Abstract
Intravenous leiomyomatosis (IVL) is a rare benign tumor. The study aimed to assess outcomes of patients treated surgically for IVL.
Between November 2002 and January 2015, 76 patients were treated for IVL. The stage of IVL was evaluated preoperatively by echocardiography and enhanced computerized tomography (CT) scan, and graded into 4 stages according to intravascular tumor progression. We recorded age, lower limb edema before surgery, surgical parameters, and hospitalization expenses. Patients were followed up every 6 months and tumor recurrence was assessed by CT and ultrasound. Patients were followed up for a mean of 4.5 ± 2.5 years (range 1–13 years) and there was no operative, hospital, or long-term mortality or were lost to follow-up.
The rate of lower extremity edema, amount of blood loss, postoperative transfusion, length of intensive care unit (ICU) stay, postoperative hospitalization, and hospitalization expenses differed significantly between patients at different presurgery stages. Tumors recurred in 4 of 7 patients with stage I IVL that opted for surgery that preserved the ovaries and uterus. No recurrence was observed in patients graded stage II or more, in all of which the uterus and ovaries were removed. Recurrence was observed in only 4 of 76 cases of IVL, all of whom opted for surgery that spared the ovaries and uterus.
Different surgical strategies should be decided based on the staging to completely remove the tumor and ensure the safety of patients. Removal of both ovaries is necessary for inhibiting tumor growth and avoiding recurrence.
Keywords: intravenous leiomyomatosis, surgical strategies, uterine fibroids
1. Introduction
Intravenous leiomyomatosis (IVL) is a rare but a life-threatening disease in women of childbearing age. IVL was firstly described by Birch-Hirschfeld in 1896,[1] and Dürck[2] reported the first case of extension of IVL to the heart in 1907. In 1959, Marshall and Morris published a review of 17 IVL cases,[3] describing IVL as a pathological progression of the common benign pelvic tumor uterine leiomyoma (LM) into the veins in up to 30% of cases, that can extend as far as to the right atrium and right ventricle and even pulmonary arteries in up to 10% of cases.[4–7] This enlarged smooth muscle tumor is benign, but can cause sudden death by obstruction of the total outflow tract.
Whether IVL originates from the smooth muscle of the uterus or the smooth muscle of the venae uterinae remains controversial.[8–13] IVL is a relatively rare disease with nonspecific clinical presentation, thus many IVL patients are misdiagnosed or only diagnosed after the tumor has reached the heart. Early and accurate diagnosis remains challenging, and appropriate treatments are not well characterized,[14] and IVL tends to recur.[5] However, IVL is currently most often treated surgically and with antiestrogen therapy to suppress estrogen.[15]
Complete removal of the tumor is crucial to avoid recurrence of IVL, though due to involvement with the iliac vein this is not always possible. In these cases, as estrogen and progesterone receptors are present on the surface of IVL cells, and tumor growth is closely associated with circulating estrogen levels, removal of the ovaries can further inhibit tumor growth.[16–19]
Between November 2002 and January 2015, 30,757 patients were treated for LM at Peking Union Medical College Hospital, while only 76 (0.25%) were diagnosed with IVL. Here we present a relatively large case series of a rare disease, and summarize the treatment and outcome of these 76 patients with IVL.
2. Materials and methods
2.1. Patients
We retrospectively reviewed the clinical medical records of patients diagnosed with IVL undergoing surgery at the Peking Union Medical College Hospital Department of Cardiac Surgery, Department of Vascular Surgery, and Department of Gynecology between November 2002 and January 2015, consecutively. This study was approved by the Ethics Committee of Peking Union Medical College Hospital. The need for individual consent was waived by the committee because of the retrospective nature of the study.
2.2. Diagnosis
All patients had a history of preoperative uterine fibroids. IVL was suspected following presurgery pelvic computerized tomography (CT) scan and ultrasound which identified an inferior vena cava mass originating from the iliac vein or ovarian vein. IVL was diagnosed based on postoperative pathology where the tumor was found to originate from the uterus and that grow into the vein. If the tumor did not invade the vein, then the patient was diagnosed with common uterine fibroids. Some patients had received previous surgical treatment for IVL at other hospitals which did not successfully remove the tumor.
2.3. Preoperative clinical assessment
All patients received enhanced CT scan of chest, abdomen, and pelvis (Figs. 1 and 2), ultrasound of pelvis and inferior vena cava. Patient blood type, hepatic function, renal function, clotting were assessed before surgery, and echocardiography examination was performed.
Figure 1.
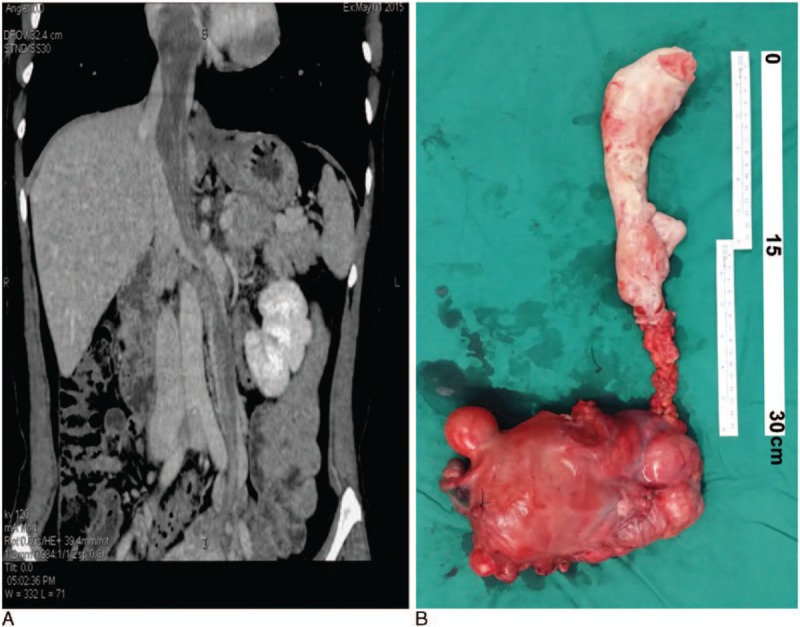
An example of stage III patient. A 44-year-old patient with stage III, IVL complained of chest tightness and shortness of breath. An enhanced CT scan was performed a week before surgery (A), and the patient underwent cardiopulmonary bypass-assisted surgery, with deep hypothermia circulation arrest. The right atrium and inferior vena cava were opened and the uterus, ovary, and tumor embolus was removed using an abdominal thoracic incision (B).
Figure 2.
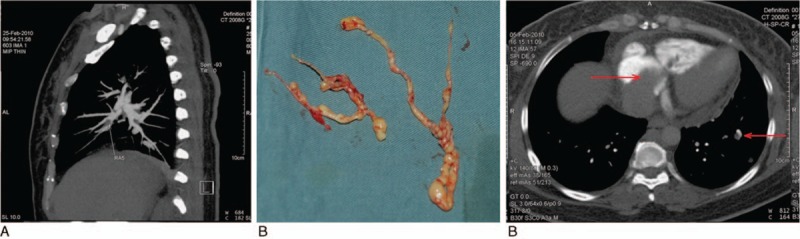
An example of stage IV patient. A 48-year-old patient, with stage IV IVL complained of chest pain and palpitations. An enhanced CT scan of the pulmonary artery performed a week before surgery (A) revealed a tumor embolus (arrow) in the right pulmonary branch. The patient underwent cardiopulmonary bypass-assisted surgery with deep hypothermia circulation arrest. The right atrium, pulmonary artery, and inferior vena cava were opened and the tumor embolus in the pulmonary artery was removed using a horizontal position, abdominal thoracic incision (B). An enhanced CT scan of the chest revealed a pulmonary metastasis. The tumor embolus was located within the right atrium, right ventricle, and a pulmonary metastasis was identified (arrow) (C).
2.4. Development of clinical staging
Patients were categorized into 4 stages reflecting presurgery tumor progression. Where tumors penetrated the uterine venous wall, but were confined to the pelvic cavity, patients were categorized as stage I. Where tumors has extended into the abdominal cavity, but had not reached the renal vein they were categorized as stage II. Where tumors reached the renal vein and inferior vena cava, and further extended into the right atrium, but had not reached the pulmonary arteries, patients were categorized as stage III. Where tumors reached the pulmonary arteries and/or lung metastases were observed, patients were categorized as stage IV (Fig. 3A). For patients graded stage 2 or above, tumor that originates in the uterus can grow into the ovarian vein, or iliac vein, or even both (Fig. 3B), then extends along the blood flow and reaching as far as the right atrium, or even pulmonary arteries.
Figure 3.
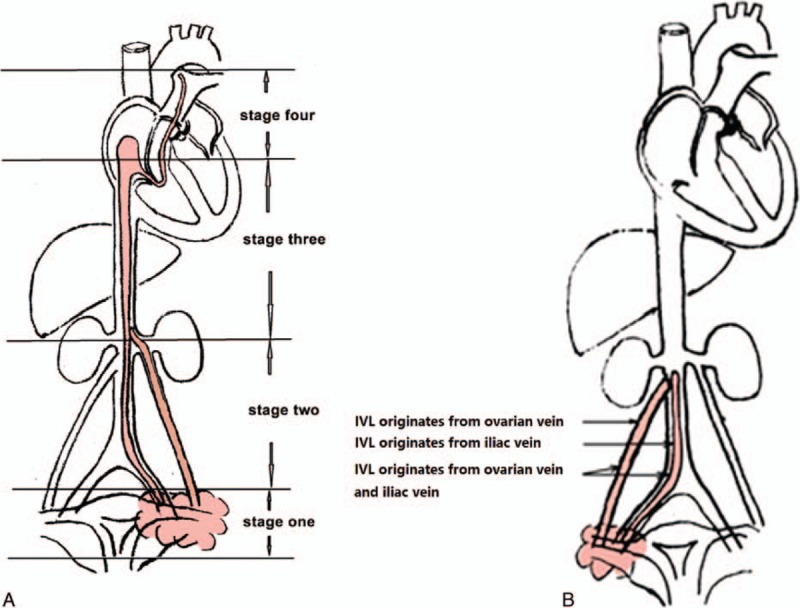
Stage and origin of intravenous leiomyomatosis. Intravenous leiomyomatosis was categorized into 4 stages reflecting presurgery tumor progression (A), and was found to originate from different tissues (B).
2.5. Surgery
Liquid diets were ordered and clysis and vaginal douche were performed the day before surgery. If the patient had not previously undergone a hysterectomy, a thin prep cytologic test (TCT) was suggested to exclude cervical cancer before surgery. Surgical strategies were chosen by a surgeon based on the progression of the leiomyoma and whether the tumor could be resected completely and safely.
Although we recommended that all IVL patients underwent oophorectomy and hysterectomy, some stage I patients refused oophorectomy or hysterectomy in order to preserve fertility. All patients graded stage II or above received total tumor resection, abdominal hysterectomy, and bilateral salpingo-oophorectomy.
For stage I patients, whose tumor was confined to the pelvis, the surgery was relatively simple and was performed by the gynecological surgeon independently. For stage II patients, where the tumor progressed to the renal vein, surgery included incision of the inferior vena cava, and removal of the tumor thrombus. This surgery involved cooperation of gynecology and vascular surgeons without cardiopulmonary bypass (CPB). For stage III and IV patients, where the tumor progressed up the renal vein, resection was difficult without CPB which was applied to avoid massive hemorrhage during tumor resection (Figs. 1 and 2). All patients graded stage III or stage IV underwent intraoperative transesophageal echocardiography examination (Fig. 4).
Figure 4.
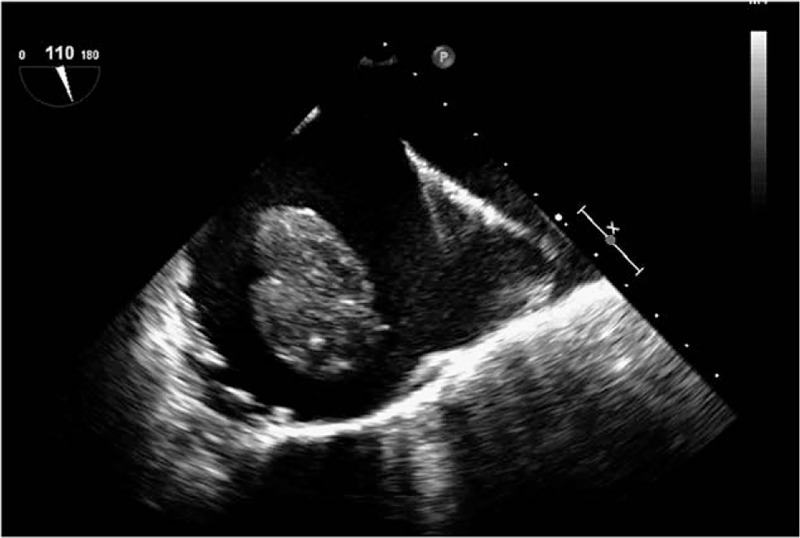
Transesophageal echocardiography (TEE) revealed a strip-type mass in the right atrium of a stage III patient. A 55-year-old patient with stage III IVL complained of palpitations and shortness of breath. The patient underwent cardiopulmonary bypass-assisted surgery with deep hypothermia circulation arrest. Intraoperative TEE revealed a strip-type mass, extending from the inferior vena cava to right atrium. The head end was enlarged to 66 mm × 25 mm, and it entered into right ventricular while ventricular diastole. The right atrium and inferior vena cava were opened and the tumor was removed via an abdominal thoracic incision.
2.6. Other treatments
Patients with large unresectable tumors were administered 3.75 mg diphereline (GnRHa, Ipsen Pharma Biotech, Boulogne-Billancourt-France) by intramuscular injection once every 28 days for at least 3 months before operation, which could inhibits the enlargement of tumor and reduce the size, so as to profit the operation. Patients that underwent oophorectomy and hysterectomy were administered 2.5 mg letrozole (Jiang Su HengRui Medicine Co. Ltd, Lianyungang, Jiangsu, China) orally daily for at least 6 months after surgery.
2.7. Follow-up
After surgery patients were scheduled to have chest and abdominal CT and gynecological ultrasound and echocardiography examination before hospital discharge. Patients were followed up every 6 months after surgery, and at each follow-up appointment received chest and abdominal CT and gynecological ultrasound and echocardiography again. If a new mass was detected or the remnant tumor was enlarged, we considered the tumor recurred.
2.8. Statistical analysis
Data were presented as n and percentage. Where data were not normally distributed, the median (interquartile range, IQR) was presented. We assessed the influence of demographic and clinical characteristics such as age, CPB time, circulatory arrest time on IVL staging and outcomes using Kruskal–Wallis test, Mann–Whitney U test were to compare bleeding, postoperative hospitalization, hospitalization expenses between patients of each stage. Cross table, Chi-square test, and Fisher exact were used to compare lower limb edema, whether the patients were sent to intensive care unit (ICU) after surgery and whether allogeneic blood transfusion was performed between patients of each stage. SPSS 12.0 was used for statistical analysis, and P values under 0.05 were considered to indicate statistical significance.
3. Results
3.1. Patient characteristics
We retrospectively reviewed the medical records of 76 patients that underwent surgery for IVL at the Peking Union Medical College Hospital between November 2002 and January 2015. Patients ranged in age from 24 to 61 years (average age 43.6 ± 8.0 years). All patients had a history of uterine fibroids, and 27 patients (35.5%) had a hysterectomy before this hospitalization (Table 1). Some patients who had previously undergone partial resection of the inferior vena cava tumor without oophorectomy at other hospitals, subsequently experienced recurrence or progression.
Table 1.
Patient characteristics.
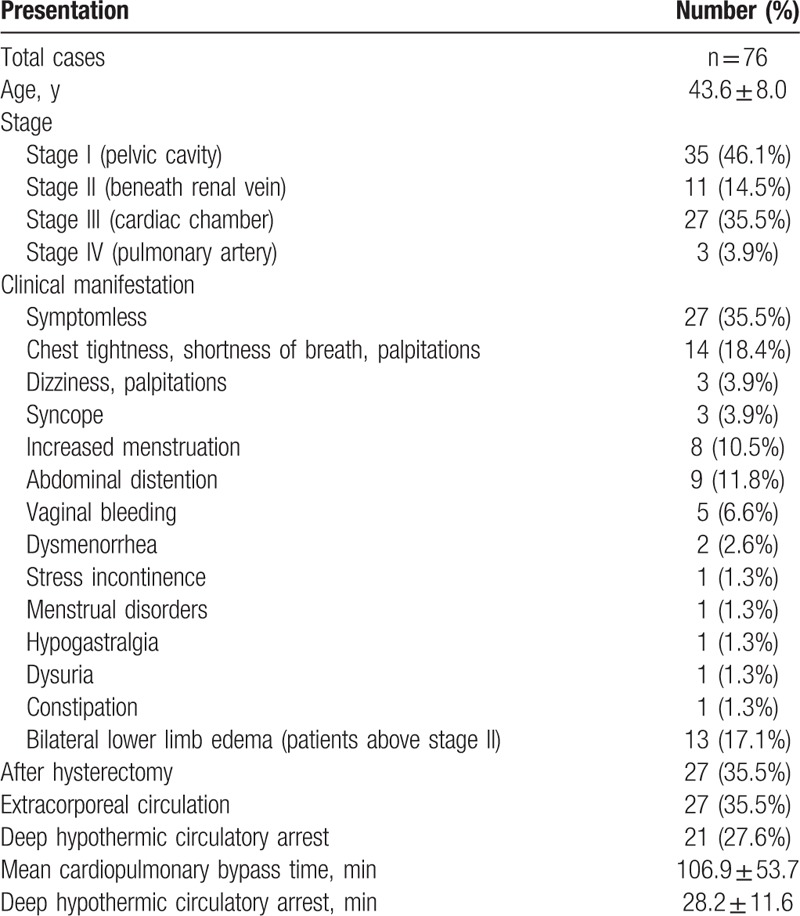
IVL was categorized into 4 stages reflecting presurgery tumor progression (Table 1). A total of 41 patients (53.9%) were categorized as stage II or above (Table 2).
Table 2.
Characteristics of patients with stage II and above disease.

The symptoms of the patients varied (Table 1). Thirteen patients (17.1%) reported bilateral lower limb edema, all of which were categorized as stage II or above. Some patients had multiple symptoms, such as chest tightness, palpitations and shortness of breath, and some patients had single symptoms. Three patients with stage IV IVL had lung metastasis and some patients had double lung metastasis.
3.2. Surgical intervention
Surgical strategies were chosen by a surgeon based on the progression of the leiomyoma and whether the tumor could be resected completely and safely. CPB was applied in 27 cases (35.5%). The average duration of CPB was 106.9 ± 53.7 minutes. Twenty-one patients (27.6%), all with stage III or IV disease, were treated with the deep hypothermic circulatory arrest (DHCA). The mean DHCA time was 28.2 ± 11.6 minutes.
The tumor growth into the ovarian veins in 14.5% (11 cases), the iliac vein in 27.6% (21 cases), and both veins in 11.8% (9 cases). Thirteen cases (17.1%) underwent staging surgery. In 8 cases (10.5%) residual tumor at the pedicle remained after surgery due to local adhesion.
For the 35 patient diagnosed with stage I IVL, a total tumor resection, hysterectomy, and bilateral salpingo-oophorectomy were suggested. However, 6 patients (17.1%) refused bilateral salpingo-oophorectomy, 3 patients (8.6%) refused hysterectomy, 7 patients (20.0%) refused bilateral salpingo-oophorectomy and hysterectomy.
Intraoperative observation revealed the surface of IVL tumors to be smooth, with endothelial coverage and no thrombosis. We found that the texture of IVL is tough, and easy to remove, and rarely causes embolisms. However, after the tumor has extended into the cardiac chamber, it can adopt a spindle shape in the heart cavity which causes congestion of tricuspid valve, obstructing flow. Alternatively the tumor can thicken in the inferior vena cava and block the lumen. However, due to the slow pathological progress and collateral circulation formation, it does not cause significant hemodynamic disorder.
3.3. Follow-up
Patients were followed every 6 months after surgery for a mean of 4.5 ± 2.5 years (range 1–13 years), there was no operative, hospital, or long-term mortality or were lost during follow-up. All the residual tumors were located in the iliac vein, and no further expansion of the tumor was seen. Three patients with pulmonary metastasis were followed up for 3 to 5 years and neither further expansion of the pulmonary nodules nor new metastases were observed (see Table 1). Of the 7 patients that refused salpingo-oophorectomy and hysterectomy, tumors recurred in 4 (11.4%).
3.4. Intraoperative blood loss, postoperative hospital stay, and hospitalization expenses
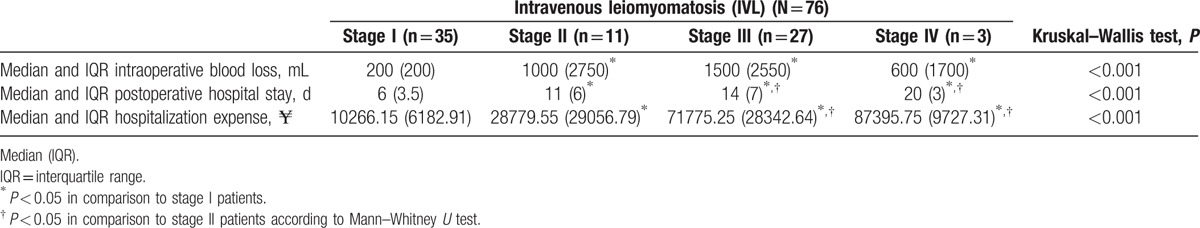
The median intraoperative blood loss, hospitalization time, and hospitalization expenses of patients differed significantly between patients in the 4 stages (all P < 0.001, Table 3). In pairwise comparisons, intraoperative blood loss volume differed significantly between patients at stage I and those at stage II, III, or IV (all P < 0.01), however did not differ significantly between those at stage II and those at stage III or IV, or between at stage III and those in stage IV. Postoperative hospital stay differed significantly between patients at stage I and those at stage II, III, or IV (all P < 0.01), and those at stage II and those at stage III or IV (both P < 0.05), however this did not differ significantly between patients at stage III and those in at stage IV. Hospitalization expenses differed significantly between patients at stage I and those at stage II, III, or IV (all P < 0.01), and those at stage II and those at stage III or IV (P < 0.01 and P < 0.05, respectively), however did not differ significantly between those at stage III and those in stage IV.
Table 3.
Intraoperative blood loss, postoperative hospital stay, hospitalization expenses in different stages.
3.5. Relationship between lower limb edema, ICU stay, and blood transfusion and IVL stage
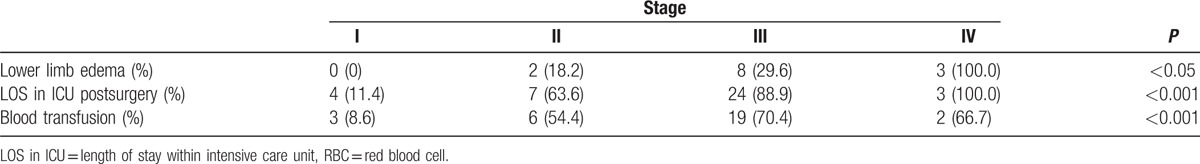
No patients at stage I reported lower limb edema; while 2 patients at stage II had lower extremity edema (18.2%); 8 patients at stage III had lower extremity edema (29.6%); and all 3 patients at stage IV had lower extremity edema (100.0%). While only 4 patients at stage I stayed in ICU postsurgery (11.4%), 7 (63.6%) patients at stage II, 24 (88.9%) patients at stage III, and 3 (100%) patients at stage IV stayed in ICU postsurgery. Three patients at stage I (8.6%), 6 at stage II (54.5%), 19 at stage III (70.4%), and 2 at stage IV (66.7%) received blood transfusion (including red blood cell or plasma or platelet). Chi-square test indicated that these factors differed significantly between the 4 stages of IVL (Table 4, P < 0.05).
Table 4.
Lower limb edema before surgery, duration in ICU postsurgery, and blood transfusion (including RBC or plasma or platelet) in at each stage.
3.6. Relationship between retention of the ovary and uterus and tumor recurrence at stage I patients
Of the 35 stage I patients, 6 (17.1%) refused bilateral salpingo-oophorectomy, 3 (8.6%) refused hysterectomy, 7 (20.0%) refused both bilateral salpingo-oophorectomy and hysterectomy. Patients were followed up every 6 months after surgery for a mean of 4.5 ± 2.5 years (range 1–13 years), and no patients died or were lost to follow-up. Although 4 (11.4%) of the patients who refused both bilateral salpingo-oophorectomy and hysterectomy experienced tumor recurrence, no further vascular invasion was found during follow-up. No recurrence was found in patients at stage II or above stage II during the follow-up.
3.7. Postoperative complications
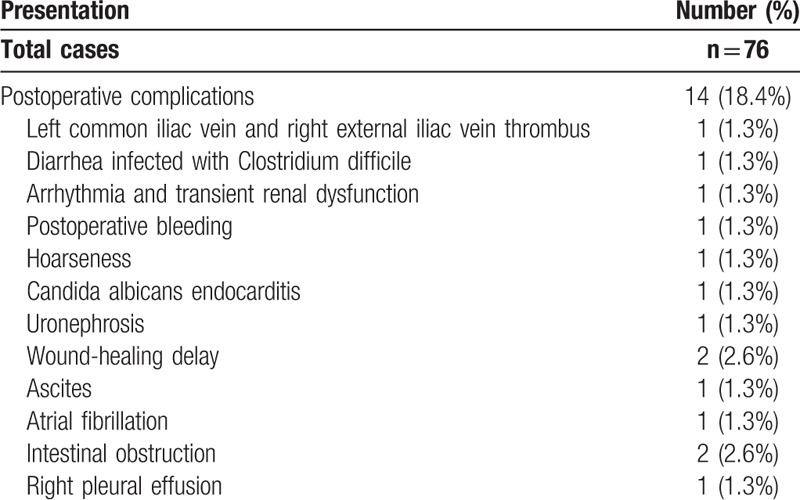
Fourteen patients (18.4%), all scored stage II or above, had postoperative complications within 2 weeks of surgery. Most occurred while the patient was still in the ICU (Table 5). All complications were improved after treatment.
Table 5.
Postoperative complications.
3.8. Pathology
We observed that tumor growth was more advanced and the rate of mucoid degeneration was higher in younger patients. In postmenopausal women tumors were often gray and white and appeared shrunken.
HE staining of tumor embolus specimens indicated bland spindle cells, immunohistochemical staining indicated Desmin and SMA-positive cells, while no CD10, CD31 or CD34 staining was detected, and the specimens had a Ki-67 index of 2% (Fig. 5).
Figure 5.
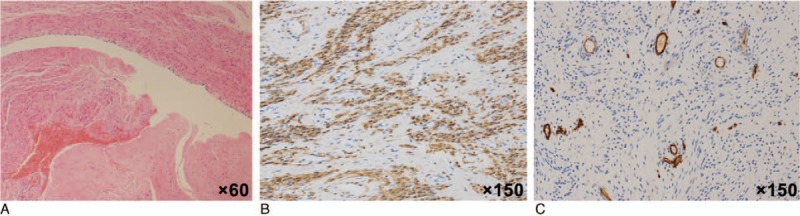
Tumor embolus specimens. HE staining of tumor embolus specimens indicated bland spindle cells (A, ×60), and immunohistochemical staining indicated Desmin (FB) and SMA (+) (B, ×150). The Ki-67 index was 2%, and no CD10, CD31, or CD34+ cells were detected (C, ×150).
4. Discussion
Here we present a relatively large case series of a rare disease, and summarize the treatment and outcome of these 76 patients with IVL. Patients were categorized into 4 stages reflecting presurgery tumor progression. The rate of lower extremity edema, intraoperative bleeding volume, postoperative transfusion, stay in ICU, postoperative hospitalization time, and hospital costs differed significantly between patients rated with different presurgery stages.
Intraoperative observation revealed the surface of IVL tumors to be smooth, with endothelial coverage and no thrombosis, and in patients below stage III little adhesion to the vessel wall was observed. For patients at stage I, the surgery is relatively simple. For patients at stage II, the surgery can be performed through cooperation of gynecology and vascular surgeons, without using CPB, while for patients above stage III, CPB is highly recommended.
Thus blood loss was highest in stage II patients as patients with stage III and IV received CPB DHCA, limiting blood loss. The tumor resection and inferior vena cava repair can be completed in half an hour without significantly increasing the length of surgery, recovery time, or rate of systemic inflammatory response syndrome. However, when tumors had adhered to the inferior vena cava, not only is the tumor is difficult to resect, but it may also cause bleeding that is hard to control, which forces surgeons to change the surgical method, increasing the risk of complications.
Three patients with stage IV IVL had lung metastasis and some patients had bilateral lung metastasis. We did not perform pulmonary lobectomy since it would seriously affect pulmonary function, however the primary tumor, lesions in the inferior vena cava, and both ovaries were removed. After follow-up observation for 3 to 5 years, no expansion or metastasis of pulmonary lesions was observed.
Intraoperative esophageal echocardiography can help to locate the tumor, evaluate the inferior vena cava adhesion, and avoid injury of the inferior vena cava during surgery.[20–22] For IVL patients above stage III, whether tumors in the abdominal cavity and the inferior vena cava should be removed in a single surgery or not remains controversial.[20,23–27] Of our 30 patients at stage III or IV, 13 underwent repeated surgeries to remove the tumor in stages, while most received complete resection in a single surgery. We presume that unless the patient's general condition is poor, or they have a huge vascular tumor, or serious abdominal and pelvic adhesion, complete resection should be performed in 1 stage to reduce injury to adjacent organs and adhesion of tissues, and which can cause increased bleeding during subsequent surgeries. Although concurrent surgery might lead to larger surgical scars, no significant increase in the rate of complications or recovery time was observed. But for large tumors which are strongly adhered to surrounding tissue, staging surgery was recommended.
The incidence of postsurgical complications in stage I patients was 0, while 14 patients with stage II and above IVL experienced complications. Surgery for patients above stage II is more complicated and causes more trauma, however all complications were resolved with treatment.
We found that the texture of IVL is tough, and easy to remove, and rarely causes embolisms. However, after the tumor has extended into the cardiac chamber, it can adopt a spindle shape in the heart cavity which causes congestion of tricuspid valve, obstructing flow. Alternatively the tumor can thicken in the inferior vena cava and block the lumen. However, due to the slow pathological progress and collateral circulation formation, it does not cause significant hemodynamic disorder.
We observed that tumor growth was more advanced and the rate of mucoid degeneration was higher in younger patients. In postmenopausal women tumors were often gray and white and appeared shrunken. These observations suggest that tumor growth may related to estrogen level, as has been previously reported.[16–19] Thus it is important to remove the ovaries in patients whose tumors could not be completely removed. Our study included some patients who had previously undergone partial resection of the inferior vena cava tumor without oophorectomy at other hospitals, and subsequently experienced recurrence or progression. In addition, we included 8 patients whose tumor grow into the iliac vein. In these patients complete tumor resection was not possible due to technical issues, but oophorectomy was performed, and while residual local tumor was observed during follow-up, no obvious increase in tumor growth was observed. Tumor recurrence was detected in 4 of 7 patients graded with stage I IVL that opted for surgery that spared the ovaries and uterus. No recurrence was observed in patients graded stage II or more (in all of which the uterus and ovaries were removed). We conclude that resection of ovaries is essential to prevent recurrence. Endocrine therapy, including diphereline (GnRHa) and letrozole can reduce the estrogen secretion, and thus inhibit tumor growth, promote tumor atrophy, and improve conditions for the surgery.[5]
Whether IVL originates from the smooth muscle of the uterus or the smooth muscle of the venae uterinae remains controversial.[8–13] Immunohistochemical staining indicated Desmin and SMA-positive cells, it means that the tumor thrombus was originated from smooth muscle cell. While CD10, CD31, or CD34 staining was negative, it means that the tumor was not originated from endometrial stroma cell or vascular endothelium cell. Ki-67 index of 2% means that the tumor had a very low proliferation index. We observed that IVL tumors in the blood vessels were smooth and did not form thrombus, histological examination indicated that the tumor thrombus was covered with endothelial cells, thus we suggest that IVL and LM originate from different tissues though they are closely related.
Whilst IVL is a rare disease, between 2002 and 2015 at our hospital IVL was diagnosed in 0.25% of patients with uterine fibroids, suggesting that this hard to diagnose disease may be less rare that suspected in the Chinese population. The increasing rate of IVL diagnosis could be attributed to improvements in medical technology, diagnostic techniques, and the knowledge of this disease. Because IVL is rare, it is difficult to carry out a randomized controlled trial (RCT). To the best of our knowledge our retrospective analysis of 76 cases is so far the largest to be reported. Given that IVL is a rare case, the operation has certain challenge for team lacks of experience. The reason we suggest IVL staging is that it could be applied as a surgical guidance to regulate the surgical strategies, standardize the operation, and enhanced operability, which can improve the safety of surgery and reduce the chance of recurrence of IVL. In the future we hope that cohort studies will be able to better characterize appropriate surgical and pharmaceutical strategies to improve outcomes in IVL.
Acknowledgment
We are grateful for Dr Haiyu Pang's guidance and help on statistics.
Footnotes
Abbreviations: CPB = cardiopulmonary bypass, CT = computerized tomography, DHCA = deep hypothermic circulatory arrest, ICU = intensive care unit, IQR = interquartile range, IVL = intravenous leiomyomatosis, LM = uterine leiomyoma, LOS in ICU = length of stay within intensive care unit, PUMCH = Peking Union Medical College Hospital.
The authors have no conflicts of interest to disclose.
References
- 1.Birch-Hirschfeld FV. Lehrbuch der pathologischen anatomie. 1896; Leipzig, Germany: FCW Vogel, 226–258. [Google Scholar]
- 2.Dürck H. Ueber ein kontinvierlich durch die learned Holvene in das Herz vorwachsendes Fibromyom des Uterus. München Med Wochenschr 1907; 54:1154. [Google Scholar]
- 3.Marshall JF, Morris DS. Intravenous leiomyomatosis of the uterus and pelvis: case report. Ann Surg 1959; 149:126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi IJ, Han MS, Cha MS. A huge case of intravenous leiomyomatosis with intracardiac extension. J Women Med 2010; 3:29–31. [Google Scholar]
- 5.Li B, Chen X, Chu YD, et al. Intracardiac leiomyomatosis: a comprehensive analysis of 194 cases. Interact Cardiovasc Thorac Surg 2013; 17:132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clay TD, Dimitriou J, McNally OM, et al. Intravenous leiomyomatosis with intracardiac extension—a review of diagnosis and management with an illustrative case. Surg Oncol 2013; 22:e44–e52. [DOI] [PubMed] [Google Scholar]
- 7.Gu X, He Y, Li Z, et al. Intracardiac leiomyomatosis: clinical findings and detailed echocardiographic features—a Chinese institutional experience. J Am Soc Echocardiogr 2014; 27:1011–1016. [DOI] [PubMed] [Google Scholar]
- 8.Ordulu Z, Nucci MR, Dal Cin P, et al. Intravenous leiomyomatosis: an unusual intermediate between benign and malignant uterine smooth muscle tumors. Mod Pathol 2016; 29:500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diakomanolis E, Elsheikh A, Sotiropoulou M, et al. Intravenous leiomyomatosis. Arch Gynecol Obstet 2003; 267:256–257. [DOI] [PubMed] [Google Scholar]
- 10.Mandelbaum I, Pauletto FJ, Nasser WK. Resection of a leiomyoma of the inferior vena cava that produced tricuspid valvular obstruction. J Thorac Cardiovasc Surg 1974; 67:561–567. [PubMed] [Google Scholar]
- 11.Ohmori T, Uraga N, Tabei R, et al. Intravenous leiomyomatosis: a case report emphasizing the vascular component. Histopathology 1988; 13:470–472. [DOI] [PubMed] [Google Scholar]
- 12.Marcus SG, Krauss T, Freedberg RS, et al. Pulmonary embolectomy for intravenous uterine leiomyomatosis. Am Heart J 1994; 127:1642–1645. [DOI] [PubMed] [Google Scholar]
- 13.Du J, Zhao X, Guo D, et al. Intravenous leiomyomatosis of the uterus: a clinicopathologic study of 18 cases, with emphasis on early diagnosis and appropriate treatment strategies. Hum Pathol 2011; 42:1240–1246. [DOI] [PubMed] [Google Scholar]
- 14.Gui T, Qian Q, Cao D, et al. Computerized tomography angiography in preoperative assessment of intravenous leiomyomatosis extending to inferior vena cava and heart. BMC Cancer 2015; 16:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doyle MP, Li A, Villanueva CI, et al. Treatment of intravenous leiomyomatosis with cardiac extension following incomplete resection. Int J Vasc Med 2015; 2015:756141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quade BJ, Dal Cin P, Neskey DM, et al. Intravenous leiomyomatosis: molecular and cytogenetic analysis of a case. Mod Pathol 2002; 15:351–356. [DOI] [PubMed] [Google Scholar]
- 17.Kir G, Kir M, Gurbuz A, et al. Estrogen and progesterone expression of vessel walls with intravascular leiomyomatosis; discussion of histogenesis. Eur J Gynaecol Oncol 2004; 25:362–366. [PubMed] [Google Scholar]
- 18.Suginami H, Kaura R, Ochi H, et al. Intravenous leiomyomatosis with cardiac extension: successful surgical management and histopathologic study. Obstet Gynecol 1990; 76:527–529. [PubMed] [Google Scholar]
- 19.Biri A, Korucuoglu U, Zumrutbas N, et al. Intravenous leiomyomatosis treated with aromatase inhibitor therapy. Int J Gynaecol Obstet 2008; 101:299–300. [DOI] [PubMed] [Google Scholar]
- 20.Luciani N, Anselmi A, Glieca F, et al. Diagnostic and surgical issues in emergency presentation of a pelvic leiomyoma in the right heart. Ann Thorac Surg 2009; 87:1589–1592. [DOI] [PubMed] [Google Scholar]
- 21.Harris LM, Karakousis CP. Intravenous leiomyomatosis with cardiac extension: tumor thrombectomy through an abdominal approach. J Vasc Surg 2000; 31:1046–1051. [DOI] [PubMed] [Google Scholar]
- 22.Subramaniam B, Pawlowski J, Gross BA, et al. TEE-guided one-stage excision of intravenous leiomyomatosis with cardiac extension through an abdominal approach. J Cardiothorac Vasc Anesth 2006; 20:94–95. [DOI] [PubMed] [Google Scholar]
- 23.Castelli P, Caronno R, Piffaretti G, et al. Intravenous uterine leiomyomatosis with right heart extension: successful two-stage surgical removal. Ann Vasc Surg 2006; 20:405–407. [DOI] [PubMed] [Google Scholar]
- 24.Liu B, Liu C, Guan H, et al. Intravenous leiomyomatosis with inferior vena cava and heart extension. J Vasc Surg 2009; 50:897–902. [DOI] [PubMed] [Google Scholar]
- 25.Altinok D, Yildiz YT, Tacal T, et al. MRI of intravascular leiomyomatosis extending to the heart. Eur Radiol 2000; 10:871. [DOI] [PubMed] [Google Scholar]
- 26.Lee S, Kim DK, Narm KS, et al. Pulmonary artery embolization of intravenous leiomyomatosis extending into the right atrium. Korean J Thorac Cardiovasc Surg 2011; 44:243–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li B, Li RY, Chen X, et al. One-stage complete removal of intracardiac leiomyomatosis without cardiac arrest. Thorac Cardiovasc Surg 2013; 61:88–90. [DOI] [PubMed] [Google Scholar]


