Abstract
Cardiac resynchronisation therapy (CRT) can profoundly improve outcome in selected patients with heart failure; however, response is difficult to predict and can be absent in up to one in three patients. There has been a substantial amount of interest in the echocardiographic assessment of left ventricular dyssynchrony, with the ultimate aim of reliably identifying patients who will respond to CRT. The measurement of myocardial deformation (strain) has conventionally been assessed using tissue Doppler imaging (TDI), which is limited by its angle dependence and ability to measure in a single plane. Two-dimensional speckle-tracking echocardiography is a technique that provides measurements of strain in three planes, by tracking patterns of ultrasound interference (‘speckles’) in the myocardial wall throughout the cardiac cycle. Since its initial use over 15 years ago, it has emerged as a tool that provides more robust, reproducible and sensitive markers of dyssynchrony than TDI. This article reviews the use of two-dimensional and three-dimensional speckle-tracking echocardiography in the assessment of dyssynchrony, including the identification of echocardiographic parameters that may hold predictive potential for the response to CRT. It also reviews the application of these techniques in guiding optimal LV lead placement pre-implant, with promising results in clinical improvement post-CRT.
Keywords: speckle-tracking echocardiography, strain, cardiac resynchronisation therapy, dyssynchrony
Introduction
Heart failure can be defined as the inability of the heart to deliver oxygen to meet the metabolic requirements of the body, due to an abnormality in the cardiac structure or function (1). The characterisation of cardiac dyssynchrony has led to a better understanding of the mechanisms that underlie the compromise in cardiac stroke volume in some forms of heart failure. CRT aims to achieve inter- and intra-ventricular synchrony by pacing the right ventricle (RV) and left ventricle (LV) simultaneously, with pacing leads positioned in the right ventricle and coronary sinus, respectively. In patients with sinus rhythm, CRT also aims to restore atrioventricular (AV) synchrony. It has made a substantial improvement in the clinical outcomes (including hospitalisation and mortality) in appropriately selected patients with heart failure (2) and is recommended in cases of drug-refractory New York Heart Association class II–IV symptomatic heart failure, an ejection fraction <35% and ECG evidence of left bundle branch block (LBBB) (3). However, up to one in three patients do not show an improvement post-CRT (4, 5).
Numerous echocardiographic indices of dyssynchrony have been evaluated, based on M-mode, pulsed Doppler and tissue Doppler (TDI) methods (6). However, they have yielded mixed results in their ability to predict CRT response, with observational studies demonstrating a link between the presence of dyssynchrony and an improvement post-CRT; however, larger trials show poor technique agreement and reproducibility (7, 8). Over the last 15 years, speckle-tracking echocardiography (STE) has emerged as a method for assessing global and regional LV systolic function through the measurement of myocardial deformation and has been applied to the evaluation of dyssynchrony in potential candidates for CRT. This article describes the technique of STE and reviews its role in guiding patient selection for CRT.
Mechanisms of dyssynchrony
Cardiac dyssynchrony comprises three main components: atrioventricular (AV), inter-ventricular and intra-ventricular. AV dyssynchrony describes a delay in the normal sequential atrioventricular contraction, resulting from delayed conduction through the AV node. This leads to disordered ventricular diastolic filling and ultimately to a reduced LV preload that compromises stroke volume (due to loss of the Starling mechanism) (9). The mechanisms involved include (i) initiation of ventricular systole while the ventricular is still filling, resulting in mitral regurgitation; (ii) shortened ventricular filling time and (iii) the occurrence of atrial systole simultaneously with early passive filling (10). Inter- and intra-ventricular dyssynchrony have a relatively greater effect on ventricular pump function than AV dyssynchrony (9). Inter-ventricular dyssynchrony describes a sequential delay in activation between the RV and LV, resulting in a lack of co-ordinated contraction. In LBBB, the anterior surface of the RV is the earliest to depolarise (due to fast electrical propagation through the intact right bundle), and the posterolateral basal LV is usually the latest (due to the relatively slow propagation from cell to cell) (11). Intra-ventricular dyssynchrony can result from the temporal delay in electrical activation of one region of the LV myocardium relative to another, such as that observed in LBBB. However, it can also exist in the absence of regionally delayed electrical activation, where abnormal myocardial loading is considered instead of contributing to dyssynchrony (11).
Intra-ventricular dyssynchrony leads to LV contraction that is inferior in both effectiveness and energy efficiency. In early systole, the septum contracts, whereas the lateral wall that faces it is passively stretched. The reverse of this pattern is seen in late systole, when lateral wall contraction results in septal stretching. The late-activated septal wall contracts forcefully (due to earlier stretch), but against a high LV cavity pressure. The successive contraction and stretching wastes myocardial energy and compromises efficiency. The haemodynamic consequences of dyssynchronous LV contraction are reduced stroke volume, stroke work and slower rate of rise of LV pressure and increased LV end-systolic wall stress. In addition, the LV end-systolic pressure–volume relationship shifts to the right, denoting that the LV now operates at a larger volume in order to recruit the Frank–Starling mechanism (9, 11).
By restoring AV, inter- and intra-ventricular synchrony, CRT is able to produce acute and sustained improvements in LV contractility, and response can be gauged by the improvement in LV contractility. This is measurable acutely as an increase in dP/dT and arterial pulse pressure, and a decrease in pulmonary capillary wedge pressure (12).
Echocardiographic assessment of LV dyssynchrony
Various techniques have been explored to assess the presence of LV dyssynchrony. M-mode can be used to measure the time delay between contraction of the LV anteroseptal and posterior walls. Although this is relatively quick to perform, it is assumed that these two LV walls reflect the presence or absence of dyssynchrony within the entire ventricle. Its ability to predict echocardiographic and clinical outcome post-CRT (13) has not been reproducible between different groups (14).
TDI allows a greater number of LV segments to be sampled compared with M-mode. Pulsed Doppler method is used to sample the longitudinal shortening velocity profile of up to six basal LV segments from the apical views (15). By measuring the time delay between the onset of the QRS and the peak systolic velocity, electromechanical delay can be quantified. The dyssynchrony index is then expressed as the dispersion of regional electromechanical delays. This has been shown by various groups to predict the improvement in exercise capacity, symptoms and echocardiographic parameters including LV volumes and ejection fraction (16, 17, 18). Extending the sampling from the six basal LV segments to the six mid-LV segments provides the standard deviation of electromechanical delay (Ts-SD) in the 12 non-apical LV segments. This may be a more accurate predictor of CRT response than other TDI techniques (19). The limitations of TDI include the need for a high imaging frame rate and several separate acquisitions. Furthermore, as with any Doppler technique, the ultrasound beam must be aligned as parallel as possible to the sampling region of interest.
Real-time 3D echocardiography provides a dataset containing the entire LV. This can then be divided into 16 or 17 sub-volumes (corresponding to the standard myocardial segments) to derive time–volume curves for each segment. The standard deviation of the time to peak segmental contraction can be reproducibly quantified in patients with LV dysfunction and is an independent predictor of CRT response (20).
It is possible that conventional TDI measurements may also detect dyssynchrony in the presence of heterogeneous intra-ventricular activation sequences, which are not necessarily correctable by CRT. More novel approaches to the assessment of dyssynchrony have included focus on electromechanical (as opposed to mechanical) abnormalities. An example of this is ‘septal flash’: the abnormal brief inward septal motion that occurs during the isovolumic contraction time and which characterises LBBB-related dyssynchrony. An observational study of over 1000 patients (PREDICT-CRT) demonstrated that the presence of apical rocking and septal flash had an incremental value over clinical variables and QRS width for identifying CRT responders (21). In addition, their absence or unsuccessful correction was associated with a high risk for non-response and unfavourable long-term survival.
Using an approach that considers all three components of dyssynchrony and also incorporates the presence of a correctable mechanical abnormality is predictive of response to CRT and survival. Using pre-defined mechanical abnormalities such as septal flash, abnormalities in LV filling and prolonged inter-ventricular delay, Doltra and co-workers demonstrated that some abnormalities correlated more strongly with CRT response than others and that the absence of any mechanical abnormality greatly increased the likelihood of CRT non-response (22).
Measuring myocardial deformation using TDI and STE
TDI was one of the earliest techniques developed to measure myocardial deformation, or ‘strain’. By measuring LV longitudinal shortening velocities over time, the velocity gradient between two points in the myocardial wall can be used to calculate strain rate. This is then used to derive strain. As it is a Doppler-based method, TDI is only able to assess deformation in the plane incident with the ultrasound beam and requires acquisition of dedicated images (23).
More recently, myocardial deformation imaging has been performed through the use of speckle-tracking echocardiography (24). This uses the presence of natural acoustic markers in B mode grey-scale images that are created by interference of ultrasound beams within myocardial tissue and are denoted as ‘speckles’ (25). They are equally distributed throughout the myocardium, and tracking their relative positions through each frame of the cardiac cycle provides information on regional myocardial deformation, known as ‘strain’. Strain reflects the percentage change of the myocardial length from its original value, with thickening represented by a positive strain value and shortening represented by a negative strain value. During systole, the LV shortens in the longitudinal and circumferential planes and thickens in the radial plane.
Radial and circumferential strains are obtained from LV short-axis images at the level of the mitral valve, papillary muscles and apex. Longitudinal strain is obtained from apical 4-, 3- and 2-chamber images. In contrast to TDI, the analysis is performed offline and requires semi-automated positioning of the endocardial contour, following which the software tracks the speckles through the cardiac cycle. Operator adjustment of the tracking is possible by manual correction of the contour. The myocardium is divided into segments in each view, so the final display of strain in each plane takes the form of segmental time–strain curves. The analysis of longitudinal and radial strain is shown in Fig. 1. The advantages of STE over TDI for the determination of strain include relatively less angle dependence in the sampled area, as the speckles are tracked along the direction of movement of the myocardial wall, instead of the direction of the ultrasound beam (23, 26). However, it should be noted that STE is not entirely independent of angle, as axial versus lateral resolution remain critical for this method too. In addition, lower inter-observer variability and less time-consuming analysis have been demonstrated for STE-derived strain compared with TDI-derived strain (27). Compared with TDI, which reflects myocardial motion, but also translational movement of the heart within the thoracic cavity, strain reflects shortening of the myocardium, irrespective of the movement of the whole heart (28). Disadvantages include the requirement of STE for high-resolution image quality and the potential for different tracking algorithms to produce different results (23), although this inter-vendor variation has been largely eliminated on the latest ultrasound systems.
Figure 1.
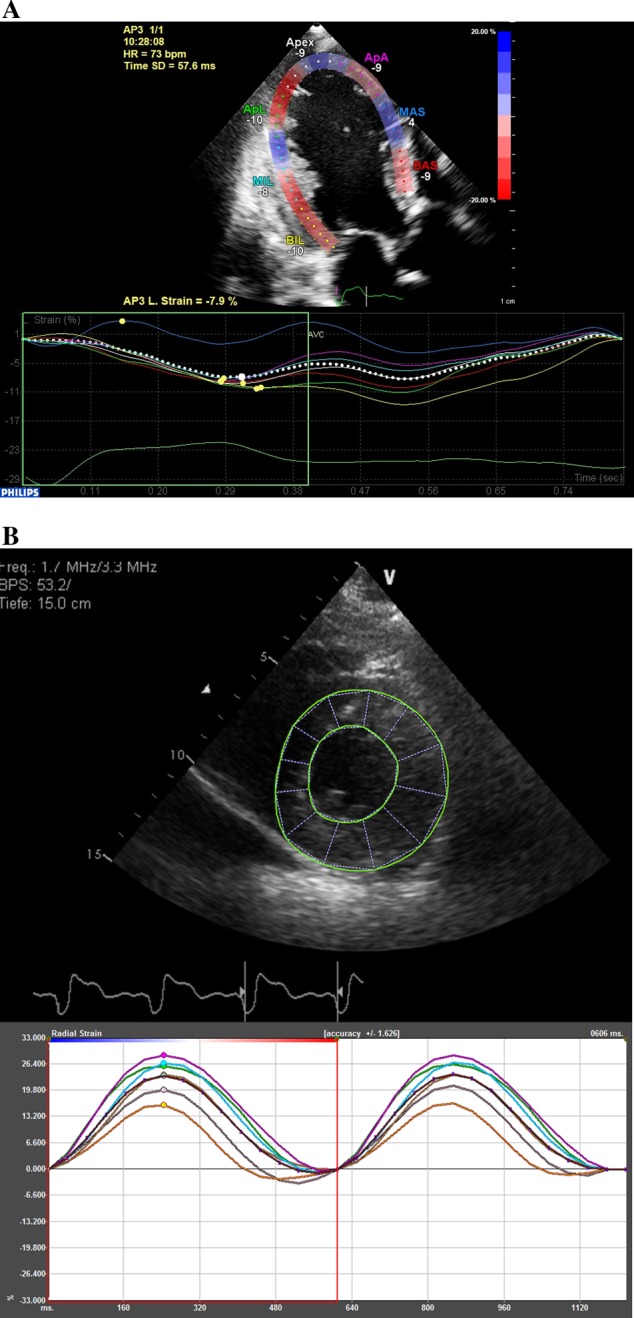
(A) Analysis of longitudinal strain. In this apical three-chamber view, the software has divided the LV into seven segments (named according to the 16-segment model nomenclature) and tracked the speckles through the cardiac cycle. Strain is displayed in the form of seven segmental time–strain curves, which are negative, reflecting myocardial shortening. To obtain global longitudinal strain, analysis would be performed on all the three apical views. BIL, basal inferolateral; MIL, mid inferolateral; ApL, apical lateral; ApA, apical anterior; MAS, mid anteroseptal; BAS, basal anteroseptal. (B) Analysis of radial strain. In this LV short-axis view (at papillary muscle level), the software has divided the LV into segments. Segmental time–strain curves are positive, reflecting radial thickening. To obtain global radial strain, analysis would be performed on all the three short-axis views. Figure kindly provided by Daan Javornik and Rolf Baumann (Tomtec, Munich, Germany) using 2D Cardiac Performance Analysis (2D CPA) software.
The assessment of myocardial dyssynchrony using STE includes measuring the maximum time delay between peak systolic strain of two segments (usually between the anteroseptal and posterolateral walls) and the dyssynchrony index of the LV (taken from the standard deviation of time to peaksystolic strain, Fig. 2). In practice, speckle tracking in the short-axis images is more reliable than in the apical images, as non-valid tracking of apical segments can present a problem. However, studies have shown that longitudinal strain is more reproducible than circumferential and radial strains (29, 30), as short-axis views are more sensitive to out-of-plane motion of the speckles.
Figure 2.
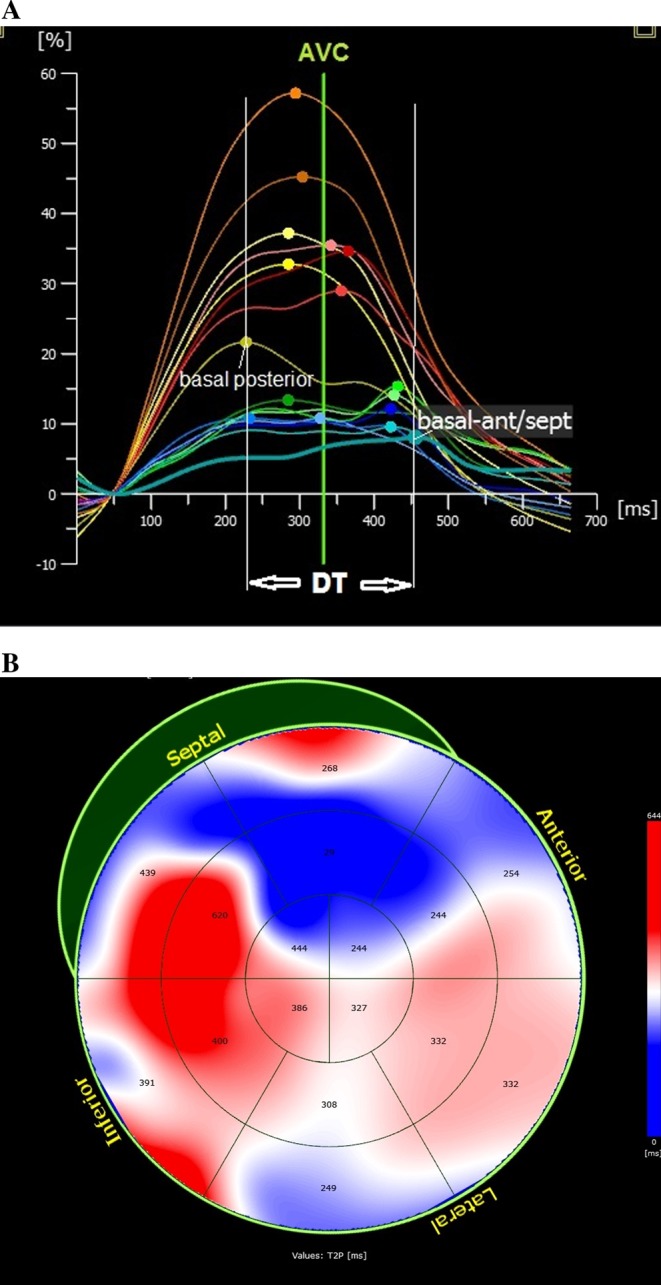
Dyssynchrony assessment using STE. The parameters used for dyssynchrony assessment are (A) maximum time delay between peak systolic strain of two segments (usually between the anteroseptal and posterolateral walls, DT) and (B) dyssynchrony index of the LV, taken from the standard deviation of time to peak systolic strain (mapping shows time to peak values for each segment). AVC, aortic valve closure; DT, time delay in peak systolic contraction between posterior wall and anteroseptal wall.
Measurements of strain and strain rate show good agreement between TDI and 2D STE (31, 32). It should be noted that a combined approach of TDI and speckle tracking to obtain longitudinal velocity and radial strain, respectively, has been shown to increase the predictive value of response to CRT compared with either technique alone (33).
Evidence for 2DSTE in predicting response to CRT
CRT involves simultaneous pacing of the RV and the posterolateral wall of the LV. Although it has made a significant impact on clinical outcomes in heart failure (2), the incidence of non-responders remains a problematic issue and has been estimated as approximately one in three patients (4, 5). In the literature, at least 17 different criteria have been suggested for defining a good response to CRT, eight of which are echocardiographic (including but not limited to): an increase in ejection fraction >5% to >15%, a decrease in LV end-systolic volume >10% to >15%, a decrease in LV end-diastolic volume >15% and an increase in stroke volume >15% (34). There is no current consensus on agreed criteria, although many recent studies have used the decrease in LV end-systolic volume >15% to define response (28, 35).
Although the electrocardiogram (ECG) has provided robust predictors of CRT response in the form of QRS width and the presence of left bundle branch block (LBBB), it is less sensitive than echocardiography in detecting lesser degrees of mechanical dyssynchrony. Over the last 15 years, various studies have sought to identify echocardiographic markers of dyssynchrony that can reliably predict the response to CRT. The multi-centre PROSPECT trial studied 12 echocardiographic parameters of dyssynchrony and found that although several parameters differed between CRT responders and non-responders, they showed only modest sensitivity and specificity. The investigators concluded that no single parameter could reliably improve patient selection for CRT (8). However, there was a wide variability in the analysis of echocardiographic measures, and a sub-analysis identified the pre-ejection interval, inter-ventricular mechanical delay and TDI longitudinal velocity as predictors of the LV end-systolic volume response and improvement in CCS (36).
Subsequent studies with longer follow-up periods (3–4 years) have identified speckle-tracking radial strain dyssynchrony (discussed above) as a predictor of clinical benefit in patients with ischaemic cardiomyopathy undergoing CRT (37, 38, 39). It is associated with outcome independently of the QRS width (38) and is an independent predictor of long-term survival (37). Using a value of ≥130ms, STE radial dyssynchrony predicts the ejection fraction response 8 months post-CRT with 89% sensitivity and 83% specificity (28). Furthermore, the absence of radial strain dyssynchrony is associated with a poorer outcome in patients with a QRS width of <150ms (38, 39).
The prospective multi-centre Speckle Tracking and Resynchronisation (STAR) study demonstrated that among the different types of strain, radial strain had the highest sensitivity for predicting ejection fraction response in patients undergoing CRT (sensitivity 86% and specificity 67%) (39). Circumferential and longitudinal strains predicted response when dyssynchrony was detected, but failed to identify dyssynchrony in one-third of patients who responded to CRT. Another group also found that radial strain dyssynchrony correlated better with long-term effects post-CRT than circumferential and longitudinal strain-based dyssynchrony (35).
The above studies enrolled patients with a mean QRS duration of 150–167ms. However, the benefit of CRT appears to be relatively smaller in patients with a QRS width <150ms (4, 40), and this is reflected in the ESC guidelines, in which LBBB with a QRS duration of 120–150ms is a class IB rather than IA indication for CRT (3). However, the complementary assessment of mechanical dyssynchrony by echocardiography can improve selection even in this patient group. This again relates to the concept that the surface ECG will not necessarily detect abnormalities of regional mechanical activation that result in mechanical dyssynchrony. Supportive of this theory are the results of the CARE-HF trial, in which patients with a QRS duration of 120–149ms experienced better survival and fewer cardiac hospitalisations when the following echocardiographic criteria for dyssynchrony were used to guide CRT implantation: aortic pre-ejection delay >140ms, inter-ventricular mechanical delay >40ms and delayed activation of the posterolateral LV wall (41). However, benefits are questionable when the QRS width is <130 ms, and the large, multi-centre, randomised Echo CRT trial was stopped early due to an apparent increase in mortality in the CRT-on group (42).
Speckle-tracking radial strain dyssynchrony has also been used to characterise dyssynchrony in CRT-D patients with severe symptomatic heart failure (43). The persistence of, or development of, radial strain dyssynchrony was strongly associated with the rate of ventricular arrhythmia and combined end point of arrhythmia, death, transplantation or LVAD implantation.
Longitudinal strain dyssynchrony is also an independent predictor of response to CRT (44, 45). Longitudinal strain is able to distinguish ischaemic from non-ischaemic LBBB (46). On bull’s eye mapping, the peak systolic strain shows a basal septal ‘horseshoe’ pattern of reduced regional strain in patients with non-ischaemic LBBB. This is of particular importance in assessing the suitability for CRT, as patients with non-ischaemic cardiomyopathy are better responders than those with ischaemic cardiomyopathy (3).
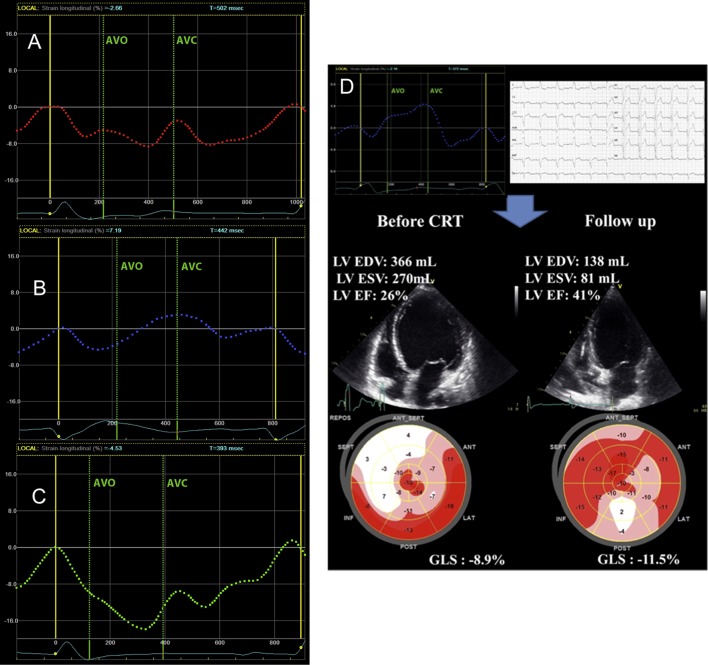
Randomised control trials have clearly demonstrated that LBBB morphology is a strong predictor of CRT response. Indeed, LBBB-induced dyssynchrony is characterised by septal flash, which strongly correlates with response to CRT (47). Recent work has demonstrated that the identification of this septal flash by longitudinal strain (LBBB longitudinal pattern) is associated with CRT response and long-term outcome (48). Three patterns of LV septal deformation have been identified (Fig. 3): double-peaked systolic (pattern 1), early pre-ejection peak followed by prominent systolic stretch (relaxation and lengthening of the myocardium; pattern 2) and pseudonormal shortening with late-systolic peak and smaller end-systolic stretch (pattern 3). Patterns 1 and 2 are associated with better improvements in echocardiographic outcomes and event-free survival post-CRT compared with pattern 3 (49).
Figure 3.
(A) Characterisation of LV septal deformation patterns using speckle-tracking longitudinal strain. Double-peaked systolic shortening (A, pattern 1), early pre-ejection shortening peak followed by prominent systolic stretch/lengthening (B, pattern 2) and pseudonormal shortening with a late-systolic shortening peak and less pronounced end-systolic stretch (C, pattern 3). Patterns 1 and 2 are associated with better improvement in echocardiographic outcomes and event-free survival post-CRT compared with pattern 3. (D) Example of a patient with severe heart failure, LBBB (QRS width 180 ms) and septal deformation pattern 2. Response to CRT with reduction in LV volumes and improvements in LV function and GLS is demonstrated. Reprinted from Journal of the American Society of Echocardiography, Vol 27, Marechaux S, Guiot A, Castel AL, Guyomar Y, Semichon M, Delelis F, Heuls S, Ennezat PV, Graux P & Tribouilloy C, Relationship between two-dimensional speckle-tracking septal strain and response to cardiac resynchronization therapy in patients with left ventricular dysfunction and left bundle branch block: a prospective pilot study, pp 501–511, Copyright (2014) American Society of Echocardiography, with permission from Elsevier.
In addition, the time delay in radial strain corresponds to the delay between the septal flash and the posterior wall. The septal-to-posterior wall motion delay is bimodal owing to the septal flash (50). Combining the strain dyssynchrony index derived from radial, circumferential and longitudinal strains holds better predictive value for CRT outcome than using a single parameter alone (51).
RV dyssynchrony parameters add incremental value to LV dyssynchrony parameters in the evaluation of candidates for CRT (52). The use of STE to assess RV dysfunction (using global longitudinal strain) has demonstrated that RV dysfunction is associated with poor short-term and long-term prognosis after CRT implantation (53). Preserved RV function is an independent predictor of long-term event-free survival after CRT (54).
The role of 2DSTE in guiding CRT lead placement
Myocardial tissue Doppler method has been used to identify the site of latest mechanical activation in the LV of patients with heart failure. When this information is used to guide the optimal position of the ‘LV’ lead during CRT, it substantially improves clinical and echocardiographic outcomes compared withdiscordant lead placement (55). Speckle-tracking echocardiography has built on this potential, and when radial strain data are used for guiding lead placement, improvements have been observed in LV ejection fraction (28), LV reverse remodelling, all-cause mortality and heart failure hospitalisations, compared with discordant lead placement (37, 56). These results have also been borne out in large, randomised clinical trials. The TARGET (Targeted Left Ventricular Lead Placement to Guide Cardiac Resynchronisation Therapy) trial (57) used speckle-tracking radial strain to guide LV lead positioning and also to identify sites of scar, with beneficial effects on LV reverse remodelling and clinical outcomes at 6 months. The STARTER (Speckle Tracking Assisted Resynchronisation Therapy for Electrode Region) trial (58) determined the latest time to peak strain in the LV basal and mid-wall short-axis segments and found a reduction in the combined end point of death and heart failure hospitalisation.
Although cardiac magnetic resonance (CMR) remains the gold standard for quantifying myocardial scar, speckle-tracking strain can also serve as a useful adjunct in patients in whom CMR is contraindicated. The ideal cut-off value for radial strain is yet to be determined, with values of <16.5% (37) and <10% (57, 59) having been used to denote scar, and holding predictive value for LV reverse remodelling and clinical outcomes.
3D speckle-tracking echocardiography
The limitations of 2D speckle-tracking echocardiography pertain to its ability to track speckles obtained from 2D images, whereas cardiac motion comprises rotation, contraction and shortening in three dimensions. Thus, some speckles may be lost in through-plane motion during the cardiac cycle (25). This applies particularly to longitudinal displacement in apical views and to radial and circumferential displacement in short-axis views. Loss in through-plane motion is more frequently found in radial and circumferential strains than in longitudinal strain.
3D speckle-tracking echocardiography allows acquisition of 3D indices of the entire LV from a 3D dataset acquired in the full-volume mode with an apical view (60). In the apical plane, three reference points are set by the user: two at the base of the LV at the mitral valve level and one at the apex. The same three points are fixed on a second orthogonal plane. The epicardial border can be entered manually or by setting a default ‘thickness’ for the myocardium. After detection of the myocardial borders at the end-diastolic reference frame, the user can correct the shape of the LV reference at the starting image. The 3D images of the LV wall are automatically divided into 16 segments. The software automatically tracks the contour on the subsequent frames in the three different strain vectors simultaneously. The feasibility of acquiring 3D volumes is approximately 70% (61).
Discordant values for strain have been demonstrated when comparing data obtained from 3D vs 2D techniques, with circumferential strain being reportedly greater and longitudinal strain being smaller with 3D speckle-tracking echocardiography (62). This has been attributed to the full appreciation of 3D cardiac motion by 3D tracking, which is not possible in the cross-sectional image plane of 2D tracking techniques. However, there appears to be a close correlation between 2D and 3D dyssynchrony indices (63). An advantage of the 3D tracking technique is the potential for faster analysis of strain compared with the 2D tracking method, as all the three strain vectors are analysed simultaneously, as opposed to sequentially (62, 64). It can also assess a greater proportion of myocardial segments compared with 2D tracking, independent of the quality of the patient’s acoustic window. A disadvantage of 3D STE is relatively low temporal resolution (65) and a high dependence on image quality. Thus, when the acoustic window is poor, dropout in the endocardial border can result in inappropriate tracking and inaccurate strain curves.
3D STE can be used to quantify the LV dyssynchrony in patients referred for CRT (63) and has identified the site of latest mechanical activation as mid and basal posterior walls in most cases, followed by mid and basal lateral walls. This agrees with the data obtained from 2D STE (56). A U-shaped propagation of LV activation is associated with LBBB and with a favourable response to CRT (66).
Controversies
Various echocardiographic methods now exist for the potential evaluation of dyssynchrony, along with a multitude of apparently robust predictors of CRT response. However, a number of challenges exist in the acquisition and interpretation of data required for dyssynchrony analysis. For example, the TDI signal shows significant sensitivity to the position of the sampling region. It can be challenging to differentiate physiological signal versus noise. Perhaps it is not surprising that considerable intra-observer and inter-observer variability exists, even in the clinical trial setting.
STE is also subject to errors in interpretation, due to loss of through-plane motion, and the potential for different tracking algorithms to produce different results. STE for CRT patient selection has not been tested rigorously in a blinded, multi-centre study. This is particularly relevant as many single-centre studies markedly overestimate the predictive effect of dyssynchrony markers compared with studies requiring formal enrolment and blinded analysis (67). However, it does appear to provide more robust, reproducible and sensitive markers of dyssynchrony than TDI. Therefore, it is reasonable to assume that it may perform better in randomised studies, should these be performed in the future.
Given the complex and multi-faceted nature of dyssynchrony, it is likely that CRT response will be best predicted by a combination of factors–echocardiographic and clinical–rather than an isolated factor. Examples of factors that are known to influence the outcome include the aetiology of heart failure, the presence of scar and the site of the LV lead.
Conclusions
STE has emerged as a clinically reproducible method of assessing LV dyssynchrony, and observational studies have shown its potential in guiding patient selection for CRT. Measurements of dyssynchrony agree with those obtained by the longer established technique of TDI; however, STE has the advantages of relatively less angle dependence and the ability to measure strain in all three planes of cardiac motion. STE is also able to provide information on patterns of myocardial activation, thus allowing the identification of optimal LV lead positioning pre-CRT implant. The limitations of STE include the need for high-resolution images. There is also the potential for speckles to drop out of the plane during cardiac motion; however, this can be overcome by the use of 3D STE, which also allows quicker analysis compared with 2D STE. Currently, no echo technique is generally accepted or guideline-endorsed for the identification of CRT responders. This is reflected in the ESC guidelines on CRT, which do not recommend using the presence of echocardiographic dyssynchrony as a selection criterion for CRT. However, they acknowledge that a number of observational studies suggest a link between dyssynchrony and the response to CRT, with the predictive value yet to be determined in randomised trials.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- 1.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. European Heart Journal 2012. 33 1787–1847. ( 10.1093/eurheartj/ehs104) [DOI] [PubMed] [Google Scholar]
- 2.Al-Majed NS, McAlister FA, Bakal JA, Ezekowitz JA. Meta-analysis: cardiac resynchronization therapy for patients with less symptomatic heart failure. Annals of Internal Medicine 2011. 154 401–412. ( 10.7326/0003-4819-154-6-201103150-00313) [DOI] [PubMed] [Google Scholar]
- 3.Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PM, et al. ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). European Heart Journal 2013. 34 2281–2329. ( 10.1093/eurheartj/eht150) [DOI] [PubMed] [Google Scholar]
- 4.Dickstein K, Vardas PE, Auricchio A, Daubert JC, Linde C, McMurray J, Ponikowski P, Priori SG, Sutton R, van Veldhuisen DJ, et al. Focused update of ESC guidelines on device therapy in heart failure: an update of the 2008 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure and the 2007 ESC guidelines for cardiac and resynchronization therapy. Developed with the special contribution of the Heart Failure Association and the European Heart Rhythm Association. European Heart Journal 2010. 31 2677–2687. ( 10.1093/eurheartj/ehq337) [DOI] [PubMed] [Google Scholar]
- 5.Stevenson WG, Hernandez AF, Carson PE, Fang JC, Katz SD, Spertus JA, Sweitzer NK, Tang WH, Albert NM, Butler J, et al. Indications for cardiac resynchronization therapy: 2011 update from the Heart Failure Society of America Guideline Committee. Journal of Cardiac Failure 2012. 18 94–106. ( 10.1016/j.cardfail.2011.12.004) [DOI] [PubMed] [Google Scholar]
- 6.Kapetanakis S, Bhan A, Monaghan MJ. Echo determinants of dyssynchrony (atrioventricular and inter- and intraventricular) and predictors of response to cardiac resynchronization therapy. Echocardiography 2008 25 1020–1030. [DOI] [PubMed] [Google Scholar]
- 7.Burri H, Muller H, Vieira I, Lerch R. Poor agreement of echographic measures of ventricular dyssynchrony. European Journal of Echocardiography 2008. 9 235–240. [DOI] [PubMed] [Google Scholar]
- 8.Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, Abraham WT, Ghio S, Leclercq C, Bax JJ, et al. Results of the predictors of response to CRT (PROSPECT) trial. Circulation 2008 117 2608–2616. ( 10.1161/CIRCULATIONAHA.107.743120) [DOI] [PubMed] [Google Scholar]
- 9.Sweeney MO, Prinzen FW. Ventricular pump function and pacing: physiological and clinical integration. Circulation: Arrhythmia and Electrophysiology 2008. 1 127–139. ( 10.1161/CIRCEP.108.777904) [DOI] [PubMed] [Google Scholar]
- 10.Bax JJ, Ansalone G, Breithardt OA, Derumeaux G, Leclercq C, Schalij MJ, Sogaard P, St John Sutton M & Nihoyannopoulos P. Echocardiographic evaluation of cardiac resynchronization therapy: ready for routine clinical use? A critical appraisal. Journal of the American College of Cardiology 2004. 44 1–9. ( 10.1016/j.jacc.2004.02.055) [DOI] [PubMed] [Google Scholar]
- 11.Cheng A, Helm RH, Abraham TP. Pathophysiological mechanisms underlying ventricular dyssynchrony. Europace 2009. 11 (Supplement 5) v10–v14. ( 10.1093/europace/eup272) [DOI] [PubMed] [Google Scholar]
- 12.Leclercq C, Cazeau S, Le Breton H, Ritter P, Mabo P, Gras D, Pavin D, Lazarus A, Daubert JC. Acute hemodynamic effects of biventricular DDD pacing in patients with end-stage heart failure. Journal of the American College of Cardiology 1998 32 1825–1831. [DOI] [PubMed] [Google Scholar]
- 13.Pitzalis MV, Iacoviello M, Romito R, Massari F, Rizzon B, Luzzi G, Guida P, Andriani A, Mastropasqua F, Rizzon P. Cardiac resynchronization therapy tailored by echocardiographic evaluation of ventricular asynchrony. Journal of the American College of Cardiology 2002 40 1615–1622. [DOI] [PubMed] [Google Scholar]
- 14.Diaz-Infante E, Mont L, Leal J, Garcia-Bolao I, Fernandez-Lozano I, Hernandez-Madrid A, Perez-Castellano N, Sitges M, Pavon-Jimenez R, Barba J, et al. Predictors of lack of response to resynchronization therapy. American Journal of Cardiology 2005 95 1436–1440. [DOI] [PubMed] [Google Scholar]
- 15.Sogaard P, Egeblad H, Kim WY, Jensen HK, Pedersen AK, Kristensen BO, Mortensen PT. Tissue Doppler imaging predicts improved systolic performance and reversed left ventricular remodeling during long-term cardiac resynchronization therapy. Journal of the American College of Cardiology 2002. 40 723–730. ( 10.1016/S0735-1097(02)02010-7) [DOI] [PubMed] [Google Scholar]
- 16.Ansalone G, Giannantoni P, Ricci R, Trambaiolo P, Laurenti A, Fedele F, Santini M. Doppler myocardial imaging in patients with heart failure receiving biventricular pacing treatment. American Heart Journal 2001. 142 881–896. ( 10.1067/mhj.2001.117324) [DOI] [PubMed] [Google Scholar]
- 17.Bax JJ, Bleeker GB, Marwick TH, Molhoek SG, Boersma E, Steendijk P, van der Wall EE, Schalij MJ. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. Journal of the American College of Cardiology 2004 44 1834–1840. [DOI] [PubMed] [Google Scholar]
- 18.Penicka M, Vanderheyden M, Geelen P, Mortier L, Goethals M, Verstreken S, Karasek J, De Bruyne B, Bartunek J. Tissue Doppler predicts long-term clinical outcome after cardiac resynchronization therapy. International Journal of Cardiology 2008. 124 40–46. ( 10.1016/j.ijcard.2006.12.035) [DOI] [PubMed] [Google Scholar]
- 19.Yu CM, Fung JW, Zhang Q, Chan CK, Chan YS, Lin H, Kum LC, Kong SL, Zhang Y, Sanderson JE. Tissue Doppler imaging is superior to strain rate imaging and postsystolic shortening on the prediction of reverse remodeling in both ischemic and nonischemic heart failure after cardiac resynchronization therapy. Circulation 2004. 110 66–73. ( 10.1161/01.CIR.0000133276.45198.A5) [DOI] [PubMed] [Google Scholar]
- 20.Kapetanakis S, Kearney MT, Siva A, Gall N, Cooklin M, Monaghan MJ. Real-time three-dimensional echocardiography: a novel technique to quantify global left ventricular mechanical dyssynchrony. Circulation 2005. 112 992–1000. ( 10.1161/CIRCULATIONAHA.104.474445) [DOI] [PubMed] [Google Scholar]
- 21.Stankovic I, Prinz C, Ciarka A, Daraban AM, Kotrc M, Aarones M, Szulik M, Winter S, Belmans A, Neskovic AN, et al. Relationship of visually assessed apical rocking and septal flash to response and long-term survival following cardiac resynchronization therapy (PREDICT-CRT). European Heart Journal Cardiovascular Imaging 2015. 17 262–269. ( 10.1093/ehjci/jev288) [DOI] [PubMed] [Google Scholar]
- 22.Doltra A, Bijnens B, Tolosana JM, Borras R, Khatib M, Penela D, De Caralt TM, Castel MA, Berruezo A, Brugada J, et al. Mechanical abnormalities detected with conventional echocardiography are associated with response and midterm survival in CRT. JACC Cardiovascular Imaging 2014. 7 969–979. ( 10.1016/j.jcmg.2014.03.022) [DOI] [PubMed] [Google Scholar]
- 23.Dandel M, Lehmkuhl H, Knosalla C, Suramelashvili N, Hetzer R. Strain and strain rate imaging by echocardiography–basic concepts and clinical applicability. Current Cardiology Reviews 2009. 5 133–148. ( 10.2174/157340309788166642) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reisner SA, Lysyansky P, Agmon Y, Mutlak D, Lessick J, Friedman Z. Global longitudinal strain: a novel index of left ventricular systolic function. Journal of the American Society of Echocardiography 2004. 17 630–633. ( 10.1016/j.echo.2004.02.011) [DOI] [PubMed] [Google Scholar]
- 25.Notomi Y, Lysyansky P, Setser RM, Shiota T, Popovic ZB, Martin-Miklovic MG, Weaver JA, Oryszak SJ, Greenberg NL, White RD, et al. Measurement of ventricular torsion by two-dimensional ultrasound speckle tracking imaging. Journal of the American College of Cardiology 2005 45 2034–2041. [DOI] [PubMed] [Google Scholar]
- 26.Amundsen BH, Helle-Valle T, Edvardsen T, Torp H, Crosby J, Lyseggen E, Stoylen A, Ihlen H, Lima JA, Smiseth OA, et al. Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. Journal of the American College of Cardiology 2006. 47 789–793. ( 10.1016/j.jacc.2005.10.040) [DOI] [PubMed] [Google Scholar]
- 27.Ingul CB, Torp H, Aase SA, Berg S, Stoylen A, Slordahl SA. Automated analysis of strain rate and strain: feasibility and clinical implications. Journal of the American Society of Echocardiography 2005. 18 411–418. ( 10.1016/j.echo.2005.01.032) [DOI] [PubMed] [Google Scholar]
- 28.Suffoletto MS, Dohi K, Cannesson M, Saba S, Gorcsan J., III Novel speckle-tracking radial strain from routine black-and-white echocardiographic images to quantify dyssynchrony and predict response to cardiac resynchronization therapy. Circulation 2006. 113 960–968. ( 10.1161/CIRCULATIONAHA.105.571455) [DOI] [PubMed] [Google Scholar]
- 29.Altman M, Bergerot C, Aussoleil A, Davidsen ES, Sibellas F, Ovize M, Bonnefoy-Cudraz E, Thibault H, Derumeaux G. Assessment of left ventricular systolic function by deformation imaging derived from speckle tracking: a comparison between 2D and 3D echo modalities. European Heart Journal Cardiovascular Imaging 2014. 15 316–323. ( 10.1093/ehjci/jet103) [DOI] [PubMed] [Google Scholar]
- 30.Armstrong AC, Ricketts EP, Cox C, Adler P, Arynchyn A, Liu K, Stengel E, Sidney S, Lewis CE, Schreiner PJ, et al. Quality control and reproducibility in M-mode, two-dimensional, and speckle tracking echocardiography acquisition and analysis: The CARDIA study, year 25 examination experience. Echocardiography 2015. 32 1233–1240. ( 10.1111/echo.2015.32.issue-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perkins EJ, Abraham T. Pharmacokinetics, metabolism, and excretion of the intestinal peptide transporter 1 (SLC15A1)-targeted prodrug (1S,2S,5R,6S)-2-[(2’S)-(2-amino)propionyl]aminobicyclo[3.1.0.]hexen-2,6-dicarboxy lic acid (LY544344) in rats and dogs: assessment of first-pass bioactivation and dose linearity. Drug Metabolism and Disposition 2007. 35 1903–1909. ( 10.1124/dmd.107.016154) [DOI] [PubMed] [Google Scholar]
- 32.Leitman M, Lysyansky P, Sidenko S, Shir V, Peleg E, Binenbaum M, Kaluski E, Krakover R, Vered Z. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. Journal of the American Society of Echocardiography 2004 17 1021–1029. [DOI] [PubMed] [Google Scholar]
- 33.Gorcsan J, III, Tanabe M, Bleeker GB, Suffoletto MS, Thomas NC, Saba S, Tops LF, Schalij MJ, Bax JJ. Combined longitudinal and radial dyssynchrony predicts ventricular response after resynchronization therapy. Journal of the American College of Cardiology 2007 50 1476–1483. [DOI] [PubMed] [Google Scholar]
- 34.Fornwalt BK, Sprague WW, BeDell P, Suever JD, Gerritse B, Merlino JD, Fyfe DA, Leon AR, Oshinski JN. Agreement is poor among current criteria used to define response to cardiac resynchronization therapy. Circulation 2010 121 1985–1991. ( 10.1161/CIRCULATIONAHA.109.910778) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delgado V, Ypenburg C, van Bommel RJ, Tops LF, Mollema SA, Marsan NA, Bleeker GB, Schalij MJ, Bax JJ. Assessment of left ventricular dyssynchrony by speckle tracking strain imaging comparison between longitudinal, circumferential, and radial strain in cardiac resynchronization therapy. Journal of the American College of Cardiology 2008 51 1944–1952. ( 10.1016/j.jacc.2008.02.040) [DOI] [PubMed] [Google Scholar]
- 36.van Bommel RJ, Bax JJ, Abraham WT, Chung ES, Pires LA, Tavazzi L, Zimetbaum PJ, Gerritse B, Kristiansen N, Ghio S. Characteristics of heart failure patients associated with good and poor response to cardiac resynchronization therapy: a PROSPECT (Predictors of response to CRT) sub-analysis. European Heart Journal 2009 30 2470–2477. ( 10.1093/eurheartj/ehp368) [DOI] [PubMed] [Google Scholar]
- 37.Delgado V, van Bommel RJ, Bertini M, Borleffs CJ, Marsan NA, Arnold CT, Nucifora G, van de Veire NR, Ypenburg C, Boersma E, et al. Relative merits of left ventricular dyssynchrony, left ventricular lead position, and myocardial scar to predict long-term survival of ischemic heart failure patients undergoing cardiac resynchronization therapy. Circulation 2011. 123 70–78. ( 10.1161/CIRCULATIONAHA.110.945345) [DOI] [PubMed] [Google Scholar]
- 38.Gorcsan J, III, Oyenuga O, Habib PJ, Tanaka H, Adelstein EC, Hara H, McNamara DM, Saba S. Relationship of echocardiographic dyssynchrony to long-term survival after cardiac resynchronization therapy. Circulation 2010 122 1910–1918. ( 10.1161/CIRCULATIONAHA.110.954768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka H, Nesser HJ, Buck T, Oyenuga O, Janosi RA, Winter S, Saba S, Gorcsan J., III Dyssynchrony by speckle-tracking echocardiography and response to cardiac resynchronization therapy: results of the Speckle Tracking and Resynchronization (STAR) study. European Heart Journal 2010 31 1690–1700. ( 10.1093/eurheartj/ehq213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sipahi I, Chou JC, Hyden M, Rowland DY, Simon DI, Fang JC. Effect of QRS morphology on clinical event reduction with cardiac resynchronization therapy: meta-analysis of randomized controlled trials. American Heart Journal 2012. 163 260–267. ( 10.1016/j.ahj.2011.11.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L, Cardiac Resynchronization-Heart Failure Study I The effect of cardiac resynchronization on morbidity and mortality in heart failure. New England Journal of Medicine 2005. 352 1539–1549. ( 10.1056/NEJMoa050496) [DOI] [PubMed] [Google Scholar]
- 42.Ruschitzka F, Abraham WT, Singh JP, Bax JJ, Borer JS, Brugada J, Dickstein K, Ford I, Gorcsan J, III, Gras D, et al. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. New England Journal of Medicine 2013 369 1395–1405. ( 10.1056/NEJMoa1306687) [DOI] [PubMed] [Google Scholar]
- 43.Haugaa KH, Marek JJ, Ahmed M, Ryo K, Adelstein EC, Schwartzman D, Saba S, Gorcsan J., III Mechanical dyssynchrony after cardiac resynchronization therapy for severely symptomatic heart failure is associated with risk for ventricular arrhythmias. Journal of the American Society of Echocardiography 2014. 27 872–879. ( 10.1016/j.echo.2014.04.001) [DOI] [PubMed] [Google Scholar]
- 44.D’Andrea A, Caso P, Scarafile R, Riegler L, Salerno G, Castaldo F, Gravino R, Cocchia R, Del Viscovo L, Limongelli G, et al. Effects of global longitudinal strain and total scar burden on response to cardiac resynchronization therapy in patients with ischaemic dilated cardiomyopathy. European Journal of Heart Failure 2009. 11 58–67. ( 10.1093/eurjhf/hfn010) [DOI] [PubMed] [Google Scholar]
- 45.Ghani A, Delnoy PP, Adiyaman A, Ottervanger JP, Ramdat Misier AR, Smit JJ, Elvan A. Response to cardiac resynchronization therapy as assessed by time-based speckle tracking imaging. Pacing and Clinical Electrophysiology 2015. 38 455–464. ( 10.1111/pace.12589) [DOI] [PubMed] [Google Scholar]
- 46.Magne J, Habib G, Cosyns B, Donal E, Miller O, Neglia D, Petersen SE, Lancellotti P. EuroEcho-Imaging 2014: highlights. European Heart Journal of Cardiovascular Imaging 2015 16 703–711. ( 10.1093/ehjci/jev084) [DOI] [PubMed] [Google Scholar]
- 47.Parsai C, Bijnens B, Sutherland GR, Baltabaeva A, Claus P, Marciniak M, Paul V, Scheffer M, Donal E, Derumeaux G, et al. Toward understanding response to cardiac resynchronization therapy: left ventricular dyssynchrony is only one of multiple mechanisms. European Heart Journal 2009. 30 940–949. ( 10.1093/eurheartj/ehn481) [DOI] [PubMed] [Google Scholar]
- 48.Risum N, Tayal B, Hansen TF, Bruun NE, Jensen MT, Lauridsen TK, Saba S, Kisslo J, Gorcsan J, III, Sogaard P. Identification of typical left bundle branch block contraction by strain echocardiography is additive to electrocardiography in prediction of long-term outcome after cardiac resynchronization therapy. Journal of the American College of Cardiology 2015. 66 631–641. ( 10.1016/j.jacc.2015.06.020) [DOI] [PubMed] [Google Scholar]
- 49.Marechaux S, Guiot A, Castel AL, Guyomar Y, Semichon M, Delelis F, Heuls S, Ennezat PV, Graux P, Tribouilloy C. Relationship between two-dimensional speckle-tracking septal strain and response to cardiac resynchronization therapy in patients with left ventricular dysfunction and left bundle branch block: a prospective pilot study. Journal of the American Society of Echocardiography 2014. 27 501–511. ( 10.1016/j.echo.2014.01.004) [DOI] [PubMed] [Google Scholar]
- 50.Lumens J, Leenders GE, Cramer MJ, De Boeck BW, Doevendans PA, Prinzen FW, Delhaas T. Mechanistic evaluation of echocardiographic dyssynchrony indices: patient data combined with multiscale computer simulations. Circulation Cardiovascular Imaging 2012. 5 491–499. [DOI] [PubMed] [Google Scholar]
- 51.Tatsumi K, Tanaka H, Matsumoto K, Kaneko A, Tsuji T, Ryo K, Fukuda Y, Norisada K, Onishi T, Yoshida A, et al. Relation between strain dyssynchrony index determined by comprehensive assessment using speckle-tracking imaging and long-term outcome after cardiac resynchronization therapy for patients with heart failure. American Journal of Cardiology 2012 109 1187–1193. ( 10.1016/j.amjcard.2011.11.057) [DOI] [PubMed] [Google Scholar]
- 52.Vitarelli A, Franciosa P, Nguyen BL, Capotosto L, Ciccaglioni A, Conde Y, Iorio G, De Curtis G, Caranci F, Vitarelli M, et al. Additive value of right ventricular dyssynchrony indexes in predicting the success of cardiac resynchronization therapy: a speckle-tracking imaging study. Journal of Cardiac Failure 2011. 17 392–402. ( 10.1016/j.cardfail.2010.12.004) [DOI] [PubMed] [Google Scholar]
- 53.Nagy VK, Szeplaki G, Apor A, Kutyifa V, Kovacs A, Kosztin A, Becker D, Boros AM, Geller L, Merkely B. Role of right ventricular global longitudinal strain in predicting early and long-term mortality in cardiac resynchronization therapy patients. PLoS ONE 2015. 10 e0143907 ( 10.1371/journal.pone.0143907) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sade LE, Ozin B, Atar I, Demir O, Demirtas S, Muderrisoglu H. Right ventricular function is a determinant of long-term survival after cardiac resynchronization therapy. Journal of the American Society of Echocardiography 2013. 26 706–713. ( 10.1016/j.echo.2013.03.013) [DOI] [PubMed] [Google Scholar]
- 55.Ansalone G, Giannantoni P, Ricci R, Trambaiolo P, Fedele F, Santini M. Doppler myocardial imaging to evaluate the effectiveness of pacing sites in patients receiving biventricular pacing. Journal of the American College of Cardiology 2002. 39 489–499. ( 10.1016/S0735-1097(01)01772-7) [DOI] [PubMed] [Google Scholar]
- 56.Ypenburg C, van Bommel RJ, Delgado V, Mollema SA, Bleeker GB, Boersma E, Schalij MJ, Bax JJ. Optimal left ventricular lead position predicts reverse remodeling and survival after cardiac resynchronization therapy. Journal of the American College of Cardiology 2008 52 1402–1409. [DOI] [PubMed] [Google Scholar]
- 57.Khan FZ, Virdee MS, Palmer CR, Pugh PJ, O’Halloran D, Elsik M, Read PA, Begley D, Fynn SP, Dutka DP. Targeted left ventricular lead placement to guide cardiac resynchronization therapy: the TARGET study: a randomized, controlled trial. Journal of the American College of Cardiology 2012 59 1509–1518. ( 10.1016/j.jacc.2011.12.030) [DOI] [PubMed] [Google Scholar]
- 58.Saba S, Marek J, Schwartzman D, Jain S, Adelstein E, White P, Oyenuga OA, Onishi T, Soman P, Gorcsan J., III Echocardiography-guided left ventricular lead placement for cardiac resynchronization therapy: results of the speckle tracking assisted resynchronization therapy for electrode region trial. Circulation Heart Failure 2013. 6 427–434. ( 10.1161/CIRCHEARTFAILURE.112.000078) [DOI] [PubMed] [Google Scholar]
- 59.Khan FZ, Virdee MS, Read PA, Pugh PJ, O’Halloran D, Fahey M, Elsik M, Begley D, Fynn SP, Dutka DP. Effect of low-amplitude two-dimensional radial strain at left ventricular pacing sites on response to cardiac resynchronization therapy. Journal of the American Society of Echocardiography 2010 23 1168–1176. ( 10.1016/j.echo.2010.08.023) [DOI] [PubMed] [Google Scholar]
- 60.Seo Y, Ishizu T, Enomoto Y, Sugimori H, Yamamoto M, Machino T, Kawamura R, Aonuma K. Validation of 3-dimensional speckle tracking imaging to quantify regional myocardial deformation. Circulation Cardiovascular Imaging 2009. 2 451–459. ( 10.1161/CIRCIMAGING.109.858480) [DOI] [PubMed] [Google Scholar]
- 61.Thavendiranathan P, Liu S, Verhaert D, Calleja A, Nitinunu A, Van Houten T, De Michelis N, Simonetti O, Rajagopalan S, Ryan T, et al. Feasibility, accuracy, and reproducibility of real-time full-volume 3D transthoracic echocardiography to measure LV volumes and systolic function: a fully automated endocardial contouring algorithm in sinus rhythm and atrial fibrillation. JACC Cardiovascular Imaging 2012. 5 239–251. ( 10.1016/j.jcmg.2011.12.012) [DOI] [PubMed] [Google Scholar]
- 62.Saito K, Okura H, Watanabe N, Hayashida A, Obase K, Imai K, Maehama T, Kawamoto T, Neishi Y, Yoshida K. Comprehensive evaluation of left ventricular strain using speckle tracking echocardiography in normal adults: comparison of three-dimensional and two-dimensional approaches. Journal of the American Society of Echocardiography 2009 22 1025–1030. ( 10.1016/j.echo.2009.05.021) [DOI] [PubMed] [Google Scholar]
- 63.Tanaka H, Hara H, Saba S, Gorcsan J., III Usefulness of three-dimensional speckle tracking strain to quantify dyssynchrony and the site of latest mechanical activation. American Journal of Cardiology 2010. 105 235–242. ( 10.1016/j.amjcard.2009.09.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perez de Isla L, Balcones DV, Fernandez-Golfin C, Marcos-Alberca P, Almeria C, Rodrigo JL, Macaya C & Zamorano J. Three-dimensional-wall motion tracking: a new and faster tool for myocardial strain assessment: comparison with two-dimensional-wall motion tracking. Journal of the American Society of Echocardiography 2009. 22 325–330. ( 10.1016/j.echo.2009.01.001) [DOI] [PubMed] [Google Scholar]
- 65.Thebault C, Donal E, Bernard A, Moreau O, Schnell F, Mabo P, Leclercq C. Real-time three-dimensional speckle tracking echocardiography: a novel technique to quantify global left ventricular mechanical dyssynchrony. European Journal of Echocardiography 2011. 12 26–32. ( 10.1093/ejechocard/jeq095) [DOI] [PubMed] [Google Scholar]
- 66.Seo Y, Ishizu T, Kawamura R, Yamamoto M, Kuroki K, Igarashi M, Sekiguchi Y, Nogami A, Aonuma K. Three-dimensional propagation imaging of left ventricular activation by speckle-tracking echocardiography to predict responses to cardiac resynchronization therapy. Journal of the American Society of Echocardiography 2015. 28 606–614. ( 10.1016/j.echo.2015.02.003) [DOI] [PubMed] [Google Scholar]
- 67.Nijjer SS, Pabari PA, Stegemann B, Palmieri V, Leyva F, Linde C, Freemantle N, Davies JE, Hughes AD, Francis DP. The limit of plausibility for predictors of response: application to biventricular pacing. JACC Cardiovascular Imaging 2012 5 1046–1065. ( 10.1016/j.jcmg.2012.07.010) [DOI] [PubMed] [Google Scholar]



 This work
is licensed under a
This work
is licensed under a