to −0.13, P < 0.01. CI = confidence interval, CRBD = catheter-related bladder discomfort, RR = risk ratio.
Figure 5.

Ketamine decreased the incidence of CRBD at 2 hours after operation. RR = 0.31, 95%CI = 0.17 to 0.55, P < 0.01. CI = confidence interval, CRBD = catheter-related bladder discomfort, RR = risk ratio.
Figure 6.
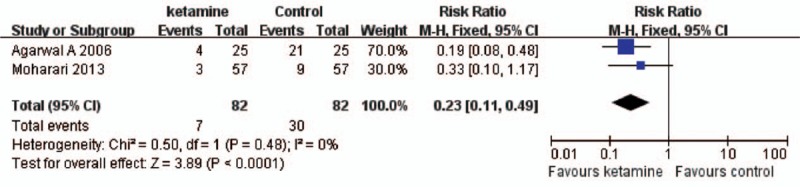
Ketamine decreased the incidence of CRBD at 6 hours after operation. RR = 0.23, 95%CI = 0.11 to 0.49, P < 0.01. CI = confidence interval, CRBD = catheter-related bladder discomfort, RR = risk ratio.
Category 4: Effect of oxybutynin on the incidence of CRBD. 2 RCTs[5,6] with 202 patients tested the incidence of CRBD compared oxybutynin with placebo. Meta-analysis using random effect model (χ2 = 2.83, P = 0.09, I2 = 65%) found no difference (RR = 0.46, 95%CI = 0.20–1.03, P = 0.06) between the 2 groups (Fig. 7).
Figure 7.

The incidence of CRBD was not affected by oxybutynin. RR = 0.46, 95%CI = 0.20 to 1.03, P = 0.06. CI = confidence interval, CRBD = catheter-related bladder discomfort, RR = risk ratio.
Category 5: Effect of anticholinergic drugs on the incidence of CRBD. 3 studies[2,6,14] using anticholinergic drugs concerning the effect of butylscopolamine, tolterodine, and oxybutynin on CRBD with 506 patients were included for meta-analysis. Meta-analysis using fixed effect model (χ2 = 9.92, P < 0.01, I2 = 80%) found anticholinergic drugs significantly reduced the incidence of CRBD (RR = 0.52, 95%CI = 0.38–0.71, P < 0.01) comparing with placebo at 0 hour in the postanesthesia care unit (Fig. 8). Although at 1 hour fixed effect model (χ2 = 3.56, P = 0.17, I2 = 44%) was applied and the result demonstrated that there was significantly difference (RR = 0.66, 95% CI = 0.51–0.86, P < 0.01) between anticholinergic drugs group and placebo group (Fig. 9). In addition, meta-analysis using fixed effect model (χ2 = 4.13, P = 0.13, I2 = 52%) reveling that anticholinergic drugs decreasing the incidence of CRBD significantly (RR = 0.62, 95%CI = 0.46–0.84, P < 0.01) at 2 houre in the postanesthesia care unit (Fig. 10). Meta-analysis using fixed effect model (χ2 = 0.79, P = 0.67, I2 = 0%) showing that there was significantly difference (RR = 0.56, 95%CI = 0.38–0.81, P < 0.01) between anticholinergic drugs group and placebo group on the incidence of CRBD at 6 hours postoperatively (Fig. 11).
Figure 8.
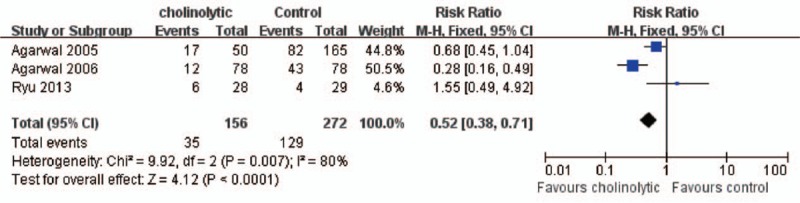
Anticholinolytic drugs reduced the incidence of CRBD at 0 hour after operation. RR = 0.52, 95%CI = 0.38 to 0.71, P < 0.01. CI = confidence interval, CRBD = catheter-related bladder discomfort, RR = risk ratio.
Figure 9.

Anticholinolytic drugs reduced the incidence of CRBD at 1 hour after operation. RR = 0.66, 95%CI = 0.51 to 0.86, P < 0.01. CI = confidence interval, CRBD = catheter-related bladder discomfort, RR = risk ratio.
Figure 10.

Anticholinolytic drugs reduced the incidence of CRBD at 2 hours after operation. RR = 0.62, 95%CI = 0.46 to 0.84, P < 0.01. CI = confidence interval, CRBD = catheter-related bladder discomfort, RR = risk ratio.
Figure 11.

Anticholinolytic drugs reduced the incidence of CRBD at 6 hours after operation. RR = 0.56, 95%CI = 0.38 to 0.81, P < 0.01. CI = confidence interval, CRBD = catheter-related bladder discomfort, RR = risk ratio.
4. Discussion
The presentation of CRBD was the main complaint from patients with indwelling urinary catheters during the postoperative period.[18] Although CRBD was defined as an urge to void or discomfort in the suprapubic region. The major characteristic of CRBD presented at the overactive bladder with the elevated symptoms of urinary frequency and urgency. It had been founded that the involuntary contractions of bladder are mainly mediated by muscarinic receptors directly.[2,7] In other words, antimuscarinic reagents would be used as the mainstay for the treatment of CRBD. In the present systematic review and meta-analysis, many antimuscarinic reagents including gabapentin, oxybutynin, tolterodine, and ketamine can be used to prevent or reduce the incidence of CRBD.
Tolterodine, a competitive pure muscarinic receptor antagonist, showed functional selectivity for the bladder with its metabolite 5-HM. In the present systematic review and meta-analysis, tolterodine combined with other anticholinergic drugs including butylscopolamine and oxybutynin decreasing the incidence of CRBD significantly compared with placebo at 1 and 2 hours on the arrival at the PACU. However, oxybutynin which is a muscarinic receptor antagonist did not take effect in treating the incidence of CRBD in the present systematic review and meta-analysis. Because of the spasmolytic and antimuscarinic properties, oxybutynin has a direct relaxant effect on the bladder theoretically. Due to the bias of administrated process in the studies,[5,6] the effect was not definite.
Recently, an RCT conducted by Nam et al[19] in Republic of Korea, recruited 99 patients, demonstrated that butylscopolamine decreased the incidence and severity of CRBD for 6 hours postoperatively without adverse effects. It had been demonstrated that the diameter of the bladder catheter and gender were identified as independent predictors of CRBD.[4] In the present systematic review and meta-analysis, the differences of diameter of the Foley catheter in men or women were not significant. There were no differences between genders in the present systematic review and meta-analysis. However, there were no accurate techniques or agents to resolve the complaints of CRBD.
A recent review[20] demonstrated the similar results to the present systematic review and meta-analysis; however, the review focused on the systematic review and lacking meta results to supporting the conclusion. In the present systematic review and meta-analysis, we focused on the meta-analysis of the data and concluded the outcome.
There were several limitations in the present systematic review and meta-analysis. First, population participated in the included studies was adult but not restricted to male or female. As studies had shown that gender may affect the incidence of CRBD, which may sharpen the bias of the results. Second, all patients in the included studies were treated with 16G or18G Foley catheter; however, reagents used in the included studies were not at the same time point. Third, the location of the included studies were mainly from India conducted by Agarwal, which may sharpen the bias of the results.
5. Conclusion
The present systematic review and meta-analysis had shown that reagents including oxybutynin, tolterodine, and ketamine could be choices in preventing the incidence of CRBD perioperatively.
Acknowledgements
The authors thank Young Medical Talents Training Program of Pudong Health Bureau of Shanghai (Grant No. WRq2015-17) for the support.
Footnotes
Abbreviations: CENTRAL = Cochrane Central Database of Controlled Trials, CI = confidence interval, CRBD = catheter-related bladder discomfort, RCT = randomized clinical trial, RR = risk ratio.
BH, CL, and MP contributed equally to this work.
Funding/support: This work was collectively supported by Young Medical Talents Training Program of Pudong Health Bureau of Shanghai (Grant No. WRq2015-17).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
All authors have no conflicts of interest to disclose.
References
- 1.Kunin CM. Nosocomial urinary tract infections and the indwelling catheter: what is new and what is true? Chest [Comment Editorial] 2001; 120:10–12. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal A, Raza M, Singhal V, et al. The efficacy of tolterodine for prevention of catheter-related bladder discomfort: a prospective, randomized, placebo-controlled, double-blind study. Anesth Analg 2005; 101:1065–1067.table of contents. [DOI] [PubMed] [Google Scholar]
- 3.Akca B, Aydogan-Eren E, Canbay O, et al. Comparison of efficacy of prophylactic ketamine and dexmedetomidine on postoperative bladder catheter-related discomfort. Saudi Med J 2016; 37:55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binhas M, Motamed C, Hawajri N, et al. Predictors of catheter-related bladder discomfort in the post-anaesthesia care unit. Annales francaises d’anesthesie et de reanimation. [Clinical Trial] 2011; 30:122–125. [DOI] [PubMed] [Google Scholar]
- 5.Tauzin-Fin P, Sesay M, Svartz L, et al. Sublingual oxybutynin reduces postoperative pain related to indwelling bladder catheter after radical retropubic prostatectomy. Br J Anaesth 2007; 99:572–575. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal A, Dhiraaj S, Singhal V, et al. Comparison of efficacy of oxybutynin and tolterodine for prevention of catheter related bladder discomfort: a prospective, randomized, placebo-controlled, double-blind study. Br J Anaesth 2006; 96:377–380. [DOI] [PubMed] [Google Scholar]
- 7.Anderson KE. Pharmacology of lower urinary tract smooth muscles and penile erectile tissues. Pharmacol Rev 1993; 45:253–308. [PubMed] [Google Scholar]
- 8.Appell RA, Sand P, Dmochowski R, et al. Prospective randomized controlled trial of extended-release oxybutynin chloride and tolterodine tartrate in the treatment of overactive bladder: results of the OBJECT Study. Mayo Clin Proc 2001; 76:358–363. [PubMed] [Google Scholar]
- 9.Shariat Moharari R, Lajevardi M, Khajavi M, et al. Effects of intra-operative ketamine administration on postoperative catheter-related bladder discomfort: a double-blind clinical trial. Pain Pract 2014; 14:146–150. [DOI] [PubMed] [Google Scholar]
- 10.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson KA, Dickersin K. Development of a highly sensitive search strategy for the retrieval of reports of controlled trials using PubMed. Int J Epidemiol 2002; 31:150–153. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal A, Yadav G, Gupta D, et al. Evaluation of intra-operative tramadol for prevention of catheter-related bladder discomfort: a prospective, randomized, double-blind study. Br J Anaesth 2008; 101:506–510. [DOI] [PubMed] [Google Scholar]
- 14.Ryu JH, Hwang JW, Lee JW, et al. Efficacy of butylscopolamine for the treatment of catheter-related bladder discomfort: a prospective, randomized, placebo-controlled, double-blind study. Br J Anaesth 2013; 111:932–937. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal A, Dhiraaj S, Pawar S, et al. An evaluation of the efficacy of gabapentin for prevention of catheter-related bladder discomfort: a prospective, randomized, placebo-controlled, double-blind study. Anesth Analg 2007; 105:1454–1457.table of contents. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal A, Gupta D, Kumar M, et al. Ketamine for treatment of catheter related bladder discomfort: a prospective, randomized, placebo controlled and double blind study. Br J Anaesth 2006; 96:587–589. [DOI] [PubMed] [Google Scholar]
- 17.Shariat Moharari R, Lajevardi M, Khajavi M, et al. Effects of intra-operative ketamine administration on postoperative catheter-related bladder discomfort: a double-blind clinical trial. Pain Pract 2013; Apr 8. [DOI] [PubMed] [Google Scholar]
- 18.Li JY, Yi ML, Liao R. Dorsal penile nerve block with ropivacaine-reduced postoperative catheter-related bladder discomfort in male patients after emergence of general anesthesia: a prospective, randomized, controlled study. Medicine 2016; 95:e3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nam K, Seo JH, Ryu JH, et al. Randomized, clinical trial on the preventive effects of butylscopolamine on early postoperative catheter-related bladder discomfort. Surgery 2015; 157:396–401. [DOI] [PubMed] [Google Scholar]
- 20.Bai Y, Wang X, Li X, et al. Management of catheter-related bladder discomfort in patients who underwent elective surgery. J Endourol 2015; 29:640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]


