Graphical Abstract
Introduction
Type I diabetes is an autoimmune disorder characterized by a lack of insulin production by the beta cells of the pancreas [Yoon, 2005 #48]. This lack of insulin causes a variety of systemic effects on whole body metabolism. Poorly managed type 1 diabetes can lead to cardiovascular disease, diabetic neuropathy, and diabetic retinopathy [Sowers, 2001 #70;Said, 2007 #71;Barber, 2003 #72;Vinik, 1992 #73]. Increasingly, even well managed Type 1 diabetic patients show damage to peripheral organs related to complications from the disease. Insulin’s central role in energy homeostasis renders it an important signaling factor in the reproductive tract as well. Type 1 diabetes has now been shown to cause defects in sperm and testes, and the aim of this review is to present the known effects of insulin’s role in the function of the male reproductive tract. These effects may be mediated through hormonal alterations in the hypothalamic pituitary gonadal axis, or through the direct interaction of insulin on the testes and sperm cells. Although fertility complications also occur in Type II diabetics, this review will focus on the defects specifically linked with the lack of insulin seen in Type I diabetes.
Many diabetic animal models and human diabetes patients experience problems with spermatogenesis and/or fertility. The link between Type I diabetes has long been established. Accounts dating as far back as the 11th century have described the disease as, “a collapse of sexual functions”, highlighting the importance of insulin in the reproductive system. Currently, many animal diabetic models and human diabetic patients experience problems with spermatogenesis and/or fertility. Even after treatment of Type 1 diabetes with insulin, there continues to be a detrimental effect of diabetes on reproductive capacity.
Diabetes is associated with reduced sperm parameters in affected males. It is yet unclear whether the damage is due to local effects from hyperglycemia or by alterations in hormone levels which disrupt the hypothalamic-pituitary gonadal axis. The recent discovery that both the testes and sperm produce insulin brings a new perspective on how diabetes may contribute to subfertility. Indeed, insulin expression in the testes also seems to be affected by diabetes, with streptozocin-induced diabetic rats expressing less than half of the insulin protein compared to controls [Gomez, 2009 #29]. This suggests that insulin may have an important role in spermatogenesis. In addition to the testes, sperm cells have also been shown to contain both insulin mRNA and protein [Aquila, 2005 #50]. These cells are activated by insulin to induce pAKT phosphorylation, suggesting a functional role in insulin signaling. Additionally, these cells have been shown to secrete insulin in response to glucose administration. These contributions open a new avenue of research into the functions of insulin in the reproductive tract as the specific role of insulin in the process of spermatogenesis and sperm motility and/or capacitation has not been determined.
In light of new data on the possible autocrine role of insulin in the testes and sperm, it is unclear how the pathogenesis of diabetic subfertility is mediated. Diabetes has numerous systemic effects, notably disruptions in the hypothalamic pituitary gonadal axis, which may ultimately contribute to a loss in fertility. Alternatively, disruptions in local testicular insulin signaling may also play a part in these fertility defects.
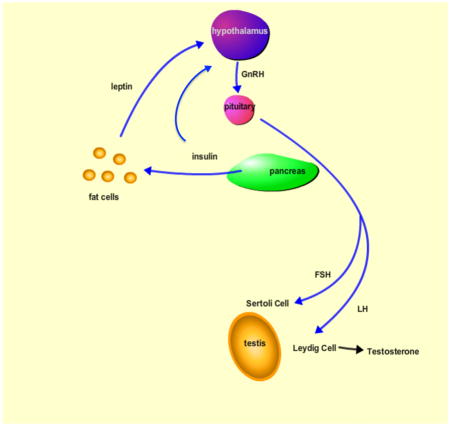
In a normally functioning hypothalamic pituitary gonadal axis, the hypothalamus releases GnRH pulses that stimulate the pituitary to secrete both luteinizing hormone (LH) and follicular stimulating hormone (FSH). LH and FSH act on the Sertoli cells and the Leydig cells, respectively, to stimulate the process of spermatogenesis. The onset of Type I diabetes is known to disrupt the HPG axis, resulting in impaired spermatogenesis and subsequent subfertility. Disruptions in any part of the HPG axis impair fertility, and this review will focus on how these disruptions lead to infertility. Specifically, we will focus on the effects of T1D on: 1) insulin and leptin levels, 2) GnRH pulses from the hypothalamus, 3) LH and FSH secretion from the pituitary, 4) testosterone secretion from the Leydig cells, and 5) sperm quality. Additionally, we will address the possibility of local insulin signaling within the testes and how T1D may locally affect the gonads.
Insulin Levels Mediate the Function of the HPG axis
Serum insulin has long been known to affect the central nervous system, and these effects could mediate whole body energy homeostasis, including the reproductive axis through further signaling to the pituitary and ultimately, the gonads. A study in 1977 by [Porte, 2005 #46;Porte, 2005 #46] showed the peripheral insulin injection caused an increase in insulin levels in cerebral spinal fluids, suggesting that insulin could potentially be a signal to the brain regarding energy stores and promoting whole-body energy homeostasis. Thus, a lack of insulin would signal to the brain a lack of energy supply and the central nervous system could potentially shut down extraneous energy-consuming processes such as reproductive function.
Indeed, insulin levels have dramatic effects on the regulation of the HPG axis. The effects of diabetes on the reproductive axis are mediated at least in part, by signaling in the brain. Insulin mediates its effects through binding with the insulin receptor, resulting in a signaling cascade. Through interactions with the insulin receptor substrate proteins, notably IRS-2, insulin potentiates signaling through PI 3-kinase, which then activates AKT, an important mediator of energy signaling [Boura-Halfon, 2009 #27].
Insulin signaling in the brain can happen at multiple sites. Insulin receptor expression has been detected in the hypothalamus, the olfactory bulb, and the pituitary [Havrankova, 1981 #22]. Additionally, insulin concentrations in the brain are markedly higher than plasma insulin levels, suggesting that insulin in the brain is not simply a reflection of serum levels but that there is a critical function of this signaling hormone in the central nervous system[Havrankova, 1978 #23].
Exactly how insulin in the brain impacts changes in the reproductive axis is unknown. An experiment using a brain-specific insulin receptor knockout revealed the connection between insulin signaling in the brain and fertility. The development of a neuron-specific insulin receptor knockout mouse (NIRKO) resulted in subfertile male mice. NIRKO mice display a significant reduction in fertility, with 46% of male mating leading to offspring versus 76% for controls. Male NIRKO mice display impaired spermatogenesis, which may account for this decline in fecundity. Histological examination revealed that although many of the seminiferous tubules appeared normal, about 20% did not have a lumen and had little or no mature sperm cells. Additionally, the Leydig cells appeared shrunken, suggesting that the lack of proper insulin signaling in the brain reduced the hormonal output necessary to retain the Leydig cell population to successfully promote spermatogenesis in all tubules. Also, there was a 60% reduction in circulating LH, indicating that the lack of insulin response in the brain reduces hypothalamic-pituitary axis function [Bruning, 2000 #24].
Other studies examining the mechanism of insulin signaling in the brain have found that insulin signaling in the brain is required for inhibition of glucose production. Injection of insulin directly into the brain resulted in decreased production of glucose independently of serum insulin levels. Injection of insulin signaling inhibitors resulted in an increased glucose production, despite circulating serum insulin levels. This study further demonstrates that neuronal insulin action is regulated separately from plasma insulin and may be involved in overall energy homeostasis mediated by the central nervous system. [Obici, 2002 #45]
Defects in the IRS signaling proteins can also have detrimental effects on fertility, since the insulin receptor substrate proteins are activated by the binding of insulin to its receptor. IRS-2 knockout males develop severe glucose intolerance and inadequate carbohydrate metabolism. Despite being initially fertile, IRS-2 knockout mice become infertile after the onset of diabetes. This suggests that it is the diabetic state and not the lack of IRS-2 that is responsible for the impairment in male reproductive capacity. Therefore, any number of hormones or glucose response pathways could be responsible for the subfertility in IRS-2 knockout males [Burks, 2000 #62]. IRS-2 protein may also mediate its effects through signaling to the brain. IRS-2 protein has been localized to the AR hypothalamus along with insulin receptor. Activation with insulin results in tyrosine phosphorylation of IRS-2 corresponding to activation of PI3-kinase, indicating that insulin signaling via the [Porte, 2005 #46]
T1D Males have decreased leptin levels, correlating to HPG Axis Dysfunction
Insulin’s effects on the reproductive axis are not solely mediated by insulin interactions with receptors in the brain. Insulin levels are also known to directly correlate to circulating levels of leptin, an important molecule involved in maintaining energy homeostasis. Leptin is an important signaling molecule secreted by the fat cells that signals to the hypothalamus that regulates the reproductive system. Leptin likely serves as a metabolic symbol that informs the brain of nutritional status and provides information regarding an animal’s ability to meet the energy demands of reproduction [Barash, 1996 #19].
Insulin Levels are Correlated to Leptin Levels
In Type I diabetes, the decreased insulin levels can also affect leptin levels. Leptin is a hormone secreted by fat cells which signals to the brain the amount of energy availability. In states of decreased fat levels such as starvation, leptin signals to the brain to regulate metabolism by shutting down reproductive pathways. Insulin has been shown in vitro to have direct effects on leptin synthesis. [Cammisotto, 2002 #36;Barr, 1997 #35;Wabitsch, 1996 #47]. In fat cells, administration of insulin promotes an increase in leptin production in vitro. In vivo, long-term exposure to hyperinsulinemia promoted an increase in circulating insulin levels [Kolaczynski, 1996 #42]. However, this effect was not seen instantaneously, but only in the final 24 hours of the study, suggesting that the hypothalamic leptin response to insulin is an indirect mechanism acting through adipose cells. In humans, leptin levels also are affected by Type 1 diabetes. Leptin levels were decreased in newly diagnosed Type 1 diabetic patients before the administration of insulin treatment, but these levels normalized after the onset of insulin treatment [Azar, 2002 #33]. In another study of newly diagnosed children with Type 1 diabetes, leptin levels were low prior to insulin treatment, and became elevated after only 1 day of insulin therapy. This leads to the conclusion that serum leptin is not simply a readout of body fat stores, but that it is regulated by insulin levels as well. [Hanaki, 1999 #39]
Further studies have established that both insulin and leptin potentiate insulin signaling in the brain. Injections of either insulin or leptin into the intracerebroventricular region of the brain elicited overlapping but distinct signaling mechanisms. Both hormones were able to affect the IRS and PI3-kinase signaling pathways, while insulin alone resulted in the phosphorylation of AKT serine. [Carvalheira, 2005 #37]
Uncontrolled Type 1 Diabetes is Associated with decreased Leptin Levels
It now seems clear that leptin plays an important role is the development of Type 1 diabetes. Low leptin levels are found in uncontrolled type 1 diabetic patients. Decreased levels of leptin are associated with insulin resistance and other markers of metabolic syndrome, suggesting an interplay between the two molecules. [German, 2010 #63] Additionally, decreased leptin levels in streptozocin-induced diabetic Wistar rats contributes to insulin resistance and metabolic syndrome prior to the development of hyperglycemia.[German, 2010 #63] Another study showed that leptin therapy alone in a nonobese diabetic mouse restored normal blood glucose levels as well as reversed the catabolic state of the mice.[Wang, 2010 #65]. These data suggest that leptin effects may be downstream of insulin effects on metabolism, and thus restoring leptin levels in Type 1 diabetics may be able to reverse many of the effects insulin deficiency.
Disturbances in Leptin Signaling Cause Subfertility
Through interactions with the hypothalamus, leptin signaling regulates fertility. Both the ob/ob and the db/db mouse models, which lack the leptin protein and the leptin receptor protein, respectively, exemplify the central role of leptin in the HPG axis. Both the ob/ob mouse and the db/db mouse have impaired fertility, and this defect is rescued by the administration of exogenous leptin. However, the defect in fertility is not restored with food restriction, suggesting that it is the lack of leptin, and not obesity, that is responsible for this infertility [Mounzih, 1997 #67]. Ob/ob males without leptin treatment have smaller testes, reduced numbers of sperms within the seminiferous tubules, and also have shrunken Leydig cells, indicative of impaired steroidogenesis. [Fujikawa, 2010 #64]. Another study found that free testosterone levels were not altered in the leptin deficient ob/ob mice. [Bhat, 2006 #68] However, the authors speculated that this may be because the leptin deficient mice produce a binding protein which lowers free biologically active testosterone.
Leptin may mediate its effects by direct interactions with the hypothalamus. Leptin is known to impact the GnRH pulses by the brain. Injections of leptin directly into the rat hypothalamus stimulate the release of GnRH, suggesting that leptin directly acts within the hypothalamus to stimulate gonadotropin-releasing hormone secretion in vivo [Watanobe, 2002 #31]. Another study found that leptin indirectly regulates the GnRH neurons [Quennell, 2009 #10]. The authors deleted leptin receptor either in the forebrain or in the GnRH neurons. Male infertility resulted only from deletion in the forebrain, suggested that there is an indirect mechanistic relationship between leptin and GnRH pulses. These experiments display how leptin signaling to the brain is critical for reproduction.
Leptin receptors are found in the rat and mouse brain, notably in the hypothalamus as well as in the pituitary, indicating a potential for leptin regulation at both sites. Leptin receptors are also expressed in the testis, suggesting that leptin secreted by fat cells can directly signal to the gonads [Zamorano, 1997 #20]. Leptin receptor expression in the testes seems to be stage-specific, indicating a role in spermatogenesis. Expression is restricted to spermatocyes in stage IX and X of the testes, corresponding to the time just after sperm release [El-Hefnawy, 2000 #21]. The authors postulate that mature sperm may suppress leptin receptor expression through a negative feedback loop that prevents expression of the receptor in the other spermatogenic stages. Thus, disruptions in leptin production during the pathogenesis of diabetes may impact the gonadal axis at multiple levels, through signaling to the hypothalamus affecting GnRH pulses, through signaling to the pituitary affecting LH and FSH secretion, and through direct interactions with developing sperm cells in the testes.
GnRH Signaling from the Hypothalamus is Impaired in Type 1 Diabetic Males
Both leptin and insulin are known to interact with the hypothalamus to ultimately regulate the output of GnRH from the hypothalamus. These GnRH pulses subsequently affect hormones important in spermatogenesis, notably luteinizing hormone and follicular stimulating hormone. Follicular stimulating hormone acts on the Sertoli cells of the testes to stimulate germ cell progression through mitosis and entry into meiosis [O’Shaughnessy, 2010 #51;Selice, 2011 #52] Luteinizing hormone acts on the Leydig cells of the testes to stimulate testosterone synthesis (Amory and Bremner 2003). Disruption of the hypothalamic pituitary gonadal axis at any point in this hormonal loop results in a disruption of spermatogenesis.
Diabetic men are known to have decreased fertility, but the precise mechanism remains unclear. Subfertility could be due either to local effects of the hyperglycemia on the testes resulting in disruptions in spermatogenesis, or due to hormonal alterations impacting the hypothalamic pituitary axis and subsequent spermatogenesis. A human study by attempted to examine the function of the HPG axis in diabetic men by measuring LH and FSH secretion in in response to GnRH as well as TEM microscopy of sperm cells to look for abnormalities. The authors found that LH secretion in response to GnRH pulses was lower in diabetics than in healthy controls (48 ± 8 versus 59 ± 10 mIU/ml; P < 0.05). Upon examination of sperm ultrastructure, sperm from diabetic patients had a greater percentage of abnormally shaped acrosomes (Baccetti et al. 2002), Another group found that impotent diabetic males had a lower LH response to GnRH pulses (Zeidler et al. 1982). LH secretion in response to GnRH administration was also shown to be impaired in Type 1 diabetic men in a study conducted by (Lopez-Alvarenga et al. 2002). This study examined young diabetic men without systemic complications of diabetes for an average of 3.7 years. The authors found that patients with poorly controlled diabetes had lower endogenous LH pulses as well as a decreased LH response to pulsatile GnRH administration. The authors concluded that the lack of LH response stemmed from the acute effects of diabetes including hypoinsulinemia and hyperglycemia and not from long-term systemic complications of the disease. These data together suggest that the pathogenesis of Type 1 diabetes affects the hypothalamic pituitary axis resulting in decreased LH secretion. The low LH response may have negative implications on steroidogenesis from the Leydig cells, resulting in impaired spermatogenesis.
Disruptions in Follicular Hormone Signaling Impair Spermatogenesis
Disturbances in FSH levels have severe impacts on the male reproductive tract. Disturbances in the HPG axis impact both LH and FSH levels, impairing spermatogenesis. Scientists have attempted to isolate the roles of these hormones by developing mouse models lacking either hormone or its receptor. Mice lacking the FSH receptor (FORKO mice) have underdeveloped testes and a 50% reduction in the number of Sertoli cells. (Sairam and Krishnamurthy 2001). FORKO mice also show a decreased seminiferous tubule diameter and reduced sperm count corresponding to low testosterone levels (Krishnamurthy et al. 2000). FORKO mice also display a delay in puberty, suggesting that FSH signaling is important in the onset of spermatogenesis (Krishnamurthy et al. 2001). These mice remain fertile, however, suggesting that FSH is required for normal spermatogenesis but not critical for fertility. In humans, mutations in the FSH or its receptor are associated with fertility abnormalities. Three males with FSH beta mutations have been described, all leading to subfertility including symptoms of small testes and azoospermia. Mutations in the FSH receptor resulted in low sperm counts or low sperm volume, but none of these patients were infertile. (Meduri et al. 2008)
Disturbances in Luteinizing Hormone Signaling Impair Late Stages of Spermatogenesis
A lack of luteinizing hormone seems to disrupt the later stages of spermatogenesis, and this corresponds to a decreased production of testosterone by the Leydig cells. A study of mice lacking the luteinizing hormone receptor demonstrated that these mice were still capable of producing normal numbers of Sertoli cells, spermatogonia, and early spermatocytes, however the Leydig cell population was decreased and the numbers of later spermatocytes and round spermatids were reduced and the elongated spermatid population was completely absent. Testosterone implants in LH receptor null mice rescued the process of spermatogenesis and produced mature sperm cells (Lei et al. 2004). A human study showed that human LH replacement in gonadotropin deficient men restored spermatogenesis(Matsumoto et al. 1984). After FSH and LH depletion via exogenous testosterone administration and subsequent confirmation of azoospermia, men were treated with human LH, which resulted in a significant increase in sperm concentration, although not to normal levels. The authors concluded that LH, but not FSH was required for normal spermatogenesis.
Mouse models also present important data regarding gonadotropin deficiency within the testes. Mice lacking the luteinizing hormone beta-subunit are completely infertile. The testis size is reduced and hormones involved in the biosynthesis for testosterone are reduced or absent. Additionally, the seminiferous tubules of these mice contain spermatogonia, spermatocytes, and round spermatids, but they lack any elongated and late-stage spermatids. Whether this phenotype can be rescued with exogenous testosterone remains to be determined (Ma et al. 2004). Mice lacking the luteinizing hormone receptor are also infertile. Spermatogensis is arrested at the round spermatid stage and testosterone levels are severely reduced. Testosterone replacement therapy was able to resume spermatogenesis (Lei et al. 2004). This suggests that FSH alone is able to support the Sertoli cell population and the early stages of spermatogenesis, but that LH is required for the progression and/or maintenance of a mature sperm cell population.
Testosterone Levels are Decreased in Uncontrolled Type 1 Diabetic Males
Given that diabetes impacts LH levels, the effects of endogenous testosterone production may also be affected. One group conducted a study of age-matched diabetic and control patients. This study found that men with diabetes had reduced testosterone levels compared to controls, while LH and FSH levels remained similar (M. Maneesh 2006). Another study found that free testosterone levels were decreased while total testosterone levels were similar in Type 1 diabetic men versus controls. However, LH levels were not different while FSH levels were slightly increased (van Dam et al. 2003). A study of newly diagnosed Type 1 diabetic patients revealed that plasma testosterone concentrations were decreased in diabetic patients but returned to normal levels after four days of insulin treatment (Gluud et al. 1982). Similarly, a study of Type 1 diabetic patients measured androgens before and after insulin withdrawal. While testosterone levels were similar before insulin withdrawal, testosterone levels decreased below control significantly after 4 hours of insulin withdrawal and remained lower throughout the 12-hour study (Madsbad et al. 1986). Together, this data suggests that testosterone levels are impaired by Type 1 diabetes in poorly controlled subjects, which could inhibit the process of spermatogenesis.
Diabetes is Associated with Poor Sperm Quality
Diabetes mellitus is associated with spermatogenic defects in afflicted males as a result, at least in part, of the known perturbations in the HPG axis. The characterization of these spermatogenic defects is an area of active research as new technology for assaying the quality of sperm cells continues to be developed. Rather recently, researchers have found that men with diabetes have sperm with significantly higher amounts of DNA damage (Agbaje et al. 2007). Additionally, sperm from diabetic men was shown to have higher DNA fragmentation as well as an increase in RAGE, a receptor protein important in the oxidative stress response (Karimi et al. 2011; Mallidis et al. 2007). Additionally, sperm from Type 1 diabetic men has an increased number of mitochondrial DNA deletions as well as an increase in sperm nuclear DNA fragmentation (Agbaje et al. 2007). Sperm from diabetic men also correlates to decreased embryo quality. In vitro fertilization using sperm from diabetic men produced far fewer pregnancies compared to controls. However, two-cell embryo fertilization rates from using sperm from diabetic men was not impaired(Mulholland et al. 2011). This indicates that the sperm cells are able to fertilize embryo but there may be DNA damage that prevents competent embryo development. Additionally, work by Sung Tae Kim in both streptozocin-induced and genetically diabetic Akita mice showed that sperm from these mice produced fewer 2-cell embryos as well as fewer 2-cell embryos developing to blastocysts. This suggests that the severe diabetes in these Type I models affects sperm competence, thereby impairing proper embryo development (Kim and Moley 2008).
A study by (Ballester et al. 2004) sought to establish the linkage between diabetes and fertility by examining a Type 1 diabetic rat model for defects in testicular signaling. The authors treated male Wistar rats with a single dose of streptozocin (70mg/kg). After 3 months, many of the rats were infertile, had smaller testes and decreased numbers of Leydig cells. Additionally, the serum levels of LH, FSH and testosterone were all decreased. The authors also suggested that the mechanism between insulin and LH to be an indirect one, as they did not find a significant correlation between serum insulin and LH levels. This study shows an important relationship between Type 1 diabetes and pituitary hormone signaling to the testes leading to infertility.
Insulin also may Act Directly in the Testes to Regulate Spermatogenesis
Aside from disruptions in the HPG axis, diabetes could directly impact fertility by perturbing insulin signaling in the testes and sperm. Recent research has identified insulin expression in the testes and sperm (Gomez et al. 2009). This has implications on the pathogenesis of diabetes, and how the effects of diabetes on fertility are mediated. Insulin mRNA and protein expression was detected in human sperm cells and these cells respond to increasing concentrations of glucose with increasing secretion of insulin. Additionally, sperm cells released more insulin upon capacitation, the process by which sperm acquire the ability to fertilize. This provides a potential mechanism by which insulin might have an autocrine role in sperm cells, which could serve to mediate sperm maturation and/or fertilization events. Additionally, insulin transcripts and protein have been detected in the testes as well, raising the possibility that insulin may interact directly with receptors in the testes to promote the process of spermatogenesis.
A study of cultured cells derived from chicken testes showed that incubation of testicular cells with insulin increased proliferation, as demonstrated by the incorporation of 3H-thymidine into the cells. This indicated that insulin may have a mitogenic effect in the testes (Bobes et al. 2001). Detection of insulin in control and diabetic rat testes had also been established. Histological examination localized insulin to the spermatids and Leydig cells. Additionally, insulin receptor was localized to the cytoplasmic droplets of elongating spermatids just prior to release into the lumen (Gomez et al. 2009). Together, these studies demonstrate the importance of local insulin signaling within the testes in addition to systemic effects.
Conclusion
Disruptions in the hypothalamic pituitary gonadal axis have severe reproductive consequences. Type 1 diabetes can impact many aspects of the functional axis, resulting in subfertility. Low insulin levels due to Type 1 diabetes lead to decreased leptin levels, leading to decreased GnRH secretion, and subsequent decreased in LH and FSH signaling to the testes. However, it is probable that not all of the diabetic outcomes on fertility are mediated through the HPG axis, but also by the detrimental effects of hyperglycemia and oxidative DNA damage to the testes and sperm cells. Additionally, the presence of insulin transcripts in testes and sperm brings up the possibility that insulin signaling may be important within the testes and may play a part in the diabetic pathogenesis of infertility. Additional work in the field is necessary to establish the precise role of direct insulin signaling in the testes and sperm cells, and to determine whether diabetes has an impact on this local signaling.
References
- Agbaje IM, Rogers DA, McVicar CM, McClure N, Atkinson AB, et al. Insulin dependant diabetes mellitus: implications for male reproductive function. Hum Reprod. 2007;22:1871–1877. doi: 10.1093/humrep/dem077. [DOI] [PubMed] [Google Scholar]
- Amory JK, Bremner WJ. Regulation of testicular function in men: implications for male hormonal contraceptive development. J Steroid Biochem Mol Biol. 2003;85:357–361. doi: 10.1016/s0960-0760(03)00205-x. [DOI] [PubMed] [Google Scholar]
- Aquila S, Gentile M, Middea E, Catalano S, Ando S. Autocrine regulation of insulin secretion in human ejaculated spermatozoa. Endocrinology. 2005;146:552–557. doi: 10.1210/en.2004-1252. [DOI] [PubMed] [Google Scholar]
- Armstrong SP, Caunt CJ, Fowkes RC, Tsaneva-Atanasova K, McArdle CA. Pulsatile and sustained gonadotropin-releasing hormone (GnRH) receptor signaling: does the Ca2+/NFAT signaling pathway decode GnRH pulse frequency? J Biol Chem. 2009;284:35746–35757. doi: 10.1074/jbc.M109.063917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar ST, Zalloua PA, Zantout MS, Shahine CH, Salti I. Leptin levels in patients with type 1 diabetes receiving intensive insulin therapy compared with those in patients receiving conventional insulin therapy. J Endocrinol Invest. 2002;25:724–726. doi: 10.1007/BF03345107. [DOI] [PubMed] [Google Scholar]
- Baccetti B, La Marca A, Piomboni P, Capitani S, Bruni E, et al. Insulin-dependent diabetes in men is associated with hypothalamo-pituitary derangement and with impairment in semen quality. Hum Reprod. 2002;17:2673–2677. doi: 10.1093/humrep/17.10.2673. [DOI] [PubMed] [Google Scholar]
- Ballester J, Munoz MC, Dominguez J, Rigau T, Guinovart JJ, et al. Insulin-dependent diabetes affects testicular function by FSH- and LH-linked mechanisms. J Androl. 2004;25:706–719. doi: 10.1002/j.1939-4640.2004.tb02845.x. [DOI] [PubMed] [Google Scholar]
- Barash IA, Cheung CC, Weigle DS, Ren H, Kabigting EB, et al. Leptin is a metabolic signal to the reproductive system. Endocrinology. 1996;137:3144–3147. doi: 10.1210/endo.137.7.8770941. [DOI] [PubMed] [Google Scholar]
- Barber AJ. A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:283–290. doi: 10.1016/S0278-5846(03)00023-X. [DOI] [PubMed] [Google Scholar]
- Barr VA, Malide D, Zarnowski MJ, Taylor SI, Cushman SW. Insulin stimulates both leptin secretion and production by rat white adipose tissue. Endocrinology. 1997;138:4463–4472. doi: 10.1210/endo.138.10.5451. [DOI] [PubMed] [Google Scholar]
- Bhat GK, Sea TL, Olatinwo MO, Simorangkir D, Ford GD, et al. Influence of a leptin deficiency on testicular morphology, germ cell apoptosis, and expression levels of apoptosis-related genes in the mouse. J Androl. 2006;27:302–310. doi: 10.2164/jandrol.05133. [DOI] [PubMed] [Google Scholar]
- Bobes RJ, Castro JI, Miranda C, Romano MC. Insulin modifies the proliferation and function of chicken testis cells. Poult Sci. 2001;80:637–642. doi: 10.1093/ps/80.5.637. [DOI] [PubMed] [Google Scholar]
- Boura-Halfon S, Zick Y. Phosphorylation of IRS proteins, insulin action, and insulin resistance. Am J Physiol Endocrinol Metab. 2009;296:E581–591. doi: 10.1152/ajpendo.90437.2008. [DOI] [PubMed] [Google Scholar]
- Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- Burks DJ, Font de Mora J, Schubert M, Withers DJ, Myers MG, et al. IRS-2 pathways integrate female reproduction and energy homeostasis. Nature. 2000;407:377–382. doi: 10.1038/35030105. [DOI] [PubMed] [Google Scholar]
- Cammisotto PG, Bukowiecki LJ. Mechanisms of leptin secretion from white adipocytes. Am J Physiol Cell Physiol. 2002;283:C244–250. doi: 10.1152/ajpcell.00033.2002. [DOI] [PubMed] [Google Scholar]
- Carvalheira JB, Torsoni MA, Ueno M, Amaral ME, Araujo EP, et al. Cross-talk between the insulin and leptin signaling systems in rat hypothalamus. Obes Res. 2005;13:48–57. doi: 10.1038/oby.2005.7. [DOI] [PubMed] [Google Scholar]
- El-Hefnawy T, Ioffe S, Dym M. Expression of the leptin receptor during germ cell development in the mouse testis. Endocrinology. 2000;141:2624–2630. doi: 10.1210/endo.141.7.7542. [DOI] [PubMed] [Google Scholar]
- Fujikawa T, Chuang JC, Sakata I, Ramadori G, Coppari R. Leptin therapy improves insulin-deficient type 1 diabetes by CNS-dependent mechanisms in mice. Proc Natl Acad Sci U S A. 2010;107:17391–17396. doi: 10.1073/pnas.1008025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Jones JE, Olson D, Hill J, Lee CE, et al. Monitoring FoxO1 localization in chemically identified neurons. J Neurosci. 2008;28:13640–13648. doi: 10.1523/JNEUROSCI.4023-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German JP, Wisse BE, Thaler JP, Oh IS, Sarruf DA, et al. Leptin deficiency causes insulin resistance induced by uncontrolled diabetes. Diabetes. 2010;59:1626–1634. doi: 10.2337/db09-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluud C, Madsbad S, Krarup T, Bennett P. Plasma testosterone and androstenedione in insulin dependent patients at time of diagnosis and during the first year of insulin treatment. Acta Endocrinol (Copenh) 1982;100:406–409. doi: 10.1530/acta.0.1000406. [DOI] [PubMed] [Google Scholar]
- Gomez O, Ballester B, Romero A, Arnal E, Almansa I, et al. Expression and regulation of insulin and the glucose transporter GLUT8 in the testes of diabetic rats. Horm Metab Res. 2009;41:343–349. doi: 10.1055/s-0028-1128146. [DOI] [PubMed] [Google Scholar]
- Hanaki K, Becker DJ, Arslanian SA. Leptin before and after insulin therapy in children with new-onset type 1 diabetes. J Clin Endocrinol Metab. 1999;84:1524–1526. doi: 10.1210/jcem.84.5.5653. [DOI] [PubMed] [Google Scholar]
- Havrankova J, Brownstein M, Roth J. Insulin and insulin receptors in rodent brain. Diabetologia. 1981;20(Suppl):268–273. [PubMed] [Google Scholar]
- Havrankova J, Schmechel D, Roth J, Brownstein M. Identification of insulin in rat brain. Proc Natl Acad Sci U S A. 1978;75:5737–5741. doi: 10.1073/pnas.75.11.5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JW, Elmquist JK, Elias CF. Hypothalamic pathways linking energy balance and reproduction. Am J Physiol Endocrinol Metab. 2008;294:E827–832. doi: 10.1152/ajpendo.00670.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi J, Goodarzi MT, Tavilani H, Khodadadi I, Amiri I. Increased receptor for advanced glycation end products in spermatozoa of diabetic men and its association with sperm nuclear DNA fragmentation. Andrologia. 2011 doi: 10.1111/j.1439-0272.2011.01178.x. [DOI] [PubMed] [Google Scholar]
- Kim ST, Moley KH. Paternal effect on embryo quality in diabetic mice is related to poor sperm quality and associated with decreased glucose transporter expression. Reproduction. 2008;136:313–322. doi: 10.1530/REP-08-0167. [DOI] [PubMed] [Google Scholar]
- Kolaczynski JW, Nyce MR, Considine RV, Boden G, Nolan JJ, et al. Acute and chronic effects of insulin on leptin production in humans: Studies in vivo and in vitro. Diabetes. 1996;45:699–701. doi: 10.2337/diab.45.5.699. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy H, Babu PS, Morales CR, Sairam MR. Delay in sexual maturity of the follicle-stimulating hormone receptor knockout male mouse. Biol Reprod. 2001;65:522–531. doi: 10.1095/biolreprod65.2.522. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy H, Danilovich N, Morales CR, Sairam MR. Qualitative and quantitative decline in spermatogenesis of the follicle-stimulating hormone receptor knockout (FORKO) mouse. Biol Reprod. 2000;62:1146–1159. doi: 10.1095/biolreprod62.5.1146. [DOI] [PubMed] [Google Scholar]
- Lei ZM, Mishra S, Ponnuru P, Li X, Yang ZW, et al. Testicular phenotype in luteinizing hormone receptor knockout animals and the effect of testosterone replacement therapy. Biol Reprod. 2004;71:1605–1613. doi: 10.1095/biolreprod.104.031161. [DOI] [PubMed] [Google Scholar]
- Lopez-Alvarenga JC, Zarinan T, Olivares A, Gonzalez-Barranco J, Veldhuis JD, et al. Poorly controlled type I diabetes mellitus in young men selectively suppresses luteinizing hormone secretory burst mass. J Clin Endocrinol Metab. 2002;87:5507–5515. doi: 10.1210/jc.2002-020803. [DOI] [PubMed] [Google Scholar]
- Maneesh M, HJ, Singh TA, Chakrabarti Amit. IMPAIRED HYPOTHALAMIC-PITUITARY-GONADAL AXIS FUNCTION IN MEN WITH DIABETES MELLITUS. Indian Journal of Clinical Biochemistry. 2006;21:165–168. doi: 10.1007/BF02913088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Dong Y, Matzuk MM, Kumar TR. Targeted disruption of luteinizing hormone beta-subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proc Natl Acad Sci U S A. 2004;101:17294–17299. doi: 10.1073/pnas.0404743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsbad S, Gluud C, Bennett P, Krarup T. Rapid changes in plasma androgens during insulin withdrawal in male type 1 (insulin-dependent) diabetics. J Endocrinol Invest. 1986;9:21–25. doi: 10.1007/BF03348056. [DOI] [PubMed] [Google Scholar]
- Mallidis C, Agbaje I, Rogers D, Glenn J, McCullough S, et al. Distribution of the receptor for advanced glycation end products in the human male reproductive tract: prevalence in men with diabetes mellitus. Hum Reprod. 2007;22:2169–2177. doi: 10.1093/humrep/dem156. [DOI] [PubMed] [Google Scholar]
- Matsumoto AM, Paulsen CA, Bremner WJ. Stimulation of sperm production by human luteinizing hormone in gonadotropin-suppressed normal men. J Clin Endocrinol Metab. 1984;59:882–887. doi: 10.1210/jcem-59-5-882. [DOI] [PubMed] [Google Scholar]
- Meduri G, Bachelot A, Cocca MP, Vasseur C, Rodien P, et al. Molecular pathology of the FSH receptor: new insights into FSH physiology. Mol Cell Endocrinol. 2008;282:130–142. doi: 10.1016/j.mce.2007.11.027. [DOI] [PubMed] [Google Scholar]
- Mounzih K, Lu R, Chehab FF. Leptin treatment rescues the sterility of genetically obese ob/ob males. Endocrinology. 1997;138:1190–1193. doi: 10.1210/endo.138.3.5024. [DOI] [PubMed] [Google Scholar]
- Mulholland J, Mallidis C, Agbaje I, McClure N. Male diabetes mellitus and assisted reproduction treatment outcome. Reprod Biomed Online. 2011;22:215–219. doi: 10.1016/j.rbmo.2010.10.005. [DOI] [PubMed] [Google Scholar]
- O’Shaughnessy PJ, Monteiro A, Verhoeven G, De Gendt K, Abel MH. Effect of FSH on testicular morphology and spermatogenesis in gonadotrophin-deficient hypogonadal mice lacking androgen receptors. Reproduction. 2010;139:177–184. doi: 10.1530/REP-09-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8:1376–1382. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- Porte D, Jr, Baskin DG, Schwartz MW. Insulin signaling in the central nervous system: a critical role in metabolic homeostasis and disease from C. elegans to humans. Diabetes. 2005;54:1264–1276. doi: 10.2337/diabetes.54.5.1264. [DOI] [PubMed] [Google Scholar]
- Quennell JH, Mulligan AC, Tups A, Liu X, Phipps SJ, et al. Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology. 2009;150:2805–2812. doi: 10.1210/en.2008-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said G. Diabetic neuropathy--a review. Nat Clin Pract Neurol. 2007;3:331–340. doi: 10.1038/ncpneuro0504. [DOI] [PubMed] [Google Scholar]
- Sairam MR, Krishnamurthy H. The role of follicle-stimulating hormone in spermatogenesis: lessons from knockout animal models. Arch Med Res. 2001;32:601–608. doi: 10.1016/s0188-4409(01)00328-9. [DOI] [PubMed] [Google Scholar]
- Selice R, Ferlin A, Garolla A, Caretta N, Foresta C. Effects of endogenous FSH on normal human spermatogenesis in adults. Int J Androl. 2011;34:e511–517. doi: 10.1111/j.1365-2605.2010.01134.x. [DOI] [PubMed] [Google Scholar]
- Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension. 2001;37:1053–1059. doi: 10.1161/01.hyp.37.4.1053. [DOI] [PubMed] [Google Scholar]
- van Dam EW, Dekker JM, Lentjes EG, Romijn FP, Smulders YM, et al. Steroids in adult men with type 1 diabetes: a tendency to hypogonadism. Diabetes Care. 2003;26:1812–1818. doi: 10.2337/diacare.26.6.1812. [DOI] [PubMed] [Google Scholar]
- Vinik AI, Holland MT, Le Beau JM, Liuzzi FJ, Stansberry KB, et al. Diabetic neuropathies. Diabetes Care. 1992;15:1926–1975. doi: 10.2337/diacare.15.12.1926. [DOI] [PubMed] [Google Scholar]
- Wabitsch M, Jensen PB, Blum WF, Christoffersen CT, Englaro P, et al. Insulin and cortisol promote leptin production in cultured human fat cells. Diabetes. 1996;45:1435–1438. doi: 10.2337/diab.45.10.1435. [DOI] [PubMed] [Google Scholar]
- Wang MY, Chen L, Clark GO, Lee Y, Stevens RD, et al. Leptin therapy in insulin-deficient type I diabetes. Proc Natl Acad Sci U S A. 2010;107:4813–4819. doi: 10.1073/pnas.0909422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanobe H. Leptin directly acts within the hypothalamus to stimulate gonadotropin-releasing hormone secretion in vivo in rats. J Physiol. 2002;545:255–268. doi: 10.1113/jphysiol.2002.023895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JW, Jun HS. Autoimmune destruction of pancreatic beta cells. Am J Ther. 2005;12:580–591. doi: 10.1097/01.mjt.0000178767.67857.63. [DOI] [PubMed] [Google Scholar]
- Zamorano PL, V, Mahesh B, De Sevilla LM, Chorich LP, Bhat GK, et al. Expression and localization of the leptin receptor in endocrine and neuroendocrine tissues of the rat. Neuroendocrinology. 1997;65:223–228. doi: 10.1159/000127276. [DOI] [PubMed] [Google Scholar]
- Zeidler A, Gelfand R, Tamagna E, Marrs R, Chopp R, et al. Pituitary gonadal function in diabetic male patients with and without impotence. Andrologia. 1982;14:62–68. doi: 10.1111/j.1439-0272.1982.tb03096.x. [DOI] [PubMed] [Google Scholar]


