Abstract
Data on the long-term efficacy and safety of abacavir/lamivudine (ABC/3TC) and nevirapine (NVP) are scarce. This combination has the advantage of simplifying treatment and improving long-term tolerance. The aim of this study was to compare the rate of any discontinuation of antiretroviral (ARV) regimen because of virologic failure (VF), and/or adverse drug reaction (ADR) among patients receiving stable ARV regimens for at least 6 months.
ABC/3TC/NVP was compared to ABC/3TC with either ritonavir-boosted darunavir (DRV/r) or ritonavir-boosted atazanavir (ATV/r), unboosted ATV, or tenofovir/emtricitabine (TDF/FTC) with either one of the following: ATV/r, unboosted ATV, DRV/r, efavirenz (EFV), or NVP, in the French prospective multicenter Dat’AIDS cohort.
The study enrolled 16,511 patients treated with following ARV regimens: ABC/3TC/NVP (n = 1089), TDF/FTC/NVP (n = 1542), ABC/3TC/DRV/r (n = 1065), ABC/3TC/ATV/r (n = 1847), ABC/3TC/ATV (n = 563), TDF/FTC/ATV/r (n = 3519), TDF/FTC/DRV/r (n = 2767), TDF/FTC/ATV (n = 419), and TDF/FTC/EFV (n = 3700). Mean follow-up was 36 ± 24 months. Patients treated with ABC/3TC/NVP received this regimen as a switch regimen in 97% of cases. By multivariable analysis, the risk of treatment discontinuation due to VF was similar between ABC/3TC/NVP and other ARV regimens, except for TDF/FTC/ATV and ABC/3TC/ATV, which were associated with a higher risk of treatment interruption due to VF (hazard ratio [HR] 1.99; 95% confidence interval [CI] 1.29–3.06 and HR 2.19; 95% CI 1.51–3.18, respectively). Treatment discontinuation due to ADR was lowest with the ABC/3TC/NVP regimen. Other ARV regimens were associated with a 1.80- to 3.19-fold increase in the risk of treatment discontinuation due to ADR (P < 0.0001 for all comparisons).
ABC/3TC/NVP as a simplification regimen is a long-term effective regimen with lower discontinuation due to long-term toxicity compared with other standard ARV regimens.
Keywords: abacavir/lamivudine, abacavir/lamivudine/nevirapine, antiretroviral treatment, ARV long-term efficacy, ARV long-term tolerance, ARV long-term toxicity, Dat’AIDS cohort, nevirapine
1. Introduction
In the majority of recent guidelines for antiretroviral (ARV) therapy of HIV-1 infection in adults, nevirapine (NVP)-containing regimens are no longer recommended in treatment-naïve patients due to the risk of hypersensitivity, hepatotoxicity, and insufficient evidence in favor of efficacy.[1,2] However, it remains an option for treatment optimization in patients with viral suppression.[1,2] Several randomized trials have shown the efficacy of such a treatment strategy with NVP.[3] Among the most frequently used nucleoside/nucleotide reverse transcriptase inhibitors backbones, abacavir/lamivudine (ABC/3TC) may have a better long-term safety profile than tenofovir/emtricitabine (TDF/FTC) and lacks renal or bone toxicity.[4,5] Data on the long-term efficacy and toxicity of ABC/3TC/NVP are scarce.[6] With the availability of NVP extended release, this combination has the advantage of being a simple treatment, with 2 tablets once daily. It could also potentially improve long-term safety (e.g., lipid profile and cardiovascular risk for NVP and renal and bone risk for ABC/3TC).[4,5,7–10] Thanks to these perceived advantages, the combination of ABC/3TC/NVP has been extensively used as a long-term simplification regimen in France, despite few data regarding its benefits.
The aim of this study was to compare the long-term efficacy and tolerability of ABC/3TC/NVP to 8 other frequently prescribed ARV regimens in France before 2013, namely ABC/3TC and ritonavir-boosted darunavir (DRV/r) or ritonavir-boosted or not atazanavir (ATV); TDF/FTC/NVP, TDF/FTC/ATV (ritonavir-boosted or not) or TDF/FTC/DRV/r, and TDF/FTC/efavirenz (EFV) in the French prospective Dat’AIDS cohort. As the rate of treatment switching due to adverse cutaneous and hepatic events is particularly high with NVP in the first few months,[11] to allow for long-term evaluation, we included only patients receiving each regimen for a duration of at least 6 months. The primary outcome was defined as any discontinuation of the ARV regimen due to virologic failure (VF) and/or adverse drug reaction (ADR).
2. Methods
2.1. Cohort and patients
Dat’AIDS is a French multicenter prospective cohort involving 12 large HIV reference centers in France.[12] Data are collected in real time during clinical visits, and data collection is ongoing since 2000 using a computerized medical record (Nadis, Fedialis Medica, Marly le Roi, France). The data collection has been approved by the French national commission on informatics and liberty.[12]
Dat’AIDS collects demographic and clinical data, ARV history, HIV RNA, and CD4 cell counts at regular 3- to 6-month intervals during routine clinical assessment.[12] Automated checks are performed during data capture. For example, if the treatment is changed during a visit, the prescription and the treatment history have to be coherent, and the reason for the discontinuation must be clearly indicated. The reasons for the discontinuation of the ARV regimen are given by the physician. For each of the following entries, quality control aims for 100% completeness and correctness: date of first HIV-positive test, CDC stage, date of first AIDS-defining event (if any), full ARV treatment history including all treatment discontinuations or changes with date and cause, and medical history. The ADRs are listed under specific organ system sections including neurological, renal, cardiovascular, osteoarticular, liver, cutaneous, psychiatric, dyslipidemia, lipodystrophy, and others.
Based on frequency of use in the cohort during the study period, we selected 8 ARV regimens for this study: ABC/3TC/NVP, TDF/FTC/NVP, ABC/3TC/DRV/r, ABC/3TC/ATV/r, ABC/3TC/ATV, TDF/FTC/DRV/r, TDF/FTC/ATV/r, TDF/FTC/ATV, and TDF/FTC/EFV. Patients were included if they started one of these combinations between January 1, 2000 and July 31, 2013. Because the main objective of the study was long-term tolerability and efficacy, we restricted the population to patients treated at least 6 months with one of these regimens. A sensitivity analysis was performed restricted to non-naïve patients who had undetectable HIV viral load at each ARV initiation.
2.2. Data collection
The following data were collected: age, gender, risk categories for HIV infection, duration of HIV infection, prior duration of ARV therapy, AIDS status, and CD4 lymphocyte count at the initiation of each prior duration of ARV therapy, number of prior ARV regimen, and death rate for each ARV regimen.
2.3. Statistical analysis
The primary study endpoints were time to discontinuation of ARV regimen due to VF, defined by any modification of the ARV regimen due to lack of efficacy, and time to discontinuation of ARV regimen due to ADR, defined by any modification of the ARV regimen due to poor tolerability.
Population characteristics were described as mean (standard deviation) or number (%), as appropriate. Baseline comparisons were calculated using Pearson chi-squared test or analysis of variance, as appropriate.
For each patient, time from initiation of an eligible ARV regimen to the primary endpoint was calculated. If no event occurred, follow-up was censored at the last follow-up visit or July 31, 2013, whichever came first.
Bivariable and multivariable analysis were performed using Cox proportional hazard model. Variables with a P value <0.20 by bivariable analysis were included in the multivariable model. Hazard ratios (HRs) and 95% confidence interval (CI) were estimated. Statistical analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, NC). Tests were considered as significant at a P value <0.05.
3. Results
Our analysis included 16,511 patients who were treated for at least 6 months between 2000 and 2013 with the following regimens: ABC/3TC/NVP (n = 1089), TDF/FTC/NVP (n = 1542), ABC/3TC/DRV/r (n = 1065), ABC/3TC/ATV/r (n = 1847), ABC/3TC/ATV (n = 563), TDF/FTC/ATV/r (n = 3519), TDF/FTC/DRV/r (n = 2767), TDF/FTC/ATV (n = 419), and TDF/FTC/EFV (n = 3700). Mean follow-up was 36 ± 24 months.
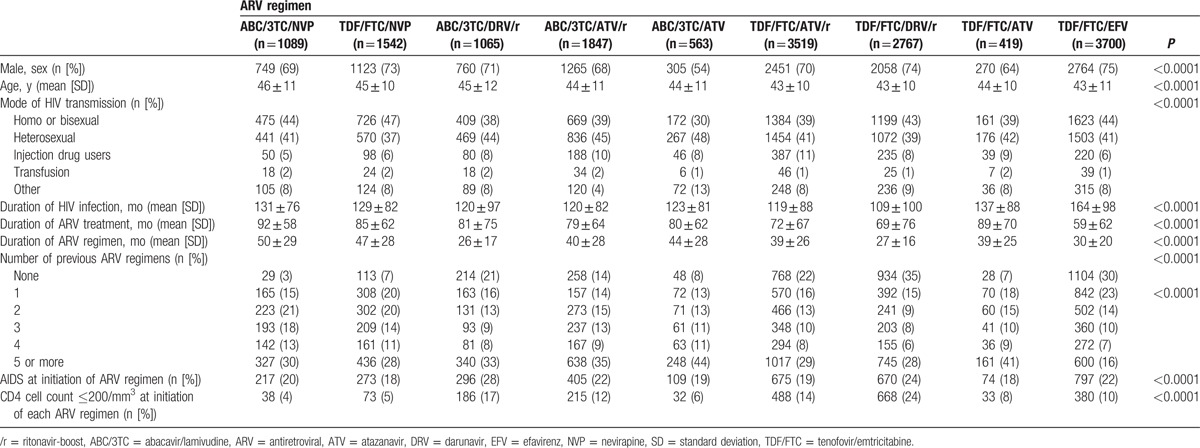
The baseline characteristics of the patients are presented in Table 1. All baseline characteristics differed significantly between ARV regimen groups (age, gender, risk categories for HIV infection, duration of HIV infection, number of prior ARV regimen, AIDS status, and CD4 count at initiation of each regimen). Patients treated with ABC/3TC/NVP were oldest and were less-frequent drug users than the other patients. The mean duration of ABC/3TC/NVP regimen was significantly longer than all other regimens. In addition, ABC/3TC/NVP was rarely given as the first ARV regimen (only 3%) or in patients with CD4 cell count ≤200/mm3 (4%).
Table 1.
Patient characteristics at the time of treatment initiation in each ARV regimen (n = 16,511).
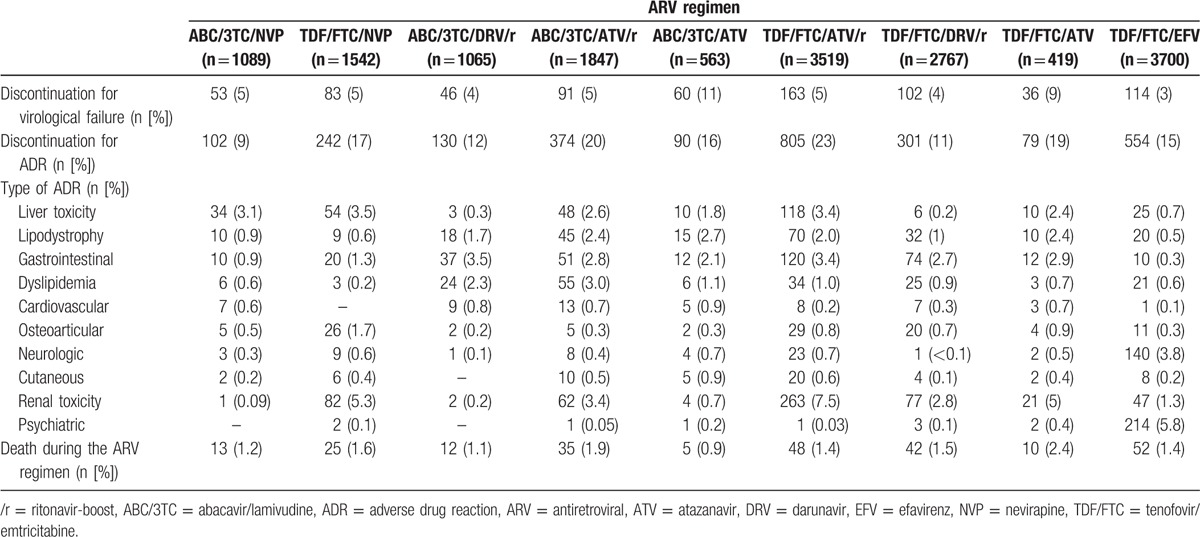
Discontinuation of the ARV regimen due to VF was observed in 748/16,511 patients overall (4.5%). The frequency of VF among the different ARV regimens is given in Table 2 (≤5% for all regimens except TDF/FTC/ATV [9%] and ABC/3TC/ATV [11%]).
Table 2.
Rate and reasons for discontinuation for each ARV regimen.
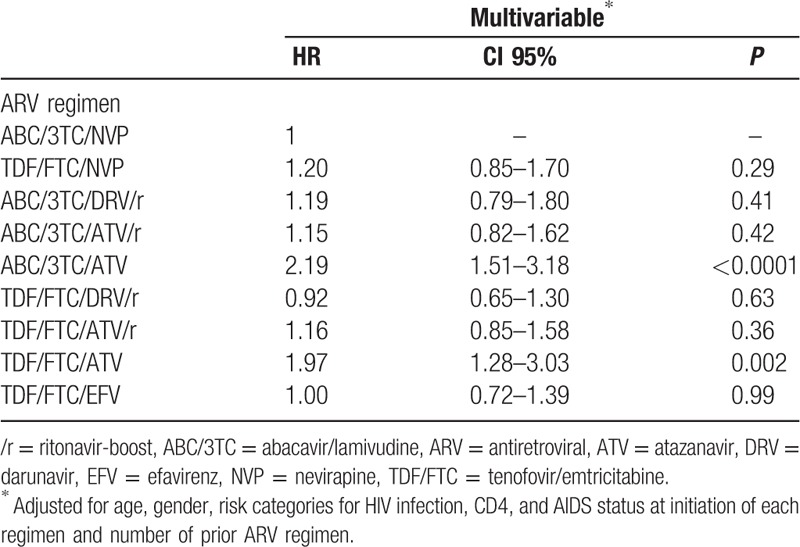
By multivariable analysis, after adjusting for age, gender, risk categories for HIV infection, CD4, and AIDS status at initiation of each regimen and number of prior ARV regimens, the time to discontinuation of ARV regimen due to VF was not significantly different between ARV regimens, except for TDF/FTC/ATV (HR 1.99; 95% CI 1.29–3.06; P = 0.002) and ABC/3TC/ATV (HR 2.19; 95% CI 1.51–3.18; P < 0.0001) (Table 3).
Table 3.
Factors associated with time to discontinuation due to virological failure by multivariable analysis.
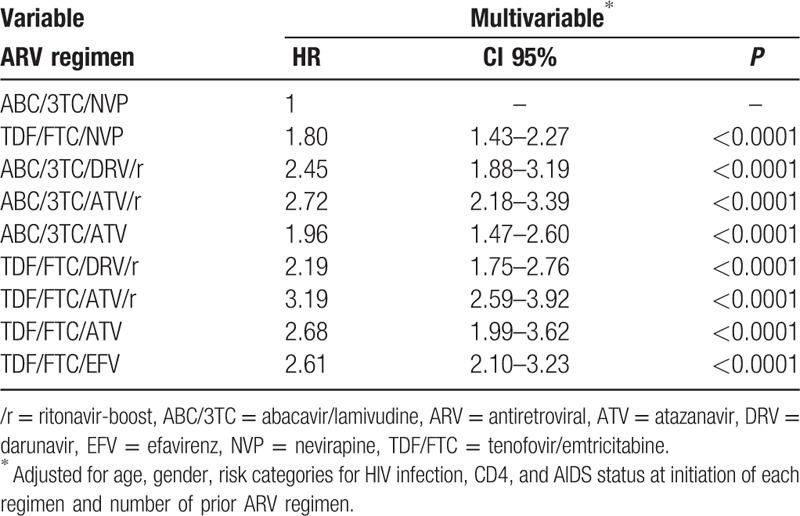
Discontinuation of the ARV regimen due to ADR was reported in 2677 (16.2%) patients. The lowest rate of discontinuation was observed with the ABC/3TC/NVP regimen (9%) and the highest with the TDF/FTC/ATV/r regimen (23%). By multivariable analysis, ABC/3TC/NVP was the regimen associated with the lowest rate of discontinuation due to ADR (P < 0.0001) (Table 4).
Table 4.
Factors associated with time to discontinuation due to ADR by multivariable analysis.
As discontinuation of the ARV regimen due to specific ADRs was very rare with some regimens, the sample size was too small to compare specific ADRs between the different regimens and/or ADRs. Specific ADRs leading to ARV discontinuation differed between regimens: liver toxicity was the main reason for discontinuation of ABC/3TC/NVP (>1/3 of discontinuations). In patients who discontinued this regimen, normalization of alanine aminotransferase and aspartate aminotransferase levels was observed in, respectively, 76% and 82% of patients at 3 months after discontinuation. In contrast, renal toxicity was one of the main reasons for discontinuation of TDF or ATV-based regimens, with the highest rate observed with TDF/FTC/ATV/r (n = 263; 7.5%), while it was rare with ABC/3TC + NVP (n = 1) or +DRV/r (n = 2) (Table 2). Overall, discontinuation of the ARV regimen due to renal toxicity was reported in 20.8% (n = 559) of all cases of discontinuation for ADR. Discontinuation of ARV due to digestive disorders was mostly observed in protease inhibitor (PI) regimens (2.1%–3.5%). Discontinuation of ARV therapy due to dyslipidemia was rarely observed with the NVP or EFV regimens (0.2%–0.6%), as compared to ritonavir-boosted PI regimens (0.9%–3.0%). Discontinuation for cardiovascular events was rare in all groups (<1%), while discontinuation due to psychiatric or neurological events was almost solely observed with the TDF/FTC/EFV regimen, that is, n = 214 (5.8%) and n = 140 (3.8%), respectively. Sensitivity analysis restricted to non-naïve patients who had undetectable HIV viral load at ARV initiation (n = 6997) confirmed the results of the overall sample, except for TDF/FTC/EFV (HR 2.31; 95% CI 1.35–3.97; P = 0.002). By sensitivity analysis, which had also a higher risk of discontinuation for VF, ABC/3TC/NVP was still found to be associated with the lowest rate of discontinuation due to ADR (P < 0.0001).
4. Discussion
This study is the largest to date to compare time to any discontinuation of ARV therapy due to VF and/or ADR, between 8 frequently prescribed ARV regimens before 2013, with ABC/3TC or TDF/FTC and either a nonnucleoside reverse transcriptase inhibitor (NNRTI) or a PI. Based on real-life data from this large, multicenter prospective cohort of HIV patients (n = 16,511) receiving the same ARV regimen for at least 6 months, the rate of discontinuation for VF was not different between ABC/3TC/NVP and other ARV regimens, except for TDF/FTC/ATV and ABC/3TC/ATV, which had a higher risk of discontinuation, while discontinuation for ADR was significantly lower with ABC/3TC/NVP regimen compared to all other 8 regimens, that is, TDF/FTC with either DRV/r, ATV/r, ATV, EFV, or NVP; ABC/3TC with either ATV/r, ATV, or DRV/r.
Patients treated with ABC/3TC/NVP were significantly older, less frequently ARV treatment-naïve (3%), and had the longest regimen duration (50 ± 29 months). Almost all of them received this regimen as the result of a switch in treatment (97%). The rate of treatment discontinuation due to VF with ABC/3TC/NVP was low (5%) and similar to the other frequently prescribed regimens, after adjustment for age, gender, risk categories for HIV infection, CD4, and AIDS status at initiation of each regimen and number of prior ARV regimens. Such a low rate could be partially explained by the inclusion of patients who stayed on the same treatment for more than 6 months, since the objective of the study was to assess the long-term effect of the different ARV regimens. Our results are in line with the rate of VF (1%) observed in a retrospective study including 98 patients receiving ABC/3TC/NVP as a simplification with a median follow-up of 27 months.[6] In another study evaluating TDF/FTC/NVP as a switch regimen, only 2.9% of 341 patients experienced VF at 1 year.[13] In contrast, TDF/FTC and ABC/3TC and unboosted ATV were associated with a 2-fold increase in the risk of VF (HR 1.99; 95% CI 1.29–3.06; P = 0.002 and HR 2.19; 95% CI 1.51–3.18; P < 0.0001, respectively). Drug–drug interactions between TDF and unboosted ATV might compromise the clinical activity of ATV, especially in ARV-experienced patients and could partly explain our findings.[14] In the 2013 French guidelines for ARV therapy of HIV-1 infection, these regimens could be considered as optimization of ARV therapy in order to improve tolerance; however, only in patients without a history of prior VF and with a recommendation for ATV dosage in patients receiving TDF/FTC as a backbone.[1]
The ABC/3TC/NVP regimen was associated with the lowest rate of treatment discontinuation due to ADR; with all other regimens being associated with a 1.80- to 3.19-fold increase in the risk of treatment discontinuation due to ADR (P < 0.0001). These results confirm that, when considering long-term safety, beyond the first 6 months of exposure—therefore, excluding patients with early toxicity—ABC/3TC/NVP has one of the most favorable profiles of current ARV regimens.
Overall, renal toxicity was the most frequent reason of discontinuation of ARV regimen due to ADR. Among HIV patients, the prevalence of chronic kidney disease (defined as glomerular filtration rate [GFR] <60 mL/min/1.73 m2) ranges in industrialized countries from 4.7% to 9.7% and up to 20% in patients aged 60 years or over.[4,15] HIV infection and ARVs themselves are associated with a higher risk of renal impairment.[4,16] Furthermore, the age-standardized and relative risks of chronic kidney disease increase substantially with time after ARV initiation.[17] The frequently prescribed ARV drugs TDF and ATV have mostly been associated with renal function impairment.[4,18] TDF has also been associated with greater declines in GFR when combined with ATV or a ritonavir-boosted PI in some studies, suggesting an increased risk for nephrotoxicity with these combinations.[4,19] In our study, renal toxicity was one of the main reasons for discontinuation due to ADR among all TDF-based regimens, particularly in association with ATV, whether ritonavir-boosted or not. In addition, the rate of discontinuation of the TDF/FTC/NVP regimen due to renal toxicity was high, but was rare with ABC/3TC/NVP, highlighting the role of TDF in the renal toxicity. It was also very rare when ABC/3TC was given in combination with DRV/r but was higher in combination with ATV/r, suggesting a role of ATV/r in the occurrence of renal toxicity.
NVP has been reported to be associated with a protective effect on cardiovascular risk, but studies of the impact of ABC have given conflicting results.[7,17,20] NVP was shown to have a favorable lipid profile when compared with EFV and PIs, but this was documented in studies conducted with PIs, which are no longer recommended.[7,8,21] In our study, discontinuation of the ARV regimen due to cardiovascular events was very rare across all groups. However, dyslipidemia, which was the cause of 6.6% of ARV discontinuations, was nonetheless mainly observed in boosted PI-based regimens, that is, DRV/r and ATV/r in combination with ABC/3TC.
The major treatment-limiting side effect of NVP is the drug hypersensitivity syndrome, characterized by fever, skin rash, and/or hepatitis occurring during the first few weeks of therapy. This phenomenon is well described, but studies on the long-term liver tolerance of NVP are scarce.[11] In 1 study evaluating the long-term NVP and EFV liver tolerance with a mean follow-up of 6 years, no significant difference in liver toxicity was observed between patients receiving NNRTI or PI regimens or between patients prescribed EFV or NVP.[22] In this same study, twice as many hepatotoxic events occurred during the first year of NVP or EFV therapy as compared with the entire period after 1 year.[22] Hepatitis C and hepatitis B virus coinfections have been shown to be associated with a higher risk of hepatotoxicity, which increases steadily over time during NNRTI therapy.[11,22] Liver toxicity was the most frequent reason for discontinuation of NVP regimens either in association with ABC/3TC (3.1%) or TDF/3TC (3.5%). One of the limitations of our study is that we could not ascertain the exact cause of liver toxicity. However, the toxicity was probably related to NVP, since normalization of alanine aminotransferase and aspartate aminotransferase was observed in, respectively, 76% and 82% of patients 3 months after discontinuation of the ABC/3TC/NVP of this regimen. Liver toxicity was also more frequently observed with ritonavir-boosted or unboosted ATV regimens (1.8%–2.4%), where it was likely related to hyperbilirubinemia compared with DRV/r regimens (0.2%–0.3%). These results confirm those of a large randomized study in first-line therapy, where maximum treatment-emergent alanine aminotransferase increase (>3 times the upper limit of normal) was 2% with DRV/r + 2 NRTIs at week 48.[23]
In keeping with other studies, discontinuation of ARV regimens due to neuropsychiatric adverse events was almost only observed with the EFV regimen. A switch from EFV to NVP in subjects with virological suppression has been shown to be effective in resolving neuropsychiatric symptoms.[24] Many alternatives among NNRTI or other classes are now available, with the result that EFV is no longer preferred, mainly because of these long-term ADRs.[25]
During the early years after the advent of potent ARV therapy, gastrointestinal toxicity was one of the most frequent adverse events and was often a reason for treatment discontinuation, particularly grades 2 to 4 diarrhea with lopinavir/r and fosamprenavir/r.[9] Interestingly, in our study, the rate of discontinuation for gastrointestinal problems was very low with NNRTIs, as expected and around 3% across all 5 PI-containing regimens. The implication is that physicians should keep in mind that currently used PIs, although associated with less digestive discomfort, may still cause problems in the long term. Although this does not strictly qualify as toxicity, ARV-associated gastrointestinal symptoms should prompt physicians to discuss a potential switch in treatment.
The major strength of this study is that it provides data from a large nationwide prevalent cohort of over 16,000 patients followed in routine practice in France. Since early discontinuation of ARV regimens due to short-term tolerability problems is high in many cohort studies (>25% at 1 year), we included in our analyses only patients who received the same regimen for at least 6 months, whatever the ARV type, in order to evaluate both long-term tolerance and virological effectiveness.[26,27] Nonetheless, our study suffers from some limitations that deserve to be acknowledged. Although the multivariable model was adjusted for major patient and ARV characteristics known to be associated with better adherence to HIV therapy, such as age, gender, HIV route of transmission, CD4, and AIDS status at initiation of each regimen, and number of prior ARV regimen, other sociodemographic factors and/or medical factors that drive the physician's decision in selecting the ARV regimen were not evaluated in our study. Therefore, we cannot exclude the possibility of indication and channeling biases or other confounding factors. However, except for TDF/FTC/EFV, our results were confirmed by sensitivity analysis restricted to non-naïve patients who had undetectable HIV viral load at ARV initiation. The difference observed with TDF/FTC/EFV could be due to a selection bias arising from the exclusion of naïve subjects. Indeed, TDF/FTC/EFV is used both as first-line treatment and as simplification. In contrast, ATV/r or DRV/r-based regimens are more often prescribed in naïve patients than as simplification. Second, the ADRs reported in the Dat’AIDS database are not graded or detailed other than by the main organ-specific items, and the exact diagnosis is not systematically recorded. Moreover, we only evaluated adverse events leading to ARV discontinuation, and therefore we cannot evaluate the prevalence of the various ADRs. As in all large observational multicenter cohorts, criteria to discontinue for ADR were decided by each physician, with a certain degree of subjectivity. However, due to the very large number of patients in each subgroup, and the long follow-up, our results reflect real-life experience and practice. Furthermore, the findings are in line with the well known toxicity of each ARV drug.
In conclusion, in this representative French cohort of HIV patients receiving the same ARV regimen for at least 6 months, ABC/3TC/NVP was associated with the same risk of long-term VF as TDF/FTC/NVP, ABC/3TC/DRV/r, ABC/3TC/ATV/r, TDF/FTC/DRV/r, TDF/FTC/ATV/r, and TDF/FTC/EFV, and had the lowest rate of treatment discontinuation due to ADR. Therefore, ABC/3TC/NVP is an effective long-term option for treatment simplification in patients with viral suppression. Furthermore, this regimen could yield significant cost savings in the current economic climate, where many Western countries are seeking to limit public financing of national health systems.
Acknowledgments
We thank Fiona ECARNOT (EA3920, University Hospital Jean Minjoz and University of Franche-Comté, Besancon, France) for editorial assistance.
Footnotes
Abbreviations: ABC/3TC = abacavir/lamivudine, ADR = adverse drug reaction, ARV = antiretroviral, ATV = atazanavir, ATV/r = ritonavir-boosted atazanavir, CI = confidence interval, DRV/r = ritonavir-boosted darunavir, EFV = efavirenz, GFR = glomerular filtration rate, HR = hazard ratio, NNRTI = nonnucleoside reverse transcriptase inhibitor, NVP = nevirapine, PI = protease inhibitor, TDF = tenofovir, TDF/FTC = tenofovir/emtricitabine, VF = virologic failure.
Dat’AIDS scientific committee: P Dellamonica, P Pugliese, IP-M, L Cuzin, Y Yazdanpanah, FR, AC, RG, C Delpierre, C Allavena, C Katlama, MS Valantin, C Duvivier, B Hoen, G Peytavin, C Jacomet, DR, PD, A Cheret, C Chidiac, C Isnard-Bagnis, LC, D Peyramond, FB-S, V Joly, T Jovelin, K Saune, PM Roger, C Chirouze, and T May.
Dat’AIDS Study Group: P Enel, V Obry-Roguet, O Faucher, S Bregigeon, A Ménard, I Poizot-Martin (Marseille); P Delobel, B Marchou, P Massip, M Alvarez, L Porte, A Debard, G Martin-Blondel, L Cuzin, N Biezunski, C Fourcade, I Lepain (Toulouse); P Pugliese, L Bentz, C Ceppi, E Cua, J Cottalorda, J Durant, S Ferrando, JG Fuzibet, R Garraffo, V Mondain, A Naqvi, I Perbost, S Pillet, B Prouvost-Keller, C Pradier, S Wehrlen-Pugliese, P-M Roger, E Rosenthal, P Dellamonica (Nice); C Allavena, E Billaud, S Bouchez, T Jovelin, S Pineau, V Reliquet, F Raffi (Nantes); A Cheret, P Choisy (Tourcoing); C Duvivier (Pasteur Necker), MA Valantin, R Agher, C Katlama (Paris Pitié Salpétriere); A Cabié, S Abel, S Pierre-François, B Liautaud (Fort de France); D Rey, P Fischer, M Partisani, C Cheneau, ML Batard, C Bernard-Henry, M Priester, E de Mautort (Strasbourg); C Chirouze, Q Gardiennet (Besançon); F Bani-Sadr, C Rouger, JL Berger, Y N’Guyen, D Lambert, I Kmiec, M Hentzien, D Lebrun, C Migault (Reims); L Cotte, C Chidiac, T Ferry, F Ader, F Biron, A Boibieux, P Miailhes, T Perpoint, I Schlienger, F Dahoud, J Lippmann, E Braun, J Koffi, C Longuet, V Guéripel, C Augustin-Normand, and S Degroodt (Lyon).
The Nadis EMR is developed and maintained with support from Fedialis Medica and ViiV Healthcare.
Author contributions: All authors participated in the design of the study protocol and data collection. PdB and MD performed statistical analyses. PdB, MD, and FB-S wrote the first manuscript draft. All authors participated in interpretation of the data and writing of the final manuscript, and all authors approved the final manuscript. FB-S and MD were responsible for the overall supervision of the study.
The authors have no conflicts of interest to disclose.
References
- 1.Hoen B, Bonnet F, Delaugerre C, et al. French 2013 guidelines for antiretroviral therapy of HIV-1 infection in adults. J Int AIDS Soc 2014; 17:19034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Günthard HF, Aberg JA, Eron JJ, et al. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society-USA Panel. JAMA 2014; 312:410–425. [DOI] [PubMed] [Google Scholar]
- 3.Ena J, Leach A, Nguyen P. Switching from suppressive protease inhibitor-based regimens to nevirapine-based regimens: a meta-analysis of randomized controlled trials. HIV Med 2008; 9:747–756. [DOI] [PubMed] [Google Scholar]
- 4.Lucas GM, Ross MJ, Stock PG, et al. Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 59:e96–e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Escota GV, Mondy K, Bush T, et al. High prevalence of low bone mineral density and substantial bone loss over 4 years among HIV-infected persons in the era of modern antiretroviral therapy. AIDS Res Hum Retroviruses 2016; 32:59–67. [DOI] [PubMed] [Google Scholar]
- 6.Cabello Úbeda A, Sanz Moreno J, Williams F, et al. Efficacy and safety of nevirapine + Kivexa (abacavir/lamivudine) as a simplification strategy for HIV patients with undetectable viral load. J Acquir Immune Defic Syndr 2011; 58:e95–e96. [DOI] [PubMed] [Google Scholar]
- 7.Maggi P, Bellacosa C, Carito V, et al. Cardiovascular risk factors in patients on long-term treatment with nevirapine- or efavirenz-based regimens. J Antimicrob Chemother 2011; 66:896–900. [DOI] [PubMed] [Google Scholar]
- 8.Boccara F, Lang S, Meuleman C, et al. HIV and coronary heart disease: time for a better understanding. J Am Coll Cardiol 2013; 61:511–523. [DOI] [PubMed] [Google Scholar]
- 9.Hill A, Balkin A. Risk factors for gastrointestinal adverse events in HIV treated and untreated patients. AIDS Rev 2009; 11:30–38. [PubMed] [Google Scholar]
- 10.Reliquet V, Allavena C, Morineau-Le Houssine P, et al. Twelve-year experience of nevirapine use: benefits and convenience for long-term management in a French cohort of HIV-1-infected patients. HIV Clin Trials 2010; 11:110–117. [DOI] [PubMed] [Google Scholar]
- 11.Bonnet F, Lawson-Ayayi S, Thiébaut R, et al. A cohort study of nevirapine tolerance in clinical practice: French Aquitaine Cohort, 1997–1999. Clin Infect Dis 2002; 35:1231–1237. [DOI] [PubMed] [Google Scholar]
- 12.Pugliese P, Cuzin L, Cabié A, et al. A large French prospective cohort of HIV-infected patients: the Nadis cohort. HIV Med 2009; 10:504–511. [DOI] [PubMed] [Google Scholar]
- 13.Llibre JM, Bravo I, Ornelas A, et al. Effectiveness of a treatment switch to nevirapine plus tenofovir and emtricitabine (or lamivudine) in adults with HIV-1 suppressed viremia. PLoS One 2015; 10:e0128131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonora S, Gonzalez de Requena D, D’Avolio A, et al. Pharmacokinetics of switching unboosted atazanavir coadministered with tenofovir disoproxil fumarate from 400 mg once daily to 200 mg twice daily in HIV-positive patients. Antivir Ther 2011; 16:499–504. [DOI] [PubMed] [Google Scholar]
- 15.Hentzien M, Dramé M, Allavena C, et al. Impact of age-related comorbidities on five-year overall mortality among elderly HIV-infected patients in the late HAART era—role of chronic renal disease. J Nutr Health Aging 2016; 20:408–414. [DOI] [PubMed] [Google Scholar]
- 16.Althoff KN, McGinnis KA, Wyatt CM, et al. Comparison of risk and age at diagnosis of myocardial infarction, end-stage renal disease, and non-AIDS-defining cancer in HIV-infected versus uninfected adults. Clin Infect Dis 2015; 60:627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasmussen LD, May MT, Kronborg G, et al. Time trends for risk of severe age-related diseases in individuals with and without HIV infection in Denmark: a nationwide population-based cohort study. Lancet HIV 2015; 2:e288–e298. [DOI] [PubMed] [Google Scholar]
- 18.Ryom L, Mocroft A, Kirk O, et al. Association between antiretroviral exposure and renal impairment among HIV-positive persons with normal baseline renal function: the D:A:D study. J Infect Dis 2013; 207:1359–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morlat P, Vivot A, Vandenhende M-A, et al. Role of traditional risk factors and antiretroviral drugs in the incidence of chronic kidney disease, ANRS CO3 Aquitaine cohort, France, 2004–2012. PLoS One 2013; 8:e66223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cruciani M, Zanichelli V, Serpelloni G, et al. Abacavir use and cardiovascular disease events: a meta-analysis of published and unpublished data. AIDS 2011; 25:1993–2004. [DOI] [PubMed] [Google Scholar]
- 21.Islam FM, Wu J, Jansson J, et al. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta-analysis. HIV Med 2012; 13:453–468. [DOI] [PubMed] [Google Scholar]
- 22.Van Welzen B, Mudrikova T, Arends J, et al. No increased risk of hepatotoxicity in long-term use of nonnucleoside reverse transcriptase inhibitors in HIV-infected patients. HIV Med 2012; 13:448–452. [DOI] [PubMed] [Google Scholar]
- 23.Clotet B, Feinberg J, van Lunzen J, et al. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet 2014; 383:2222–2231. [DOI] [PubMed] [Google Scholar]
- 24.Pedrol E, Llibre JM, Tasias M, et al. Outcome of neuropsychiatric symptoms related to an antiretroviral drug following its substitution by nevirapine: the RELAX study. HIV Med 2015. [DOI] [PubMed] [Google Scholar]
- 25.Raffi F, Pozniak AL, Wainberg MA. Has the time come to abandon efavirenz for first-line antiretroviral therapy? J Antimicrob Chemother 2014; 69:1742–1747.24603962 [Google Scholar]
- 26.Cuzin L, Pugliese P, Allavena C, et al. Comparative effectiveness of first antiretroviral regimens in clinical practice using a causal approach. Medicine (Baltimore) 2015; 94:e1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abgrall S, Ingle SM, May MT, et al. Durability of first ART regimen and risk factors for modification, interruption or death in HIV-positive patients starting ART in Europe and North America 2002–2009. AIDS 2013; 27:803–813. [DOI] [PubMed] [Google Scholar]


