Abstract
The aetiology of sudden cardiac arrest can often be identified to underlying cardiac pathology. Mitral valve prolapse is a relatively common valvular pathology with symptoms manifesting with increasing severity of mitral regurgitation (MR). It is unusual for severe MR to be present without symptoms, and there is growing evidence that this subset of patients may be at increased risk of sudden cardiac arrest or death. The difficulty lies in identifying those patients at risk and applying measures that are appropriate to halting progression to cardiac arrest. This article examines the association of mitral valve prolapse with cardiac arrests, the underlying pathophysiological process and the strategies for identifying those at risk.
Keywords: mitral valve prolapse, mitral regurgitation, cardiac arrest, sudden cardiac death
Case
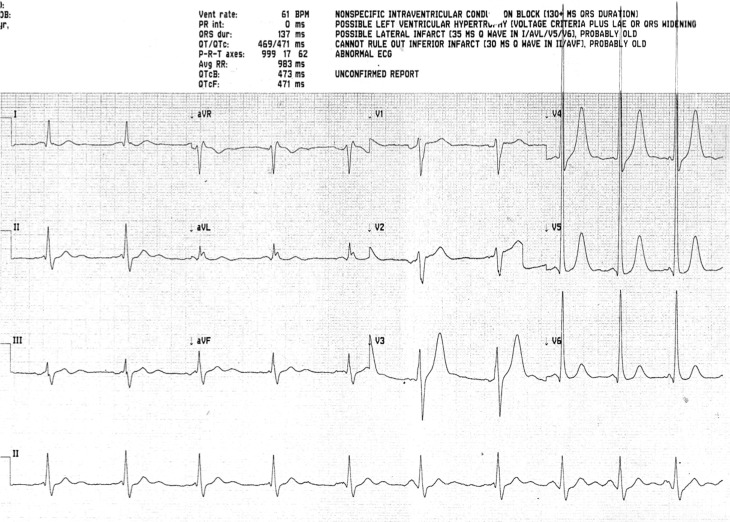
A 45-year-old male had a sudden collapse at home, witnessed by their partner who started bystander cardiopulmonary resuscitation (CPR). The initial observed cardiac rhythm was pulseless ventricular tachycardia on arrival of the emergency medical services. A direct current shock was delivered resulting in asystole. The patient underwent a further 10 min of CPR prior to the return of spontaneous circulation, during which time endotracheal intubation and positive pressure ventilation were commenced. The total low-flow time was between 20 and 30 min. The patient was transported by air ambulance to a tertiary cardiac arrest centre. The patient was transferred to the accident and emergency department where intravenous sedation was started and maintained. The body temperature was measured at 34.9°C. Initial blood investigations were as follows: troponin I 350 ng/mL, C-reactive protein <4 mg/mL, leucocyte count 18 103/µL, sodium 140 mmol/L, potassium 3.1 mmol/L, urea 6.6 mmol/L, creatinine 66µmol/L and glucose 12 mmol/L. Initial arterial blood gas sampling demonstrated a pH 7.31, PaCO2 6.41 kPa, PaO2 62.9 kPa, base excess −1.7 and lactate 3.0 mmol/L. The ECG showed sinus rhythm, there were no signs of ischaemia and it fulfilled electrical criteria for left ventricular hypertrophy (Fig. 1). A chest X-ray showed the presence of an endotracheal tube, but it was otherwise unremarkable. A CT scan of the head was obtained which was reported as normal.
Figure 1.
Initial ECG on admission to hospital.
Collateral history determined that the patient had no significant co-morbidities and that the patient was healthy prior to the sudden cardiac event. A bedside transthoracic echocardiogram was obtained (Videos 1 and 2). This reported a thickened and prolapsing anterior mitral valve leaflet with associated severe mitral regurgitation (MR). The left ventricular ejection fraction was inappropriately normal, but not hyperdynamic; although impaired when the severe MR was taken into account. There was no evidence of a systolic regional wall motion abnormality. On this evidence, the patient was treated empirically with vancomycin and gentamicin for suspected infective endocarditis and transferred to the intensive care unit.
Parasternal long-axis TTE zoomed in on the mitral valve showing a prolapsing anterior leaflet with evidence of cordal rupture. View Video 1 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-15-0020/video-1.
Download Video 1 (455KB, wmv)
Apical 2/3 chamber TTE zoomed in on the mitral valve showing the anterior leaflet. View Video 2 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-15-0020/video-2.
Download Video 2 (667.4KB, wmv)
In line with current protocols, the patient’s temperature was allowed to increase to 36°C and maintained at this level for the first 24 h. An infusion of norepinephrine was commenced to maintain a mean arterial blood pressure greater than 65 mmHg. A transoesophageal echocardiography (TOE) was performed 12 h after admission (Videos 3 and 4). This confirmed a flail A2 segment secondary to chordal rupture with evidence of myxomatous degeneration. The mitral annulus was dilated at 5.2 cm. No vegetations were observed on the mitral valve. Doppler interrogation confirmed the presence of severe MR. The left ventricle (LV) diastolic dimension was 7.2 cm, the systolic dimension was 5.3 cm and the ejection fraction (Simpson’s biplane) was 58%. A coronary angiogram was performed and showed normal unobstructed coronary arteries. Empirical antibiotic therapy for bacterial endocarditis was discontinued following TOE.
TOE showing a truncated commissural view with a prolapsing A2 segment of the anterior leaflet. View Video 3 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-15-0020/video-3.
Download Video 3 (430.6KB, wmv)
TOE showing the 2D appearance from Video 3 with colour flow Doppler added. There is regurgitation through the centre of the valve corresponding to A2. This is difficult to visualise due to the hyperdynamic ventricular function. View Video 4 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-15-0020/video-4.
Download Video 4 (293.9KB, wmv)
Following antibiotic therapy for hospital-acquired pneumonia, mechanical ventilation was successfully weaned and the trachea was successfully extubated 1 week after admission. Although the patient experienced an initial deficit in short-term memory function, they continue to make a good neurological recovery. Prior to hospital discharge, a single lead implantable cardiac defibrillator was inserted given the significant risk of further malignant ventricular rhythms. Three weeks later, a cardiac magnetic resonance imaging scan was performed, which showed a severely dilated LV with mild impairment of left ventricular function and severe MR. Further inquiry revealed no family history of sudden cardiac death, cardiac disease or connective tissue disorders. Genetic testing is yet to be performed. The patient was discharged home from hospital 3 weeks after admission with referral for cardiac surgery to repair the mitral valve prolapse.
Review of sudden cardiac arrest with coexisting mitral valve prolapse
Introduction
We report a case of out-of-hospital cardiac arrest (OOHCA) secondary to ventricular tachycardia (VT) likely related to mitral valve prolapse (MVP) and severe mitral regurgitation (MR).
Sudden cardiac death (SCD) is an unexpected natural death from a cardiac cause within a short time period (1). In most epidemiologic studies, this short period is defined within 1 h from the onset of symptoms. UK data show that an incidence of SCD is 100,000 adults per year (2). In the USA, it accounts for about 300,000 cases annually representing about 50% of mortality from cardiac causes (3). The overall incidence is about 50 100:100,000 people per year. On average, the survival with good neurologic recovery after OOHCA is about 5 10% (4). Due to the short time period from the onset of symptoms to arrest, identification of the high-risk population and prevention is the most effective strategy. Causes of SCD include coronary artery disease, cardiomyopathies, structural heart disease and primary electrophysiologic abnormalities. In some patients, the cause remains unclear, and hence, the term ‘idiopathic ventricular fibrillation’ is used (4).
Definition
MVP is defined as displacement of mitral leaflet tissue into the left atrium past the mitral annular plane during systole (5). It was first described by Barlow in the 1960s as an auscultatory and cine-angiocardiographic phenomenon, prior to the availability of diagnostic echocardiography (6, 7, 8). Advances in echocardiography (e.g. transoesophageal echocardiography (TOE) and three-dimensional imaging) have made it possible for accurate diagnosis and quantification of MR (9).
The role of echocardiography in MVP
Echocardiography can be used for diagnosis, surveillance and assessment of interventions in MVP. Carpentier’s functional classification of MR described MVP (Type II classification) as an abnormality of leaflet motion, where one or several components of the valve protrude into the left atrium (LA) during ventricular systole (10). Two-dimensional (2D) echocardiography can be used to divide MVP into classical and non-classical criteria for diagnosis (11). Classical MVP describes >2 mm displacement of the mitral valve leaflets into the LA in long-axis view during ventricular systole, with a leaflet thickness of ≥5 mm. Non-classical MVP describes >2 mm leaflet displacement with a leaflet thickness of <5 mm.
Classical MVP will have either a symmetrical or an asymmetrical point of coaptation. Both leaflet tips meet at the same point on the mitral valve annulus in symmetrical MVP. Asymmetrical coaptation results in one leaflet being displaced towards the LA in relation to the other leaflet. Asymmetrical coaptation is more likely to deteriorate and develop flail prolapse and result in greater severity of MR. Flail segment or prolapse describes the presence of leaflet tips that turn outwards and point into the LA. Flail prolapse can involve a single segment, multiple segments, one leaflet, or both leaflets (likely secondary to cordal rupture).
Both 2D TTE and TOE can be used to evaluate mitral valve morphology (Table 1). TOE will more reliably provide superior views across the LA window and should be considered in all cases of MVP assessment. The diagnosis of MVP using TTE should only be made in the parasternal long-axis view and/or the apical long-axis view as the hyperbolic paraboloid shape of the mitral valve annulus can give a false-positive diagnosis of MVP (12). In addition, a description of the leaflet thickness or redundancy, annular dilatation and chordal length should be included. Visual accuracy of mitral valve shape and deformity may be improved using 3D echocardiography techniques, especially for anterior leaflet or commissural involvement (13, 14).
Table 1.
TOE and TTE views required for assessing the location of MVP.
| View | Segments viewed | TOE | TTE |
|---|---|---|---|
| 1 | A2 and P2, four-chamber view | Sagittal view at 0° | Parasternal long-axis view |
| 2 | A2 and P2 | 120° | |
| 3 | A1 and P1 | As for 1 with angulation towards AV | As for 1 with angulation towards AV |
| 4 | A3 and P3 | As for 1 with angulation towards TV | As for with angulation towards TV |
| 5 | P3, A2 and P1 (left to right, bi-commisural view) | 40–60° | Bicommisural view |
| 6 | A1, A2, A3, P1, P2 and P3 | Transgastric view at 0° | Parasternal short-axis view |
Doppler imaging is essential to determine the severity of MR. This should involve quantitative measures to determine disease progression, predict outcome and assess suitability for intervention. It is recommended that the colour flow Doppler area should not be used to quantify the severity of MR. Where feasible, the vena contracta or proximal isovelocity surface area should be used as a measure of severity (12). Both the pulsed Doppler mitral-to-aortic time velocity–integral ratio and the systolic pulmonary flow reversal can be used as adjuncts to assist with quantifying the severity of MR (12).
The downstream effects of MR, including LA dilatation, LV dilatation, LV dysfunction, pulmonary vein flow reversal, pulmonary hypertension, RV dilatation and tricuspid regurgitation will also help determine the severity of MR. LV dilatation is a particularly important marker of progression in asymptomatic regurgitation, with monitoring of LV-end systolic diameter used to suggest when surgical intervention may be indicated.
Intraoperatively 2D TOE and/or 3D TOE is recommended to assist surgical repair or replacement of the valve (14). Three-dimensional TOE has been shown to be more reliable than surgical inspection at accurately diagnosing the cleft-like indentations of the posterior mitral valve leaflet of myxomatous mitral valve disease and aiding repair (15). It is also important in percutaneous methods of mitral valve repair such as with MitraClip (16).
Epidemiology
According to recent figures, the MVP prevalence is 1 2.4% (17, 18). This is down from previous estimations of up to 35%. This difference can be explained by better understanding the anatomy of the mitral valve, stricter diagnostic criteria and better diagnostic technology. Nevertheless, MVP remains the most common cause of MR in developed countries.
Pathophysiology of MVP
Several pathological processes may cause prolapse of the mitral valve, including rheumatic heart disease, Marfan’s syndrome, endocarditis and myocardial ischemia, but degenerative MVP refers specifically to a specific spectrum of primary lesions (5, 19). On the two extremes of the spectrum are fibroelastic deficiency (FED) and Barlow’s disease. FED is a fibrillin deficiency leading to rupture of one of the chordae. In this case, the mitral valve leaflets are thinned and the annular size is normal (18, 20). At the other end of the spectrum is Barlow’s disease affecting younger patients. The mitral annulus may become calcified and dilated with thickened leaflets secondary to myxomatous degeneration (19, 20).
Arrhythmias
Both supraventricular (SVA) and ventricular arrhythmias (VA) are associated with complications of MVP. This high incidence rate has been recognised for more than 20 years. A study in 1994 reported an incidence of SVA and VA associated with MVP of 14 and 30%, respectively (21). When tested with continuous ECG monitoring, MVP patients had a prevalence of VA as high as 34% with premature ventricular contractions as the most common pattern (66% of cases). Moderate-to-severe MR has been shown to be an independent risk factor to developing arrhythmias (22). Ikeda studied patients with idiopathic VT and found a high prevalence of MVP (12 out of 35 patients). VT originated from the LV in most of these cases in contrast to non-MVP (91.7 vs 69.6%) (23). Abnormal ventricular repolarisation, including early repolarisation, has also been linked to the presence of MVP (24, 25). A cross-sectional study of 100 patients with MVP showed a high incidence of early repolarisation (represented by notch in the descending arm of QRS and J-point and/or ST segment changes) compared with healthy individuals without MVP (25).
Left ventricular remodelling
The presence of a dilated left ventricle in the context of severe MR may indicate a period of LV remodelling. In acute primary MR, afterload may initially decrease due to the alternate pathway for ejection. However, with volume loading of the LV over time, the relatively thin-walled LV may dilate and hypertrophy. Consequently, the afterload in chronic compensated MR will be normal and elevated in chronic decompensated MR (26). Remodelling of the LV may allow MR to be tolerated with mild or no symptoms by increasing the stroke volume. However, progression to heart failure and possibly cardiac arrest can occur rapidly often in the presence of myocyte dysfunction and sympathetic activation (27). LV remodelling has been associated with evidence of VAs. However, there is a paucity of evidence assessing this link in the context of MVP without coronary artery disease (28).
Incidence of SCD
Despite the general impression of a benign course, several case reports since the 1980s describe SCD in MVP patients, with a significant proportion of young and previously asymptomatic individuals (29, 30). A debate continues as to whether MVP is the cause of SCD or merely an association. Data from forensic autopsy examinations have reported floppy mitral valves in 5% of specimens (99 out of 2007 specimens). From those, MVP was considered directly responsible for the death of 17 patients (0.8% of cases) (31). This would rank MVP as the most common congenital and valvular cardiac cause of SCD. A consensus statement in 1997 from American and European societies does not attribute the cause of SCD to MVP, unless the prolapse is associated with valve redundancy, thickening and regurgitation, QT prolongation or ST-T wave changes (32).
It is not known if the mitral valve repair or replacement will have a preventive effect on VAs. Subsequently, the American Heart Association/European Society of Cardiology guidelines for VAs and SCD have no distinctive recommendations for the management of VA or SCD in mitral valvular heart disease (33). Moreover, neither the recent American nor the European valvular heart disease guidelines mention a criterion for predicting or assuming SCD secondary to MVP (33, 34).
The 10-year mortality of asymptomatic MVP patients is reported to be 19%, with greater than moderate MR and impaired LV function as the most important risk factors (35). This draws similar comparisons to the all-cause mortality from MR with a flail leaflet (MR-FL). Collective data from the all-cause mortality from MR-FL showed an annual mortality of 1.8%. Not surprisingly, left ventricular ejection fraction and NYHA class were the risk factors for mortality. However, in the subgroup of patients with SCD, 40% of patients were categorised as NYHA class I (36). This highlights a subgroup of patients with SCD predominantly due to cardiac arrhythmia and not related to the severity of MR or LV failure in MVP. These patients were mostly young and asymptomatic. The subgroup of patients with NYHA class I had a yearly risk of SCD of 1% (36). This is equal to the overall mortality in hypertrophic obstructive cardiomyopathy (HCM, a pathology considered as one of the most common causes of SCD in young people (37). Consequently, it is logical to identify a high-risk group of MVP similar to the recommendation for primary prevention in HCM.
Risk of SCD
As the incidence of SCD is very low in patients with MVP, studies have been directed towards identifying a high-risk subgroup that could benefit from primary or secondary prevention. Autopsy evaluation of SCD patients associated with MVP identified a subgroup of patients with isolated MVP. These patients were younger, mostly females and with less prevalence of MR (38). The high prevalence of MVP makes a strategy based on primary prevention feasible if the high-risk group could be strictly defined. Advances in echocardiography, cardiac magnetic resonance (CMR) and electrophysiology are promising in the identification of this group. Currently, no clear strategy is recommended for this small subgroup. Being young and asymptomatic makes it difficult to detect patients for primary prevention. However, we know that the incidence of SCD is much lower in young adults than in the general population (i.e. about 1:100,000). This incidence doubles if athletic (39). It is not known whether exercise increases the risk of SCD in MVP patients. Till now, MVP-associated SCD has been categorised as idiopathic VF. The real question is whether it is possible to prospectively identify risk factors for SCD in individuals with MVP.
A retrospective American study identified 12 out of 50 SCD patients with idiopathic VF as having MVP. A quarter of MVP patients had a family history of SCD (40). Another study by Vohra and coworkers in 1993 prospectively studied seven patients with MVP associated with mild MR and normal LV function. All patients initially presented with syncope or OOHCA and the mean follow-up period was 2.5 years. They had proven VAs on Holter or electrophysiological study (EPS). Patients were treated with anti-arrhythmic pharmacotherapy, although with limited efficacy as there were two who suffered sudden death. Two patients underwent reparative surgery, but the arrhythmias were re-inducible on repeat EPS (41). This is in contrast to another study in which surgical correction had a protective effect (36).
Prevention of SCD
A cohort study that reviewed 24 patients who had idiopathic VF/VT OOHCA found echocardiographic evidence of bileaflet MVP in 10 patients (42%). The author named it malignant bileaflet MVP. Those patients with MVP had higher incidence of ECG abnormalities and VAs (42). Electrolyte disturbances can be an aggravating factor contributing to the occurrence of VAs and subsequently SCD (43). Screening programmes, technical availability (ECG, echocardiography) and cost remain obstacles for a wide application of primary prevention. Nevertheless, if MVP had been detected accidentally or in a screening program (e.g. athletes or relatives of patients with SCD), it may be easier to reconsider recommending a strategy for those patients depending on clear risk criteria. However, an implantable cardiac defibrillator (ICD) is not without risk, especially for young people. EPS, MRI and echocardiography may be helpful in identifying the right group.
Secondary prevention is an easier question. In patients diagnosed with idiopathic VF, ICD is a class I recommendation for survivors (44). A follow-up of 24 patients with OOHCA with VF/VT showed patients with bileaflet MVP had a significantly better ICD appropriateness compared with non-MVP patients (80 vs 36%, P = 0.04). However, this may had been affected by a longer follow-up period in the MVP group (42).
Cardiac magnetic resonance imaging
The advent of CMR has shed further light on this condition. CMR has shown an association between MVP and papillary muscle fibrosis. In a study of 16 patients, 8 had a history of VA on previous Holter monitoring (couples of VPCs or non-sustained VT) and 63% of the MVP patients had papillary muscle fibrosis on late gadolinium enhancement (45). Basal LV hypertrophy is another abnormality, which can be better detected by CMR in such patients. However, its significance in cases of arrhythmias and SCD remains unknown (46).
Other causes of SCD with MVP
It should be noted that other possible mechanisms of cardiac arrest should not be ignored. Primary spontaneous chordal rupture is one of the recognised complications of MVP. This may cause acute MR and cardiogenic pulmonary oedema (47). Intramyocardial small vessel disease has a known association with SCD (48). One of its variants, fibromuscular dysplasia, had been observed by pathologic examination more frequently in MVP than in the controls (75 vs 25%). This variant of small vessel disease in MVP cases was associated with fibrosis of the basal interventricular septum (49).
Conclusion
In this case, the patient was previously asymptomatic and presented with an aborted SCD. Severe MR was demonstrated with echocardiography. The presence of a dilated LV at presentation and later on during CMR suggests chronic severe MR with extensive LV remodelling in the absence of symptoms. It is possible that the haemodynamic effects of the acute regurgitation may have caused syncope, which progressed to dysrhythmia and cardiac arrest due to reduced coronary flow. It is equally possible that there was a primary dysrhythmia associated with MVP or left ventricular remodelling. Early TOE and CMR are important, as is a greater understanding of the possible electrophysiological mechanisms of primary arrhythmogenesis.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this article.
Funding
This case report did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
References
- 1.Zipes DP, Wellens HJ. 1998. Sudden cardiac death. Circulation 98 2334–2351. ( 10.1161/01.CIR.98.21.2334) [DOI] [PubMed] [Google Scholar]
- 2.Walker WM. 2010. Sudden cardiac death in adults: causes, incidence and interventions. Nursing Standard 24 50–56. ( 10.7748/ns2010.05.24.38.50.c7803) [DOI] [PubMed] [Google Scholar]
- 3.Myerburg RJ, Kessler KM, Castellanos A. 1993. Sudden cardiac death: epidemiology, transient risk, and intervention assessment. Annals of Internal Medicine 119 1187–1197. ( 10.7326/0003-4819-119-12-199312150-00006) [DOI] [PubMed] [Google Scholar]
- 4.Wever EF. & Robles de Medina EO 2004. Sudden death in patients without structural heart disease. Journal of the American College of Cardiology 43 1137–1144. ( 10.1016/j.jacc.2003.10.053) [DOI] [PubMed] [Google Scholar]
- 5.Shah PM. 2010. Current concepts in mitral valve prolapse – diagnosis and management. Journal of Cardiology 56 125–133. ( 10.1016/j.jjcc.2010.06.004) [DOI] [PubMed] [Google Scholar]
- 6.Barlow JB, Pocock WA. 1963. The significance of late systolic murmurs and mid-late systolic clicks. Maryland State Medical Journal 12 76–77. [PubMed] [Google Scholar]
- 7.Barlow JB, Pocock WA, Marchand P, Denny M. 1963. The significance of late systolic murmurs. American Heart Journal 66 443–452. [Google Scholar]
- 8.Barlow JB, Bosman CK. 1966. Aneurysmal protrusion of the posterior leaflet of the mitral valve. An auscultatory-electrocardiographic syndrome. American Heart Journal 71 166–178. [DOI] [PubMed] [Google Scholar]
- 9.Cheng TO, Wang XF, Zhang J, Xie MX. 2010. Recent advances in the echocardiographic diagnosis of mitral valve prolapse. International Journal of Cardiology 140 1–11. ( 10.1016/j.ijcard.2009.12.030) [DOI] [PubMed] [Google Scholar]
- 10.Carpentier A. 1983. Cardiac valve surgery—the “French correction.” Journal of Thoracic and Cardiovascular Surgery 86 323–337. [PubMed] [Google Scholar]
- 11.Freed LA, Levy D, Levine RA, Larson MG, Evans JC, Fuller DL, Lehman B, Benjamin EJ. 1999. Prevalence and clinical outcome of mitral-valve prolapse. New England Journal of Medicine 341 1–7. ( 10.1056/NEJM199907013410101) [DOI] [PubMed] [Google Scholar]
- 12.Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, Badano L, Zamorano JL. 2013. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. European Heart Journal Cardiovascular Imaging 14 611–644. ( 10.1093/ehjci/jet105). [DOI] [PubMed] [Google Scholar]
- 13.Pepi M, Tamborini G, Maltagliati A, Galli CA, Sisillo E, Salvi L, Naliato M, Porqueddu M, Parolari A, Zanobini M, et al. 2006. Head-to-head comparison of two- and three-dimensional transthoracic and transesopha-geal echocardiography in the localization of mitral valve prolapse. Journal of the American College of Cardiology 48 2524–2530. ( 10.1016/j.jacc.2006.02.079) [DOI] [PubMed] [Google Scholar]
- 14.La Canna G, Arendar I, Maisano F, Monaco F, Collu E, Benussi S, De Bonis M, Castiglioni A, Alfieri O. 2011. Real-time three-dimensional trans-esophageal echocardiography for assessment of mitral valve functional anatomy in patients with prolapse-related regurgitation. American Journal of Cardiology 107 1365–1374. ( 10.1016/j.amjcard.2010.12.048) [DOI] [PubMed] [Google Scholar]
- 15.Mantovani F, Clavel MA, Vatury O, Mankad SV, Malouf J, Michelena HI, Jain S, Badano LP, Enriquez-Sarano M. 2015. Cleft-like indentations in myxomatous mitral valves by three-dimensional echocardiographic imaging. Heart 101 1111–1117. ( 10.1136/heartjnl-2014-307016) [DOI] [PubMed] [Google Scholar]
- 16.Lang RM, Badano LP, Tsang W, Adams DH, Agricola E, Buck T, Faletra FF, Franke A, Hung J, de Isla LP. 2012. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. Journal of the American Society of Echocardiography 25 3–46. ( 10.1016/j.echo.2011.11.010) [DOI] [PubMed] [Google Scholar]
- 17.Freed LA, Levy D, Levine RA, Larson MG, Evans JC, Fuller DL, Lehman B, Benjamin EJ. 1999. Prevalence and clinical outcome of mitral-valve prolapse. New England Journal of Medicine 341 1–7. ( 10.1056/NEJM199907013410101) [DOI] [PubMed] [Google Scholar]
- 18.Hepner AD, Ahmadi-Kashani M, Movahed MR. 2007. The prevalence of mitral valve prolapse in patients undergoing echocardiography for clinical reason. International Journal of Cardiology 123 55–57. ( 10.1016/j.ijcard.2006.11.130) [DOI] [PubMed] [Google Scholar]
- 19.Adams DH, Rosenhek R, Falk V. 2010. Degenerative mitral valve regurgitation: best practice revolution. European Heart Journal 31 1958–1966. ( 10.1093/eurheartj/ehq222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anyanwu AC, Adams DH. 2007. Etiologic classification of degenerative mitral valve disease: Barlow’s disease and fibroelastic deficiency. Seminars in Thoracic and Cardiovascular Surgery 19 90–96. ( 10.1053/j.semtcvs.2007.04.002) [DOI] [PubMed] [Google Scholar]
- 21.Zuppiroli A, Mori F, Favilli S, Barchielli A, Corti G, Montereggi A, Dolara A. 1994. Arrhythmias in mitral valve prolapse: relation to anterior mitral leaflet thickening, clinical variables, and color Doppler echocardiographic parameters. American Heart Journal 128 919–927. ( 10.1016/0002-8703(94)90590-8) [DOI] [PubMed] [Google Scholar]
- 22.Turker Y, Ozaydin M, Acar G, Ozgul M, Hoscan Y, Varol E, Dogan A, Erdogan D, Yucel H. 2010. Predictors of ventricular arrhythmias in patients with mitral valve prolapse. International Journal of Cardiovascular Imaging 26 139–145. ( 10.1007/s10554-009-9514-6) [DOI] [PubMed] [Google Scholar]
- 23.Ikeda T. 1991. Mitral valve prolapse in idiopathic ventricular tachycardia: clinical and electrophysiological characteristics. Journal of Cardiology 21 717–726. ( 10.1016/j.ijcard.2006.08.073) [DOI] [PubMed] [Google Scholar]
- 24.Digeos-Hasnier S, Copie X, Paziaud O, Abergel E, Guize L, Diebold B, Jeunemaître X, Berrebi A, Piot O, Lavergne T. 2005. Abnormalities of ventricular repolarization in mitral valve prolapse. Annals of Noninvasive Electrocardiology 10 297–304. ( 10.1111/j.1542-474X.2005.00630.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peighambari MM, Alizadehasl A, Totonchi Z. 2014. Electrocardiographic changes in mitral valve prolapse syndrome. Journal of Cardiovascular and Thoracic Research 6 21–23. ( 10.5681/jcvtr.2014.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corin WJ, Monrad ES, Murakami T, Nonogi H, Hess OM, Krayenbuehl HP. 1987. The relationship of afterload to ejection performance in chronic mitral regurgitation. Circulation 76 59–67. ( 10.1161/01.CIR.76.1.59) [DOI] [PubMed] [Google Scholar]
- 27.Tsutsui H, Spinale FG, Nagatsu M, Schmid PG, Ishihara K, DeFreyta G, Cooper G, IV, Carabello BA. 1994. Effects of chronic beta-adrenergic blockade on the left ventricular and cardiocyte abnormalities of chronic mitral regurgitation. Journal of Clinical Investigation 93 2639–2648. ( 10.1172/JCI117277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Draper TS, Jr, Silver JS, Gaasch WH. 2015. Adverse structural remodeling of the left ventricle and ventricular arrhythmias in patients with depressed ejection fraction. Journal of Cardiac Failure 21 97–102. ( 10.1016/j.cardfail.2014.10.018) [DOI] [PubMed] [Google Scholar]
- 29.Pocock WA, Bosman CK, Chesler E, Barlow JB, Edwards JE. 1984. Sudden death in primary mitral valve prolapse. American Heart Journal 107 378–382. ( 10.1016/0002-8703(84)90389-2) [DOI] [PubMed] [Google Scholar]
- 30.Anders S, Said S, Schulz F, Püschel K. 2007. Mitral valve prolapse syndrome as cause of sudden death in young adults. Forensic Science International 171 127–130. ( 10.1016/j.forsciint.2006.10.011) [DOI] [PubMed] [Google Scholar]
- 31.Waller BF, Catellier MJ, Clark MA, Hawley DA, Pless JE. 1992. Cardiac pathology in 2007 consecutive forensic autopsies. Clinical Cardiology 15 760–765. ( 10.1002/clc.4960151014) [DOI] [PubMed] [Google Scholar]
- 32.Consensus Statement of the Joint Steering Committees of the Unexplained Cardiac Arrest Registry of Europe and of the Idiopathic Ventricular Fibrillation Registry of the United States 1997 Survivors of out-of-hospital cardiac arrest with apparently normal heart. Need for definition and standardized clinical evaluation. Circulation 95 265–267. ( 10.1161/01.CIR.95.1.265) [DOI] [PubMed] [Google Scholar]
- 33.Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, et al. 2006. American College of Cardiology/ American Heart Association Task Force; European Society of Cardiology Committee for Practice Guidelines; European Heart Rhythm Association and the Heart Rhythm Society. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death – executive summary. European Heart Journal 27 2099–2140. ( 10.1093/eurheartj/ehl199) [DOI] [PubMed] [Google Scholar]
- 34.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, III, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P. 2014. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Journal of the American College of Cardiology 63 e57–e185. ( 10.1016/j.jacc.2014.02.536) [DOI] [PubMed] [Google Scholar]
- 35.Avierinos JF, Gersh BJ, Melton LJ III, Bailey KR, Shub C, Nishimura RA, Tajik AJ, Enriquez-Sarano M. 2002. Natural history of asymptomatic mitral valve prolapse in the community. Circulation 106 1355–1361. ( 10.1161/01.CIR.0000028933.34260.09) [DOI] [PubMed] [Google Scholar]
- 36.Grigioni F, Enriquez-Sarano M, Ling LH, Bailey KR, Seward JB, Tajik AJ, Frye RL. 1999. Sudden death in mitral regurgitation due to flail leaflet. Journal of the American College of Cardiology 34 2078–2085. ( 10.1016/S0735-1097(99)00474-X) [DOI] [PubMed] [Google Scholar]
- 37.Maron BJ, Olivotto I, Spirito P, Casey SA, Bellone P, Gohman TE, Graham KJ, Burton DA, Cecchi F. 2000. Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large non-referral-based patient population. Circulation 102 858–864. ( 10.1161/01.CIR.102.8.858) [DOI] [PubMed] [Google Scholar]
- 38.Dollar AL, Roberts WC. 1991. Morphologic comparison of patients with mitral valve prolapse who died suddenly with patients who died from severe valvular dysfunction or other conditions. Journal of the American College of Cardiology 17 921–931. ( 10.1016/0735-1097(91)90875-A) [DOI] [PubMed] [Google Scholar]
- 39.Corrado D, Basso C, Pavei A, Michieli P, Schiavon M, Thiene G. 2006. Trends in sudden cardiovascular death in young competitive athletes after implementation of a preparticipation screening program. JAMA 296 1593–1601. ( 10.1001/jama.296.13.1593) [DOI] [PubMed] [Google Scholar]
- 40.Topaz O, Edwards JE. 1985. Pathologic features of sudden death in children, adolescents, and young adults. Chest 87 476–482. ( 10.1378/chest.87.4.476) [DOI] [PubMed] [Google Scholar]
- 41.Vohra J, Sathe S, Warren R, Tatoulis J, Hunt D. 1993. Malignant ventricular arrhythmias in patients with mitral valve prolapse and mild mitral regurgitation. Pacing and Clinical Electrophysiology 16 387–393. [DOI] [PubMed] [Google Scholar]
- 42.Sriram CS, Syed FF, Ferguson ME, Johnson JN, Enriquez-Sarano M, Cetta F, Cannon BC, Asirvatham SJ, Ackerman MJ. 2013. Malignant bileaflet mitral valve prolapse syndrome in patients with otherwise idiopathic out-of-hospital cardiac arrest. Journal of the American College of Cardiology 62 222–230. ( 10.1016/j.jacc.2013.02.060) [DOI] [PubMed] [Google Scholar]
- 43.Rajani AR, Murugesan V, Baslaib FO, Rafiq MA. 2014. Mitral valve prolapse and electrolyte abnormality: a dangerous combination for ventricular arrhythmias. BMJ Case Reports 14 2014. ( 10.1136/bcr-2014-205055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA III, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL. 2008. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Journal of the American College of Cardiology 51 e1–e62. ( 10.1016/j.jacc.2008.02.032) [DOI] [PubMed] [Google Scholar]
- 45.Han Y, Peters DC, Salton CJ, Bzymek D, Nezafat R, Goddu B, Kissinger KV, Zimetbaum PJ, Manning WJ, Yeon SB. 2008. Cardiovascular magnetic resonance characterization of mitral valve prolapse. JACC: Cardiovascular Imaging 1 294–303. ( 10.1016/j.jcmg.2008.01.013) [DOI] [PubMed] [Google Scholar]
- 46.Zia MI, Valenti V, Cherston C, Criscito MC, Uretsky S, Wolff SD. 2012. Severity of mitral valve prolapse is associated with basal left ventricular hypertrophy: a cardiac magnetic resonance study. Journal of Cardiovascular Magnetic Resonance 14 (Supplement 1) P99 ( 10.1016/j.amjcard.2011.12.029) [DOI] [PubMed] [Google Scholar]
- 47.Hickey AJ, Wilcken DE, Wright JS, Warren BA. 1985. Primary (spontaneous) chordal rupture: relation to myxomatous valve disease and mitral valve prolapse. Journal of the American College of Cardiology 5 1341–1346. ( 10.1016/S0735-1097(85)80346-6) [DOI] [PubMed] [Google Scholar]
- 47.Hickey AJ, Wilcken DE, Wright JS, Warren BA. 1985. Primary (spontaneous) chordal rupture: relation to myxomatous valve disease and mitral valve prolapse. Journal of the American College of Cardiology 5 1341–1346. ( 10.1016/S0735-1097(85)80346-6) [DOI] [PubMed] [Google Scholar]
- 48.Veinot JP, Johnston B, Acharya V, Healey J. 2002. The spectrum of intramyocardial small vessel disease associated with sudden death. Journal of Forensic Sciences 47 384–388. [PubMed] [Google Scholar]
- 49.Burke AP, Farb A, Tang A, Smialek J, Virmani R. 1997. Fibromuscular dysplasia of small coronary arteries and fibrosis in the basilar ventricular septum in mitral valve prolapse. American Heart Journal 134 282–291. ( 10.1016/S0002-8703(97)70136-4) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Parasternal long-axis TTE zoomed in on the mitral valve showing a prolapsing anterior leaflet with evidence of cordal rupture. View Video 1 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-15-0020/video-1.
Download Video 1 (455KB, wmv)
Apical 2/3 chamber TTE zoomed in on the mitral valve showing the anterior leaflet. View Video 2 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-15-0020/video-2.
Download Video 2 (667.4KB, wmv)
TOE showing a truncated commissural view with a prolapsing A2 segment of the anterior leaflet. View Video 3 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-15-0020/video-3.
Download Video 3 (430.6KB, wmv)
TOE showing the 2D appearance from Video 3 with colour flow Doppler added. There is regurgitation through the centre of the valve corresponding to A2. This is difficult to visualise due to the hyperdynamic ventricular function. View Video 4 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-15-0020/video-4.
Download Video 4 (293.9KB, wmv)



 This work
is licensed under a
This work
is licensed under a