Supplemental Digital Content is available in the text
Keywords: gastric cancer, laparoscopy-assisted distal gastrectomy, learning curve
Abstract
Laparoscopy-assisted distal gastrectomy (LADG) is widely used for gastric cancer (GC) patients nowadays. This study aimed to investigate the time trend of outcomes so as to describe the learning curve for GC patients with LADG at a single medical institution in western China over a 6-year period.
A total of 246 consecutive GC patients with LADG were divided into 5 groups (group A: 46 patients from 2006 to 2007; group B: 47 patients in 2008; group C: 49 patients in 2009; group D: 73 patients in 2010; and group E: 31 patients in 2011). All surgeries were conducted by the same surgeon. Comparative analyses were successively performed by Mann–Whitney U test or Student t test among the 5 different groups for the clinical data, including clinicopathologic characteristics, surgical parameters, postoperative course, and survival outcomes, through which the learning curve was described.
There were no differences in the baseline information among the 5 groups (P > 0.05), and the proportion of advanced GC patients with LADG slightly increased from 58.7% to 77.4% during the 6 years. Besides, the proportion of D2/D2+ lymphadenectomy and the number of retrieved lymph nodes gradually grew from 60.9% to 80.6% and from 20.0 to 28.8, respectively. In addition, the operation time decreased from 299.2 to 267.8 minutes, while the estimated blood loss dropped from 175.2 to 146.8 mL. Furthermore, some surgical parameters (surgical duration and blood loss) and postoperative course (such as postoperative complications, the time to ambulation, to first flatus, and to first liquid intake as well as the length of hospital stay) were all observed to be significantly different between group A and other groups (P < 0.05), illustrating a similar downward trend and remaining stable to form a plateau after 46 cases in group A. However, no difference on overall survival was found among these 5 groups, and multivariate analysis indicated that factors, such as age, tumor differentiation, tumor size, and T stage as well as N stage, were independent prognostic factors for patients with LADG.
Improvement on surgical parameters and postoperative course can be seen over the past years, and the cutoff value of the learning curve of LADG for surgeons with rich experience in open operation might be 46 cases.
1. Introduction
With the development of advanced laparoscopic instrument, improved techniques, and accumulated experience of laparoscopic surgery as well, laparoscopy-assisted distal gastrectomy (LADG) has nowadays become a widely used procedure of choice for patients with gastric cancer (GC) located in the lower third of stomach in the Eastern countries, especially in Japan, Korea, and China,[1–6] where there is a high prevalence of GC.[7,8] The benefits of LADG, such as better visualization, less-estimated blood loss,[9] lower complication rate,[9,10] shorter length of hospital stays,[10,11] and better survival outcomes[1,6] have been well documented. In addition to being safe and technically feasible, LADG has been demonstrated in several studies to yield acceptable oncologic outcomes, not only for early GC patients,[3,9,10,12] but also for those who were with advanced GC.[4–6,11,13,14]
However, the fact is that, though LADG is regarded as an acceptable choice and feasible technique for treating GC, previous findings relating to tumor features, surgical parameters, survival outcomes, and learning curve vary from study to study. Even so, there are few studies which reported time-related trend on tumor features and survival prognosis in Chinese patients with GC.[15,16] Therefore, this study was conducted to investigate the time trend of clinicopathologic characteristics, surgical parameters, postoperative course as well as survival outcomes, and to evaluate the learning curve, with the aim of further identifying the safety and feasibility of LADG for resectable GC patients at a single medical institution in western China.
2. Patients and methods
2.1. Patients
The West China Hospital Research Ethics Committee approved the retrospective analysis of anonymous data involved in this study. Patient records were anonymized and deidentified before analysis, and signed patient informed consent was waived as per the committee approval because it was a retrospective analysis.
From January 2006 to June 2011, a total of 276 consecutive patients with GC who received LADG at the Department of Gastrointestinal Surgery, West China Hospital, were retrospectively evaluated in this study. The diagnosis of gastric adenocarcinoma for all patients was confirmed by upper endoscopy and biopsy. Patients were excluded if they had any of the following situations: pathological examination confirmed that they had not received R0 resection,[17] a curative resection with negative residual margins; with any preoperative chemotherapy or radiotherapy; with another malignancy or any other life-threatening diseases diagnosed during 3 years before the operation; and with surgical findings of distant metastasis.[18] Finally, 246 patients were enrolled in this study as shown in Fig. 1.
Figure 1.
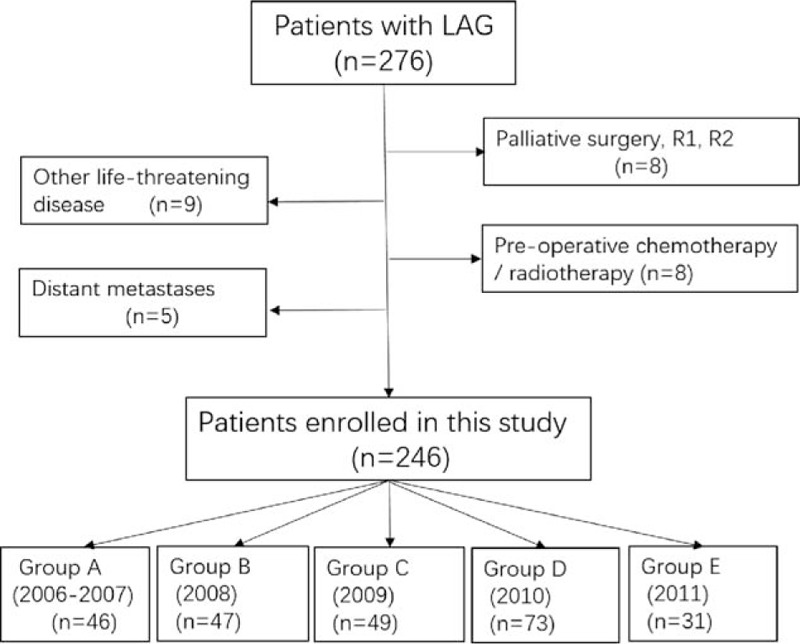
The flowchart of patients enrolled in this study.
2.2. Clinicopathologic characteristics analysis
The patients’ clinicopathologic features (such as age, gender, tumor size, tumor location, differentiation, T stage, N stage, and TNM stage),[18] surgical parameters (such as lymphadenectomy, reconstruction types, mean number of retrieved lymph nodes, surgical duration, and estimated blood loss), postoperative course (time to ambulation, time to first flatus, time to first liquid intake and postoperative stay, surgical postoperative complications including morbidity and mortality which were recorded according to the Clavien–Dindo classification),[19] and survival outcomes as well were included in the data and analyzed in this study. Tumor size was divided into 3 subgroups by the cutoff points, 2.5 and 4.5 cm, produced by X-tile with the strongest discriminatory capacity (figure S1).
2.3. Treatment
All patients underwent LADG with D1, D1+, D2, or D2+ lymphadenectomy for GC, defined by the Japanese Classification of Gastric Carcinoma.[17] Billroth I, Billroth II, or Roux-en-Y anastomosis with hand-sewn or mechanical staples was used to reconstruct the digestive tract after distal subtotal gastrectomy according to surgeon's preference. All the operations were conducted by a surgeon who is specialized in gastrointestinal surgery, with abundant experience in open surgery. The surgeon intended to perform D2 lymphadenectomy routinely, but D1 was conducted only when the depth of invasion was less than T2 without lymph nodes metastasis through preoperative evaluation. The patient was placed in the reverse Trendelenburg position with the 2 legs apart, and the operator stood on the right side of the patient. Five abdominal trocar ports were used: 2 left 5-mm assistant ports, 2 right operator ports (5-mm upper and 12-mm lower port), and 1 umbilical port for laparoscope insertion. Lymphadenectomy was performed intracorporeally with laparoscope, while the anastomosis and reconstruction were performed extracorporeally through a minilaparotomy with a 5- to 6-cm midline upper abdominal incision at the epigastrium. With pneumoperitoneum of 12 to 14 mm Hg, gastric dissection was started by dividing the greater omentum and by moving toward the left gastroepiploic area. After completing perigastric lymph nodes dissection, the duodenum was transected using endoscopic linear staplers intracorporeally, whereas the distal two-thirds of the stomach were then resected using a linear stapler extracorporeally.
2.4. Follow-up and survival
All patients, after undergoing laparoscopy-assisted resection of GC, were periodically followed up by outpatient visits, telephone interviews, and letters as well. Overall survival (OS) was the primary end point and the survival time was calculated from the date of operation to the date of death or the last follow-up time, June 2016. Patients who were lost to the long-term follow-up were also recorded and the main reasons for follow-up losses were patients’ refusal to the outpatients visit and changed contact address or telephone numbers. Of the 246 patients, 233 (94.7%) were followed up.
2.5. Statistical analysis
Data were presented as mean ± standard deviation for continuous variables and as numbers (%) for categorical variables. Chi-square test in the SPSS version 19.0 for windows (SPSS, Inc., Chicago, IL) was performed to evaluate differences in proportions, whereas Mann–Whitney U test and student t test were applied to analyze continuous variables. X-tile program (Version 3.1.2, Yale University, USA) was used to calculate the optimal cutoff points for tumor size using minimum P value from log-rank χ2 statistics. Univariate and multivariate survival analyses were performed by Cox proportional hazard regression model with conditional backward stepwise in the SPSS version 19.0. The OS rates were calculated using the Kaplan–Meier method, with subgroups compared by the log-rank test through GraphPad Prism 5. A P value of <0.05 (2-sided) was defined to be statistically significant.
3. Results
3.1. Clinicopathologic characteristics of patients
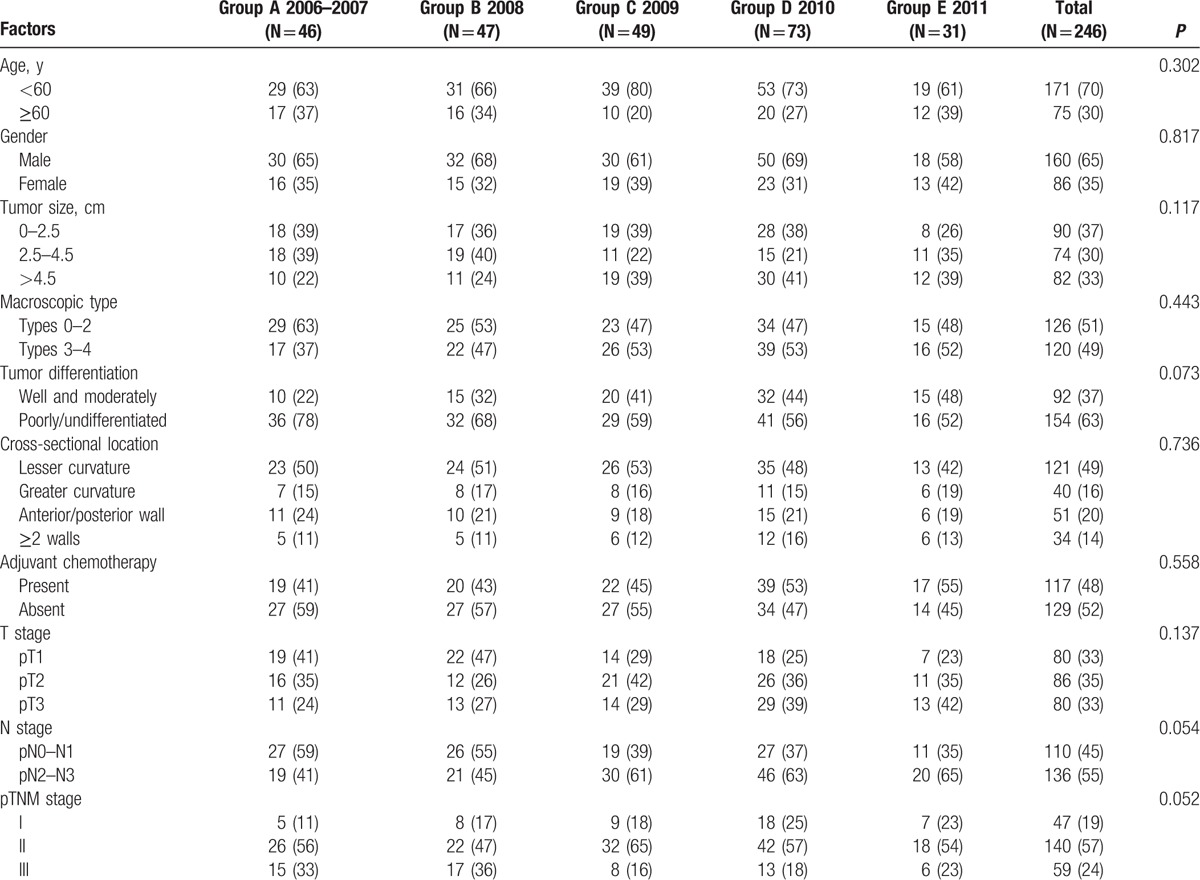
A total of 246 consecutive patients with GC were divided into 5 groups (group A: 46 patients from 2006 to 2007; group B: 47 patients in 2008; group C: 49 patients in 2009; group D: 73 patients in 2010; and group E: 31 patients in 2011) according to the time they received LADG. Clinicopathologic characteristics of patients are summarized by each group from 2006 to 2011 in Table 1. There was no significant difference between these 5 groups in terms of various clinicopathologic characteristics, such as age, gender, tumor size, tumor location, macroscopic type, tumor differentiation, cross-sectional location, adjuvant chemotherapy, T stage, N stage, and TNM stage, so that the baseline between these groups was balanced. There were 160 male and 86 female GC patients with a mean age of 54.5 years, and it revealed a slight increase in tumor size from 3.9 cm in 2006 to 4.4 cm in 2011. Besides, the proportion of macroscopic types 3 to 4 grew from 37.0% to 53.4%, whereas types 0 to 2 fell from 63.0% in 2006 to 48.3% in 2011. Tumor was more likely to be located at the lesser curvature (49%), and there was no obvious changing in terms of years. In addition, in terms of T stage, it presented a decreasing tendency for patients with stage T1, while there were an increasing number of patients who were postoperative pathologically diagnosed in stage T3 and received adjuvant chemotherapy, which means the proportion of advanced GC patients with LADG was increasing from 58.7% in 2006 to 77.4% in 2011. It also revealed that patients with stages N0 and N1 had a downward trend, but patients with stages N2 and N3 increased from 41.3% in 2006 to 64.5% in 2011.
Table 1.
Clinicopathologic characteristics of GC patients who underwent LADG from 2006 to 2011 (n, %).
3.2. Surgical parameters and postoperative course
As it can be seen in Table 2, of the 246 patients in this study, Billroth II reconstruction after gastrectomy was performed on 123 patients (50.0%), while Roux-en-Y anastomosis was conducted on 96 patients (39.0%). The total percentages of D1/D1+ and D2/D2+ lymphadenectomy of the 6 years were 25.2% and 74.8%, respectively. In addition, the proportion of patients with D2/D2+ lymphadenectomy gradually grew from 60.9% in 2006 to 80.6% in 2011 (Fig. 2A). Besides, the mean number of retrieved lymph nodes was 28.6, growing up abruptly from 20.0 in 2006 to 28.8 in 2007, and then with a slight increase, reaching 30.5 in 2011 (Fig. 2C). However, the surgical duration decreased from 299.2 minutes in 2006 to 267.8 minutes in 2011 (Fig. 2B and figure S2). Likewise, the estimated blood loss dropped from 175.2 mL in 2006 to 146.8 mL in 2011, being significantly different between groups A and B (P = 0.011), but without any significant difference in the comparison of groups B, C, D, and E (P = 0.218), indicating that it reached a plateau in 2008 after a decreasing trend in 46 cases from 2006 to 2007 (Fig. 2D and figure S2).
Table 2.
Surgical parameters and postoperative course of patients underwent LADG from 2006 to 2011 (n, %).
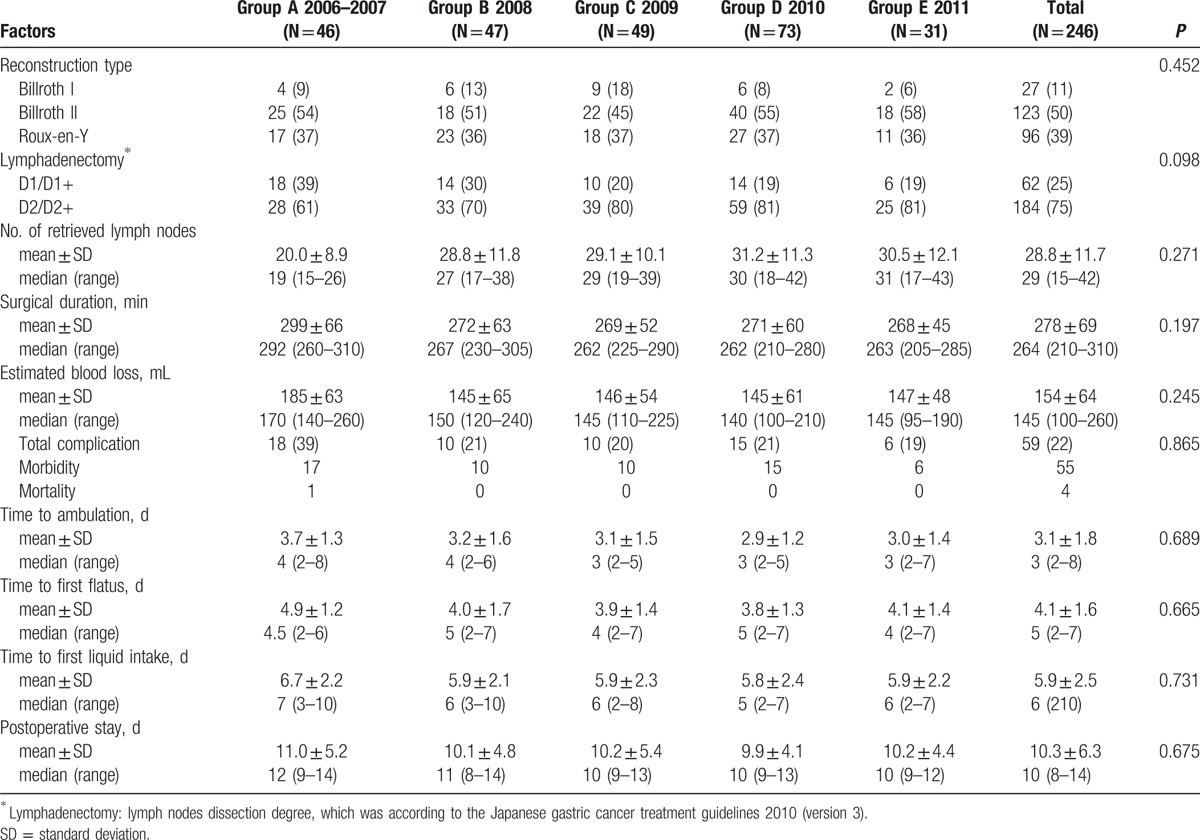
Figure 2.
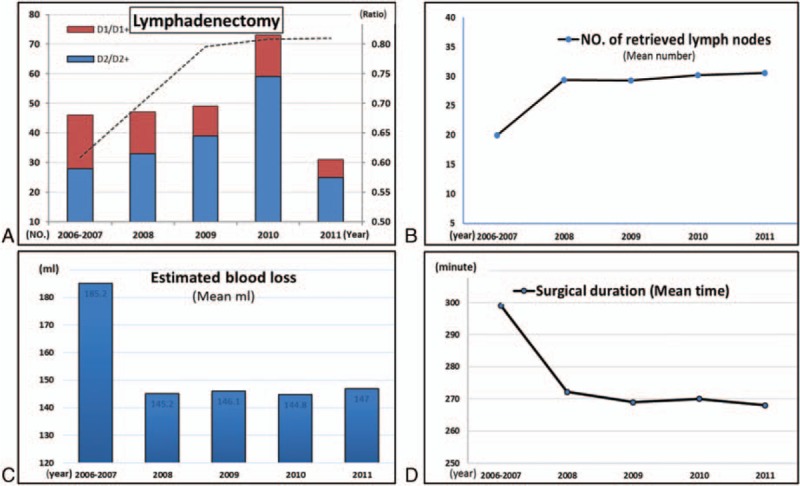
Time trend of clinicopathologic characteristics and surgical parameters for patients with LADG.
The same trend could be found in postoperative course in Table 2. The 6 years witnessed a stable decline in the postoperative complications from 39.1% to 19.4%. The list of detailed postoperative complications and Clavien–Dindo classification are shown in table S1. There was only 1 patient who died postoperatively in group A because of anastomotic leakage combined with pulmonary infection and intra-abdominal infection. Figure 3 demonstrated that the time to ambulation, the time to first flatus, and the time to first liquid intake during the 6 years declined from 3.7 to 3.0 days, from 4.9 to 4.1 days, and from 6.7 to 5.9 days, respectively, and there seemed to be a plateau after a sharp decline from 2006 to 2008. Furthermore, a decreasing time that patients with LADG spent on postoperative stay could be easily noticed, from 11.0 days in 2006 to 10.2 days in 2011.
Figure 3.
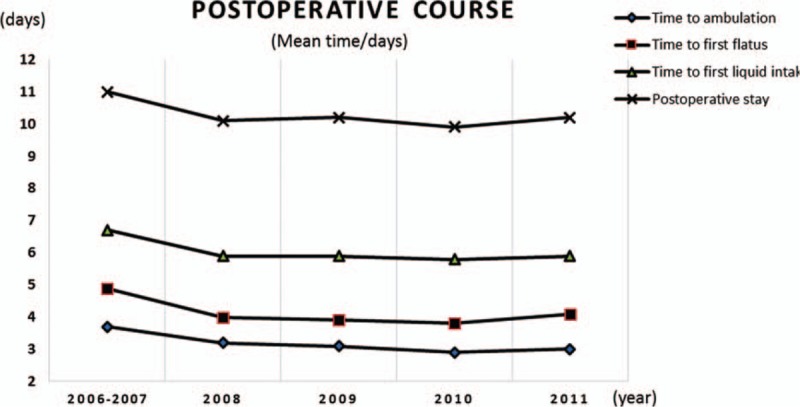
Time trend of postoperative course for patients with LADG.
3.3. Prognosis of patients and independent prognostic factors
At the time of the last follow-up (June 10, 2016), 160 patients were alive, while 73 patients were dead with 13 patients being lost to follow-up. The median length of follow-up was 66 (range, 1–98) months for all patients. Cox regression analyses in Table 3 showed that age, tumor differentiation, tumor size, and T stage as well as N stage were significantly independent prognostic factors, while gender, reconstruction type, cross-sectional location, lymphadenectomy, and year of operation were not independently associated with the survival prognosis. Furthermore, the survival curves for each factor mentioned above were shown in Figs. 4 and 5, and subgroups were compared by log-rank test.
Table 3.
Univariate and multivariate analyses of the patients’ clinicopathologic factors by Cox regression model.
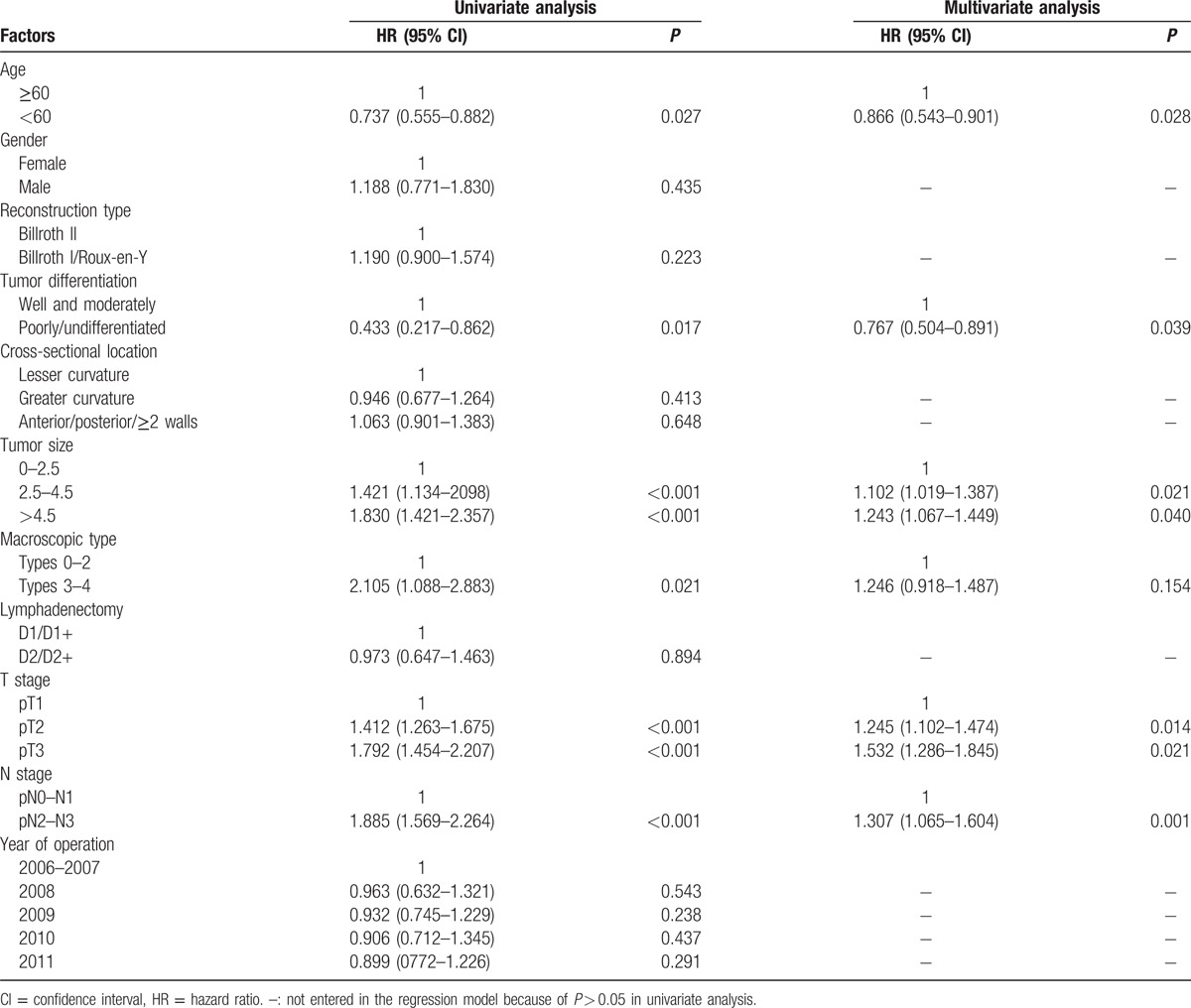
Figure 4.
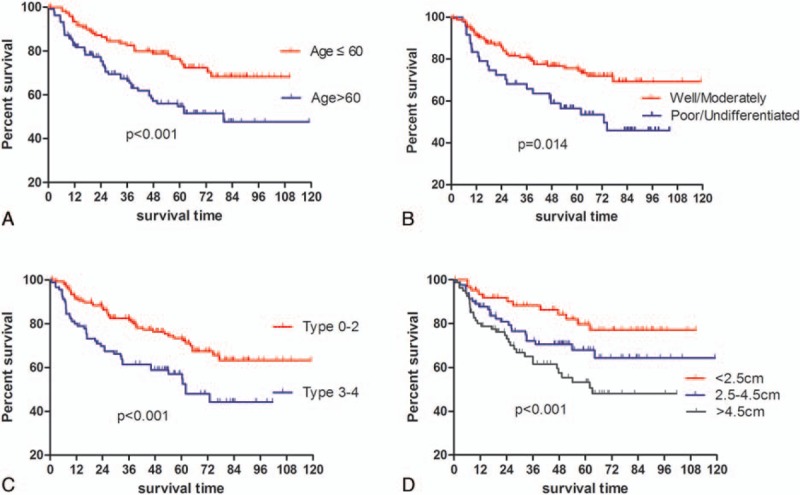
Kaplan–Meier survival analysis regarding age, tumor differentiation, macroscopic type, and tumor size.
Figure 5.
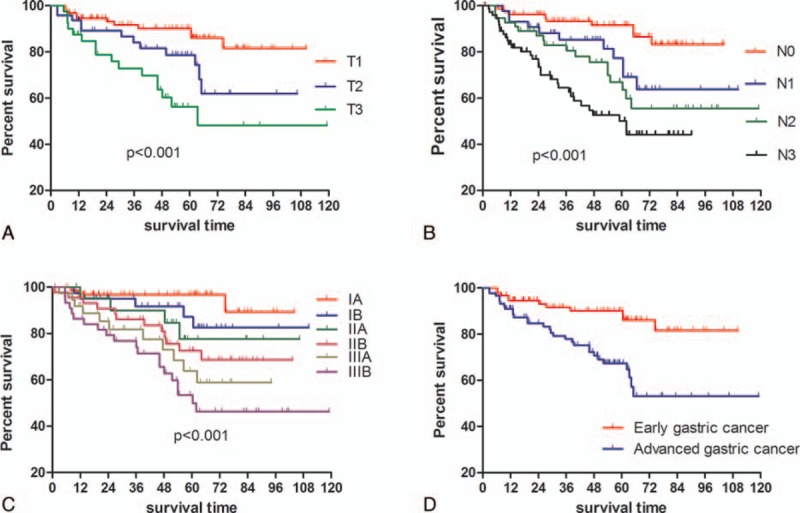
Kaplan–Meier survival analysis in terms of different stages.
4. Discussion
LADG, as a minimally invasive surgical approach, has gained increasing popularity since it was introduced in the surgical treatment for GC by Kitano et al[20] in the early 1990s. This study investigated our hospital experience with resectable GC patients who underwent LADG over a 6-year period, from the year 2006 to 2011. First of all, despite no significant difference among the 5 groups, a slight increasing proportion of advanced GC patients with LADG was found in our study due to the development of surgeon's accumulated experience, and because of the safety and feasibility confirmed by previous studies, which resulted in the expansion of operative indication for GC patients.[4,5,11,14] Normally, patients in advanced stage featured with more aggressive tumor behavior than those in early stage, which might be the reason why tumor size was increasing and the proportion of early macroscopic type was decreasing during the 6 years in this study.
Debate on the lymphadenectomy in surgical treatment for GC has been existing for decades, and no consensus has reached by far between the Western and Eastern countries. In the Western countries, D2 lymph node dissection is regarded as a recommended, but not required, procedure despite its contribution to accurate staging of the disease, given that several large randomized trials conducted in the Western countries failed to demonstrate a significant survival benefit for D2 over D1 lymphadenectomy.[21,22] However, in eastern Asia, gastrectomy with D2 lymph node dissection is the standard treatment for curable GC in advanced stage. D2 lymphadenectomy, particularly in Japan and Korea, has been performed for resectable GC since 1980.[23] Although laparoscopic D2 lymphadenectomy requires a significant degree of training and expertise, and a relatively long operative time,[11] the percentage of D2 lymphadenectomy in our study increased during the 6-year period, and D2 lymphadenectomy had been performed on more than 80% of patients in our study since 2010, being consistent with previous studies,[4,11] which is due to the downward trend of early GC patients enrolled in our study and because patients with advanced GC were majorly selected in the laparoscopic treatment with the extended operation indications.
The number of lymph nodes retrieved by laparoscopy-assisted gastrectomy varies broadly from the West to East. Previous studies showed that the number of retrieved lymph nodes differed from 27 to 35,[2,13,14,24] and the number of lymph nodes retrieved by laparoscope was significantly lower than that of open surgery, but lymph nodes staging appeared with no difference.[10] In this study, the number of retrieved lymph nodes grew gradually from 20 to 31.2, with a mean number of 28.6, which was similar to the results of the studies mentioned above. Actually, the number of retrieved lymph nodes can be influenced by many factors, for example, race, gender, age, tumor characteristics, and surgeons’ expertise.[25] There is uniform agreement that dissection of a sufficient number of lymph nodes (15 or greater) is of great benefit to provide adequate and accurate postoperative N staging,[8,17] which is mainly due to the consideration that N stage might be incorrect unless a sufficient number of lymph nodes was harvested,[26] and that the number of examined lymph nodes as an independent factor was potentially associated with the prognosis of GC.[27] Furthermore, the type of lymphadenectomy was also regarded as an important reason for the upward trend of the number of retrieved lymph nodes in this study, because that the extent of D2 lymphadenectomy was larger than that of D1 lymphadenectomy.
Surgical duration, estimated blood loss, incidence of morbidity and mortality, as significant indices for evaluating surgical safety, are closely correlated with the laparoscopic experience of surgeons. Thanks to the development of advanced surgical equipment, improved expertise, and accumulated experience of laparoscopic surgery, the 6 years witnessed a decreasing trend on the operation time, estimated blood loss, morbidity, and mortality. Our postoperative morbidity was within the 6.6% to 24.2% range of rates described in previous studies.[2,11,14] In addition, the indices mentioned before, together with the postoperative course, such as the time to ambulation, the time to first flatus, and the time to first liquid intake, presented a similar downward trend when LADG performed for the 46 patients was finished in the Group A in our study, and thereafter, a plateau was reached during the following years, which may indicate that the operation skill reached a mature and stable level after the first 46 operations, being similar to previous studies.[28–30] Therefore, the learning curve for LADG might be 46 cases in this study, and LADG, being a safe and feasible procedure in a certain degree, could help patients with quick postoperative recovery, resulting in short hospital stay.
We also focused on the evaluation of prognosis and prognostic factors of the patients with LADG, given that the long-term outcome is normally one of the extremely important issues to be concerned in the surgical treatment of GC. The 3- and 5-year OS rates were 78.1% and 56.2%, respectively, which were slightly higher than the OS rates for all patients regardless of surgical methods (open or laparoscopic surgery),[31] being still lower than that in Japan or Korea. In addition, no difference in OS was found among these 5 groups, though the proportion of advanced GC patients was slightly increasing. We attributed this to that this change could not be large enough to affect the OS because there was no difference among these 5 groups regarding T stage, which is showed in Table 1. Apart from age, T stage, N stage, and tumor differentiation as well, tumor size, which has been already demonstrated to be significantly associated with survival in our previous study,[32] when divided into 3 groups by the cutoff points, 2.5 and 4.5 cm in this study, was also illustrated to be an independent prognostic factor of OS.
There are also several limitations in our study. First of all, its retrospective design has the drawbacks of being observational or nonexperimental in nature, and the selection criteria bias of patients undergoing the laparoscopic approach needs to be considered. Second, as a nonrandomized single-center study, our findings are also limited by information bias, and the samples are not relatively large enough. Moreover, description of temporal trends only over 6 years might be not informative enough.
5. Conclusion
In conclusion, on one hand, some changes and improvements on tumor features, surgical parameters, and postoperative course in this study can be seen over the last years in the laparoscopic treatment of the GC. On the other hand, the learning curve for LADG for surgeons with rich experience in open operation might be 46 cases, and LADG can be safely adopted without increasing operative risk during the learning process even for the advanced GC.
Acknowledgments
The authors thank the Volunteer Team of Gastric Cancer Surgery (VOLTGA), West China Hospital, Sichuan University, China, for the substantial work in data collection and follow-up of the database.
Supplementary Material
Footnotes
Abbreviations: GC = gastric cancer, LADG = laparoscopy-assisted distal gastrectomy, OS = overall survival.
L-YZ and W-HZ contributed equally to the article.
Funding sources: Domestic support from (1) National Natural Science Foundation of China (nos. 81372344 and 81301867); (2) Sichuan Province Youth Science & Technology Innovative Research Team (no. 2015TD0009); (3) 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Kitano S, Shiraishi N, Uyama I, et al. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg 2007; 245:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JH, Nam BH, Ryu KW, et al. Comparison of outcomes after laparoscopy-assisted and open total gastrectomy for early gastric cancer. Br J Surg 2015; 102:1500–1505. [DOI] [PubMed] [Google Scholar]
- 3.Yamashita K, Sakuramoto S, Kikuchi S, et al. Laparoscopic versus open distal gastrectomy for early gastric cancer in Japan: long-term clinical outcomes of a randomized clinical trial. Surg Today 2015; 46:741–749. [DOI] [PubMed] [Google Scholar]
- 4.Lin JX, Huang CM, Zheng CH, et al. Laparoscopy-assisted gastrectomy with D2 lymph node dissection for advanced gastric cancer without serosa invasion: a matched cohort study from South China. World J Surg Oncol 2013; 11:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ben-David K, Tuttle R, Kukar M, et al. Laparoscopic distal, subtotal gastrectomy for advanced gastric cancer. J Gastrointest Surg 2015; 19:369–374. [DOI] [PubMed] [Google Scholar]
- 6.Hu Y, Ying M, Huang C, et al. Oncologic outcomes of laparoscopy-assisted gastrectomy for advanced gastric cancer: a large-scale multicenter retrospective cohort study from China. Surg Endosc 2014; 28:2048–2056. [DOI] [PubMed] [Google Scholar]
- 7.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011; 61:69–90. [DOI] [PubMed] [Google Scholar]
- 8.Ajani JA, Bentrem DJ, Besh S, et al. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2013; 11:531–546. [DOI] [PubMed] [Google Scholar]
- 9.Ohtani H, Tamamori Y, Noguchi K, et al. A meta-analysis of randomized controlled trials that compared laparoscopy-assisted and open distal gastrectomy for early gastric cancer. J Gastrointest Surg 2010; 14:958–964. [DOI] [PubMed] [Google Scholar]
- 10.Vinuela EF, Gonen M, Brennan MF, et al. Laparoscopic versus open distal gastrectomy for gastric cancer: a meta-analysis of randomized controlled trials and high-quality nonrandomized studies. Ann Surg 2012; 255:446–456. [DOI] [PubMed] [Google Scholar]
- 11.Shinohara T, Satoh S, Kanaya S, et al. Laparoscopic versus open D2 gastrectomy for advanced gastric cancer: a retrospective cohort study. Surg Endosc 2013; 27:286–294. [DOI] [PubMed] [Google Scholar]
- 12.Kosuga T, Ichikawa D, Okamoto K, et al. Impact of age on early surgical outcomes of laparoscopy-assisted gastrectomy with suprapancreatic nodal dissection for clinical stage I gastric cancer. Anticancer Res 2015; 35:2191–2198. [PubMed] [Google Scholar]
- 13.Moisan F, Norero E, Slako M, et al. Completely laparoscopic versus open gastrectomy for early and advanced gastric cancer: a matched cohort study. Surg Endosc 2012; 26:661–672. [DOI] [PubMed] [Google Scholar]
- 14.Park do J, Han SU, Hyung WJ, et al. Long-term outcomes after laparoscopy-assisted gastrectomy for advanced gastric cancer: a large-scale multicenter retrospective study. Surg Endosc 2012; 26:1548–1553. [DOI] [PubMed] [Google Scholar]
- 15.Liu K, Yang K, Zhang W, et al. Changes of esophagogastric junctional adenocarcinoma and gastroesophageal reflux disease among surgical patients during 1988–2012: a single-institution, high-volume experience in China. Ann Surg 2016; 263:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W, Zheng C, Fang C, et al. Time trends of clinicopathologic features and surgical treatment for gastric cancer: results from 2 high-volume institutions in southern China. Surgery 2015; 158:1590–1597. [DOI] [PubMed] [Google Scholar]
- 17.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011; 14:101–112. [DOI] [PubMed] [Google Scholar]
- 18.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol 2010; 17:3077–3079. [DOI] [PubMed] [Google Scholar]
- 19.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg 2009; 250:187–196. [DOI] [PubMed] [Google Scholar]
- 20.Kitano S, Iso Y, Moriyama M, et al. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 1994; 4:146–148. [PubMed] [Google Scholar]
- 21.Hartgrink HH, van de Velde CJ, Putter H, et al. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol 2004; 22:2069–2077. [DOI] [PubMed] [Google Scholar]
- 22.Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010; 11:439–449. [DOI] [PubMed] [Google Scholar]
- 23.Hyung WJ, Kim SS, Choi WH, et al. Changes in treatment outcomes of gastric cancer surgery over 45 years at a single institution. Yonsei Med J 2008; 49:409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shinohara T, Kawano S, Tanaka Y, et al. Comparison of the cost and outcomes following totally laparoscopic and laparoscopy-assisted distal gastrectomies for gastric cancer: a single-institution comparison. Surg Endosc 2015; 30:3573–3581. [DOI] [PubMed] [Google Scholar]
- 25.Luna A, Rebasa P, Montmany S, et al. Learning curve for d2 lymphadenectomy in gastric cancer. ISRN Surg 2013; 2013:508719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coburn NG, Swallow CJ, Kiss A, et al. Significant regional variation in adequacy of lymph node assessment and survival in gastric cancer. Cancer 2006; 107:2143–2151. [DOI] [PubMed] [Google Scholar]
- 27.Biffi R, Botteri E, Cenciarelli S, et al. Impact on survival of the number of lymph nodes removed in patients with node-negative gastric cancer submitted to extended lymph node dissection. Eur J Surg Oncol 2011; 37:305–311. [DOI] [PubMed] [Google Scholar]
- 28.Jin SH, Kim DY, Kim H, et al. Multidimensional learning curve in laparoscopy-assisted gastrectomy for early gastric cancer. Surg Endosc 2007; 21:28–33. [DOI] [PubMed] [Google Scholar]
- 29.Kim MC, Jung GJ, Kim HH. Learning curve of laparoscopy-assisted distal gastrectomy with systemic lymphadenectomy for early gastric cancer. World J Gastroenterol 2005; 11:7508–7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu WG, Ma JJ, Zang L, et al. Learning curve and long-term outcomes of laparoscopy-assisted distal gastrectomy for gastric cancer. J Laparoendosc Adv Surg Tech A 2014; 24:487–492. [DOI] [PubMed] [Google Scholar]
- 31.Zhang WH, Chen XZ, Liu K, et al. Outcomes of surgical treatment for gastric cancer patients: 11-year experience of a Chinese high-volume hospital. Med Oncol 2014; 31:150. [DOI] [PubMed] [Google Scholar]
- 32.Zhao LY, Zhang WH, Chen XZ, et al. Prognostic significance of tumor size in 2405 patients with gastric cancer: a Retrospective Cohort study. Medicine (Baltimore) 2015; 94:e2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


