Abstract
Background
Herbochip® technology is a high throughput drug screening platform in a reverse screening manner, in which potential chemical leads in herbal extracts are immobilized and drug target proteins can be used as probes for screening process [BMC Complementary and Alternative Medicine (2015) 15:146]. While herbal medicines represent an ideal reservoir for drug screenings, here a molecular chaperone GRP78 is demonstrated to serve as a potential target for antiviral drug discovery.
Methods
We cloned and expressed a truncated but fully functional form of human GRP78 (hGRP781-508) and used it as a probe for anti-HBV drug screening on herbochips. In vitro cytotoxicity and in vitro anti-HBV activity of the herbal extracts were evaluated by MTT and ELISA assays, respectively. Finally, anti-HBV activity was confirmed by in vivo assay using DHBV DNA levels in DHBV-infected ducklings as a model.
Results
Primary screenings using GRP78 on 40 herbochips revealed 11 positives. Four of the positives, namely Dioscorea bulbifera, Lasiosphaera fenzlii, Paeonia suffruticosa and Polygonum cuspidatum were subjected to subsequent assays. None of the above extracts was cytotoxic to AML12 cells, but P. cuspidatum extract (PCE) was found to be cytotoxic to HepG2 2.2.15 cells. Both PCE and P. suffruticosa extract (PSE) suppressed secretion of HBsAg and HBeAg in HepG2 2.2.15 cells. The anti-HBV activity of PSE was further confirmed in vivo.
Conclusion
We have demonstrated that GRP78 is a valid probe for anti-HBV drug screening on herbochips. We have also shown that PSE, while being non-cytotoxic, possesses in vitro and in vivo anti-HBV activities. Taken together, our data suggest that PSE may be a potential anti-HBV agent for therapeutic use.
Electronic supplementary material
The online version of this article (doi:10.1186/s13020-017-0132-2) contains supplementary material, which is available to authorized users.
Keywords: Paeoniaceae, Paeonia suffruticosa, Moutan Cortex Radicis, GRP78, Anti-HBV, Herbochip
Background
Liver infection by hepatitis B virus (HBV) can cause both acute and chronic diseases, which puts human at high risk of death from cirrhosis and liver cancer leading to life-threatening consequences. According to the data of World Health Organization (WHO), hepatitis B remains a major global health problem despite of the availability of highly effective vaccines since 1982 [1]. There are more than 4 million clinical cases of acute HBV infection every year. In low-endemic countries, such as North America and Northern Europe, the estimated prevalence of HBV surface antigen (HBsAg)-positive subjects is less than 2%, while that of high-endemic countries, such as sub-Saharan Africa and China, is greater than 8% [1, 2]. Globally, WHO estimates that approximately 240 million people are chronically infected with hepatitis B (defined as HBsAg-positive for longer than 6 months), and 780 thousand persons die each year for HBV infection—650 thousand from cirrhosis and hepatocellular carcinoma (HCC) due to chronic infection and another 130 thousand from acute hepatitis B [1]. In addition, it is well documented that HCC is one of the most common primary cancers and the third leading cause of death worldwide [3]; and, about 80% of HCC is attributed to HBV and/or HCV infections [4]. In fact, most HCC patients are infected by HBV, but the mechanisms underlying chronic HBV infection and such chronic disease progression remain largely unknown [5]. Early HCC is clinically silent and often well advanced at the first manifestation, only 10–20% of patients are suitable for surgical treatment [6]. Even having undergone surgical treatment, the prognosis of patients with HCC is still poor largely due to the invasion at early stage in HCC [7].
Initial infection of HBV can be prevented by vaccination, while progression of the disease in infected subjects can be prevented by drug treatments. Currently, peginterferon and nucleos(t)ide analogues (NUCs) are common frontline antiviral agents against HBV [2, 8, 9]. However, significant side-effect profile of interferon and drug resistance in NUCs both limits its long-term use and drug resistance in NUCs could limit long-term monotherapy. Moreover, combination therapy with NUCs and peginterferon or two NUCs given for 1 year does not show superiority in durability of response as compared to monotherapy [10]. Therefore, development of novel antiviral drugs and more effective therapies for the treatments of chronic hepatitis B, thus HCC, are urgently needed. In China and Eastern Asian countries including Japan, Korea, Taiwan and Hong Kong, traditional Chinese medicine represents a vast reservoir for anti-HBV drug discoveries [11, 12].
Our previous studies show that ethanolic extract of Boehmeria nivea root (BNE) can significantly suppress production of HBV in vitro and in vivo [13–15]. Detailed analysis of the inhibitory mechanism points to the fact that the molecular chaperone 78-kDa glucose-regulated protein (GRP78) is involved. Interestingly, viral core and large surface proteins accompanied with the encapsidated viral DNA were observed to accumulate within the BNE-treated cells. Although GRP78 was suppressed by BNE, stimulation of GRP78 expression by thapsigargin could rescue virus production initially inhibited by BNE. Furthermore, GRP78 siRNA knockdown led to suppression of HBV secretion [14]. Several lines of additional evidence also indicated that GRP78 is involved in the maturation of the large surface protein (HBsAg) and the viral particle of HBV in infected cells [16–19]. Taken together, these observations lead us to conclude that GRP78 may be a target protein for anti-HBV drug discovery and development. However, a competent GRP78 inhibitor or regulator in clinical application is not yet identified. In this study, we aim to identify GRP78 regulators from natural Chinese herbs and potential drug leads for further targeted anti-HBV therapy using the Herbochip® screening platform.
Herbochip®, an array-based high throughput screening platform, is an enabling technology for screening active compounds from herbal extracts in the reverse manner. That is, chemical compounds from each herb are firstly fractionated and arrayed onto a surface-activated plastic chip, and hybridization is performed using a biotinylated protein target coupled with streptavidin-cy5 visualization system [20–22]. The platform has been successfully employed to screen herbal fractions that bind to protein targets such as cytochrome P450 3A4 (CYP450 3A4) and tumor necrosis factor-alpha (TNF-α) [21, 22]. These results lead to identification of CYP450 3A4 and TNF-α inhibitory activities in Sophora flavescens and Geranium wilfordii, respectively, which will then be used in the design of regimens for the treatment of HIV infection and rheumatoid arthritis [21, 22]. In the most recent study, we describe the latest version of Herbochip® screening platform and report successful identification of the binding activity toward TNF-α in 46 out of 82 selected herbal extracts. The anti-TNF-α and anti-inflammatory effects of the G. wilfordii extract were, respectively, confirmed by in vitro and in vivo assays [22]. Herein, we present another screening endeavor using GRP78 as a target, hoping to identify antiviral/HBV activities in the medicinal herbs. Accordingly, we have further validated effectiveness of the Herbochip® drug screening platform using GRP78 as a molecular target, and subsequent experiments on ethanolic extract of Paeonia suffruticosa (PSE) lead us to conclude that the extract can be used for the treatment of HBV infection.
Methods
Preparation of plant extracts
Forty herbs were used for the primary screening (Table 1). Two of the herbs, namely Camptotheca acuminata and Polygonatum kingianum, were collected in wild and provided to us by Prof. GD Tao of Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences (Mengla, Yunnan, China). The plants were identified by Prof. Tao according to The Pharmacopoeia Commission of PR China [23]. The rest of the plant materials, including P. suffruticosa, were more common Chinese herbal medicines and were obtained from the Yunnan Baiyao Group Tianzihong Pharmaceutical Co. Ltd. (Kunming, Yunnan, China). Voucher specimens of all plant materials were stored in our laboratory. For large-scale preparation, plant material of P. suffruticosa Andr. (Cortex Moutan Radicis, the root cortex of P. suffruticosa, also known as mu-dan-pi in Chinese herbal medicine) was used. For small-scale preparation, 50 g of plant materials was extracted with 1 l 50% ethanol (1:20 w/v) at room temperature for 3 days. The ethanolic extracts were evaporated to 25 ml under reduced pressure (R200, Buchi, Swiss) at 60 °C and then adjusted to a concentration of 1 g/ml raw drug in 50% ethanol before used. For large amount of P. suffruticosa extract (PSE) to be used in subsequent experiments, the starting material was increased to 3 kg and processed according to the same protocol.
Table 1.
Hybridization reactivityof herbal extracts using GRP78 as a probe on herbochips
| Herbal extracts | Parts used | Specimen number | Hybridization reactivity |
|---|---|---|---|
| Acanthopanax senticosus | Root and stem | A1208A0350 | − |
| Agrimonia pilosa | Whole plant | G0709A0368 | +++ |
| Atractylodes chinensis | Root and stem | A0604A0084 | ++ |
| Bupleurum candollei | Stem and leaf | H0909B0187 | − |
| Camptotheca acuminata | Stem | B1108C0462 | − |
| Cassia obtusifolia | Seed | F1004C0160 | − |
| Cnidium monnieri | Seed | F1104C0161 | − |
| Coptis chinensis | Root | A0804C0142 | − |
| Cornus officinalis | Peel | M0303C0139 | ++ |
| Cuscuta chinensis | Seed | F0305C0168 | − |
| Dioscorea bulbifera | Tuber | B0204D0052 | ++ |
| Eclipta prostrata | Stem and leaf | H0909E0159 | − |
| Equisetum hiemale | Stem | B0507E0160 | − |
| Ganoderma lucidum | Sporophore | G0509G0132 | − |
| Herbal extracts | Parts used | Specimen number | Hybridization reactivity |
| Hedyotis diffusa | Stem and leaf | H0109H0148 | − |
| Isatis indigotica | Root | A0703I0013 | − |
| Isatis indigotica | Leaf | C1003I0015 | − |
| Lasiosphaera fenzlii | Sporophore | G0707L0177 | ++ |
| Ligustrum lucidum | Fruit | E0704L0041 | − |
| Lycium barbarum | Fruit | E1009L0162 | − |
| Lycium chinense | Root cortex | A1004L0044 | − |
| Onosma paniculatum | Root cortex | A1204O0013 | ++ |
| Paeonia lactiflora | Root | A1204P0095 | ++ |
| Paeonia suffruticosa | Root cortex | A0305P0100 | ++ |
| Phellodendron amurense | Bark | J0704P0079 | − |
| Picrorhiza scrophulariiflora | Root | A0604P0082 | − |
| Pleione bulbocodioides | Bulb | B0704P0099 | − |
| Polygonatum kingianum | Root | A0308P0239 | − |
| Polygonum cuspidatum | Root | A0904P0092 | ++ |
| Polyporus umbellatus | Sclerotium | G1208P0337 | − |
| Prunus persica | Seed | F0105P0111 | − |
| Psoralea corylifolia | Fruit | E0609P0361 | + |
| Pueraria thomsonii | Root | A0904P0080 | − |
| Rhaponticum uniflorum | Root | A0903R0025 | − |
| Rubia cordifolia | Whole plant | G0309R0113 | ++ |
| Scrophularia ningpoensis | Root | A0904S0074 | − |
| Scutellaria baicalensis | Root | A0609S0290 | − |
| Semiaquilegia adoxoides | Root | A1003S0067 | − |
| Sophora tonkinensis | Root and stem | A0709S0294 | − |
| Trichosanthes kirilowii | Root | A0105T0045 | − |
−, negative; +, weak positive, fluorescence value (FV) < 200; ++, moderate positive, FV between 200 and 1000; +++, strong positive, FV > 1000
Preparation of herbochips
Herbochips were fabricated as previous described [22]. Briefly, each plant extract obtained above was fractionated by a HPLC system (Model 1100, Agilent Technologies, USA) into 96 fractions, which were then collected into 96-well microplates. The samples were then vacuum-dried (Savant, Thermo Scientific, USA) and stored at 4 °C until used. For arraying, the samples in microplates were dissolved in Optifix I buffer [22], spotted onto surface-activated polystyrene plastic chips alongside with positive controls (Cy5-labeled streptavidin) by an automatic arrayer (Biodot A101, Shuai Ran Precision, Taiwan) according to the format specified (Fig. 1 of Ref. [22]). The slides were then dried, blocked with blocking buffer (0.1 M ethanolamine, 0.1 M sodium tetraborate), washed with TBST (50 mM Tris–HCl, pH 7.5; 0.15 M NaCl, 0.05% Tween 20) and water, then dried again [22]. The resulted slides, with HPLC fractions arrayed, were designated as herbochips and named with the herbs used. For instance, PSE-herbochip is a herbochip that was arrayed with PSE. The herbochips were sealed and stored at 4 °C until used.
Preparation of the drug target GRP78 (hGRP781-508)
DNA fragment of hGRP78 was amplified from human lung cDNA library (Stratagene, USA) as described previously [24]. Fragment encoding truncated form of hGRP781-508 was amplified by polymerase chain reaction (PCR) with forward primer XhoI-grp78 (5′-CCG CTC GAG GAG GAG GAC AAG AAG GAG-3′) and reverse primer grp78-BamHI (5′-CGG ATC CCT AGA TTG TGA TCT TAT TTT TGT TCC C-3′). Amplified PCR product was purified, digested with XhoI and BamHI, and then subcloned into XhoI/BamHI cut plasmid pET15b (Novagen, Germany) in frame with a hexahistidine N-terminal tag to generate pET15b-grp781-508. The plasmid was transformed into Escherichia coli BL21 Gold (DE3) strain. A single colony was selected to grow in 1 l Luria–Bertani (LB) medium (1% tryptone, 0.5% yeast extract, and 0.5% sodium chloride) containing 100 μg/ml ampicillin at 37 °C until OD600 reached 0.4–0.6. Protein expression was induced by addition of isopropyl b-d-1-thiogalactopyranoside (IPTG) to a final concentration of 0.5 mM and incubation at 37 °C for 3 h. Cells were harvested by centrifugation and resuspended in 80 ml of equilibrium buffer (20 mM Tris–HCl, 100 mM NaCl, pH 8.0) supplemented with 1 mM protease inhibitor phenylmethylsulfonyl fluoride (PMSF) and disrupted by a three passages through EmulsFlex-C3 high-pressure homogenizer (Avestin, Canada) at 15,000 psi. Cell lysate was centrifuged at 16,000×g for 30 min at 4 °C and the resulting supernatant was purified by Ni-Sepharose 6 Fast Flow (GE Healthcare, USA) equilibrated with 50 ml of equilibrium buffer. Subsequently, the column was washed with 200 ml equilibrium buffer supplemented with 5 mM imidazole, followed by 100 ml equilibrium buffer supplemented with 20 mM to 150 mM imidazole. Protein purity was analyzed by 12% (w/v) SDS-PAGE. Finally, the fraction of His-hGRP781-508 eluted with 150 mM imidazole-containing equilibrium buffer was concentrated and buffer-exchanged to coupling buffer (100 mM NaHCO3, pH 8.0) using an Amicon Ultra-15 centrifugal filter unit (Millipore, Germany), and protein concentration was determined by bicinchoninic acid (BCA) protein assay reagent kit (Thermo Scientific, USA).
Biotinylation of GRP78 and screening for its binding activity on herbochips
For biotinylation, 50 μl BNHS solution (2 mg/ml biotinamidohexanoic acid N-hydroxysuccinimide ester in DMSO) was added into 1 mg purified His-hGRP781-508 in 1 ml coupling buffer. The biotinylation reaction was carried out for 1 h on ice and then stopped by adding equal volume of glycine (50 mM in coupling buffer). The biotinylated His-hGRP781-508 (designated as b-GRP78) was then dialyzed against coupling buffer to remove unreacted BNHS and excess glycine. For hybridization, each cave on the chips was covered by a cover glass (22 × 22 mm) under which 20 μl b-GRP78 in TBST (50 mM Tris–HCl, pH 7.5; 0.15 M NaCl, 0.05% Tween 20) or TBST alone was added. The slides were then incubated at 37 °C for 1 h. While placed in a slide washing jar, the slides were washed in TBST (4 × 2 min), rinsed in water (4 × 2 min) on a horizontal shaker at 80 rpm at room temperature, and then dried at 37 °C (30 min). The same hybridization procedure was used in the subsequent reaction with Cy5-labeled streptavidin (SA-Cy5) where 20 μl 2.5 μg/ml SA-Cy5 in TBST was used. The dried slides were scanned by a laser scanner (GenePix4100 A, Axon, USA) and the fluorescent intensity of red spots in the image was analyzed at emission wavelength of 635 nm by GenePix 5.0 Software. The fluorescence value (FV) of each hybridization reaction was then recorded.
Cells and cell culture
HepG2 (ATCC HB-8065) and AML12 (ATCC CRL-2254) cells were purchased from American Type Culture Collection (ATCC). HepG2 2.2.15 cells were kindly provided by Dr. M.-S. Ho (Academia Sinica, Taiwan). The cells were maintained in Dulbecco’s Modified Eagle Medium containing 4.5 g/l d-glucose and l-glutamine (Gibco, Invitrogen, USA) supplemented with 10% fetal bovine serum containing 1.5 g/l sodium bicarbonate, 0.1 mM nonessential amino acids, 1.0 mM sodium pyruvate, and 100 units/ml penicillin G and 100 μg/ml streptomycin. Cells were incubated at 37 °C in a humidified atmosphere containing 5% CO2. G418 was added to the medium for maintenance of HepG2 2.2.15 cells at a final concentration of 200 μg/ml. Cells grown in various culture dishes at 80% confluence were used in all experiments.
Determinations of cytotoxicity and in vitro anti-HBV activity of the herbal extracts
The cytotoxicity effect of herbal extracts on growth of cells was determined by MTT method (Sigma, USA). The cells were seeded in 96-well culture plates at a density of 5 × 103 cells per well and cultured at 37 °C for 24 h. The culture medium was then removed and replaced with fresh medium supplemented with 50 μg/ml herbal extracts, and cultured for 72 h. Negative control of cell growth was carried out by adding the same volume of 50% ethanol in the medium (final concentration of 0.25%). Three hours prior to termination of the culture, 100 μl MTT (0.5 mg/ml in serum free medium) was added to the monolayer of cells. After incubation at 37 °C for 3 h, medium was removed and 100 μl DMSO was added to each well at 37 °C for 20 min to solubilize the formazan. Cell growth was monitored by measuring absorbance at 570 nm (ImageQuant, GE Healthcare, USA). Cell survivals after treatments were presented as % viability = A Treatment/A Control × 100%, where A Treatment and A Control indicates the absorbance of the treated and the control samples, respectively. In vitro anti-HBV activity was determined by inhibition of HBsAg and HBeAg secretion in HepG2 2.2.15 cells. HepG2 2.2.15 cells were plated at a density of 5 × 103 cells per well on 96-well plate, cultured, and treated exactly as described above. After treatments, the supernatants were collected, centrifuged at 5000 rpm for 10 min to remove cellular debris, and then immediately used for HBsAg or HBeAg detection. The levels of HBsAg and HBeAg in the culture medium were, respectively, measured by SURASE B-96 (TMB) and EASE BN-96 (TMB) ELISA kits (General Biological Corp. Taiwan) according to the manufacturer’s instructions. Absorbance at 450 nm was measured, and the data were calculated as the percentage of antigen levels by the formula: relative levels (%) = A Treatment/A Control × 100%, where A Treatment and A Control indicates the absorbance of the treated and the untreated control samples, respectively. All samples were examined in triplicates.
Animal, animal treatments and measurements of in vivo anti-DHBV activity
The duck hepatitis B virus infection model was employed for assessing the in vivo anti-HBV effects of PSE [25, 26]. Thirty 1-day-old DHBV-negative Pekin Aylesbury ducks (Anas domesticus platyrhyncos) were obtained from a commerical hatchery and were held in the animal house facilities of the School of Pharmacology, Fudan University (Shanghai, China). All animal handling procedures were approved by Fudan University animal ethics committee (Additional files 2 and 3). The ducklings were maintained under normal daylight and fed with a standard commercial diet with water ad libitum. After intravenously infected with duck hepatitis B virus (DHBV) for 7 days, 30 DHBV-positive duck were selected and separated into five groups (n = 6). Three groups were administered orally in diet with 150, 300, and 600 mg/kg/day PSE. Diet mixed with PBS or ADV (10 mg/kg/day) was served, respectively to the other two groups as negative and positive control. In vivo anti-HBV activity was determined by reduction of DHBV DNA (viral load) in serum and liver in the DHBV-challenged ducklings. Serum samples were collected from treated ducks on days 5, 10, and 13 (3 days after cessation of treatment for 10 days, referred to as day P3 hereafter). Fifty microliters of serum sample were used for DHBV DNA measurement by dot blot analysis using DIG High Prime DNA Labeling and Detection Starter Kit I (Roche Life Science, USA). DHBV DNA in liver was measured by Southern blotting. Five hundred milligrams of duck liver tissues were ground in 6 ml of a buffer [10 mM Tris–HCl, pH 7.6, 100 mM EDTA, 0.5% (w/v) SDS], and then digested with protease K at 55 °C overnight, followed by centrifugation at 13,000×g for 10 min. The supernatant was treated with RNase A at 37 °C overnight, and then extracted with phenol/chloroform. DNA was precipitated with ethanol, dissolved in 100 μl ddH2O, separated on a 1% agarose gel, and analyzed by Southern blotting using DIG High Prime DNA Labeling and Detection Starter Kit I (Roche Life Science, USA).
Statistical analysis
All data were expressed as mean ± SD. Comparison between groups was carried out by One-way ANOVA. Results with p < 0.05 were considered statistically significant.
Information of experimental design and resources
Details of the experimental design, and statistics, and resources used in this study were included in the Minimum Standards of Reporting Checklist (Additional file 1).
Results
Preparation of GRP78 and herbochip screening
Preparation of biotinylated hGRP781-508 (b-GRP78)—Full length hGRP78 was not able to be expressed in E. coli due to its toxicity and insolubility, thus a truncated but fully functional hGRP781-508 was used herein [27]. Figure 1a showed that hGRP781-508 with N-terminal His-tag expressed in E. coli BL21-Gold (DE3) was successfully expressed upon induction with 0.5 mM IPTG. The protein was purified by a nickel-affinity column chromatography with a yield of 30 mg/l of culture medium and purity of 90% (analyzed by Gel-Pro Analyzer software, data not shown). The molecular weight of recombinant His-hGRP781-508 was determined to be 58,193 Da by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) (Fig. 1b). The purified hGRP781-508 was buffer-exchanged and concentrated in coupling buffer (100 mM NaHCO3, pH 8.0) using an Amicon protein concentrator (30-kDa cut-off) and subjected to further biotinylation. One milligrams of purified His-hGRP781-508 was biotinylated, renamed as b-GRP78 and used as the probe for subsequent screening on herbochips.
Fig. 1.
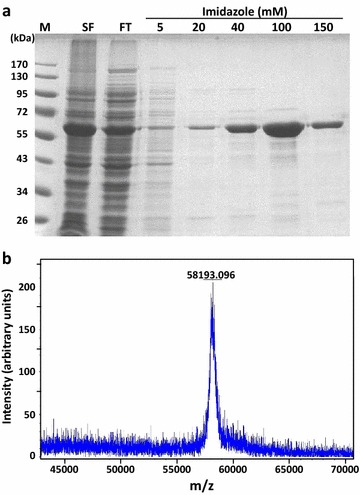
Purification and molecular weight analysis of His-hGRP781-508. a Escherichia coli culture was induced with 0.5 mM IPTG at 37 °C for 3 h, the supernatant of cell lysate containing His-hGRP781-508 was collected by centrifugation and subjected to nickel-affinity chromatography. His-hGRP781-508 was eluted with different imidazole concentration of 5, 20, 40, 100, and 150 mM. Aliquots of each fraction were analyzed by 12% (w/v) SDS-PAGE. M molecular weight marker; SF soluble fraction; FT Flow-through. b Mass determination of His-hGRP781-508 was performed by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) operating in the electrospray ionization mode. The data were acquired over the mass-to-charge ratio (m/z) range of 43,000–71,000 under normal scan resolution (x axis), the relative intensity (arbitrary units) is shown on the y axis. The data from each spectra were summed and deconvoluted
Herbochip screening for GRP78 binding activity—Ethanolic extracts of 40 Chinese herbal medicines (Table 1) were fractionated by HPLC, and 96 fractions of each herb were separately blotted on herbochips, thus a total of 40 herbochips were screened using bGRP78 as the probe. Binding activities were visualized using Cy5-conjugated streptavidin, and the spots with red fluorescent signals indicated target protein binding to the corresponding herbal fractions (Fig. 2, left panels). As mentioned in the Method section, data from four chips containing HPLC fractions from extracts of Dioscorea bulbifera (DBE), Lasiosphaera fenzlii (LFE), P. suffruticosa, and Polygonum cuspidatum (PCE) are shown in Fig. 2. The HPLC profiles (A 254) together with quantified binding signals (fluorescence intensity) were also presented (right panels) in which positive binding signals were found in spots corresponding to fractions 6–40 of DBE, 65–73 of LFE, 11–70 of PSE and 11–48 of PCE. These four herbs were then subjected to further studies as mentioned below.
Fig. 2.
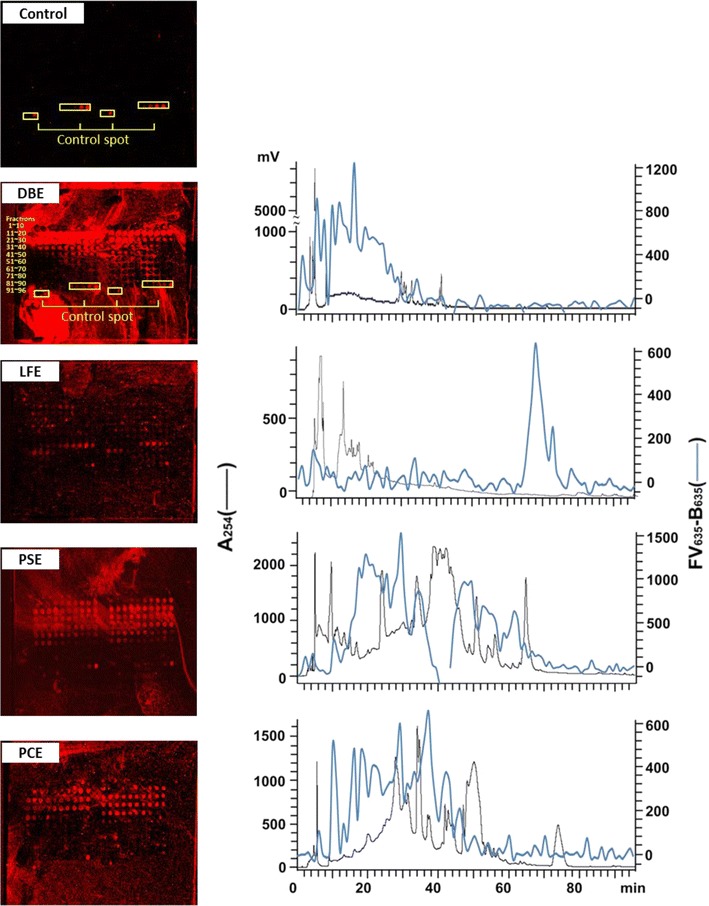
Binding signals of DBE-, LFE-, PSE-, and PCE-herbochips probed by GRP78. Left panels the images were visualized by Cy5-labeled streptavidin after the binding of b-GRP78 to DBE-, LFE-, PSE-, and PCE-herbochips, which were fabricated with extracts from Dioscorea bulbifera, Lasiosphaera fenzlii, Paeonia suffruticosa, and Polygonum cuspidatum, respectively. The spots in the control were 4, 10, 50, 250 ng/ml biotin in Optifix I, 1 μg/ml SA-Cy5 in Optifix II (positive control) and Optifix I (negative control) as those shown in Fig. 1 of Ref. [22]. Right panels the fluorescence intensity of the corresponding images were quantized by a scanner at 635 nm presented (back line) together with the original HPLC profile monitored at 254 nm (blue line). Positive signals indicated binding activity of the fractions to GRP78
Cytotoxicity of herbal extracts DBE, LFE, PSE, and PCE on AML12 and HepG2 2.2.15 cells
The effects of the above four herbal extracts on cell viability were evaluated by MTT assay using two different cell lines, AML12 and HepG2 2.2.15. AML12 is derived from alpha mouse liver cell and serves as a normal liver cell control. HepG2 2.2.15 is a widely accepted and well-established cell line for the studies of HBV life cycle and potential HBV inhibitor screening since late 1990s [19]. It is derived from hepatoma HepG2 cells and integrated with a greater than 1-unit-length HBV genome, which not only produces HBV particles but also HBsAg and HBeAg in a continuous manner. Adefovir dipivoxil (ADV), an adenosine monophosphate analog, is approved by US Food and Drug Administration for treatment of hepatitis B. It is an inhibitor of HBV DNA polymerase and competes with natural substrate deoxyadenosine triphosphate; hence, it was used as a control drug. The results showed that there is no suppression of cell viability in AML12 cells treated with extracts from D. bulbifera (DBE), L. fenzlii (LFE), P. suffruitcosa and P. cuspidatum (PCE) at a concentration of 50 μg/ml for 3 days, suggesting that these extracts were not cytotoxic to AML12 cells (Fig. 3a). In HepG2 2.2.15 cells, however, treatment with PCE at 50 μg/ml for 3 days resulted in a 33.94% reduction of cell viability; no effect was observed in the other three herbal extracts (Fig. 3b).
Fig. 3.
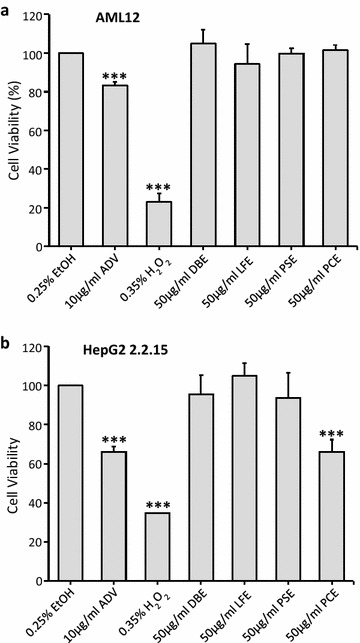
Cytotoxicity of the herbal extracts on AML12 and HepG2 2.2.15 cells. AML12 (a) and HepG2 2.2.15 (b) cells were treated for 72 h with same dosage (50 μg/ml) of ethanolic extracts of Dioscorea bulbifera, Lasiosphaera fenzlii, Paeonia suffruticosa and Polygonum cuspidatum (labeled as DBE, LFE, PSE, and PCE). EtOH and ADV were used as negative and positive controls, respectively. After treatments, cell viability was measured by MTT assay and presented as % of untreated control. The data represent the mean ± SD (n > 3). ***p < 0.001. EtOH 0.25% ethanol; ADV 10 μg/ml adefovir; H 2 O 2 0.35%
In vitro anti-HBV activity of herbal extracts in HepG2 2.2.15 cells
For anti-HBV activity assay, secretion levels of HBsAg and HBeAg in the supernatant of HepG2 2.2.15 medium were determined by ELISA using, respectively, SURASE B-96 and EASE BN-96 (TMB) ELISA Kits (General Biologicals Corp., Taiwan). Secretion of HBsAg antigen was significantly suppressed in cells treated with PSE and PCE, but not DBE and LFE at a concentration of 50 μg/ml for 3 days (Fig. 4a). The inhibition rates in PSE- and PCE-treated cells were 56.32 and 41.77%, respectively. In terms of HBeAg, the inhibition rates in PSE-, PCE-, and LFE-treated cells were 49.55, 37.00, and 56.44%, respectively, while DBE was ineffective under the same treatment protocol (Fig. 4b). However, the inhibitory effect of PCE to HBV secretion might be due to cytotoxicity to HepG2 2.2.15 cells as reported above. Taken together, PSE was non-toxic to both cell lines but inhibited secretion of both HBV antigens significantly. Hence, it may serve as a potential natural inhibitor from Chinese herbs for targeted anti-HBV therapy and is subjected to the following in vivo studies.
Fig. 4.
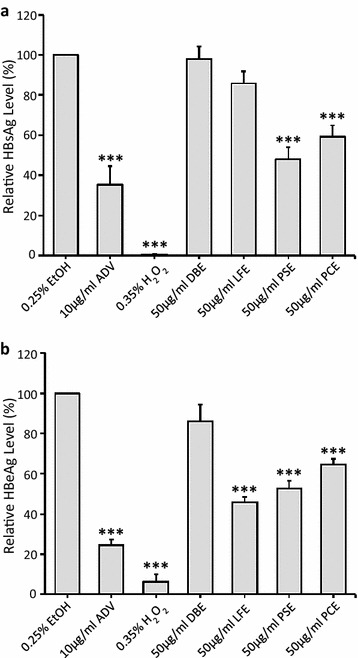
Inhibition of HBsAg and HBeAg secretion by treatment with the herbal extracts on HepG2 2.2.15 cells. HBeAg and HBsAg producing HepG2 2.2.15 cells were treated for 72 h with same dosage (50 μg/ml) of ethanolic extracts of Dioscorea bulbifera, Lasiosphaera fenzlii, Paeonia suffruticosa, and Polygonum cuspidatum (labeled as DBE, LFE, PSE, and PCE). EtOH and ADV were used as negative and positive control, respectively. After treatments, levels of HBsAg (a) and HBeAg (b) in the culture media were determined by ELISA and presented as % of untreated control. The data represent the mean ± SD (n > 3). ***p < 0.001. EtOH 0.25% ethanol; ADV 10 μg/ml adefovir; H 2 O 2 0.35%
In vivo anti-HBV activity of PSE in ducks
To further confirm antiviral activity of PSE, serum and liver DHBV DNAs of DHBV-infected ducks were measured after treatment with PSE at various dosages (Table 2). It was observed that DHBV DNA level in the serum of infected ducklings decreased upon treatment with PSE. The inhibition rate was, respectively, 24.14, 41.97, and 35.28% at the dosage of 150, 300, and 600 mg/kg/day after 5 days of treatment (Table 2, T5). At the same dosage after 10 days of treatment, the inhibition rate reduced to 14.46, 39.04, and 19.02% (Table 2, T10). Interestingly, on day P3 (3 days after the cessation of treatment), the inhibition rate was 20.01% at a dosage of 300 mg/kg/day PSE (Table 2, P3), similar to that of ADV at a dosage of 10 mg/kg/day (24.22%). Additionally, livers of infected ducks were obtained on day P3, and the DHBV DNA level was measured by Southern blotting. Densitomertic analysis of the signals showed that the inhibition rates of DHBV DNA upon treatment with 150, 300, and 600 mg/kg/day PSE were, respectively, 13.94, 28.72, and 27.55% (Table 2, the rightmost column). Although the differences were not statistically significant, the reductions of DHBV DNA levels in liver were consistent to those in serum. Taken together, these data suggested that PSE possesses in vivo anti-HBV activity with the highest inhibition rate obtained at 300 mg/kg/day. Moreover, the DHBV DNA level in infected ducks treated with PSE at this dosage rebounded to a less extent after the cessation of treatment as compared to the ADV treated group.
Table 2.
Inhibitory effects of Paeonia suffruticosa extract (PSE) on DHBV DNA level in duckling serum or liver
| Agent | Dose (mg/kg/day) | Inhibition rate on day of treatment (T) or post-treatment (P) (%) | |||
|---|---|---|---|---|---|
| Serum | Liver | ||||
| T5 | T10 | P3 | P3 | ||
| Adefovir dipivoxil (ADV) | 10 | 83.72*** | 93.11*** | 24.22** | 46.52*** |
| Paeonia suffruticosa extract | 150 | 24.14** | 14.46* | 0.69 | 13.94 |
| 300 | 41.97* | 39.04 | 20.01 | 28.72 | |
| 600 | 35.28 | 19.02 | 2.38 | 27.55 | |
* p < 0.05, ** p < 0.01, *** p < 0.001
Discussion
Herbal medicines were used in China and other ancient civilizations for thousands of years; however, most of the mechanisms of their therapeutic effects remain unknown. Herein, we further demonstrated that using the target-based Herbochip® screening platform indeed greatly facilitated discovery and development of herbal drugs for therapeutic uses. As described, the Herbochip® technology reported here is basically a reverse screening method, in which potential chemical leads in herbal extracts have been immobilized and drug target protein is used as the probe for screening process. The success of this technology may be attributed by direct interaction between herbal extracts and diseased-related target proteins, which increase higher possibility of those extracts to act as a regulator to diseases. Prior to the current study, we have examined cytochrome P450 3A5 [21] and TNF-α [22] and the results, respectively, led to the discovery of S. flavescens extract and G. wilfordii extract for possible application in anti-HIV and anti-inflammatory drug development [21, 22].
Herein, we extend the utilization of the herbochip technology employing GRP78 as a drug target for anti-HBV activity screening. We cloned and purified hGRP781-508 as a drug target to screen against a herbal library of 40 so-called heat removing medicines. Among 40 herbal species screened, 11 were found to bind hGRP78 in which four herbal extracts were subjected to further studies. At the end of the day, the ethanolic extract of the root cortex of P. suffruticosa (PSE, also known as Cortex Moutan Radicis or mu-dan-pi in Chinese herbal medicine), one of the positive samples, was demonstrated to be non-cytotoxic and able to suppress HBsAg and HBeAg secretion in HepG2 2.2.15 cells. Also, serum and liver DHBV DNA levels in DHBV-infected ducks significantly reduced after oral supplement of PSE. These results clearly revealed that PSE possessed anti-HBV activity both in vitro and in vivo. Cortex Moutan Radicis is a well-known Chinese herbal medicine for heat removing and blood cooling [23]. Modern pharmacological studies indicated that this herb possess, among others, anti-inflammatory [28, 29], anti-diabetic [29, 30], and anti-cancer [31, 32] activities. Nevertheless, this study represents the first report on anti-HBV activity of PSE. The anti-HBV activity of PSE is relatively more specific as compared to that of PCE; because of its low general cytoxicity, further development of PSE as anti-HBV agent for therapeutic used is thus warranted. It is worth to note that GRP78, a molecular chaperone, is not only a target for HBV protein maturation and assembly, but also a target/marker for oncogenesis [33–35]. Therefore, all positive signals in herbochip screening using GRP78 as the probe can be re-investigated for their anti-cancer activities. Some of the experiments are already underway and the results will be reported elsewhere.
In summary, we have demonstrated that GRP78, in addition to cytochrome P450 3A5 and TNF-α, can be used as a probe for anti-HBV drug screening on herbochips. We have also shown that one of the positive signals, namely PSE, possesses in vitro and in vivo anti-HBV activities. Our data suggest that PSE may be a potential anti-HBV agent for further therapeutic use.
Conclusion
We have validated effectiveness of the Herbochip® drug screening platform and demonstrated that GRP78 can be used as a probe for anti-HBV drug screening on the platform. Subsequent experiments on PSE, one of the positive signals, does not exhibit general cytotoxicity. However, PSE possesses in vitro and in vivo anti-HBV activities. These observations lead us to conclude that PSE may be a potential anti-HBV agent for further therapeutic use.
Additional files
Additional file 1. Minimum Standards of Reporting Checklist.
Additional file 2. Ethics approval document of Ducklings experiments.
Additional file 3. The ARRIVE Guidelines Checklist.
Authors’ contributions
YKL, XYY, MDTC, YFZ, YTC conceived and designed the studies, as well as provided financial supports for the works. MH performed plant extractions, herbochip preparation, and screening. IFL, PWY, SKN, and YCL performed the target preparation; these four authors, together with YLT and YFZ, performed all the in vitro assays. MH and XYY performed in vivo anti-HBV assays. YKL, IFL, YTC, and YCL carried out final analysis of data and manuscript preparation. All authors read and approved the final manuscript.
Acknowledgements
This study was supported in parts by a research grant from Yunnan Province Division of Technology, Kunming, Yunnan, China, and a research grant to YKL from the Ministry of Science and Technology, Taiwan (MOST 103-2320-B-007-003-MY3). SKN was supported by J.-C. Shen Tsing Hua Foundation.
Competing interests
The authors declare that they have no competing of interests.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its Additional files 2 and 3.
Funding
This study was supported in parts by a research grant from Yunnan Province Division of Technology, Kunming, Yunnan, China, and a research grant to YKL from the Ministry of Science and Technology, Taiwan (MOST 103-2320-B-007-003-MY3). SKN was supported by J.-C. Shen Tsing Hua Foundation. The funding agencies did not have any role in collection, analysis, interpretation of data, writing of the report or the decision to submit the paper for publication.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- GRP78
78 kDa glucose-regulated protein
- HBV
hepatitis B virus
- DHBV
duck hepatitis B virus
- DBE
Dioscorea bulbifera extract
- LFE
Lasiosphaera fenzlii extract
- PSE
Paeonia suffruticosa extract
- PCE
Polygonum cuspidatum extract
- HBsAg
HBV surface antigen
- HBeAg
HBV envelope antigen
- ADV
Adefovir dipivoxil
Contributor Information
Iao-Fai Lam, Email: s104080402@m104.nthu.edu.tw.
Min Huang, Email: apirl19810411@aliyun.com.
Margaret Dah-Tysr Chang, Email: dtchang@life.nthu.edu.tw.
Pei-Wun Yao, Email: her159@msn.com.
Yu-Ting Chou, Email: ytchou@life.nthu.edu.tw.
Sim-Kun Ng, Email: s100080821@m100.nthu.edu.tw.
Ying-Lin Tsai, Email: yapvictory@gmail.com.
Yu-Chang Lin, Email: w1990712w@hotmail.com.
Yun-feng Zhang, Email: zhyunfeng001@aliyun.com.
Xiao-yuan Yang, Email: yangxiaoyuan@vip.sina.com.
Yiu-Kay Lai, Email: yklai@life.nthu.edu.tw.
References
- 1.World Health Organization. Hepatitis B. Fact sheet No 204. 2015. http://www.who.int/mediacentre/factsheets/fs204/en/.
- 2.Stasi C, Silvestri C, Voller F, Cipriani F. The epidemiological changes of HCV and HBV infections in the era of new antiviral therapies and the anti-HBV vaccine. J Infect Public Health. 2016;9(4):389–395. doi: 10.1016/j.jiph.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Yang JD, Nakamura I, Roberts LR. The tumor microenvironment in hepatocellular carcinoma: current status and therapeutic targets. Semin Cancer Biol. 2011;21:35–43. doi: 10.1016/j.semcancer.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15(Suppl 4):5–13. doi: 10.1634/theoncologist.2010-S4-05. [DOI] [PubMed] [Google Scholar]
- 5.Balsano C, Alisi A. Viral hepatitis B: established and emerging therapies. Curr Med Chem. 2008;15:930–939. doi: 10.2174/092986708783955383. [DOI] [PubMed] [Google Scholar]
- 6.Clark HP, Carson WF, Kavanagh PV, Ho CP, Shen P, Zagoria RJ. Staging and current treatment of hepatocellular carcinoma. Radiographics. 2005;25(Suppl 1):S3–S23. doi: 10.1148/rg.25si055507. [DOI] [PubMed] [Google Scholar]
- 7.Yeo W, Mok TS, Zee B, Leung TW, Lai PB, Lau WY, Koh J, Mo FK, Yu SC, Chan AT, Hui P, Ma B, Lam KC, Ho WM, Wong HT, Tang A, Johnson PJ. A randomized phase III study of doxorubicin versus cisplatin/interferon α-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532–1538. doi: 10.1093/jnci/dji315. [DOI] [PubMed] [Google Scholar]
- 8.Koumbi L. Current and future antiviral drug therapies of hepatitis B chronic infection. World J Hepatol. 2015;7(8):1030–1040. doi: 10.4254/wjh.v7.i8.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tawada A, Kanda T, Yokosuka O. Current and future directions for treating hepatitis B virus infection. World J Hepatol. 2015;7:1541–1552. doi: 10.4254/wjh.v7.i11.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lau DT, Bleibel W. Current status of antiviral therapy for hepatitis B. Therap Adv Gastroenterol. 2008;1:61–75. doi: 10.1177/1756283X08093944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qi FH, Wang ZX, Cai PP, Zhao L, Gao JJ, Kokudo N, Li AY, Han JQ, Tang W. Traditional Chinese medicine and related active compounds: a review of their role on hepatitis B virus infection. Drug Discov Ther. 2013;7:212–224. doi: 10.5582/ddt.2013.v7.6.212. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Jiang HY, Shi Y, He JL, Su S, Chen Z. Chinese herbal medicine for carriers of the hepatitis B virus: an updated systematic review and meta-analysis. Pharmazie. 2014;69:723–730. [PubMed] [Google Scholar]
- 13.Huang KL, Lai YK, Lin CC, Chang JM. Inhibition of hepatitis B virus production by Boehmeria nivea root extract in HepG2 2.2.15 cells. World J Gastroenterol. 2006;12:5721–5725. doi: 10.3748/wjg.v12.i35.5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang KL, Lai YK, Lin CC, Chang JM. Involvement of GRP78 in inhibition of HBV secretion by Boehmeria nivea extract in human HepG2 2.2.15 cells. J Viral Hepat. 2009;16:367–375. doi: 10.1111/j.1365-2893.2009.01072.x. [DOI] [PubMed] [Google Scholar]
- 15.Chang JM, Huang KL, Yuan TT, Lai YK, Hung LM. The anti-hepatitis B virus activity of Boehmeria nivea extract in HBV-viremia SCID mice. Evid Based Complement Altern Med. 2010;7:189–195. doi: 10.1093/ecam/nem180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Z, Jensen G, Yen TS. Activation of hepatitis B virus S promoter by the viral large surface protein via induction of stress in the endoplasmic reticulum. J Virol. 1997;71:7387–7392. doi: 10.1128/jvi.71.10.7387-7392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim SO, Park SG, Yoo JH, Park YM, Kim HJ, Jang KT, Cho JW, Yoo BC, Jung GH, Park CK. Expression of heat shock proteins (HSP27, HSP60, HSP70, HSP90, GRP78, GRP94) in hepatitis B virus-related hepatocellular carcinomas and dysplastic nodules. World J Gastroenterol. 2005;11:2072–2079. doi: 10.3748/wjg.v11.i14.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang HC, Huang W, Lai MD, Su IJ. Hepatitis B virus pre-S mutants, endoplasmic reticulum stress and hepatocarcinogenesis. Cancer Sci. 2006;97:683–688. doi: 10.1111/j.1349-7006.2006.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma Y, Yu J, Chan HL, Chen YC, Wang H, Chen Y, Chan CY, Go MY. Glucose-regulated protein 78 is an intracellular antiviral factor against hepatitis B virus. Mol Cell Proteomics. 2009;8:2582–2594. doi: 10.1074/mcp.M900180-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang SC, Hsu LW, Chen JP, Lee JW. Herbal chip. 2003. United States Patent 6645719.
- 21.Lee SS, Zhang B, He ML, Chang VS, Kung HF. Screening of active ingredients of herbal medicine for interaction with CYP450 3A4. Phytother Res. 2007;21:1096–1099. doi: 10.1002/ptr.2149. [DOI] [PubMed] [Google Scholar]
- 22.Huang M, Yao PW, Chang MD, Ng SK, Yu CH, Zhang YF, Wen ML, Yang XY, Lai YK. Identification of anti-inflammatory fractions of Geranium wilfordii using tumor necrosis factor-alpha as a drug target on Herbochip®—an array-based high throughput screening platform. BMC Complement Altern Med. 2015;15:146. doi: 10.1186/s12906-015-0665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pharmacopoeia of Peopleʼs Republic of China, Part 1. 2010.
- 24.Shu CW, Sun FC, Cho JH, Lin CC, Liu PF, Chen PY, Chang MD, Fu HW, Lai YK. GRP78 and Raf-1 cooperatively confer resistance to endoplasmic reticulum stress-induced apoptosis. J Cell Physiol. 2008;215:627–635. doi: 10.1002/jcp.21340. [DOI] [PubMed] [Google Scholar]
- 25.Foster WK, Miller DS, Marion PL, Colonno RJ, Kotlarski I, Jilbert AR. Entecavir therapy combined with DNA vaccination for persistent duck hepatitis B virus infection. Antimicrob Agents Chemother. 2003;47:2624–2635. doi: 10.1128/AAC.47.8.2624-2635.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen ZY, Cheng AC, Wang MS, Xu DW, Zeng W, Li Z. Antiviral effects of PNA in duck hepatitis B virus infection model. Acta Pharmacol Sin. 2007;28:1652–1658. doi: 10.1111/j.1745-7254.2007.00641.x. [DOI] [PubMed] [Google Scholar]
- 27.King LS, Berg M, Chevalier M, Carey A, Elguindi EC, Blond SY. Isolation, expression, and characterization of fully functional nontoxic BiP/GRP78 mutants. Protein Expr Purif. 2001;22:148–158. doi: 10.1006/prep.2001.1424. [DOI] [PubMed] [Google Scholar]
- 28.Wu M, Gu Z. Screening of bioactive compounds from moutan cortex and their anti-inflammatory activities in rat synoviocytes. Evid Based Complement Alternat Med. 2009;6:57–63. doi: 10.1093/ecam/nem066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang MH, Feng L, Zhu MM, Gu JF, Jiang J, Cheng XD, Ding SM, Wu C, Jia XB. The anti-inflammation effect of Moutan Cortex on advanced glycation end products-induced rat mesangial cells dysfunction and high-glucose–fat diet and streptozotocin-induced diabetic nephropathy rats. J Ethnopharmacol. 2014;151:591–600. doi: 10.1016/j.jep.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Lau CH, Chan CM, Chan YW, Lau KM, Lau TW, Lam FC, Law WT, Che CT, Leung PC, Fung KP, Ho YY, Lau CB. Pharmacological investigations of the anti-diabetic effect of Cortex Moutan and its active component paeonol. Phytomedicine. 2007;14:778–784. doi: 10.1016/j.phymed.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Choi HS, Seo HS, Kim JH, Um JY, Shin YC, Ko SG. Ethanol extract of paeonia suffruticosa Andrews (PSE) induced AGS human gastric cancer cell apoptosis via fas-dependent apoptosis and MDM2-p53 pathways. J Biomed Sci. 2012;19:82. doi: 10.1186/1423-0127-19-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin MY, Lee YR, Chiang SY, Li YZ, Chen YS, Hsu CD, Liu YW. Cortex Moutan induces bladder cancer cell death via apoptosis and retards tumor growth in mouse bladders. Evid Based Complement Altern Med. 2013;2013:207279. doi: 10.1155/2013/207279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang J, Guo YS, Zhang Y, Yu XL, Li L, Huang W, Li Y, Chen B, Jiang JL, Chen ZN. CD147 induces UPR to inhibit apoptosis and chemosensitivity by increasing the transcription of Bip in hepatocellular carcinoma. Cell Death Differ. 2012;19:1779–1790. doi: 10.1038/cdd.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Z, Zhao C, Li Z, Zhao Y, Shan S, Shi T, Li J. Reconstructed mung bean trypsin inhibitor targeting cell surface GRP78 induces apoptosis and inhibits tumor growth in colorectal cancer. Int J Biochem Cell Biol. 2014;47:68–75. doi: 10.1016/j.biocel.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 35.Nagelkerke A, Bussink J, Sweep FC, Span PN. The unfolded protein response as a target for cancer therapy. Biochim Biophys Acta. 2014;1846:277–284. doi: 10.1016/j.bbcan.2014.07.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Minimum Standards of Reporting Checklist.
Additional file 2. Ethics approval document of Ducklings experiments.
Additional file 3. The ARRIVE Guidelines Checklist.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its Additional files 2 and 3.


