Abstract
Patients with high risk myelodysplastic syndromes (MDS) and acute myelogenous leukemia (AML) are commonly older with multiple co-morbidities, rendering them unsuitable for intensive induction chemotherapy or transplantation. We report preliminary cellular immune profiling of four cases receiving sequential clofarabine and lenalidomide for high risk MDS and AML in a phase I study. Our results highlight the potential of immune profiling for monitoring immune-modifying agents in high risk MDS and AML.
Keywords: Clofarabine, Lenalidomide, Immune profiling
1. Introduction
High risk MDS and AML are heterogeneous myeloid malignancies and usually have a very poor prognosis. Advanced age and co-morbidities often make the patients unsuitable candidates for intensive chemotherapy or allogeneic stem cell transplantation (allo-SCT) [1]. New less toxic treatments that improve response rates and extend survival are needed for such patients. In this phase I study, we sought to determine safety and efficacy of sequential therapy with low-dose clofarabine and lenalidomide in high risk MDS and AML. Both clofarabine lenalidomide have partial efficacy as single agents for MDS and AML [2], [3]. Although the immunomodulatory effects of lenalidomide are well described [4], [5], little is known about the effect of clofarabine on cellular immunity. We hypothesized that the lymphocyte depleting effect of clofarabine would create a favorable immunological microenvironment for subsequent lenalidomide therapy promoting T cell and NK cell reconstitution. Here we report cellular immune profiles of the four cases receiving sequential clofarabine and lenalidomide for high risk MDS and AML.
2. Materials and methods
The study was designed as an open label, single institution phase I study at the National Heart, Lung, Blood Institute, National Institutes of Health and was approved by Institutional Review Board (NCT01629082). All patients signed an informed consent prior to enrollment and the study was conducted in compliance with the Declaration of Helsinki. The eligibility criteria included patients with a diagnosis of high risk MDS, CMML, and AML who were not candidates for standard intensive chemotherapy or allo-SCT and had failed at least one prior therapy. Subjects received a single course of intravenous low-dose clofarabine 5 mg/m2/day for five days. At 28 days after induction therapy, oral lenalidomide therapy was initiated with dose escalation from 25 mg to 50 mg daily for 21 days of 28 days for up to 12 cycles. Response assessment was performed according to International Working Group (IWG) response criteria [6]. Detailed study design was described in Supplementary material. Flow cytometric analysis was performed to characterize T cell and natural killer (NK) cell subsets, with functional markers of T cell exhaustion and activating and inhibitory NK cell receptors. Antibodies used in the panel are listed in Supplementary Table 1. Relative changes in RNA expressions of various genes related to cancer immunology were evaluated by custom made 384 well PrimePCR™ Assay Panels for Real-Time PCR (Bio-Rad Laboratories, Hercules, CA, USA).
3. Results
Table 1.
Clinical and Hematological Characteristics of study population.
| Subject ID | UPN1 | UPN2 | UPN3 | UPN4 |
|---|---|---|---|---|
| Age (years), gender | 79, male | 70, male | 60, female | 64, male |
| Disease | RAEB-I IPSS intermediate |
RAEB-II IPSS high risk |
AML with multilineage dysplasia | AML with multilineage dysplasia |
| Cytogenetic abnormality | del(12)(p11.2p13), +8 |
-Y | normal | −7, inv (9)(p11q13), |
| Prior treatment | Azacitidine, Decitabine | Azacitadine, Revlimid (10 mg) growth factors | Azacitadine, hydroxyurea | Azacitadine, HIDAC MEC FLAG Auto BMT |
| Response to clofarabine | Stable Disease | Stable Disease | Partial Response | Refractory Disease |
| Off treatment reason | Disease progression | Disease progression | DLT-liver | Disease progression |
Abbreviations: RAEB, refractory anemia with excess blasts; AML, acute myeloid leukemia; DLT, dose limiting toxicity; HIDAC, High dose cytarabine; MEC, mitoxantrone, etoposide, cytarabine; FLAG, fludarabine, high dose cytarabine, G-CSF; IPSS, International Prognostic Scoring System; WHO, World Health Organization
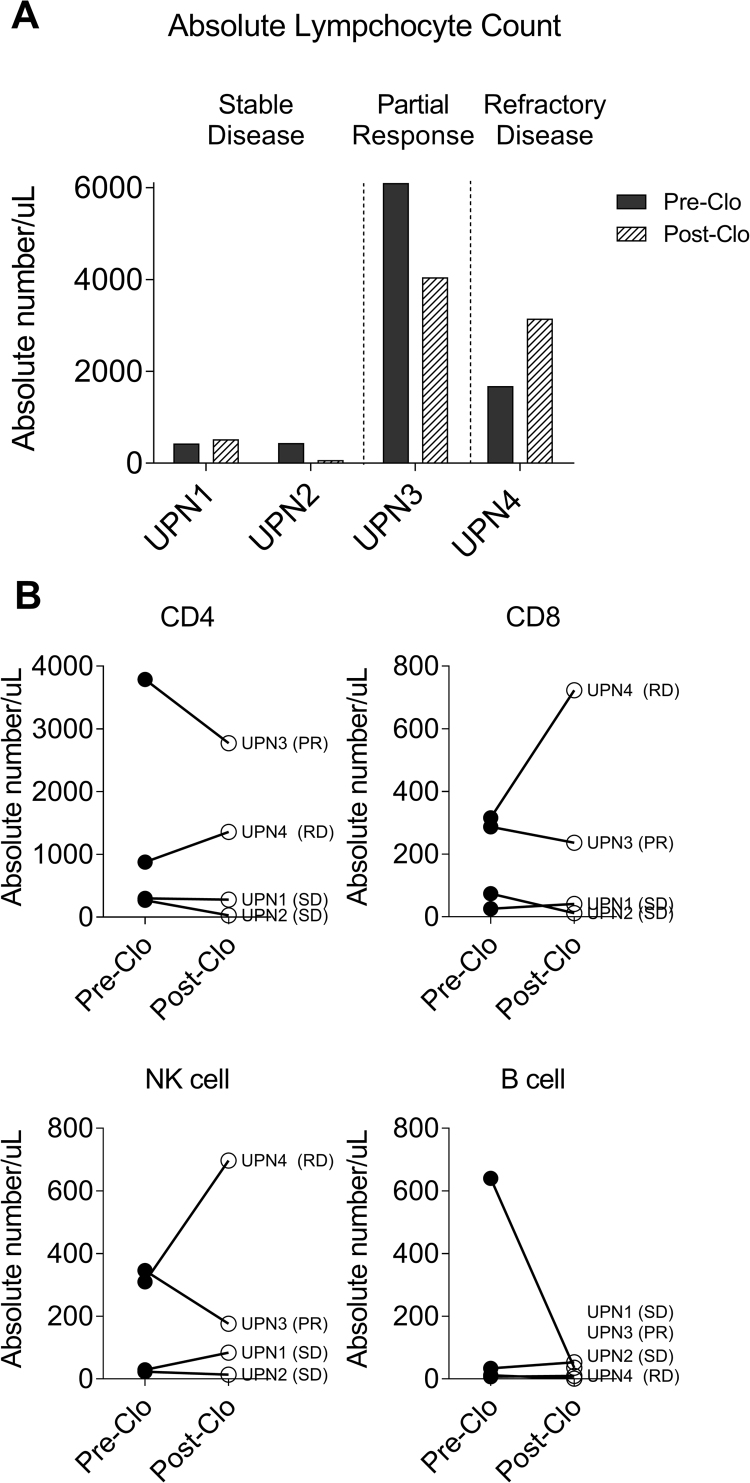
Fig. 1.
Lympho-depleting effect of clofarabine. A. PBMC samples (n=4), were collected pre clofarabine(pre-clo) and 28–42 days post clofarabine(post clo). Analysis of post clofarabine samples showed persistently low (UPN1 and UPN2, stable disease) and decreased (UPN3, partial response) absolute lymphocyte counts. In contrast absolute lymphocyte count was increased in UPN4 (refractory disease). B. All lineages of lymphocytes, CD4 T cells, CD8 T cells, NK cells and B cells were decreased in subjects with stable disease (SD) and partial responder (PR),however CD4 T cells, CD8 T cells, and NK cells were increased in subject with resistant disease (RD).
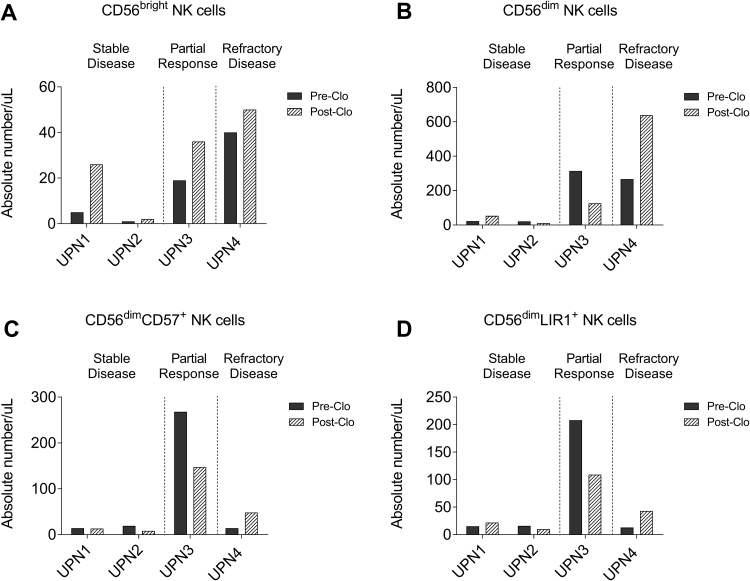
Fig. 2.
Restoration of NK cell repertoires after clofarbine treatment. A.CD56brightNK cells were increased in all subjects. B. CD56dimNK cells were unchanged or decreased in subjects with stable disease and partial responder. In contrast, CD56dimNK cells were increased in subject with refractory disease. C. CD56dimCD57+NK cells were increased in only subject with refractory disease (UPN4). D. CD56dimLIR1+NK cells were increased in only subject with refractory disease (UPN4).
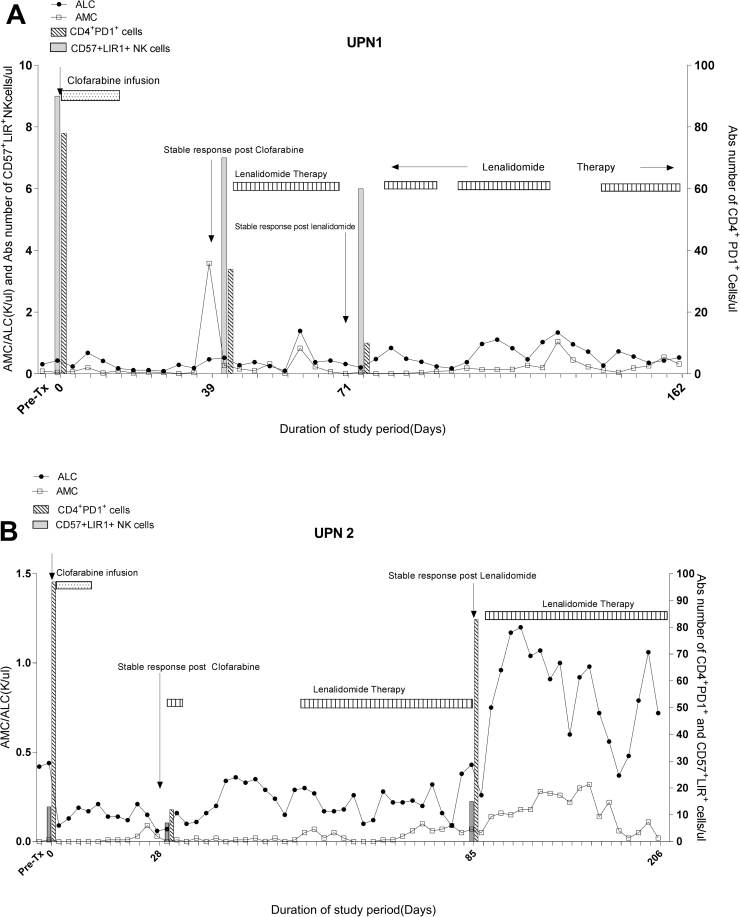
Fig. 3.
Trend in absolute lymphocyte count (ALC), absolute monocyte count (AMC), PD1+CD4 T cells, and CD57+LIR+NK cells through the study period. A. Peripheral blood counts in UPN1 showed lympho-depletion post clofarabine with stable recovery of lymphocytes and monocyte counts during lenalidomide therapy. In addition, steady decline in absolute number of PD1+CD T cells was found through the lenalidomide therapy. B. In UPN2, lenalidomide therapy was delayed due to neutropenia. There was a reciprocal rise in absolute number of PD1+CD4 T cells after lenalidomide therapy.
4. Discussion
In this pilot study, we report that sequential treatment of low dose clofarabine and lenalidomide is safe and feasible for elderly patients with high risk MDS and AML. Only one subject developed dose limiting toxicity (liver dysfunction) from lenalidomide consolidation therapy. Pre-treatment samples uniformly demonstrated high expression of the exhaustion markers (PD1, LAG3, and TIM3) in T cells which are known to cause functional impairment of T cells. A recent study showed that cancer specific T cells were highly enriched in the PD1+ T cell population [7]. We also found that NK cells had higher expression of LIR1, an inhibitory molecule known to induce impairment of leukemia killing [8]. These findings suggest that both T cells and NK cells are functionally impaired in high risk MDS or AML, possibly due to immune editing by the malignant cells [9], [10]. In responders and stable disease subjects clofarabine induced significant lympho-depletion and decreased the numbers of exhausted CD4 T cells and terminally differentiated NK cells with inhibitory markers. However, the dynamics of T cell exhaustion markers were variable during lenalidomide therapy and further study will be needed to elucidate the role of T cell subset profiles in high risk MDS and AML. Small sample size limits the generalization of our findings to clinical practice. Nonetheless our study sheds light on the immune-modulating effects of clofarabine and lenalidomide and suggests that immune profiling can be used to guide treatments aimed at boosting immunity to leukemia in high risk MDS and AML.
Conflict of interest
MB, AJB obtained the research funding through Material Cooperative Research and Development Agreement (MCRADA) between Celgene Corporation (New Jersey, USA) and National, Heart, Lung, and Blood Institute, National Institutes of Health.
All other authors declare no competing financial interests.
Author's contributions
Study concept and design (JK, ND, MB, SI and AJB); in vitro experiment and data collection (PJ, FC, QY, KK, SW, PM, SI); analysis and interpretation of data (PJ, SI, PM, AJB, MB); drafting of the manuscript (PJ, PM, AJB, SI, MB); statistical analysis (PJ, SI, MB); clinical data collection (JK, ND, KL, EK, DD, JS, AJB, SI, MB); and obtained funding and study supervision (MB, AJB). MB and SI contributed equally.
Acknowledgements
We thank all patients who donated the samples to this study. This research was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute at the National Institutes of Health (Z01-HL002342-14). Lenalidomide was obtained through Material Cooperative Research and Development Agreement (MCRADA) between Celgene Corporation (New Jersey, USA) and National, Heart, Lung, and Blood Institute, National Institutes of Health.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.lrr.2017.04.003.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Luger S.M. Treating the elderly patient with acute myelogenous leukemia. Hematol.: Am. Soc. Hematol. Educ. Program. 2010;2010:62–69. doi: 10.1182/asheducation-2010.1.62. [DOI] [PubMed] [Google Scholar]
- 2.Kantarjian H.M., Erba H.P., Claxton D., Arellano M., Lyons R.M., Kovascovics T., Gabrilove J., Craig M., Douer D., Maris M., Petersdorf S., Shami P.J., Yeager A.M., Eckert S., Abichandani R., Faderl S. Phase II study of clofarabine monotherapy in previously untreated older adults with acute myeloid leukemia and unfavorable prognostic factors. J. Clin. Oncol. 2010;28(4):549–555. doi: 10.1200/JCO.2009.23.3130. [DOI] [PubMed] [Google Scholar]
- 3.Fehniger T.A., Uy G.L., Trinkaus K., Nelson A.D., Demland J., Abboud C.N., Cashen A.F., Stockerl-Goldstein K.E., Westervelt P., DiPersio J.F., Vij R. A phase 2 study of high-dose lenalidomide as initial therapy for older patients with acute myeloid leukemia. Blood. 2011;117(6):1828–1833. doi: 10.1182/blood-2010-07-297143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gribben J.G., Fowler N., Morschhauser F. Mechanisms of action of lenalidomide in B-cell non-hodgkin lymphoma. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 2015;33(25):2803–2811. doi: 10.1200/JCO.2014.59.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ades L., Fenaux P. Immunomodulating drugs in myelodysplastic syndromes. Hematol./Am. Soc. Hematol. Educ. Program. 2011;2011:556–560. doi: 10.1182/asheducation-2011.1.556. [DOI] [PubMed] [Google Scholar]
- 6.Cheson B.D., Greenberg P.L., Bennett J.M., Lowenberg B., Wijermans P.W., Nimer S.D., Pinto A., Beran M., de Witte T.M., Stone R.M., Mittelman M., Sanz G.F., Gore S.D., Schiffer C.A., Kantarjian H. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 7.Gros A., Parkhurst M.R., Tran E., Pasetto A., Robbins P.F., Ilyas S., Prickett T.D., Gartner J.J., Crystal J.S., Roberts I.M., Trebska-McGowan K., Wunderlich J.R., Yang J.C., Rosenberg S.A. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat. Med. 2016;22(4):433–438. doi: 10.1038/nm.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godal R., Bachanova V., Gleason M., McCullar V., Yun G.H., Cooley S., Verneris M.R., McGlave P.B., Miller J.S. Natural killer cell killing of acute myelogenous leukemia and acute lymphoblastic leukemia blasts by killer cell immunoglobulin-like receptor-negative natural killer cells after NKG2A and LIR-1 blockade. Biol. Blood Marrow Transplant.: J. Am. Soc. Blood Marrow Transplant. 2010;16(5):612–621. doi: 10.1016/j.bbmt.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlsten M., Baumann B.C., Simonsson M., Jadersten M., Forsblom A.M., Hammarstedt C., Bryceson Y.T., Ljunggren H.G., Hellstrom-Lindberg E., Malmberg K.J. Reduced DNAM-1 expression on bone marrow NK cells associated with impaired killing of CD34+ blasts in myelodysplastic syndrome. Leukemia. 2010;24(9):1607–1616. doi: 10.1038/leu.2010.149. [DOI] [PubMed] [Google Scholar]
- 10.Epling-Burnette P.K., Bai F., Painter J.S., Rollison D.E., Salih H.R., Krusch M., Zou J., Ku E., Zhong B., Boulware D., Moscinski L., Wei S., Djeu J.Y., List A.F. Reduced natural killer (NK) function associated with high-risk myelodysplastic syndrome (MDS) and reduced expression of activating NK receptors. Blood. 2007;109(11):4816–4824. doi: 10.1182/blood-2006-07-035519. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material