Abstract
Patients with cancer often experience a high symptom burden prior to the start of treatment. As disease and treatment-related neurotoxicities appear to be additive, targeting disease related symptoms may attenuate overall symptom burden for cancer patients and improve the tolerability of treatment. It has been hypothesized that disease-related symptoms are a consequence of tumor-induced inflammation. We tested this hypothesis using a syngeneic heterotopic murine model of human papilloma virus (HPV)-related head and neck cancer. This model has the advantage of being mildly aggressive and not causing cachexia and weight loss. We previously showed that this tumor leads to increased IL-6, IL-1β, and TNF-α expression in the liver and increased IL-1β expression in the brain. The current study confirmed these features and demonstrated that the tumor itself exhibits high inflammatory cytokine expression (e.g., IL-6, IL-1β, and TNF-α) compared to healthy tissue. While there is a clear relationship between cytokine levels and behavioral deficits in this model, the behavioral changes are surprisingly mild. Therefore, we sought to confirm the relationship between behavior and inflammation by amplifying the effect using a low dose of lipopolysaccharide (LPS, 0.1 mg/kg). In tumor-bearing mice LPS induced deficits in nest building, tail suspension, and locomotor activity approximately 24h after LPS. However, these mice did not display an exacerbation of LPS-induced weight loss, anorexia, or anhedonia. Further, while heightened serum IL-6 was observed there was minimal priming of liver or brain cytokine expression. Next we sought to inhibit tumor-induced burrowing deficits by reducing inflammation using minocycline. Minocycline (~50 mg/kg/day in drinking water) was able to attenuate tumor-induced inflammation and burrowing deficits. These data provide evidence in favor of an inflammatory-like mechanism for the behavioral alterations associated with tumor growth in a syngeneic murine model of HPV-related head and neck cancer. However, the inflammatory state and behavioral changes induced by this tumor clearly differ from other forms of inflammation-induced sickness behavior.
Keywords: cancer, human papilloma virus, inflammation, cytokines, sickness behavior, fatigue
1. Introduction
Patients with cancer often report high levels of symptom prior to the start of cancer therapy. It is these symptoms that often drive patients to seek medical attention. While disease-related symptom expression is diverse, patients often report centrally mediated symptoms such as fatigue (Jacobsen et al., 1999; Stone et al., 2000). For example, it has been reported that up to 75% of patients with advanced cancer report severe fatigue (Stone et al., 1999). Unfortunately, the mechanisms by which the tumor induces fatigue is still unclear. A better understanding of these mechanisms may allow us to provide more direct symptom relief to advanced cancer patients as well as reduce the additive neurotoxic effects of cancer therapy.
Neuroinflammatory processes are well known to underlie symptoms of sickness and fatigue in inflammatory disorders and immune challenges (Dantzer et al., 2008; Morris et al., 2015). Many tumors induce local inflammation, which promotes tumor progression and dissemination (Hanahan and Weinberg, 2011). Based on these known factors, the primary hypothesis concerning disease-driven symptoms in patients with cancer is that tumor-induced inflammation propagates to the brain where it affects the neural networks responsible for the feelings of sickness and fatigue. While this hypothesis is well-accepted, the supporting data is rather scarce. Specifically, there is limited data indicating that local tumor-induced inflammation can propagate from the tumor to the brain to induce symptoms. Additionally, while there are studies showing a relationship between serum cytokine levels and symptom expression in cancer patients (Bower et al., 2011; Bower et al., 2009; Mills et al., 2005; Wang et al., 2010; Wang et al., 2012), other studies report high symptom expression in the absence of detectable cytokines (Ahlberg et al., 2004; Geinitz et al., 2001; Pusztai et al., 2004; Ray et al., 2011). Given the limitations of clinical research and the difficulty in studying treatment naïve patients, studies with animal models can be informative. Previous research has shown that tumor-bearing laboratory animals have elevated expression of IL-1β mRNA expression within the brain (Braun et al., 2011; Norden et al., 2015; Pyter et al., 2014; Vichaya et al., 2016). Further, experimentally-induced tumors can induce depressive-like behavior, reduce voluntary wheel running, and decrease home cage behavior although many of these effects are difficult to dissociate from the anorexia/cachexia syndrome that develops often in tumor bearing animals (Braun et al., 2011; Norden et al., 2015; Ropelle et al., 2007; Wood et al., 2006). There is also evidence that reducing central inflammation via administration of minocycline may reduce depressive-like behavior but this was observed in the context of possible disease-related alterations in physiology and metabolism (Norden et al., 2015).
In the current study we explored the hypothesis that tumor growth is associated with inflammation within the brain and that this brain inflammation is responsible for disease-related behavioral changes using a syngeneic heterotopic murine model of human papilloma virus (HPV)-related head and neck cancer. We selected this tumor model based on its mild aggressiveness despite its inflammatory nature, its lack of associated cachexia/anorexia, and its association between increased expression of liver and brain inflammatory cytokines and reduced motivated behavior (as evaluated by burrowing, an important species specific behavior in the repertoire of rodents) (Vichaya et al., 2016).
2. Methods
2.1 Experimental Subjects
Male C57BL/6 mice housed in temperature controlled environments on 12 h light-dark cycles (light on from 7:00 to 19:00 h) were used for all experiments. Food and water were available ad libitum. The Institutional Animal Care and Use Committees of the University of Texas MD Anderson Cancer Center and Sanford Research approved all procedures described.
2.2 Tumor Model
These studies used a heterotopic syngeneic murine model of HPV-related head and neck cancer (Spanos et al., 2008; Spanos et al., 2009). Tumors were induced by injecting mice intramuscularly with 1x106 tumor cells into the right hind leg. These tumor cells were derived from C57BL/6 male mouse oropharyngeal epithelial cells transfected with HPV E6/E7 oncogenes and hRAS. Tumor volume was estimated from three mutually orthogonal tumor diameters assessed by Vernier calipers [volume = (π/6(d1*d2*d3)] as previously described (Mason et al., 2005; Suit et al., 1976).
2.3 Drugs and Treatments
Lipopolysaccharide (LPS; serotype 0127:B8) was purchased from Sigma-Aldrich (St. Louis, MO). It was prepared in PBS vehicle and administered by intraperitoneal (IP) injection at a dose of 0.1 mg/kg between day 27–29 post tumor implantation. After treatment mice were monitored at 2, 6, and 24 h.
Minocycline (M-9511; Sigma-Aldrich, St. Louis, MO) was administered orally via the drinking water. It was dissolved in water at a concentration of 0.375 mg/mL to achieve a dose of approximately 50 mg/kg/day. The minocycline was introduced to the home cage, in place of regular drinking water, starting on day 10 after tumor implantation. Due to an observed decrease in drinking immediately following concurrent cisplatin and radiation treatment (CRT) after the first cycle, mice were injected with minocycline the day of and the day after the second and third treatment (tumor day 18, 19, 25, and 26), at which time the mice were provided with regular drinking water. To assess the specificity of the minocycline effect, a second experiment was conducted in which mice were treated with vehicle or minocycline in the context of CRT, previously shown to suppress inflammation (Vichaya et al., 2015). CRT was administered on day 12, 19, and 26 post-tumor implantation in mice used for the follow-up minocycline experiment. The regimen of cisplatin plus localized radiation selected for this study has previously been used to effectively treat MEER tumors (Spanos et al., 2008). Cisplatin (Calbiochem, EMD Millipore, Billerica, MA) was dissolved in a vehicle of sterile saline and administered at a dose of 5.28 mg/kg by IP injection once weekly for 3 weeks. Local radiation was administered immediately following cisplatin treatment at a dose of 8 Gy via a small animal cesium137 irradiator that collimates the parallel opposed radiation beams to a 3 cm field and, thereby, avoids irradiation of surrounding tissue.
2.4 Behavioral Assessments
General health was evaluated by assessing body weight, food consumption, and locomotor activity. To assess locomotor activity mice were placed in new environment (i.e., an empty 18.4 x 29.2 cm translucent shoebox cage) and video recorded from above for 5 min. Distance traveled was quantified using Ethovision XT Software (Noldus Information Technology, Leesberg, VA). Burrowing and nest building were used as measures of motivated behavior. In the burrowing task mice were provided access to as slightly elevated tube constructed from PVC and filled with 230 +/− 1g of standard chow for a 30 minute time period. The amount of food displaced from the tube during this time was recorded (Deacon, 2009). In the nest building task we used a procedure adapted from that described by Negus et al. (2015); a nestlet was divided into 6 pieces that were placed in 6 areas of the cage. The number of pieces consolidated by the mouse were evaluated at 1, 3, 5, 10, 30, and 60 minutes. Finally, depression-like behavior was evaluated by measuring duration of immobility in the tail suspension task. In this task mice were suspended by their tail for 6 min using tape placed approximately 2 cm from the tip of their tail. The time immobile was scored by a trained researcher blinded to experimental conditions.
2.5 Tissue Collection and Analysis
At the end of the study mice were euthanized with CO2, blood was collected by cardiac puncture, mice were perfused intracardially with saline, and tissue was collected. Samples were stored at −80ºC until analysis. Serum was analyzed for IL-6 levels using an IL-6 ELISA (cat number: 431304; BioLegend, San Diego, CA) according to manufacturer’s instructions. Brain (or microdissected brain regions), liver, and tumor samples were evaluated for changes in inflammation-related gene expression. To ensure a representative sample, whole brain and tumor tissue were crushed with a mortar and pestle on liquid nitrogen prior to RNA extraction using the E.Z.N.A. RNA Isolation Kit II (Omega Bio-Tek, Norcross, GA). A High Capacity cDNA Reverse Transcription Kit (Applied Biosystems by Life Technologies, Grand Island, NY) was used, and Real time RT-PCR was carried out using TaqMan gene expression assays. Targets evaluated include IL-6 (cat number: Mm.PT.51.12387735), IL-1β (cat number: Mm.PT.51.17212823), TNF-α (cat number: Mm.PT.51.16622079) and IL-1 receptor antagonist (IL-1RA; cat number: Mm.PT.58.31759585) from Integrated DNA Technologies (Coralville, IA). Itgam/CD11b (cat number: Mm01271259), which is expressed by many activated innate immune cells including macrophages and microglia, was purchased from Life Technologies (Grand Island, NY). GAPDH (cat number: Mm.PT.39.1; Integrated DNA Technologies, Coralville, IA) served as the endogenous housekeeping control. All reactions were performed in duplicate, and the fold difference for each gene was calculated using the 2−Δ ΔCT method.
2.6 Experimental Designs
Experiment 1
To investigate if the tumor is inflammatory and to verify that it is associated with inflammation in the liver and brain we compared control mice (n=6) to mice implanted with a tumor (n=8). All mice were single housed in this experiment. Locomotor activity and burrowing were assessed at baseline and at the end of the study (day 25–27). Mice were terminated on day 28 and tissue was collected.
Experiment 2
To determine if treatment with LPS can unmask a more severe behavioral and inflammatory profile we used a 2 (control vs tumor) x 2 (vehicle vs LPS) experimental design (n=6 mice/group). All mice were single housed in this experiment. Tumor volume and body weight were monitored weekly. On day 27 post-tumor implantation mice were treated with 0.1 mg/kg LPS and behavior was monitored for 24h. Body weight and food consumption were evaluated at baseline, 2, 5, and 24h post LPS. Sucrose preference was evaluated from 6–22h post tumor implantation. From 22–23 hours the nest building task was conducted. Locomotor activity immediately followed by the tail suspension test was evaluated at approximately 24h. Tissue and sera was collected immediately after completion of behavioral testing.
Experiment 3a
To explore the causal role of inflammation in tumor-induced behavioral deficits we administered minocycline or water to tumor-bearing mice (n=10/group). As burrowing has been shown to be a sensitive, reliable assessment of tumor-induced behavioral deficits it served as our primary endpoint. Mice were group housed in this experiment and moved to individual testing chambers for burrowing data collection. Minocycline, at a dose of approximately 50 mg/kg, was administered via the drinking water starting 10 days after tumor-implantation (a time when tumors can be reliably palpated). Tumor volume, body weight, and burrowing were assessed weekly following tumor injection. Mice were terminated on day 28 and tissue and sera were collected.
Experiment 3b
Finally, we sought to test the specificity of the effect of minocycline by treating mice in a paradigm lacking elevations in inflammation. We have previously shown that chemoradiation suppressed tumor-induced inflammation without suppressing behavioral effects (Vichaya et al., 2016). Therefore, we administered minocycline (n=8) or water (n=9) to tumor-bearing mice treated with chemoradiation on day 12, 19, and 26. As in the previous study tumor volume, body weight, and burrowing were assessed weekly following tumor injection.
2.7 Statistical Analyses
The Statistical Package for Social Sciences (SPSS; version 22) was used for analysis of the data. The data are expressed as mean +/− the standard error of the mean (SEM). One- and two-way analysis of variances (ANOVAs) as well as repeated measures ANOVAs were conducted based on experimental design. Post hoc analyses were conducted, as appropriate, to clarify group differences. Differences were considered significant if p ≤ 0.05.
3. Results
3.1 The tumor is inflammatory and is associated with increased inflammation in the liver and brain
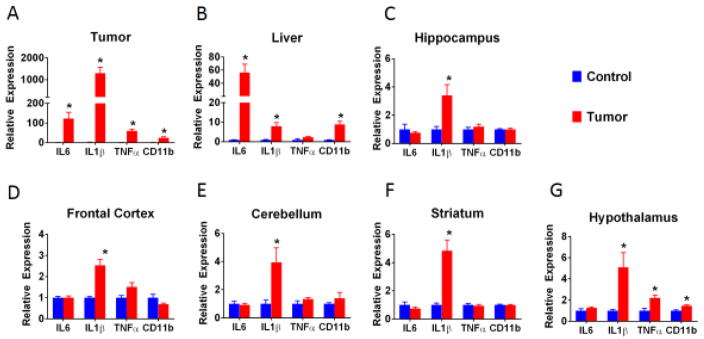
In comparison to healthy control leg tissue, tumor tissue extracted from the leg after 28 days of growth expressed significantly elevated levels of pro-inflammatory cytokine mRNA, including IL-1β, IL-6, and TNF-α, as well as increased levels of CD11b (Figure 1A; all Fs > 14.38; ps < 0.01). In a different cohort of mice cytokine expression levels within the liver and brain regions were evaluated. As was previously described (Vichaya et al., 2016), we observed an elevation in liver pro-inflammatory cytokine expression (Figure 1B). Specifically, IL-6, IL-1β, and CD11b were significantly elevated within the liver of tumor-bearing mice (all Fs>10.65; ps<0.01) and a trend was observed for TNF-α (F(1,13)=4.43, p<0.10). Further, we observed that IL-1β mRNA expression was universally upregulated within all brain regions evaluated (i.e., hippocampus, frontal cortex, cerebellum, hypothalamus, and striatum, all Fs>6.12, ps<0.05) (Figure 1C–G). TNF-α and CD11b mRNA levels were significantly elevated only in the hypothalamus (F(1,13)=9.65 and F(1,13)=11.12, p<0.01) with a trend toward elevated TNF-α levels in the frontal cortex (F(1,13)=4.02, p<0.1). IL-6 was not elevated in any of the evaluated brain regions. Prior to tissue collection we sought to confirm tumor-induced behavioral deficits in these mice. As previously described (Vichaya et al., 2016) we observed a deficit in burrowing in tumor-bearing mice, such that tumor-bearing mice burrowed an average of 125 +/− 15.4 g in comparison to 163 +/− 1.7 g in control mice, F(1,13)=6.68, p<0.05. Further, there was a trend toward a reduction in locomotor activity, from 1207 +/− 77.5 cm in controls to 980 +/− 72.3 cm in tumor-bearing mice, F(1,13)=4.49, p<0.1 (supplemental figure 1).
Fig 1.
Inflammation from the tumor propagates to the liver and brain. Tumor tissue expressed high mRNA levels for IL6, IL1β, and TNFα compared to healthy tissue (A). Within tumor-bearing mice, this local inflammation resulted in increased IL6, IL1β, and CD11b mRNA expression in the liver (B) and increased IL1β mRNA within all brain regions tested (C–G). n=6–8/mice group. * p<0.05
3.2 LPS-induced behavioral changes are enhanced in tumor-bearing mice
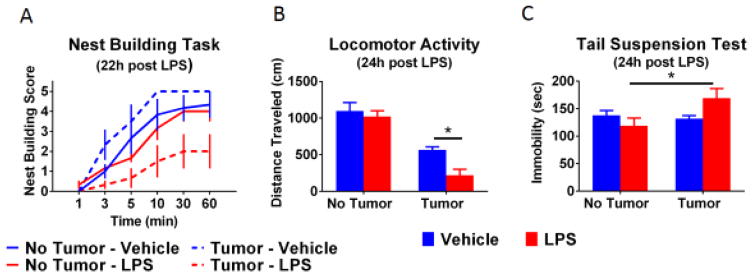
If inflammation is involved in cancer associated symptoms we would anticipate that tumor-bearing mice would show an enhanced behavioral and inflammatory response to an immune challenge. Therefore, control and tumor-bearing mice were challenged with 0.1 mg/kg LPS approximately 4 weeks after tumor implantation. At this time tumors were well established, but below threshold for euthanasia, and body weight was steady (see supplemental figure 2). The dose of LPS selected was sufficient to induce acute reduction in body weight, food consumption, and sucrose preference (see supplemental figure 3). However, the magnitude of these reductions was not influenced by the tumor. Approximately 24 h after LPS treatment nest building, locomotor activity, and immobility in the tail suspension test were assessed (Figure 2). Non-tumor-bearing mice treated with LPS showed no behavioral alterations at this time point. Further, tumor-bearing mice treated with vehicle had levels of nest building behavior and tail suspension immobility equal to that of non-tumor-bearing control mice. However, decreased nest building and increased immobility in the tail suspension test were observed only in tumor-bearing mice who were treated with LPS. Specifically, for nest building, there was a main effect of LPS (F(1,20)=10.91, p<0.01), an LPS x time interaction (F(5,100)=4.55, p<0.001), and a tumor x LPS interaction (F(1,20)=6.29, p<0.05). For the tail suspension test, there was a significant tumor x LPS interaction (F(1,20)=5.25, p<0.05). Further, locomotor activity deficits were exacerbated following LPS treatment in tumor-bearing mice at a time when non-tumor-bearing mice had fully recovered (main effect of tumor, F(1,20)=65.99, p<0.001, and LPS significant, F(1,20)=6.59, p<0.05).
Fig 2.
Behavioral changes are enhanced in tumor-bearing mice following administration of LPS. An LPS-induced suppression in the rate of nest building was only observed in tumor-bearing mice (A). As we anticipated, tumor-bearing mice showed a reduction in locomotor activity (B). In the non-tumor-bearing mice LPS did not exert a significant effect at 24h, however, an LPS-induced suppression was still observed in the tumor-bearing mice. Immediately after the locomotor activity task, mice were tested in the tail suspension test to assess depressive-like behavior (C). Increased immobility was only observed in the tumor-bearing mice treated with LPS. n=6 mice/group. * p<0.05
These behavioral changes were accompanied by an increased expression of some inflammatory markers within the liver and brain. There was a main effect of the tumor and LPS on many of the inflammatory markers evaluated (Figure 3). Further, there was a significant tumor by LPS interaction for several markers. In the liver there was a significant interaction for CD11b (F(1,19)=16.07 p<0.001) and IL1RA (F(1,19)=7.22, p<0.05) mRNA expression (Figure 3A). Within the cerebellum there was a significant interaction for IL-6 mRNA (F(1,20)=4.54, p<0.05) (Figure 3B). Further, there was a significant trend toward increased IL1RA mRNA in the cerebellum (F(1,20)=3.14, p<0.10; Figure 3B) and hippocampus (F(1,20)=3.23, p<0.10, Figure 3D). Finally, increased protein levels of serum IL-6 were observed in tumor bearing mice 24 h after treatment with LPS (significant main effects of tumor, F(1,20)=14.36, p<0.01, and LPS, F(1,20)=13.89, p<0.01, as well as a tumor x LPS interaction, , F(1,20)=13.00, p<0.01; Figure 3E).
Fig 3.
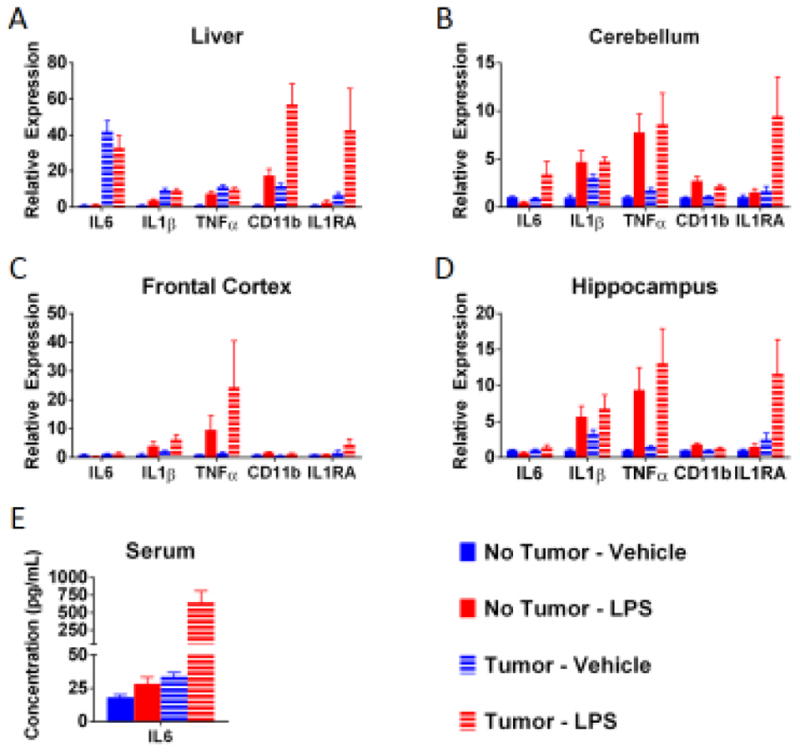
Tumor-bearing mice had increased expression of some inflammatory markers following administration of LPS. Within the liver (A), there was effect of the tumor on IL6, IL1β, TNFα, CD11b, and IL1RA; an effect of LPS for TNFα and CD11b, with a trend for IL1RA. Tumor-bearing mice treated with LPS has the highest levels of CD11b (and a trend toward higher IL1R1). Additionally, there was a significant effect of LPS in the non-tumor-bearing mice on TNFα, but this effect was not observed in the already elevated tumor-bearing mice. A similar non-significant trend was observed for IL-1β. Within the cerebellum (B), there was a trend toward an effect of tumor on IL6 and IL1RA as well as an effect of LPS for IL1β, TNFα, and CD11b (a trend for IL1RA). Further, there were higher levels of IL6 and a trend for IL1RA in tumor-bearing mice treated with LPS. Within the frontal cortex of the brain (C), there was an effect of LPS for IL1β and CD11b as well as a trend for TNFα. In tumor-bearing mice there is a trend toward an increase in IL1RA and a trend toward a suppression of CD11b. Within the hippocampus (D), tumor-bearing mice had increased IL1RA and suppressed CD11b, and LPS induced an increase in IL1β, TNFα, and CD11b mRNA expression (trend for IL1RA). There was also a trend toward a greater elevation in IL1RA following LPS in tumor-bearing mice. Within the serum (E), here was a significant effect of tumor on IL6 protein and an effect of LPS, however the LPS effect was more marked in the tumor-bearing mice. n=6 mice/group. Post hoc analyses were conducted when statistically significant main effects or interactions were noted. Lowercase letters in the figure indicate homogenous groups.
3.3 Minocycline attenuates tumor growth and burrowing deficits
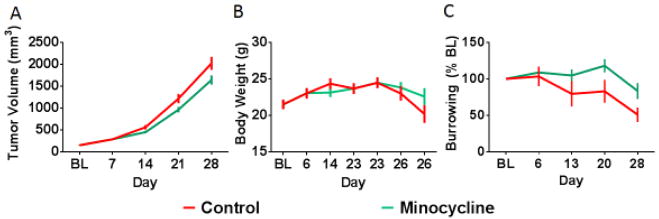
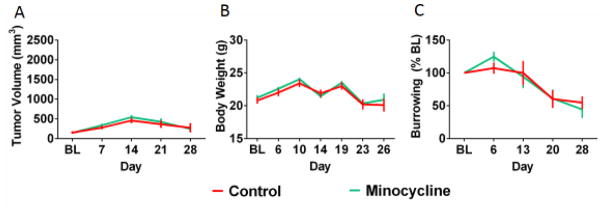
While the tumor only induces a limited set of behavioral changes (e.g., reduced locomotor activity and burrowing), the data above confirm that the inflammatory nature of the tumor has behavioral implications. Therefore, we more directly tested the role of inflammation in tumor-induced burrowing deficits by treating control and tumor-bearing mice with minocycline, a well-known anti-inflammatory drug. We selected a dose already known to decrease peripheral and central inflammation (Henry et al., 2008; Norden et al., 2015; O’Connor et al., 2009). Daily treatment with minocycline, starting 10 days after tumor implantation, mildly reduced tumor growth (Figure 4A; F(4,90)=2.60, p<0.05), had no significant effect on body weight (Figure 4B; F(1,18)=0.13, p>0.05), and significantly attenuated tumor-induced burrowing deficits (F(1,18)=4.52, p<0.05; Figure 4C).
Fig 4.
Tumor growth and burrowing deficits are attenuated by minocycline treatment. Minocycline treatment resulted in a mild reduction in rate of tumor growth reaching significance on day 21 (A). There was no significant effect of minocycline on body weight (B). Minocycline treatment also significantly attenuated tumor-induced burrowing deficits with effects emerging on day 20 and 28 (C). Two mice, one from each group, required early termination due to rapid tumor growth; missing data values (for day 26/28) were replaced with group means to allow for repeated measures analyses. n=10 mice/group. * p<0.05
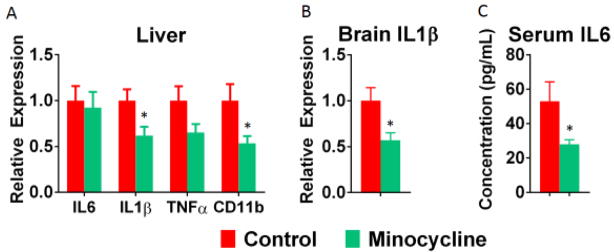
To verify the anti-inflammatory effects of minocycline, inflammatory cytokine expression was evaluated. As anticipated, minocycline treatment attenuated expression of inflammatory markers in the liver, specifically, IL-1β (F(1,17)=5.97, p<0.05) and CD11b (F(1,17)=5.60, p<0.05) (Figure 5A), and reduced IL-1β mRNA expression (F(1,17)=6.90, p<0.05) within the brain (Figure 5B). Further, minocycline treatment reduced serum IL-6 levels (F(1,17)=4.64, p<0.05; Figure 5C).
Figure 5.
Tumor-induced inflammation is attenuated by minocycline treatment. Minocycline treatment significantly reduced IL1β within the liver with a trend toward a reduction in TNFα (A). Further, minocycline resulted in a significant reduction in the expression of CD11b, a marker of activated monocytes, within the liver (likely kupffer cells in this model; Vichaya et al., 2016) (A). Within the brain, minocycline significantly reduced the expression of IL1β mRNA expression (B). Finally, circulating levels of IL6 were significantly reduced following minocycline treatment. n=9 mice/group. * p<0.05
To determine if minocycline would attenuate similar behavioral alterations not associated with inflammation, minocycline was administered to tumor bearing mice given a curative regimen of CRT. We have previously demonstrated that CRT suppresses tumor-induced inflammation in this cancer model without suppressing behavioral deficits (Vichaya et al., 2016). Neither body weight (Figure 6A; F(1,15)=0.30, p>0.05) nor tumor growth (Figure 6B; F(1,15)=0.46, p>0.05) were affected by minocycline. While we recognize that, in the absence of a study specific control group of untreated tumor-bearing mice, we cannot directly test the hypothesis that minocycline does not interfere with the effects of chemoradiation on the tumor, these data strongly support this possibility. As anticipated a reduction in burrowing was observed following the onset of CRT (main effect of time, F(4,60)=22.17, p<0.001) and this effect was not attenuated by minocycline (F(1,15)=1.54, p>0.05, Figure 6C). These findings show that the beneficial effect of minocycline is restricted to the context of inflammation.
Fig 6.
There is no effect of minocycline on tumor growth, body weight, or burrowing behavior in tumor-bearing mice exposed to chemoradiation (CRT). CRT treatment occurred on day 12, 19, and 26 (delineated by arrows in the graphs). As was anticipated, CRT suppressed tumor growth (A); this effect was not modulated by minocycline treatment. Further, there was no effect of minocycline on body weight (B) or on the burrowing deficit induced by Tumor + CRT (C). n=8–9 mice/group.
4. Discussion
The present experiments were carried out to determine if a neuroinflammation hypothesis can explain the behavioral alterations that develop in tumor bearing mice in the murine model of HPV-related head and neck cancer that was selected for this study. The neuroinflammation hypothesis posits the propagation of inflammation from the original site of inflammation to the central nervous system as the mechanism responsible for associated behavioral alterations. In accordance with this hypothesis the molecular signature of inflammation that was present at the level of the tumor was found to also be represented in the liver and to be partly represented in the brain. The molecular signature of inflammation was associated with burrowing and activity deficits reminiscent of sickness. Further, using mild inflammatory challenge we were able to unmask more behavioral and inflammatory deficits within tumor-bearing mice. Finally, this data demonstrate that minocycline, a prototypical anti-inflammatory treatment, was able to attenuate the molecular and behavioral signatures of the tumor.
The beneficial effect of minocycline in untreated tumor-bearing mice aligns with the work of Norden and colleagues (2015), showing that minocycline treatment reduced IL-1β and IL-6 mRNA expression within the brain and reduce depressive-like symptoms in a murine model of colon cancer (colon26 adenocarcinoma). In contrast to the colon cancer model, depressive symptoms were not apparent in the MEER tumor model under basal conditions. However, minocycline attenuated cytokine expression in the liver and brain and improved motived behavior, as assessed by burrowing. Despite this evidence favoring a role for inflammation in tumor-induced behavioral alterations, a careful look at the results of the current investigation reveal that this inflammatory condition induced by the tumor differs from that observed in other chronic inflammatory models. While the liver showed an increase in expression of IL-6, IL-1β, and TNF-α, the brain revealed only a consistent increase in IL-1β mRNA expression with no consistent effect on other inflammatory cytokines. Not only is IL-1β elevated consistently within the brain in the current study, but also across other murine models of cancer (Braun et al., 2011; Norden et al., 2015; Pyter et al., 2014; Vichaya et al., 2016). In the present study this increase in IL-1β mRNA was not associated with increased activation of microglia as CD11b mRNA was only increased in the hypothalamus of tumor-bearing mice and, in some cases it was actually slightly down-regulated. The relatively mild behavioral alterations observed in tumor bearing mice also support a more limited neuroinflammatory response. The behavioral profile associated with the tumor resembles what could be expected in mildly fatigued subjects (i.e., reduced burrowing and locomotor activity) while a traditional chronic inflammatory condition is usually associated with obvious signs of sickness (e.g., anorexia) and depression-like behaviors, none of which were observed in this model. However, treatment of tumor-bearing mice with LPS unmasked additional behavioral deficits that corresponded to mildly enhanced inflammation. This finding aligns with previous data showing an exacerbation of the inflammatory response (i.e., CD11b mRNA in the frontal cortex, IκBα mRNA in the hippocampus, and IDO1 mRNA in the hypothalamus) 4 hours after treatment with LPS in rats with mammary carcinomas (Pyter et al., 2014). While this points to inflammation-mediated behavioral deficits in tumor-bearing mice, the inflammation differs from other forms of inflammation in the sense that the magnitude of acute sickness was not affected and a relatively limited number of inflammatory genes within the liver and brain showed a priming effect.
An important advantage of using this HPV+ head and neck cancer model for the study of tumor induced behavioral deficits is that behavioral alterations in this model develop independent of any obvious cachexia. In addition, our tumor model is non-metastatic (Vermeer et al., 2016; Vichaya et al., 2016). Many of the previous findings of tumor-induced neuroinflammation were obtained in model systems that are highly cachectic (Braun et al., 2011; Norden et al., 2015; Ropelle et al., 2007). Despite the lack of cachexia our HPV+ tumor model has a highly inflammatory nature, which aligns with the highly metabolic profile of the HPV+ tumor (Coppock and Lee, 2016; Coppock et al., 2016), in agreement with what is observed in HPV-related head and neck cancer (Krupar et al., 2014). A final advantage of our current model system is that the tumor responds to a regimen of cisplatin-based chemoradiation that is similar to the treatment regimen employed in patient populations. This allows us to ensure that any potential therapeutic strategies do not interfere with tumor treatment. In the case of the current study we demonstrated that treatment with minocycline did not compromise the ability of chemoradiotherapy to reduce tumor growth. Given that we have previously demonstrated that tumor-bearing mice treated with CRT exhibit behavioral deficits in the absence of inflammation (Vichaya et al., 2015), it is not unexpected that minocycline was unable to reverse the behavioral effects in this context. However, it does contrast with studies showing that minocycline can reverse cancer therapy-induced pain, ototoxicity, and cognitive impairment (Boyette-Davis and Dougherty, 2011; Boyette-Davis et al., 2011; Cata et al., 2008; Di Cesare Mannelli et al., 2014; Du et al., 2011; Lee et al., 2011; Liu et al., 2010; Zhang et al., 2014). These last results can be interpreted a contrario to indicate that pain is unlikely to be a significant factor in the behavioral alterations that develop in tumor-bearing mice treated with CRT.
There is little information concerning the mechanism by which inflammation propagates from the tumor to the rest of the body to induce behavioral alterations. It is generally assumed that cytokine producing cells in the tumor and its microenvironment release cytokines that induce CNS inflammation via the humoral or neural pathway (Dantzer et al., 2012). However, given that serum IL-6 protein levels were relatively low and IL-1β and TNF-α protein could not be detected in circulation (data not shown), other mechanisms have come under consideration. One possibility is that this signal is propagated by tumor-induced microvesicles and/or exosomes. Tumors release high numbers of these microvesicles that can impact immune function (Iero et al., 2007; Taylor and Gercel-Taylor, 2011; Thery et al., 2009). In the case of breast cancer, these exosomes can induce inflammation in distant organs including the liver and brain (Chow et al., 2014). Chow et al. also demonstrated that palmitoylated proteins on the exosome surface contribute to the stimulation of macrophages via toll-like receptor 2 (TLR2). Whether a similar mechanism is involved in the propagation of inflammation from the tumor to distant target organs and, thereby, induces behavioral deficits is currently under investigation in our laboratory. Another unknown is whether tumor-induced brain inflammation is related to direct signal propagation to the brain or it is secondary to the liver induced inflammation. It is known that chronic liver inflammation can induce behavioral alterations. This can occur by afferent signaling via the vagal nerve (Bluthe et al., 1996) or via the interaction between liver induced CCR2+ monocytes and cerebral endothelial cells (D'Mello and Swain, 2014).
In summary, our findings support the hypothesis that a neuroinflammatory-like mechanism could be at the origin of tumor-associated behavioral alterations that resemble fatigue. However, the mechanisms by which tumor-induced inflammation induce brain inflammation appears to be different from the neuroinflammatory process that develops in the context of other models of systemic inflammation. This calls for further investigations on the mechanism of tumor-induced behavioral alterations to more precisely assess the limitations of the current neuroinflammation hypothesis.
Supplementary Material
Highlights.
Tumor inflammation is associated with increased liver and brain inflammation.
The tumor-induced a behavioral phenotype resembling mild fatigue.
Lipopolysaccharide exacerbates tumor-induced behavioral and inflammatory profile.
Minocycline treatment attenuates tumor-induced burrowing deficits and inflammation.
However, the inflammation profile differs from classical inflammatory models.
Acknowledgments
Funding
This work was supported by the National Cancer Institute of the National Institutes of Health [Grants R01 CA193522]. Additional support came from the University of Texas MD Anderson Cancer Center and the National Institutes of Health MD Anderson Cancer Center Support Grant [CA016672]. The content is solely the responsibility of the authors and does not necessarily represent the official view of the funding sources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlberg K, Ekman T, Gaston-Johansson F. Levels of fatigue compared to levels of cytokines and hemoglobin during pelvic radiotherapy: a pilot study. Biological research for nursing. 2004;5:203–210. doi: 10.1177/1099800403259500. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Michaud B, Kelley KW, Dantzer R. Vagotomy attenuates behavioural effects of interleukin-1 injected peripherally but not centrally. Neuroreport. 1996;7:1485–1488. doi: 10.1097/00001756-199606170-00008. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:3517–3522. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Tao ML, Hu W, Belin TR, Sepah S, Cole S, Aziz N. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:5534–5540. doi: 10.1158/1078-0432.CCR-08-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyette-Davis J, Dougherty PM. Protection against oxaliplatin-induced mechanical hyperalgesia and intraepidermal nerve fiber loss by minocycline. Experimental neurology. 2011;229:353–357. doi: 10.1016/j.expneurol.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyette-Davis J, Xin W, Zhang H, Dougherty PM. Intraepidermal nerve fiber loss corresponds to the development of taxol-induced hyperalgesia and can be prevented by treatment with minocycline. Pain. 2011;152:308–313. doi: 10.1016/j.pain.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun TP, Zhu X, Szumowski M, Scott GD, Grossberg AJ, Levasseur PR, Graham K, Khan S, Damaraju S, Colmers WF, Baracos VE, Marks DL. Central nervous system inflammation induces muscle atrophy via activation of the hypothalamic-pituitary-adrenal axis. The Journal of experimental medicine. 2011;208:2449–2463. doi: 10.1084/jem.20111020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cata JP, Weng HR, Dougherty PM. The effects of thalidomide and minocycline on taxol-induced hyperalgesia in rats. Brain research. 2008;1229:100–110. doi: 10.1016/j.brainres.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A, Zhou W, Liu L, Fong MY, Champer J, Van Haute D, Chin AR, Ren X, Gugiu BG, Meng Z, Huang W, Ngo V, Kortylewski M, Wang SE. Macrophage immunomodulation by breast cancer-derived exosomes requires Toll-like receptor 2-mediated activation of NF-κB. Scientific Reports. 2014;4:5750. doi: 10.1038/srep05750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppock JD, Lee JH. mTOR, metabolism, and the immune response in HPV-positive head and neck squamous cell cancer. World Journal of Otorhinolaryngology-Head and Neck Surgery. 2016;2:76–83. doi: 10.1016/j.wjorl.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppock JD, Vermeer PD, Vermeer DW, Lee KM, Miskimins WK, Spanos WC, Lee JH. mTOR inhibition as an adjuvant therapy in a metastatic model of HPV+ HNSCC. Oncotarget. 2016;7:24228–24241. doi: 10.18632/oncotarget.8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Mello C, Swain MG. Liver-brain interactions in inflammatory liver diseases: implications for fatigue and mood disorders. Brain, behavior, and immunity. 2014;35:9–20. doi: 10.1016/j.bbi.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Meagher MW, Cleeland CS. Translational approaches to treatment-induced symptoms in cancer patients. Nature reviews Clinical oncology. 2012;9:414–426. doi: 10.1038/nrclinonc.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon RM. Burrowing: a sensitive behavioural assay, tested in five species of laboratory rodents. Behavioural brain research. 2009;200:128–133. doi: 10.1016/j.bbr.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Di Cesare Mannelli L, Pacini A, Micheli L, Tani A, Zanardelli M, Ghelardini C. Glial role in oxaliplatin-induced neuropathic pain. Experimental neurology. 2014;261:22–33. doi: 10.1016/j.expneurol.2014.06.016. [DOI] [PubMed] [Google Scholar]
- Du B, Zhang Y, Tang Y, Wang P. Minocycline attenuates ototoxicity and enhances antitumor activity of cisplatin treatment in vitro. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2011;144:719–725. doi: 10.1177/0194599810395090. [DOI] [PubMed] [Google Scholar]
- Geinitz H, Zimmermann FB, Stoll P, Thamm R, Kaffenberger W, Ansorg K, Keller M, Busch R, van Beuningen D, Molls M. Fatigue, serum cytokine levels, and blood cell counts during radiotherapy of patients with breast cancer. International journal of radiation oncology, biology, physics. 2001;51:691–698. doi: 10.1016/s0360-3016(01)01657-1. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. Journal of Neuroinflammation. 2008;5:1–14. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, Rivoltini L. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 2007;15:80–88. doi: 10.1038/sj.cdd.4402237. [DOI] [PubMed] [Google Scholar]
- Jacobsen PB, Hann DM, Azzarello LM, Horton J, Balducci L, Lyman GH. Fatigue in women receiving adjuvant chemotherapy for breast cancer: characteristics, course, and correlates. Journal of pain and symptom management. 1999;18:233–242. doi: 10.1016/s0885-3924(99)00082-2. [DOI] [PubMed] [Google Scholar]
- Krupar R, Robold K, Gaag D, Spanier G, Kreutz M, Renner K, Hellerbrand C, Hofstaedter F, Bosserhoff AK. Immunologic and metabolic characteristics of HPV-negative and HPV-positive head and neck squamous cell carcinomas are strikingly different. Virchows Archiv : an international journal of pathology. 2014;465:299–312. doi: 10.1007/s00428-014-1630-6. [DOI] [PubMed] [Google Scholar]
- Lee CK, Shin JI, Cho YS. Protective Effect of Minocycline Against Cisplatin-induced Ototoxicity. Clinical and experimental otorhinolaryngology. 2011;4:77–82. doi: 10.3342/ceo.2011.4.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Lu N, Cui Y, Yang T, Zhao ZQ, Xin WJ, Liu XG. Prevention of paclitaxel-induced allodynia by minocycline: Effect on loss of peripheral nerve fibers and infiltration of macrophages in rats. Molecular pain. 2010;6:76. doi: 10.1186/1744-8069-6-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason KA, Ariga H, Neal R, Valdecanas D, Hunter N, Krieg AM, Whisnant JK, Milas L. Targeting toll-like receptor 9 with CpG oligodeoxynucleotides enhances tumor response to fractionated radiotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:361–369. [PubMed] [Google Scholar]
- Mills PJ, Parker B, Dimsdale JE, Sadler GR, Ancoli-Israel S. The relationship between fatigue and quality of life and inflammation during anthracycline-based chemotherapy in breast cancer. Biol Psychol. 2005;69:85–96. doi: 10.1016/j.biopsycho.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Morris G, Berk M, Walder K, Maes M. Central pathways causing fatigue in neuro-inflammatory and autoimmune illnesses. BMC medicine. 2015;13:28. doi: 10.1186/s12916-014-0259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Neddenriep B, Altarifi AA, Carroll FI, Leitl MD, Miller LL. Effects of Ketoprofen, Morphine, and Kappa Opioids On Pain-Related Depression of Nesting in Mice. Pain. 2015;156:1153–1160. doi: 10.1097/j.pain.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Bicer S, Clark Y, Jing R, Henry CJ, Wold LE, Reiser PJ, Godbout JP, McCarthy DO. Tumor growth increases neuroinflammation, fatigue and depressive-like behavior prior to alterations in muscle function. Brain, behavior, and immunity. 2015;43:76–85. doi: 10.1016/j.bbi.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Molecular psychiatry. 2009;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusztai L, Mendoza TR, Reuben JM, Martinez MM, Willey JS, Lara J, Syed A, Fritsche HA, Bruera E, Booser D, Valero V, Arun B, Ibrahim N, Rivera E, Royce M, Cleeland CS, Hortobagyi GN. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine. 2004;25:94–102. doi: 10.1016/j.cyto.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Pyter LM, El Mouatassim Bih S, Sattar H, Prendergast BJ. Peripheral tumors alter neuroinflammatory responses to lipopolysaccharide in female rats. Brain research. 2014;1552:55–63. doi: 10.1016/j.brainres.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray MA, Trammell RA, Verhulst S, Ran S, Toth LA. Development of a mouse model for assessing fatigue during chemotherapy. Comparative medicine. 2011;61:119–130. [PMC free article] [PubMed] [Google Scholar]
- Ropelle ER, Pauli JR, Zecchin KG, Ueno M, de Souza CT, Morari J, Faria MC, Velloso LA, Saad MJ, Carvalheira JB. A central role for neuronal adenosine 5'-monophosphate-activated protein kinase in cancer-induced anorexia. Endocrinology. 2007;148:5220–5229. doi: 10.1210/en.2007-0381. [DOI] [PubMed] [Google Scholar]
- Spanos WC, Hoover A, Harris GF, Wu S, Strand GL, Anderson ME, Klingelhutz AJ, Hendriks W, Bossler AD, Lee JH. The PDZ binding motif of human papillomavirus type 16 E6 induces PTPN13 loss, which allows anchorage-independent growth and synergizes with ras for invasive growth. Journal of virology. 2008;82:2493–2500. doi: 10.1128/JVI.02188-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanos WC, Nowicki P, Lee DW, Hoover A, Hostager B, Gupta A, Anderson ME, Lee JH. Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Archives of otolaryngology--head & neck surgery. 2009;135:1137–1146. doi: 10.1001/archoto.2009.159. [DOI] [PubMed] [Google Scholar]
- Stone P, Hardy J, Broadley K, Tookman AJ, Kurowska A, A'Hern R. Fatigue in advanced cancer: a prospective controlled cross-sectional study. British journal of cancer. 1999;79:1479–1486. doi: 10.1038/sj.bjc.6690236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone P, Hardy J, Huddart R, A'Hern R, Richards M. Fatigue in patients with prostate cancer receiving hormone therapy. European journal of cancer. 2000;36:1134–1141. doi: 10.1016/s0959-8049(00)00084-8. [DOI] [PubMed] [Google Scholar]
- Suit HD, Sedlacek R, Wagner M, Orsi L, Silobrcic V, Rothman KJ. Effect of Corynebacterium parvum on the response to irradiation of a C3H fibrosarcoma. Cancer research. 1976;36:1305–1314. [PubMed] [Google Scholar]
- Taylor DD, Gercel-Taylor C. Exosomes/microvesicles: mediators of cancer-associated immunosuppressive microenvironments. Seminars in immunopathology. 2011;33:441–454. doi: 10.1007/s00281-010-0234-8. [DOI] [PubMed] [Google Scholar]
- Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- Vermeer DW, Coppock JD, Zeng E, Lee KM, Spanos WC, Onken MD, Uppaluri R, Lee JH, Vermeer PD. Metastatic model of HPV+ oropharyngeal squamous cell carcinoma demonstrates heterogeneity in tumor metastasis. Oncotarget. 2016;7:24194–24207. doi: 10.18632/oncotarget.8254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichaya EG, Chiu GS, Krukowski K, Lacourt TE, Kavelaars A, Dantzer R, Heijnen CJ, Walker AK. Mechanisms of chemotherapy-induced behavioral toxicities. Frontiers in neuroscience. 2015;9:131. doi: 10.3389/fnins.2015.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichaya EG, Molkentine JM, Vermeer DW, Walker AK, Feng R, Holder G, Luu K, Mason RM, Saligan L, Heijnen CJ, Kavelaars A, Mason KA, Lee JH, Dantzer R. Sickness behavior induced by cisplatin chemotherapy and radiotherapy in a murine head and neck cancer model is associated with altered mitochondrial gene expression. Behavioural brain research. 2016;297:241–250. doi: 10.1016/j.bbr.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XS, Shi Q, Williams LA, Mao L, Cleeland CS, Komaki RR, Mobley GM, Liao Z. Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain, behavior, and immunity. 2010;24:968–974. doi: 10.1016/j.bbi.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XS, Williams LA, Krishnan S, Liao Z, Liu P, Mao L, Shi Q, Mobley GM, Woodruff JF, Cleeland CS. Serum sTNF-R1, IL-6, and the development of fatigue in patients with gastrointestinal cancer undergoing chemoradiation therapy. Brain, behavior, and immunity. 2012;26:699–705. doi: 10.1016/j.bbi.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood LJ, Nail LM, Perrin NA, Elsea CR, Fischer A, Druker BJ. The cancer chemotherapy drug etoposide (VP-16) induces proinflammatory cytokine production and sickness behavior-like symptoms in a mouse model of cancer chemotherapy-related symptoms. Biological research for nursing. 2006;8:157–169. doi: 10.1177/1099800406290932. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li K, Sun R, Zhang Y, Ji J, Huang P, Yang H, Tian Y. Minocycline ameliorates cognitive impairment induced by whole-brain irradiation: an animal study. Radiation oncology (London, England) 2014;9:281. doi: 10.1186/s13014-014-0281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.