Abstract
This cross-sectional and exploratory study aimed to compare motor performance and electroencephalographic (EEG) attention levels in children with developmental coordination disorder (DCD) and those with typical development, and determine the relationship between motor performance and the real-time EEG attention level in children with DCD.
Eighty-six children with DCD [DCD: n = 57; DCD and attention deficit hyperactivity disorder (ADHD): n = 29] and 99 children with typical development were recruited. Their motor performance was assessed with the Movement Assessment Battery for Children (MABC) and attention during the tasks of the MABC was evaluated by EEG.
All children with DCD had higher MABC impairment scores and lower EEG attention scores than their peers (P < 0.05). After accounting for age, sex, body mass index, and physical activity level, the attention index remained significantly associated with the MABC total impairment score and explained 14.1% of the variance in children who had DCD but not ADHD (P = 0.009) and 17.5% of the variance in children with both DCD and ADHD (P = 0.007). Children with DCD had poorer motor performance and were less attentive to movements than their peers. Their poor motor performance may be explained by inattention.
Keywords: children, mental concentration, motor difficulty, rehabilitation
1. Introduction
Developmental coordination disorder (DCD) is a rather common movement disorder among primary school-aged children; its prevalence rates range between 6% and 10% worldwide.[1] The motor impairment of children with DCD (e.g., marked delays in achieving motor milestones, movement clumsiness, poor performance in sports, or poor handwriting) inevitably interferes with their activities of daily living and academic achievement. The motor impairment is not due to any general medical condition and does not meet the criteria for a Pervasive Developmental Disorder (e.g., Asperger's disorder or autistic disorder).[1] Due to its negative impact on daily activities and academic performance, this developmental motor disorder—DCD—has received a great deal of attention from researchers and clinicians over the years.
Several studies have investigated the sensorimotor contribution to motor clumsiness in children with DCD,[2–4] but relatively fewer studies have investigated the mental and behavioral contributions to motor performance in this group of children.[5,6] However, children born with DCD have a high prevalence of mental-behavioral disorders such as attention deficits.[7] It is therefore crucial to understand the possible effects of attention on motor performance in children with DCD.
DCD is a heterogeneous condition. About 50% of children with DCD also have a diagnosis of attention deficit hyperactivity disorder (ADHD).[8] Even those without a formal diagnosis of ADHD, they demonstrate more attention problems in daily life than children with typical development.[6] It is known that attention could influence neuromuscular performance such as motor unit recruitment pattern.[9] So, it is possible that attention might also affect motor performance.
In fact, a number of studies have attempted to establish the link between motor abilities and cognitive functions (executive functions including attention) in children with and without developmental disorders.[10–12] For example, Alesi and her research team found that with an improvement in motor skills after physical training, executive functions (attention) improved correspondingly in a group of children with typical development.[10,11] The same motor-cognitive connection was observed in children with atypical development (Down syndrome).[13] This connection could actually be explained by the neural underpinnings of movement and cognition. During the acquisition and execution of motor skills, coactivation of prefrontal cortex (a key structure for performing executive functions), cerebellum, and basal ganglia (key structures for movement control) was observed in children with normal development.[14] As children with DCD have atypical activation of the cerebellum and basal ganglia during movements,[15] it is plausible that their executive functions including attention would also be affected. However, to date, evidence supporting this motor-cognitive relationship in children with DCD is scarce.[5,6,16]
To the best of our knowledge, only 3 research groups have studied the motor-cognitive relationship in children with DCD thus far. Leonard et al[16] reported that both executive functioning and motor performance are inferior in children with DCD compared with typically developing controls. However, they did not measure attention specifically. The other 2 studies have explored the direct link between attention/inattention and motor performance deficits in children with DCD.[5,6] Piek et al[5] invited the DCD participants’ parents to complete the Child Behavior Checklist (CBC) and used the McCarron Assessment of Neuromuscular Development to assess the participants’ motor performance. They concluded that inattention problems may have an influence on the motor performance variability in children with comorbid DCD (and ADHD). A study by Wilmut et al[6] also reported that children with DCD had deficits in the allocation of attention for voluntary actions. By measuring eye-hand movement latencies, the authors concluded that attention disengagement might contribute to visual-motor integration and motion problems in children with DCD. All these evidences collectively suggest that attention may play a significant role in motor performance in children with DCD.
Although both of the aforementioned studies agreed that inattention could be associated with inferior motor performance in children with DCD, these studies measured attention or inattention indirectly using a parent questionnaire or by tracking the child's eye movements.[5,6] A direct measurement of attention or inattention during motor tasks is necessary to confirm the results.
No research group has yet directly measured attention and inattention in children with DCD by capturing brain waves using electroencephalography (EEG). In addition, no study has measured attention levels during motor tasks in children with DCD. Because attention fluctuates from task to task, instantaneous EEG recording during motor tasks is perhaps the best method to establish a link between attention and motor performance.[17]
This study aimed to compare the motor performance and the corresponding EEG attention levels in children with DCD and those with typical development and determine the relationship between motor performance and EEG attention level in children with DCD. It was hypothesized that attention level during motor tasks and motor performance would be significantly different between children with DCD and controls, and that attention level would be significantly associated with motor performance in the DCD population.
2. Methods
2.1. Participants
This was a cross-sectional and exploratory study. The sample size was calculated using G∗Power version 3.1.0 (Franz Faul, University of Kiel, Germany) on the basis of an alpha level of 0.05 (2-tailed) and statistical power of 0.8. According to Dewey et al[7], the effect size was 0.77 for the attention scores. Therefore, the minimum sample size needed to detect a significant difference between the groups in the major outcome measure of “attention” was 28 for each group (objective 1). Regarding the regression analysis (objective 2), a previous study[5] and our pilot trial suggested a large effect size (F2 = 0.56). So, a minimum sample size of 29 children with DCD was required to detect a significant association between the level of attention and motor performance, after accounting for the effects of age, sex, body mass index (BMI), and physical activity level (i.e., a total of 5 predictors).
Children with DCD and children with comorbid DCD and ADHD were recruited from local child assessment centers, primary schools, a nongovernmental organization (Heep Hong Society), parents associations, and physiotherapy clinics by convenience sampling. The inclusion criteria were a diagnosis of DCD based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR)[1]; a gross motor composite score of 42 or lower on the Bruininks-Oseretsky Test of Motor Proficiency[18] and/or a percentile score of less than 5% on the Movement Assessment Battery for Children (MABC)[19]; a total score of less than 46 (5–7 years 11 months of age), less than 55 (8–9 years 11 months of age), or less than 57 (10–15 years of age) on the 2007 version of the DCD questionnaire. This parent-report questionnaire aims to assist in the identification of DCD in children. It consists of 15 items and the parent rated the child's motor performances such as catching and throwing a small ball on a 5-point Likert scale[20]; age between 6 and 10 years; and attendance at a mainstream primary school. The exclusion criteria were a formal diagnosis of an emotional, cognitive, behavioral (comorbid DCD and ADHD, suspected autism spectrum disorder, or dyslexia were acceptable), neurological, or other movement disorder; significant congenital, musculoskeletal, or cardiopulmonary disorders that might affect motor performance; active treatments such as physiotherapy training; demonstration of excessive disruptive behavior; and an inability to follow instructions.
Children with typical development were recruited from local primary schools and the community through advertisement with posters and a website. These children had no history of DCD or ADHD, scored greater than 15% on the MABC,[19] were between 6 and 10 years of age, attended a mainstream primary school, and did not meet any of the exclusion criteria above. Screening was performed by 2 experienced physiotherapists using the above criteria before the actual testing to ensure that all participants were eligible to join the study.
Ethical approval was obtained from the Human Research Ethics Committee of the University of Hong Kong. The study was explained to each participant and his or her parent, and written informed consent was obtained from both the participant and the parent. All data collection was performed by trained research personnel and supervised by physiotherapists and all procedures were conducted in accordance with the Declaration of Helsinki for human experiments.
2.2. Outcome measurements
2.2.1. Demographic information
The data recorded for each participant included age, sex, body weight, height, exercise habits (including the type of physical activity in which the participant had most actively engaged during a typical week within the past year), comorbid conditions (e.g., ADHD and dyslexia), medications, and treatments. The BMI was calculated by dividing weight (kg) by height (m)2. The physical activity level, in metabolic equivalent hours per week, was estimated on the basis of exercise intensity, duration, frequency, and the assigned metabolic equivalent value of the activity according to the Compendium of Energy Expenditures for Youth.[21] In addition, parents were invited to fill in the DCD questionnaire 2007 version during the screening process. The total score was then calculated. Higher scores generally indicate a better parental perception of the participant's motor skills.[20]
2.2.2. Motor performance: Movement Assessment Battery for children
The MABC was used to assess and quantify the motor proficiency of the participants because it is a standardized, well-validated, and reliable instrument for the measurement of motor performance in 4- to 12-year-old children.[19,22] It consists of 8 motor tasks for each of 4 age bands (i.e., 4–6, 7–8, 9–10, and 11–12 years). The 8 tasks are divided into 3 domains: manual dexterity, ball skills, and static and dynamic balance. The manual dexterity tests include a number of fine motor tasks such as placing or shifting pegs, threading lace or beans, and drawing a continuous line following a bicycle or flower trail. The ball skills tests include, for example, bouncing and catching a tennis ball, throwing a bean bag, and rolling a ball into a goal. The balance tests include primarily gross motor tasks such as balancing on one leg, jumping over a cord, hopping in squares, walking with the heels raised, and heel-to-toe walking. The detailed assessment procedures of each task are described in the study by Henderson and Sugden.[19] Each participant was assessed with the appropriate age-band tests. The raw scores for each test item were summed to obtain a total impairment score (TIS). The raw scores of the 3 manual dexterity domain items, the 2 ball skills domain items, and the 3 balance domain items were also summed to obtain subscores for manual dexterity, ball skills, and balance, respectively. A lower score generally represents better motor performance.[19] The TIS and the 3 subscores were used for analysis.
2.2.3. Attention: prefrontal cortex electroencephalographic recording during MABC
Before the MABC began, each participant was asked to completely remove any hair from the forehead, clean the forehead region with an alcohol swab, and remove any earrings. The research assistant then helped the participant to put on a Mindwave Mobile EEG headset (NeuroSky Inc, USA). The EEG activity of the prefrontal cortex was recorded continuously during each task of the MABC.[19] Direct EEG measurement using the commercial NeuroSky EEG device was chosen because it is easy to use (feasible in children) and it provides accurate and instantaneous measurement of attention in children. The derived attention-level index is significantly correlated with the self-reported attention level in young people (r = −0.391; P = 0.022)[23] and duration of gaze fixation in children with DCD (r = 0.648; P = 0.002).[24] Indeed, our recent study showed that this single-channel EEG device can accurately measure attention level in children with DCD. It has good concurrent validity, convergent validity, discrimination validity, and known-groups validity, and the attention-level index was not significantly influenced by eye blinking artifacts.[24]
Each EEG recording was carried out using the same Mindwave Mobile headset. It incorporates a dry active electrode that was placed on the left side of the forehead (position Fp1, according to the International 10–20 System of electrode placement)[25] and a reference electrode that was clipped to the participant's left earlobe.[26] An elastic band and adhesive tape were applied to ensure that the active electrode was in constant firm contact with the Fp1 region during the different activities of the MABC. During recording, the electrical potential from the prefrontal region was supplied directly to the embedded chipset for analog filtering with band-pass (0.5–30 Hz) and notch filters to eliminate electrical noise at 50 Hz. Known noise frequencies, such as those caused by eye blinks and extraocular or muscular activity, were eliminated automatically using proprietary algorithms. The analog data were converted into a digital format in the headset circuit board and transmitted via Bluetooth to the NeuroView data acquisition software (NeuroSky Inc, USA), which was installed on a notebook computer.[26]
The NeuroView data acquisition software can convert raw prefrontal cortex EEG signals to an attention-level index using a preconfigured proportion of EEG alpha (8–12 Hz), beta (12–30 Hz), theta (4–7 Hz), and delta (0.1–3 Hz) activities, which is also part of the proprietary information of the software.[26] The index ranges from 0 to 100, and the scores were generated and recorded for every second of EEG recording (i.e., if the EEG recording lasted for 10 seconds, 10 attention-level indices were generated). This index provides a relative indication of the degree of attention, from very low (0–20), low (21–40), and average (41–60) levels to moderate (61–80) and high (81–100) levels of mental concentration.[26]
The attention-level indices (recorded per second) during each task period of the MABC were averaged to obtain an item attention index. If 2 trials were required for a particular task, the attention-level indices recorded during the second trial were used to calculate the item attention index. The item attention index (0–100) reflects the overall attention level during a particular task of the MABC. The item attention indices of the 3 manual dexterity tasks, the 2 ball skills tasks, and the 3 balance tasks were averaged to obtain the manual dexterity attention index, the ball skills attention index, and the balance attention index, respectively. These 3 domain-based attention indices (0–100) reflect the participants’ overall attention levels during the manual dexterity tasks, the ball skills tasks, and the balance tasks and were used for analysis. In addition, the total attention index (0–100), which is the average value of the 3 domain-based attention indices, was calculated and used for analysis. Higher attention indices generally indicate a higher level of attention.[26] Note that children with DCD and ADHD were tested off medication.
2.3. Statistical analysis
The following statistical analyses were performed using IBM SPSS 20.0 software (IBM, Armonk, NY) and an alpha level of 0.05 (2-tailed) was set. Descriptive statistics (mean and standard deviations) were used to describe all relevant variables. The normality of the data was checked using a Kolmogorov-Smirnov test and histogram. Continuous demographic variables were compared between groups using independent t tests and 1-way analyses of variance as appropriate, and the categorical variable (sex) was compared with a Chi-square test.
In the primary analysis of group data, an independent t test was performed to compare the MABC scores and EEG-derived attention indices between the DCD and control groups. To avoid an inflation of type I error, the level of significance was adjusted according to the number of between-group comparisons made (i.e., Bonferroni correction) in each category of outcome. Because a significant portion of the children in the DCD group had comorbid ADHD and because ADHD symptoms can possibly confound the results,[5] secondary analyses were carried out in which children with both DCD and ADHD (DCD + ADHD), children who had DCD but not ADHD (DCD – ADHD), and children with typical development (control) were analyzed separately. A 1-way analysis of variance was used to compare all of the outcome variables among these 3 groups. Post-hoc Bonferroni tests were used to identify the significant pairs as appropriate.
The degree of association between the MABC TIS and domain subscores and the corresponding EEG-derived attention indices was determined using the Pearson product-moment coefficient of correlation (r) for the DCD + ADHD and DCD – ADHD groups. Multiple regression analyses were then performed to identify the significant determinants of the TIS on the MABC among the children who had DCD with and without ADHD. The selection of the predictors was based on their biological and clinical relevance as well as the results from the bivariate correlations. First, age, sex, BMI, and physical activity level were forced into the regression model using the Enter method. Next, the EEG-derived attention indices that were found to have a significant association (P < 0.05) with the dependent variable in the bivariate correlation analysis and were clinical relevant were entered into the model. A multicollinearity problem was checked using the tolerance approach and the variance inflation factor. Any predictor variables that had a tolerance value of less than 0.1 and a variance inflation factor of greater than 10 were not included in the same regression model.
3. Results
3.1. Participant characteristics
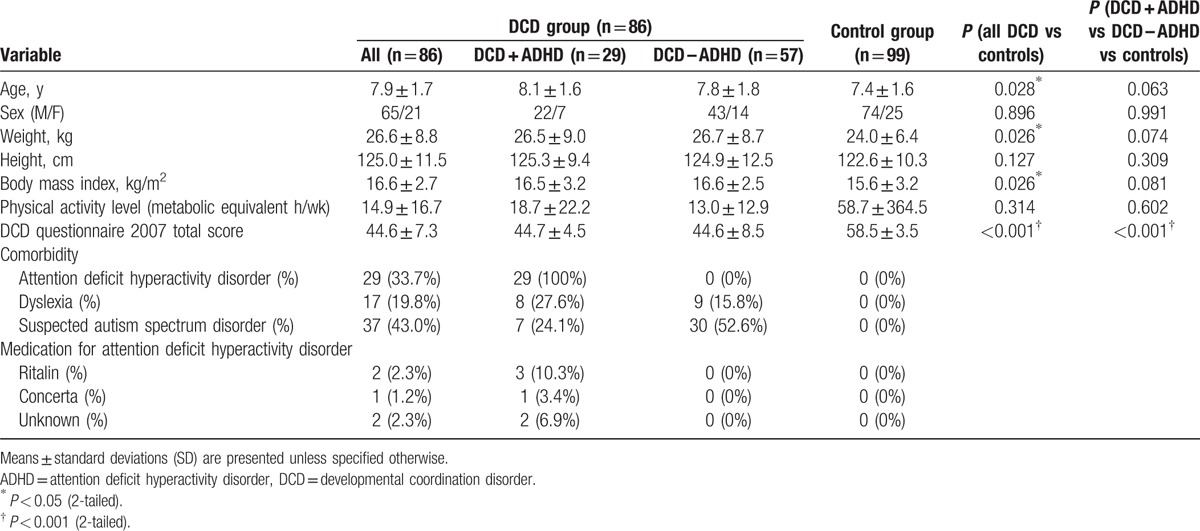
A total of 213 children were screened (DCD: n = 101 and controls: n = 112). Eighty-six children with DCD and 99 children with typical development were eligible and participated in the study. Twenty-nine of the 86 children with DCD (33.7%) also had ADHD. As expected, the children with DCD scored significantly lower on the DCD questionnaire (P < 0.001); they were 0.5 years older (P = 0.028), heavier (P = 0.026), and had higher BMIs (P = 0.026) than the children in the control group. In the ADHD-specific analysis, the 3 groups of children had comparable demographic characteristics (P > 0.05) except that both the DCD + ADHD and DCD – ADHD groups scored lower on the DCD questionnaire than the control group (P < 0.001). Detailed demographic data of the participants are presented in Table 1.
Table 1.
Characteristics of the DCD and control groups.
3.2. Comparison of MABC performances
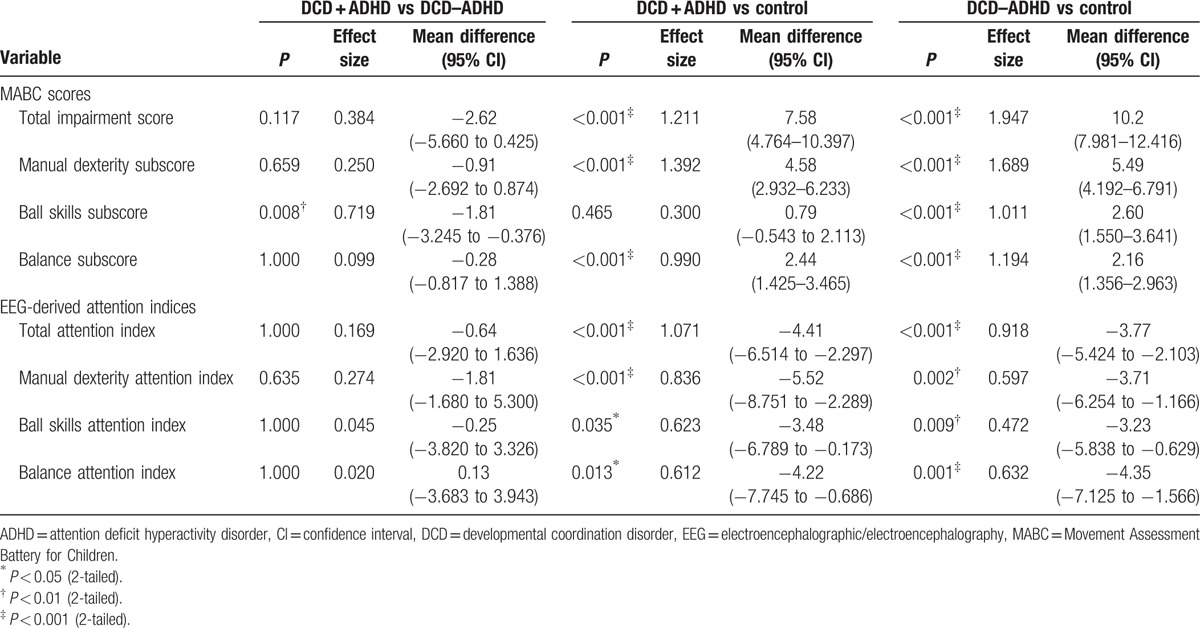
Children with DCD generally had higher TIS and domain subscores than children with typical development (P < 0.001; Table 2). Secondary analyses revealed that the DCD + ADHD and DCD – ADHD groups had higher TIS (P < 0.001), manual dexterity subscores (P < 0.001), and balance subscores (P < 0.001) than the control group. The 2 DCD groups had similar scores in the above items (P > 0.05). For the ball skills subscore, the DCD – ADHD group scored higher than the other 2 groups (P < 0.01), and the DCD + ADHD and control groups had similar scores (P = 0.465) (Table 3).
Table 2.
Comparison of outcome variables between groups.
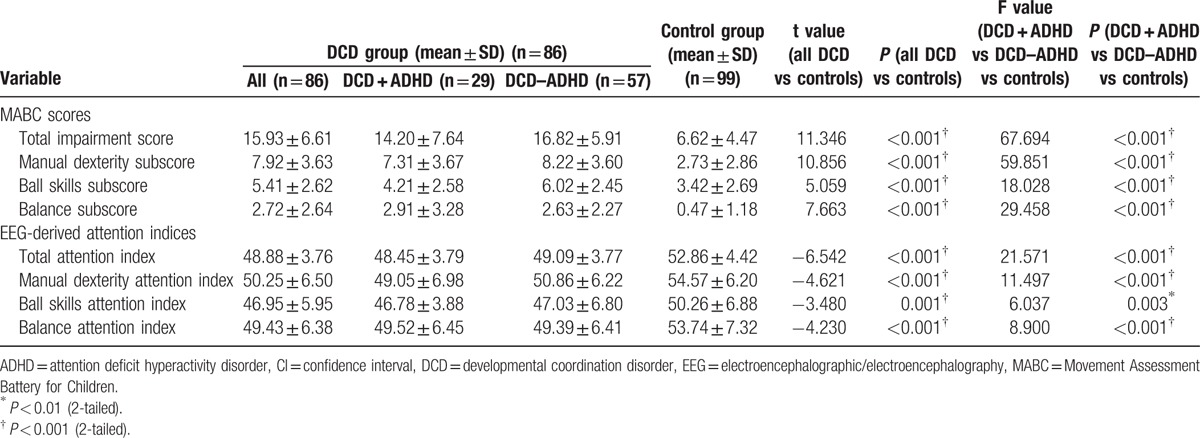
Table 3.
Post-hoc pairwise comparison of outcome variables between the DCD and ADHD, DCD with and without ADHD, and control groups.
3.3. Comparison of attention levels during MABC
The DCD group demonstrated lower attention indices during the MABC than the control group overall (P ≤ 0.001; Table 2). Secondary analyses revealed that the control group had significantly higher attention indices than the 2 DCD groups (P < 0.05) during all of the activities of the MABC. Children who had DCD with and without ADHD had similar total attention indices on the MABC and attention indices for the manual dexterity, ball skills, and balance tasks (P > 0.05) (Table 3). Our results indicated that all children with DCD had lower attention levels during the tasks of the MABC, regardless of whether they had a diagnosis of ADHD.
3.4. Associations between MABC and attention indices
Because children who had DCD with and without ADHD performed differently in some domains of the MABC (Table 3) and because ADHD can possibly confound the results,[5] bivariate correlation analyses were carried out separately in these 2 groups of children with DCD. In children with comorbid DCD and ADHD, the TIS of the MABC was significantly correlated with the total attention index (r = −0.663 and P < 0.001). The domain subscores of the MABC were also significantly correlated with the corresponding attention indices (i.e., manual dexterity subscore of the MABC and attention index, r = −0.500 and P = 0.006; ball skills subscore of the MABC and attention index, r = −0.492 and P = 0.007; and balance subscore of the MABC and attention index, r = −0.618 and P < 0.001).
In children who had DCD but not ADHD, the TIS of the MABC was also significantly correlated with the total attention index (r = −0.424 and P = 0.001). Similarly, the domain subscores of the MABC were significantly correlated with the corresponding attention indices (i.e., manual dexterity subscore of the MABC and attention index, r = −0.481 and P < 0.001; ball skills subscore of the MABC and attention index, r = −0.270 and P = 0.042; and balance subscore of the MABC and attention index, r = −0.453 and P < 0.001).
3.5. Determinants of MABC total impairment score
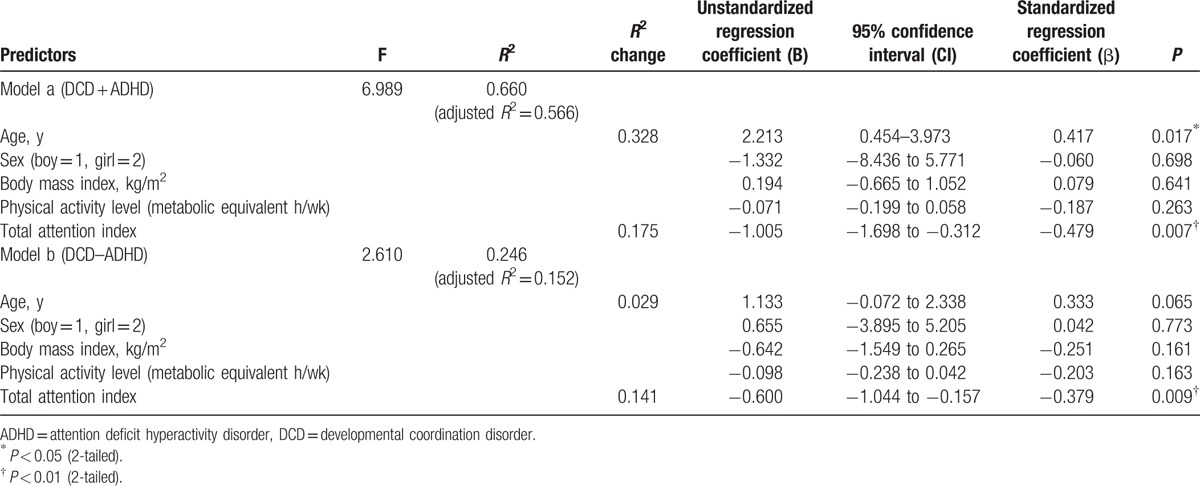
Because the strength of the relationship between the total attention index and the TIS of the MABC (magnitude of r) was different between the 2 DCD groups and because ADHD is a possible confounding factor,[5] separate regression analyses were performed to identify the determinants of the TIS of the MABC in the children with DCD and ADHD (Table 4, model a) and children who had DCD but not ADHD (Table 4, model b). In both models, the total attention index was used to predict the TIS of the MABC after adjusting for age, sex, BMI, and physical activity level. The total attention index remained independently associated with the TIS of the MABC after controlling for these factors (Table 4, model a: Fchange1,18 = 9.276, P = 0.007; Table 4, model b: Fchange1,40 = 7.476, P = 0.009). The total attention index alone explained 17.5% of the variance in the TIS of the MABC in children with DCD and ADHD (Table 4, model a) and 14.1% of the variance in the TIS of the MABC in children who had DCD but not ADHD (Table 4, model b).
Table 4.
Multiple regression analyses for determining MABC total impairment score in children with DCD and ADHD (model a); and children with DCD and without ADHD (model b).
4. Discussion
The results show that children with DCD with and without comorbid ADHD generally had poorer motor performance than the children in the control group. This finding was expected because difficulty with movement is one of the diagnostic criteria of DCD.[1] Possible causes include cerebellar and basal ganglia dysfunctions, disruption of the cerebello-cerebral network and sensory-motor deficits that may adversely affect muscle force output, reaction time,[5] execution of planned actions, visual-spatial cognition,[27] sensory organization of balance control,[2,3] and hence motor performance in functional activities.
To the best of our knowledge, this is the first study to show that children with DCD, regardless of the presence of comorbid ADHD, had lower attention levels during the MABC tasks. Our finding is basically in line with our hypothesis and previous studies that have shown that children with DCD scored higher on the attention subscale of the Child Behavioral Checklist, indicating that they were less attentive in general than their peers with typical development.[7,28] A recent neuroimaging study provided an explanation for this observation. Compared with children with typical development, children with DCD with or without ADHD exhibit alternations of functional connectivity between M1 and several brain regions involved in motor functioning (e.g., insular cortices, caudate, putamen, globus pallidus, and inferior frontal gyrus). Abnormal connectivity between these different brain regions may contribute to the attention difficulties in this group of children.[29] Further neuroimaging studies may analyze children with DCD with and without ADHD separately because these 2 types of disorders may not have the same cause.
This novel study evaluated the real-time attention level of children with DCD during gross and fine motor tasks with EEG technology. The results support our hypothesis and revealed that greater mental focus during the functional tasks of the MABC was positively associated with better motor performances in these children. Our results are not surprising. Early in 2004, Piek et al[5,30] suggested that inattention problems in children with DCD and comorbid ADHD, as measured by the attention subscale of the CBC, may have a negative influence on their gross and fine motor performance. Other studies have also hinted that attention is essential for gross motor performance.[6,31,32] For example, Laufer et al[32] and Cherng et al[31] reported that when children with DCD were distracted with a cognitive task while standing and walking, their postural sway[32] and walking pattern[31] were compromised. Regarding attention and fine motor performance, Wilmut et al[6] found that attention disengagement, as measured by the latencies of eye-hand movements, may contribute to problems of visual-motor integration and thus movement accuracy and reaction time in a look-and-hit task in children with DCD. In addition, a previous neurophysiological study showed that when attention was directed toward a motor task, beta-range cortico-muscular synchronization occurred, which indicates a close connection between attention and motor performance.[33] All of this evidence is, in principle, consistent with our current finding that attention is significantly correlated with motor performance.
Our regression analyses further showed that attention made a greater contribution to motor performance in children with comorbid DCD and ADHD (17.5%) than in children with DCD alone (14.1%). It is also a more important predictor of motor performance in children with DCD and ADHD than in children with DCD alone. Thus, our results imply that improvement in attention is particularly important to the improvement of motor performance in children with comorbid DCD and ADHD.
The link between attention and motor performance has been established, but the underpinning neurological mechanisms are not thoroughly understood. Neuroimaging studies in healthy individuals have shown that multiple brain regions and networks are routinely recruited by attentional tasks. These include the visual, left parietal and frontal (primary motor) cortices, the prefrontal regions, and frontoparietal network.[34,35] In addition, a recent study used functional magnetic resonance imaging to show that subarea 4p (posterior) within the primary motor cortex was distinctly engaged in the control of attended action.[36] Some of these regions that are responsible for attention are also important for motor control (e.g., the primary motor, parietal, and prefrontal cortices).[37,38] Disruption in these areas might result in both mental and movement disorders. Certainly, further neuroimaging and neurophysiological studies in children with DCD are necessary to confirm this postulation.
This study has clinical implications for the assessment and management of children with DCD. First, our results indicate that when the MABC is used to assess motor proficiency in children with DCD, the assessor should encourage the child to mentally attend to the motor tasks so that accurate results can be obtained. Second, our results inform treatment. The current treatment strategy for improvement of motor performance in children with DCD focuses on the remediation of neuromuscular deficits by physical training,[39] and training in attention is not usually factored into the treatment. Our results suggest that a holistic treatment protocol that includes treatment for both attention and neuromuscular deficits should be devised to improve motor performance in this particular group of children.
This study has several limitations. First, this was a cross-sectional exploratory study, and hence causality cannot be established. Second, our DCD participants were not homogenous. We did not perform diagnostic tests to exclude children with specific learning disabilities, emotional, and social problems. The presence of other comorbidities such as autism spectrum disorder, dyslexia,[40] and the different subtypes of ADHD (inattentive, hyperactive/impulsive, and combined) may have different effects on motor performance[41] and thus may confound the results. In addition, children with and without DCD presented different ages, weights, and BMIs. These could be confounding factors for the between-group comparisons of outcomes. Although using a heterogeneous sample may improve the external validity of the study, further studies should better take all the aforementioned confounding factors into account. Third, the EEG-derived attention index reported in this study cannot differentiate the different types of attentional processing such as selective attention and focused attention. Future studies better analyze the EEG frequency bands instead of a single attention index or use an event-related design (e.g., measure EEG signals at baseline vs EEG signals during motor execution) to study the neural mechanisms during motor tasks. Finally, the determinant of motor performance is undoubtedly multifaceted. Our regression models accounted for only 14.1% (DCD–ADHD) and 17.5% (DCD + ADHD) of the variance in motor performance. Future studies are needed to determine the relative contributions of attention and other sensori-motor deficits[2,3] to the motor performance of children with DCD.
5. Conclusion
This study compared the motor performance and attention level in children with and without DCD and determined the relationship between motor performance and attention in the DCD population. Findings support our hypotheses and showed that children with DCD (with and without ADHD) demonstrated deficits in attention and motor control. Their inferior motor performance was associated with their lower level of attention during motor tasks. The results imply that a holistic rehabilitation protocol that includes treatment for both attention and motor deficits (e.g., EEG assisted attention-neuromuscular training or task-specific training)[42] should be devised to enhance overall motor proficiency in this particular group of children.
Acknowledgments
The authors would like to acknowledge the Heep Hong Society, Child Assessment Service (Department of Health, Hong Kong), TWGHs Hok Shan School, SKH St Matthew's Primary School, Aplichau Kaifong Primary School, Hennessy Road Government Primary School (am and pm), Tsung Tsin Primary School and Kindergarten, Watchdog Early Education Centre, and Caritas Nursery School (Tsui Lam) for enabling the recruitment of participants.
Footnotes
Abbreviations: ADHD = attention deficit hyperactivity disorder, BMI = body mass index, CBC = Child Behavior Checklist, DCD = developmental coordination disorder, EEG = electroencephalographic/electroencephalography, MABC = Movement Assessment Battery for Children, TIS = total impairment score.
Funding: This research was partially supported by a grant (27100614) from the Research Grants Council of Hong Kong.
The authors had no conflicts of interest in conducting this research.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed.Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 2.Fong SSM, Lee VYL, Pang MYC. Sensory organization of balance control in children with developmental coordination disorder. Res Dev Disabil 2011; 32:2376–2382. [DOI] [PubMed] [Google Scholar]
- 3.Fong SSM, Tsang WWN, Ng GYF. Altered postural control strategies and sensory organization in children with developmental coordination disorder. Hum Mov Sci 2012; 31:1317–1327. [DOI] [PubMed] [Google Scholar]
- 4.Visser J. Developmental coordination disorder: a review of research on subtypes and comorbidities. Hum Mov Sci 2003; 22:479–493. [DOI] [PubMed] [Google Scholar]
- 5.Piek JP, Dyck MJ, Nieman A, et al. The relationship between motor coordination, executive functioning and attention in school aged children. Arch Clin Neuropsychol 2004; 19:1063–1076. [DOI] [PubMed] [Google Scholar]
- 6.Wilmut K, Brown JH, Wann JP. Attention disengagement in children with developmental coordination disorder. Disabil Rehabil 2007; 29:47–55. [DOI] [PubMed] [Google Scholar]
- 7.Dewey D, Kaplan BJ, Crawford SG, et al. Developmental coordination disorder: associated problems in attention, learning, and psychosocial adjustment. Hum Mov Sci 2002; 21:905–918. [DOI] [PubMed] [Google Scholar]
- 8.Barkley RA. Barkley RA. The nature of ADHD. Attention Deficit Hyperactivity Disorder. A Handbook for Diagnosis and Treatment. New York: Guilford Press; 1990. 3–39. [Google Scholar]
- 9.Lohse KR, Sherwood DE, Healy AF. Neuromuscular effects of shifting the focus of attention in a simple force production task. J Mot Behav 2011; 43:173–184. [DOI] [PubMed] [Google Scholar]
- 10.Alesi M, Bianco A, Luppina G, et al. Improving children's coordinative skills and executive functions: the effects of a football exercise program. Percept Mot Skills 2016; 122:27–46. [DOI] [PubMed] [Google Scholar]
- 11.Alesi M, Bianco A, Padulo J, et al. Motor and cognitive development: the role of karate. Muscles Ligaments Tendons J 2014; 4:114–120. [PMC free article] [PubMed] [Google Scholar]
- 12.Alesi M, Bianco A, Padulo J, et al. Motor and cognitive growth following a football training program. Front Psychol 2015; 6:1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alesi M, Battaglia G, Roccella M, et al. Improvement of gross motor and cognitive abilities by an exercise training program: three case reports. Neuropsychiatr Dis Treat 2014; 10:479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van der Fels IMJ, Wierike SCM, Hartman E, et al. The relationship between motor skills and cognitive skills in 4-16 years old typically developing children: a systematic review. J Sci Med Sport 2015; 18:697–703. [DOI] [PubMed] [Google Scholar]
- 15.Ivry RB. Cerebellar involvement in clumsiness and other developmental disorders. Neural Plast 2003; 10:141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leonard HC, Bernardi M, Hill EL, et al. Executive functioning, motor difficulties, and developmental coordination disorder. Dev Neuropsychol 2015; 40:201–215. [DOI] [PubMed] [Google Scholar]
- 17.Muthukumaraswamy SD, Johnson BW. Primary motor cortex activation during action observation revealed by wavelet analysis of the EEG. Clin Neurophysiol 2004; 115:1760–1766. [DOI] [PubMed] [Google Scholar]
- 18.Bruininks RH. Bruininks–Oseretsky Test of Motor Proficiency: Examiner's Manual. Circle Pines, MN: American Guidance Service; 1978. [Google Scholar]
- 19.Henderson SE, Sugden DA. Movement Assessment Battery for Children Manual. London, UK: The Psychological Corporation Ltd; 1992. [Google Scholar]
- 20.Wilson BN, Crawford SG, Green D, et al. Psychometric properties of the revised developmental coordination disorder questionnaire. Phys Occup Ther Pediatr 2009; 29:182–202. [DOI] [PubMed] [Google Scholar]
- 21.Ridley K, Ainsworth BE, Olds TS. Development of a compendium of energy expenditures for youth. Int J Behav Nutr Phys Act 2008; 5:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crock RV, Horvat M, McCarthy E. Reliability and concurrent validity of the Movement Assessment Battery for Children. Percept Mot Skills 2001; 93:275–280. [DOI] [PubMed] [Google Scholar]
- 23.Rebolledo-Mendez G, Dunwell I, Martinez-Miron EA, et al. Assessing NeuroSky's usability to detect attention levels in an assessment exercise. HCI N Trends 2009; 5610:149–158. [Google Scholar]
- 24.Fong SSM, Tsang WWN, Cheng YTY, et al. Single-channel electroencephalographic recording in children with developmental coordination disorder: validity and influence of eye blink artifacts. J Nov Physiother 2015; 5:1000270. [Google Scholar]
- 25.Rowan AJ. Primer of EEG: With a Mini-atlas. Philadelphia, PA: Butterworth-Heinemann; 2003. [Google Scholar]
- 26.NeuroSky. MindWave Mobile: User Guide. San Jose, USA: NeuroSky Inc; 2012. [Google Scholar]
- 27.Marien P, Wackneier P, De Surgeloose D, et al. Developmental coordination disorder: disruption of the cerebello-cerebral network evidenced by SPECT. Cerebellum 2010; 9:405–410. [DOI] [PubMed] [Google Scholar]
- 28.Tseng MH, Howe TH, Chuang IC, et al. Co-occurrence of problems in activity level, attention, psychosocial adjustment, reading and writing in children with developmental coordination disorder. Int J Rehabil Res 2007; 30:327–332. [DOI] [PubMed] [Google Scholar]
- 29.McLeod KR, Langevin LM, Goodyear BG, et al. Functional connectivity of neural motor networks is disrupted in children with developmental coordination disorder and attention-deficit/hyperactivity disorder. Neuroimage Clin 2014; 4:566–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piek JP, Dyck MJ. Sensory-motor deficits in children with developmental coordination disorder, attention deficit hyperactivity disorder and autistic disorder. Hum Mov Sci 2004; 23:475–488. [DOI] [PubMed] [Google Scholar]
- 31.Cherng RJ, Liang LY, Chen YJ, et al. The effects of a motor and a cognitive concurrent task on walking in children with developmental coordination disorder. Gait Posture 2009; 29:204–207. [DOI] [PubMed] [Google Scholar]
- 32.Laufer Y, Ashkenazi T, Josman N. The effects of a concurrent cognitive task on the postural control of young children with and without developmental coordination disorder. Gait Posture 2008; 27:347–351. [DOI] [PubMed] [Google Scholar]
- 33.Kristeva-Feige R, Fritsch C, Timmer J, et al. Effects of attention and precision of exerted force on beta range EEG-EMG synchronization during a maintained motor contraction task. Clin Neurophysiol 2002; 113:124–131. [DOI] [PubMed] [Google Scholar]
- 34.Johansen-Berg H, Matthews PM. Attention to movement modulates activity in sensori-motor areas, including primary motor cortex. Exp Brain Res 2002; 142:13–24. [DOI] [PubMed] [Google Scholar]
- 35.Pessoa L, Kastner S, Ungerleider LG. Neuroimaging studies of attention: from modulation of sensory processing to top-down control. J Neurosci 2003; 23:3990–3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Binkofski F, Fink GR, Geyer S, et al. Neural activity in human primary motor cortex areas 4a and 4p is modulated differentially by attention to action. J Neurophysiol 2002; 88:514–519. [DOI] [PubMed] [Google Scholar]
- 37.Gabbard C, Bobbio T. The inability to mentally represent action may be associated with performance deficits in children with developmental coordination disorder. Int J Neurosci 2011; 121:113–120. [DOI] [PubMed] [Google Scholar]
- 38.Zwicker JG, Missiuna C, Boyd LA. Neural correlates of developmental coordination disorder: a review of hypotheses. J Child Neurol 2009; 24:1273–1281. [DOI] [PubMed] [Google Scholar]
- 39.Mandich AD, Polatajko HJ, Macnab JJ, et al. Treatment of children with developmental coordination disorder: what is the evidence? Phys Occup Ther Pediatr 2001; 20:51–68. [PubMed] [Google Scholar]
- 40.Chaix Y, Albaret JM, Brassard C, et al. Motor impairment in dyslexia: the influence of attention disorders. Eur J Paediatr Neurol 2007; 11:368–374. [DOI] [PubMed] [Google Scholar]
- 41.Piek JP, Pitcher TM, Hay DA. Motor coordination and kinaesthesis in boys with attention deficit-hyperactivity disorder. Dev Med Child Neurol 1999; 41:159–165. [DOI] [PubMed] [Google Scholar]
- 42.Fong SSM, Guo X, Liu KPY, et al. Task-specific balance training improves the sensory organisation of balance control in children with developmental coordination disorder: a randomised controlled trial. Sci Rep 2016; 6:20945. [DOI] [PMC free article] [PubMed] [Google Scholar]


