Abstract
The default mode network (DMN) encompasses brain systems that exhibit coherent neural activity at rest. DMN brain systems have been implicated in diverse social, cognitive, and affective processes, as well as risk for forms of dementia and psychiatric disorders that associate with systemic inflammation. Areas of the anterior cingulate cortex (ACC) and surrounding medial prefrontal cortex (mPFC) within the DMN have been implicated specifically in regulating autonomic and neuroendocrine processes that relate to systemic inflammation via bidirectional signaling mechanisms. However, it is still unclear whether indicators of inflammation relate directly to coherent resting state activity of the ACC, mPFC, or other areas within the DMN. Accordingly, we tested whether plasma interleukin (IL)-6, an indicator of systemic inflammation, covaried with resting-state functional connectivity of the DMN among 98 adults aged 30–54 (39% male; 81% Caucasian). Independent component analyses were applied to resting state fMRI data to generate DMN connectivity maps. Voxel-wise regression analyses were then used to test for associations between IL-6 and DMN connectivity across individuals, controlling for age, sex, body mass index, and fMRI signal motion. Within the DMN, IL-6 covaried positively with connectivity of the sub-genual ACC and negatively with a region of the dorsal medial PFC at corrected statistical thresholds. These novel findings offer evidence for a unique association between a marker of systemic inflammation (IL-6) and ACC and mPFC functional connectivity within the DMN, a network that may be important for linking aspects of immune function to psychological and behavioral states in health and disease.
Introduction
Systemic inflammation is implicated in the pathophysiology of several chronic illnesses. Elevated levels of circulating inflammatory markers, such as interleukin (IL)-6, also confer risk for a range of adverse physical health conditions, including atherosclerotic cardiovascular and cerebrovascular diseases, diabetes, premature cognitive aging, frailty and general functional decline with age (Black, 2003; Ferrucci et al., 1999; Luc et al., 2003; Pradhan, Manson, Rifai, Buring, & Ridker, 2001; Roriz-Filho et al., 2009; Yaffe et al., 2004; Yirmiya & Goshen, 2011). Moreover, several psychosocial factors (e.g., chronic psychological stress) and psychiatric disorders (e.g., mood and anxiety disorders) associate with systemic inflammation and risk for inflammatory disease (Menard, Pfau, Hodes, & Russo, 2017), raising the possibility that inflammatory mechanisms are critical for linking psychosocial factors and related disorders to physical health (Miller, Chen, & Cole, 2009). Yet despite growing evidence supporting this possibility, there is still little direct evidence characterizing the neural systems that may plausibly account for the associations of psychosocial factors and psychiatric disorders with systemic inflammation in health and disease across the lifespan.
At present, neuroanatomical and empirical evidence suggests that areas of the anterior cingulate cortex (ACC) and surrounding medial prefrontal cortex (mPFC) might play a role in linking higher order psychosocial and behavioral processes with peripheral autonomic and endocrine control mechanisms that are known to modulate inflammation (Critchley & Harrison, 2013; Eisenberger & Cole, 2012; Gianaros & Wager, 2015). Hence, areas within the rostral portion of the ACC and adjacent areas of the ventral and dorsal mPFC issue direct and indirect neural projections to other telencephalic areas and subcortical cell groups involved in coordinating changes in peripheral physiology with cognitive, affective, and other behavioral states, including the insula, amygdala, bed nucleus of the stria terminalis, hippocampus, basal ganglia, thalamus, hypothalamus, periaqueductal gray, pons, nucleus of the solitary tract, and adjacent medullary territories (Critchley & Harrison, 2013; Öngür & Price, 2000; Vertes, 2004). Thus, ACC and mPFC areas are capable of influencing autonomic and neuroendocrine effector pathways to influence immune function and, thus, inflammatory states. In the periphery, these pathways specifically regulate inflammatory gene expression, with acute activation of the sympathetic nervous system acting through β-adrenergic receptors on immune cells to stimulate the expression and release of pro-inflammatory mediators, such as interleukin-6 (IL-6) (Eisenberger & Cole, 2012). In contrast, acute activation of the hypothalamic-pituitary-adrenal (HPA) axis and the release of glucocorticoids (cortisol in humans) acts via glucocorticoid receptors in immune cells to suppress transcription of proinflammatory cytokine genes (Eisenberger & Cole, 2012). Importantly, circulating levels of pro-inflammatory mediators also feed back to the brain to regulate central neural control of autonomic and endocrine activity, thus defining a circuit that orchestrates and coordinates immune and inflammatory activity with other physiological, psychological, and behavioral processes that are believed to confer survival advantage (Critchley & Harrison, 2013; Irwin & Cole, 2011).
Critically, several areas of the ACC and mPFC are encompassed by the default mode network (DMN), which comprises brain systems that exhibit coherent neural activity at rest and deactivation in the presence of external processing demands (Buckner, Andrews-Hanna, & Schacter, 2008). Major functional hubs of the DMN include the posterior cingulate cortex (PCC), precuneus, angular gyrus, and a large territory of the medial prefrontal cortex (mPFC) that invades the rostral portion of the ACC (Buckner et al., 2008; Raichle et al., 2001; Yeo et al., 2011). The DMN has also been functionally parcellated into a ‘dorsal medial PFC subsystem’ that is broadly implicated in interpersonal cognition (PCC, mPFC, angular gyrus, dorsal medial PFC [dmPFC], temporoparietal junction, lateral temporal cortex, and anterior temporal pole), as well as a ‘medial temporal lobe subsystem’ that is chiefly implicated in autobiographical memory, prospective thought, affective processing, and peripheral physiological control (ventromedial PFC [vmPFC]; rostral ACC; hippocampal formation, parahippocampal gyrus, retrosplenial cortex, and posterior inferior parietal lobe) (Andrews-Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010; Buckner et al., 2008; Jennings, Sheu, Kuan, Manuck, & Gianaros, 2016). Increasing evidence suggests further that the DMN may play a role in the development of psychiatric and neurological disorders that associate with both systemic inflammation and risk for inflammatory disease. For example, alterations in DMN activity have been observed in mood and anxiety disorders characterized by impairments in emotion regulation and heightened systemic inflammation (Hamilton, Farmer, Fogelman, & Gotlib, 2015; Kaiser, Andrews-Hanna, Wager, & Pizzagalli, 2015; Kaiser, Whitfield-Gabrieli, et al., 2015; Price & Drevets, 2010). Here, imaging studies of patients with clinical depression specifically show a reliable increase in the resting state metabolic activity of the sub-genual portion of the rostral ACC (sgACC), a pattern that appears to be reversible by antidepressant therapies and deep brain stimulation (Hamani et al., 2011; Mayberg et al., 2005). Finally, recent meta-analyses of studies examining the role of the DMN in major depressive disorder show consistent increases in functional connectivity between this network and sgACC and fronto-parietal regions, possibly reflecting a neural correlate of self-focused rumination (Hamilton et al., 2015; Kaiser, Andrews-Hanna, et al., 2015).
It is well established that major depression and negative mood states associate with increased peripheral markers of inflammation (Haapakoski, Mathieu, Ebmeier, Alenius, & Kivimaki, 2015; Irwin & Miller, 2007; Raison & Miller, 2011; Slavich & Irwin, 2014). Indeed, inflammatory processes are proposed to play a role in the pathogenesis of depressive disorder (Slavich & Irwin, 2014) and contribute to the increased risk for inflammatory disease that accompanies depression (Kiecolt-Glaser, Derry, & Fagundes, 2015). Pro-inflammatory cytokines are widely accepted to play a key role in immune system-to-brain signaling, accessing the brain via neural and humoral pathways to influence mood, cognition and behavior (Dantzer, O’Connor, Freund, Johnson, & Kelley, 2008; Marin & Kipnis, 2017). Interestingly, recent evidence shows an association of peripheral markers of inflammation with resting-state connectivity between the striatum and midline PFC areas, encompassing the sgACC and extending into other rostral ACC regions, that paralleled increased anhedonia and decreased motor speed among depressed patients (Felger et al., 2015). Similarly, patients with Hepatitis C showed a rapid decline in mood shortly following a first dose of interferon-alpha therapy, which associated with reductions in resting whole-brain functional connectivity and efficiency (Dipasquale et al., 2015). These findings suggest that peripheral inflammatory mediators can signal the brain to influence social, cognitive, affective and behavioral changes that are consistent with symptoms of major depressive disorder. As noted above, efferent control pathways connect the central nervous and immune systems, with negative moods activating peripheral physiologic pathways that result in the up-regulation of systemic inflammation (Slavich & Irwin, 2014). Thus, areas within the DMN that are implicated in peripheral physiological control, such as rostral ACC areas and those of the surrounding mPFC, may provide a plausible substrate for these bidirectional relationships.
Here, we aimed to better characterize the neural correlates of peripheral levels of inflammatory markers among otherwise healthy adults. We specifically tested the association between circulating IL-6 and resting state fMRI activity (connectivity) within the DMN, with a particular focus on the ACC and mPFC. The DMN was selected on the basis of evidence that areas within this network play a joint role in social, cognitive, affective, behavioral, and physiological processes that are reliably linked to immune processes underlying systemic inflammation (Critchley, 2005; Hamilton et al., 2015; Roy, Shohamy, & Wager, 2012). Based on existing evidence, it was hypothesized that functional connectivity of the DMN, particularly within the ACC and mPFC, would relate to peripheral levels of IL-6. In sensitivity analyses, we also explored whether similar associations would generalize to another indirect marker of systemic inflammation, C-reactive protein (CRP). Finally, we explored whether subclinical symptoms of depression were associated with IL-6, and whether such symptoms would relate to DMN connectivity in areas that also associated with IL-6.
Methods
Participants
Data were derived from Phase II of the Adult Health and Behavior project (AHAB-II), which assessed behavioral and biological traits among middle-aged community volunteers. Participants were recruited between March 2008 and October 2011 through mass mailings of invitation letters to individuals randomly selected from voter registration and other public domain lists. To be eligible for AHAB-II, individuals had to be between the ages of 30–54 years, working at least 25 hours per week outside of the home (a sub-study involving this cohort was focused on the association between occupational stress and coronary heart disease risk (Joseph et al., 2016)), and speak English as their first language. Individuals were excluded if they (a) had a history of cardiovascular disease, schizophrenia or bipolar disorder, chronic hepatitis, renal failure, major neurological disorder, chronic lung disease, or stage 2 hypertension (SBP/DBP ≥ 160/100); (b) consumed ≥ 5 alcoholic drinks 3–4 times (> approximately 201 grams of alcohol) per week; (c) took fish-oil supplements (because of the requirements for another sub-study (Muldoon et al., 2016)); (d) were prescribed insulin or glucocorticoid, anti-arrhythmic, antihypertensive, lipid-lowering, psychotropic, or prescription weight-loss medications; (e) were pregnant; (f) had less than 8th grade reading skills; or (g) were shift workers. Finally, all participants were screened for prior and current DSM-IV Axis-I disorders using the Mini International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998). The University of Pittsburgh Institutional Board approved the study and all participants provided informed consent in accordance with its regulations and were paid for their participation.
From the initial sample of 490 AHAB-II participants (81% Caucasian, 53% female, mean age 42.77), 461 completed a functional and structural neuroimaging protocol, with 110 participants completing an eyes-open, resting-state imaging protocol. Of the 110 who completed this resting-state protocol, 107 had useable resting fMRI BOLD signal data. Reasons for MRI exclusion (N = 3) were: participant withdrawal during imaging; failure of anatomical normalization of MRI data; and a data acquisition problem at the time of MRI. As revealed by Chi-Square analyses and t-tests, these 107 participants did not differ significantly from remaining AHAB-II participants in demographic characteristics (e.g., age gender, educational attainment) or IL-6. Of the 107 participants with complete MRI data, 98 had reliable measures of circulating IL-6 and did not have a chronic inflammatory disease or use medications known to impact immune function (cold medications/antihistamines, inhaled corticosteroids, immunosuppressants, or allergy shot within prior 2 weeks). One participant was missing depressive symptom scores. Although we have published neuroimaging findings from AHAB-II (Allen, Jennings, Gianaros, Thayer, & Manuck, 2015; Gianaros et al., 2014; Jennings, Allen, Gianaros, Thayer, & Manuck, 2015; Jennings et al., 2016; Marsland et al., 2015), the current findings are the first bearing on resting-state connectivity and inflammation in this sample.
Protocol and Measures
Measures for this study encompassed circulating levels of IL-6 and CRP, subclinical symptoms of depression, resting-state fMRI connectivity metrics, body mass index (BMI), demographic characteristics, and smoking status. Plasma levels of IL-6 and CRP were assessed from blood samples drawn between 7:30AM and 12:35PM (M = 9:16 +/− 0:54 min). Prior to the blood draw, participants were asked to fast for 8 hours, avoid vigorous exercise for 12 hours and alcohol for 24 hours, and refrain from using tobacco products that morning. The blood draw was rescheduled if the participant reported symptoms of acute infection or use of antibiotics or antivirals in the previous 2 weeks. At the blood draw visit, a registered nurse completed a medical history and medication use interview and obtained measurements of height and weight to determine body mass index (BMI in kg/m2). The nurse also drew a 40cc blood sample. Plasma samples were collected from citrated tubes, frozen at −80°C until analysis in batches. IL-6 levels were determined in duplicate by high sensitivity quantitative sandwich enzyme immunoassay kit (R & D Systems, Minneapolis, MN, standard range = 0.156–10pg/mL) run according to manufacturer’s directions. CRP was measured at the University of Vermont’s Laboratory of Clinical Biochemistry Research with the BNII nephelometer from Dade Behring utilizing a particle enhanced immunonephelometric assay. Average inter- and intra- assay coefficients of variation were <10% for both IL-6 and CRP. Natural-log transformation was applied to IL-6 and CRP to correct non-normal distributions. Subclinical depressive symptoms were assessed using the 20-item version of the Center for Epidemiologic Studies Depression Scale, CES-D (Weissman, Sholomskas, Pottenger, Prusoff, & Locke, 1977).
MRI Acquisition and Preprocessing
MRIs were conducted an average of 38 days (range = 0–107, SD=20) after the blood draw visit. In ancillary analyses, adding the length of time between MRI and blood draw assessments did not affect the direction or level of statistical significance in primary results reported below. Prior to MRI scanning, participants were required to abstain from caffeine, tobacco, and strenuous exercise for at least 3 hours and to abstain from alcohol and taking over the counter medications for at least 12 hours. MRI data were collected on a 3T Trio TIM scanner (Siemens, Erlangen, Germany), equipped with a 12-channel head coil. Resting state BOLD images were acquired during a 5 min and 6 sec period by a gradient-echo EPI sequence (FOV = 200×200 mm, matrix size = 64×64, TR = 2000 ms, TE = 32 ms, and FA = 90°). We acquired 34 slices (3 mm thick, no gap) in an inferior-to-superior direction, yielding 150 BOLD images over the period of acquisition (3 initial discarded images allowing for magnetic equilibration). For spatial co-registration of BOLD images, T1-weighted 3D magnetization-prepared rapid gradient echo (MPRAGE) images were acquired over 7 min 17 sec (FOV = 256×208 mm, matrix size = 256×208, TR = 2100 ms, inversion time (TI) = 1100 ms, TE = 3.31 ms, and FA = 8° (192 slices, 1mm thick, no gap)).
Resting-state BOLD images were first preprocessed using SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK, www.fil.ion.ucl.ac.uk). Individual images were realigned to the first image by rigid body transformation. An additional step that corrected signal distortion was also applied using the SPM unwarp procedure. Realigned and unwarped images were visually inspected and then normalized to MNI space based on the registration of individual’s gray matter image to the SPM MNI gray matter template. Each person’s gray matter image was segmented from the T1 image using the New Segmentation procedure in SPM8, co-registered with the realigned functional images and then registered to the SPM MNI gray matter template by affine transformation. After normalization, resting state images were re-sliced to 2×2×2 mm voxels and smoothed with a 6mm Gaussian kernel. The smoothed images were filtered to exclude non-resting state frequencies with a band-pass filter of 0.008 to 0.15 HZ and submitted to group probabilistic independent component analysis.
Default Mode Resting-State Connectivity
We applied group probabilistic independent component analysis (ICA) (Beckmann, DeLuca, Devlin, & Smith, 2005; Beckmann & Smith, 2004, 2005) to identify the DMN and compute individual DMN connectivity maps. ICA is a multivariate exploratory data analysis method, which examines the spatial pattern and temporal characteristics of a set of measurements to identify latent factors underlying the measurements. Accordingly, the voxel-wise BOLD signal was modeled as a linear mixture of signals from a set of ‘hidden’ structures (networks). The hidden structures were characterized by statistically independent non-Gaussian components. For group ICA, BOLD images (n=107) were concatenated to construct the spatial-temporal data matrix Yn×v,, which is modeled as the product of a mixing matrix Xn×v and a set of independent components Bp×v, namely, Yn×v = Xn×p Bp×v + en×v, where the rows of Bp×v, represent components, en×v are Gaussian noise terms, n is the number of images, v is the number of voxels in a brain, and p is the number of independent components. The data matrix was z-transformed and the independent components were determined by determining the unmixing matrix Wp×v = X̂nxv−1 that optimizes the independence of non-Gaussian components Bp×v, B̂p×v = Wp×v Yn×v. For this approach, we applied the FSL MELODIC toolbox (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/MELODIC), which utilizes the FastICA algorithm to define non-Gaussianity by negative-entropy and solves the optimization model via fixed-point iteration method (Hyvarinen & Oja, 2000). The derived DMN component was verified by comparison to that of Yeo and colleagues (Yeo et al., 2011). The overlap ratio between components was 0.55, as computed by the Dice equation (Bennett & Miller, 2010). We similarly verified our DMN component against other canonical DMN templates (http://www.fmrib.ox.ac.uk/analysis/brainmap+rsns/).
Individual functional connectivity maps were then derived by dual regression (Filippini et al., 2009) using the components computed in the group analysis described above. Individual temporal mixture weights for all components were estimated by the regression of individual BOLD images on the components, and then the individual DMN functional connectivity maps were estimated by the regression of BOLD images on the DMN mixture time series. Individual functional connectivity maps of the DMN were then used for voxel-wise regression analyses.
Control Variables
A number of control variables were examined. These included age, sex, race (coded as white [1] versus other race/ethnicities [2], years of education, smoking status (coded as current smoker [1] versus ex/non-smoker [0]), BMI, and head motion. Head motion was estimated at the subject level by first computing the frame-wise displacement (FD) of each acquired image, calculated as the sum of the absolute values of six realignment parameters produced during preprocessing (Power, Barnes, Snyder, Schlaggar, & Petersen, 2012). Average FD was calculated for each subject and tested for association with IL-6 in particular. Because FD covaried significantly with IL-6 (r (96) = 0.42; See Table S1) and may impact individual difference correlations with resting-state connectivity (Hodgson et al., 2016; Power et al., 2012; Siegel et al., 2016), it was included as an additional covariate in voxel-wise analyses (Power, Schlaggar, & Petersen, 2015).
Data Analysis
Initial correlation analyses executed in SPSS (IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp) tested for bivariate associations between IL-6, CRP, subclinical symptoms of depression and the demographic and health control variables. To test for positive and negative associations between circulating markers of inflammation (IL-6, CRP), subclinical depressive symptoms, and resting-state functional connectivity within the DMN across participants, we executed voxel-wise linear regression analyses using participants’ DMN functional connectivity maps derived by ICA as the dependent variable in SPM8, controlling for the a priori covariates, age, sex, BMI and FD. We applied a whole-brain (voxel-wise) false discovery rate (FDR) corrected threshold of q = 0.05, along with an extent threshold of k = 5 voxels. In line with our study focus, primary analyses were spatially restricted to a DMN region of interest (ROI) mask. This ROI mask was determined by a group-level, random-effects analysis of the ICA DMN component, for which we applied a voxel-wise threshold of T > 3.17 (uncorrected p < 0.001) and a cluster extent of k = 20 voxels. Accordingly, significant findings would correspond to areas within the DMN where ICA resting state functional connectivity covaries with inflammatory markers (Il-6, CRP) or subclinical depressive symptoms (CES-D). For completeness of reporting and to enable comparisons with other studies and data aggregation for meta-analyses, supplemental analyses are reported and shown for whole-brain (unmasked) regression analyses at less conservative statistical thresholds.
Results
Demographic characteristics of the sample are in Table 1, and inter-correlations between variables are in Table S1. Circulating IL-6 and CRP associated positively with BMI, age, and FD. Males exhibited lower levels of IL-6 (t (96) = −2.18, p = 0.03), but not CRP (t (96) = −0.51, p = 0.61) than females. There was a trend towards higher levels of inflammatory markers among non-whites than whites (IL-6: t (96) = −1.38, p = 0.17; CRP: t (96) = −1.91, p = 0.06) and among current smokers than former/never smokers (IL-6: t (96) = −1.36, p = 0.18; CRP: t (96) = −1.77, p = 0.08). As expected, circulating levels of IL-6 and CRP were moderately related (r (96) = 0.45, p < 0.001). No participants met criteria for current major depressive disorder on the MINI; however, 14 reported a past history of MDD. There was no significant difference in mean levels of IL-6 or CRP between those with (IL-6 = 1.30 (SD = 1.08) pg/ml; CRP = 0.99 (SD = 1.15) ng/ml) and without (IL-6 = 1.02 (0.83) pg/ml; CRP = 1.41 (2.07) ng/ml) a past history of MDD. In this otherwise healthy midlife sample of adults, there was no significant association of IL-6 or CRP with symptoms of depression as measured by the CES-D in analyses that controlled for age, sex and BMI (r (92) = −0.15, p = 0.16 and r (92) = −0.02, p = 0.76, respectively).
Table 1.
Demographic characteristics (N = 98)
| Characteristic | Mean or n | SD or % |
|---|---|---|
| Sex (N women) | 60 | 61.20% |
| Age (years) | 41.21 | 7.86 |
| Race | ||
| Caucasian | 79 | 80.60% |
| African American | 14 | 14.30% |
| Asian | 5 | 5.10% |
| Education (years) | 18.01 | 3.20 |
| Body Mass Index (kg/m2) | 25.99 | 4.84 |
| Current Smokers | 10 | 10.20% |
| Depressive Symptoms (CESD) | 8.10 | 7.2 |
| IL-6 (pg/mL) | 1.06 | 0.87 |
| CRP (ng/mL) | 1.34 | 1.96 |
| Frame-wise Displacement | 0.20 | 0.10 |
Note: IL-6, CRP, and CESD descriptive statistics are presented in raw (untransformed) values; 1 participant was missing CESD data and 1 participant was missing smoking status data.
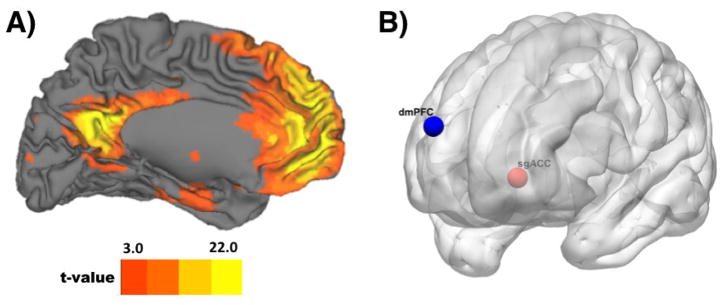
Figure 1A illustrates the DMN derived by ICA in our sample (Figure S1 also shows extended coverage). As expected, the anterior territory of this DMN component encompassed the mPFC and rostral ACC (cf., (Yeo et al., 2011), in addition to temporoparietal, hippocampal, parahippocampal, retrosplenial, and posterior cingulate areas. In a voxelwise multiple regression analysis that applied our DMN ROI mask and controlled for age, sex, BMI, and FD, we observed a positive correlation between IL-6 and DMN connectivity in Brodmann area 25 (BA 25) of the sgACC (MNI x, y, z = −4, 26, −2; k = 7; t (92) = 4.89) at a corrected FDR threshold. Conversely, we observed a negative correlation between IL-6 and DMN connectivity in the dmPFC (MNI x, y, z = 16, 68, 22; k = 16; t (92) = 5.45) at a corrected FDR threshold. No other areas besides the sgACC and dmPFC were revealed after FDR correction for multiple testing (see Figure 1B). For completeness of reporting and comparison with other studies, the results of whole-brain, unmasked, and uncorrected analyses are provided in Table S2 and Figure S2.
Figure 1.
Panel A shows the default mode network (DMN), as revealed by independent components analysis (ICA). For display purposes, the ICA component is shown at an uncorrected, whole-brain threshold of t = 3.169 (p < 0.001) with a cluster extent threshold of k = 20 voxels. Panel B shows areas within the DMN where circulating levels of IL-6 correlated positively (sub-genual anterior cingulate cortex; sgACC) and negatively (dorsal medial prefrontal cortex; dmPFC) across individuals with resting state connectivity at a corrected FDR threshold. Neuroanatomical locations of the projected spheres depicted in panel B correspond to the peak MNI coordinates within the sgACC and dmPFC clusters where IL-6 covaried with DMN connectivity.
In a parallel voxelwise multiple regression analysis with the same a priori covariates (age, sex, BMI, FD), we observed no positive or negative associations between CRP and DMN connectivity at a corrected FDR threshold or at an uncorrected threshold of p < 0.001. In addition, we similarly observed no significant positive or negative associations of depressive symptoms with DMN connectivity at a corrected FDR threshold. For completeness of reporting, Supplemental Table S3 summarizes results of CES-D analyses executed without an FDR threshold. The areas identified in these ancillary analyses did not spatially overlap with those showing positive (sgACC) and negative (dmPFC) associations with IL-6.
We note that it is unlikely that inter-individual variation in fMRI motion artifacts confounded or accounted for the associations of IL-6 with DMN connectivity, as FD was included as a covariate in our regression models. Moreover, FD itself did not associate positively or negatively with DMN connectivity at an FDR threshold (no suprathreshold voxels). Again, we conducted ancillary analyses that adjusted for number of days between the blood draw and MRI session, and all associations between IL-6 and DMN connectivity were retained.
Discussion
This study provides new evidence that circulating levels of IL-6 relate to spontaneous resting activity that is coherent with the DMN among relatively healthy, midlife adults. Within the ACC and mPFC, IL-6 covaried positively with connectivity of the sgACC and negatively with the dmPFC. These associations were independent of the covariates age, sex, BMI, and FD. In secondary analyses, we explored relationships among IL-6, connectivity within the DMN and subclinical symptoms of depression. Contrary to expectations, we did not observe a significant association of IL-6 with depressive symptoms, and there were no significant associations of depressive symptoms with DMN connectivity at corrected statistical thresholds. Finally, we observed that CRP, an acute phase reactant whose production is stimulated by IL-6, did not covary significantly with DMN connectivity.
Our findings of associations between IL-6 and resting DMN connectivity build on existing work on the neural correlates of inflammation. For example, recent studies have begun to examine changes in coordinated activity of the resting brain following experimental inflammatory challenge. Findings show that endotoxin-induced increases in IL-6 associate with increased metabolic activity of the dorsal portion of the ACC (Hannestad et al., 2012) and with resting-state connectivity of the left thalamus with the right posterior cingulate cortex (Labrenz et al., 2016). Furthermore, Dispasquale and colleagues (Dipasquale et al., 2015) used graph theory analysis of resting state fMRI data and showed a rapid reduction in whole brain network connectivity and efficiency shortly following administration of the first dose of interferon-alpha therapy to patients with Hepatitis C. In apparent contrast to the current null findings regarding CRP, Felger and colleagues (Felger et al., 2015) identified an inverse association between circulating levels of CRP and functional connectivity of the vmPFC with the ventral and dorsal striatum among patients with major depressive disorder. Reasons for divergence between our reports are unclear, but may relate to sample differences, with higher levels of inflammation known to accompany major depressive disorder (Haapakoski et al., 2015), or to evidence that CRP does not usually permeate the blood brain barrier in the absence of disease (Hsuchou, Kastin, Mishra, & Pan, 2012). In aggregate, the current findings would thus appear consistent with a growing number of studies that specifically focus on pro-inflammatory cytokines (e.g., IL-6) that modulate central inflammatory processes and may consequently influence coordinated activity of the resting brain.
In addition to relationships with resting brain activity, peripheral inflammation associates with task-induced activation, particularly within regions of the PFC that are implicated in peripheral physiological control. Indeed, we have previously shown an association of circulating IL-6 with engagement of ACC and mPFC during effortful emotion regulation among a sample of 183 participants that partly overlaps with the current sample (Gianaros et al., 2014). Similarly, increased activity of the dACC in response to a social rejection task has been shown to associate positively with stress-induced increases in peripheral inflammation (Slavich, Way, Eisenberger, & Taylor, 2010). Experimentally induced increases in peripheral IL-6 also relate to increased activity within the ACC. For example, Harrison and colleagues (Harrison, Brydon, Walker, Gray, Steptoe, Dolan, et al., 2009) showed that typhoid vaccination increased circulating levels of IL-6 and influenced stressor-evoked reactivity in the ACC and dorsolateral PFC. Similarly, Kullman et al. (Kullmann et al., 2013) showed that endotoxin-induced increases in IL-6 positively associated with activation of the inferior orbitofrontal cortex and the superior and medial PFC in response to emotionally aversive visual stimuli. Heightened dACC activity in response to a visuospatial attention task has also been observed in response to IFN-α therapy (Capuron et al., 2005). Finally, O’Connor et al (O’Connor, Irwin, & Wellisch, 2009) showed a positive association of salivary markers of inflammation with activation of the sgACC and orbitofrontal cortex in response to a grief elicitation task among recently bereaved women. In sum, converging evidence supports an association of inflammation with functional and resting state activity, particularly within the PFC and cingulate. However, there appears to be regional variation in the neural correlates of systemic inflammation that may depend on the specific marker of systemic inflammation, task assessment protocol, participant sample, and metric of neural activity examined.
Although the functions of synchronized activity of the DMN remain uncertain, growing consensus supports a role in coordinating processes relevant for survival (Kaiser, Andrews-Hanna, et al., 2015; Roy et al., 2012). The DMN is thought to operate as an intrinsic and coherent network that is activated at rest when individuals are focused internally (Buckner et al., 2008). Analysis of activity within the DMN suggests two coordinated subsystems that interact with a core set of hubs, yet exhibit distinct contributions to behavioral functions (Andrews-Hanna et al., 2010). A “medial temporal lobe subsystem” includes anterior midline and cingulate regions, posterior parietal lobule, retrosplenial cortex, parahippocampal cortex and hippocampal formation and is activated when individuals formulate episodic decisions about their future (Andrews-Hanna et al., 2010). A “dorsal medial prefrontal cortex subsystem” includes the dmPFC, tempoparietal junction, lateral temporal cortex, and temporal pole and is active when individuals consider their present mental states (Andrews-Hanna et al., 2010). Because of differences in methodology, we are reluctant to draw strong conclusions regarding how the current findings map onto the functionality of these putative subsystems. However, it is possible that the observed positive association of IL-6 with connectivity of the sgACC maps onto the medial temporal lobe subsystem and the negative association of IL-6 with dmPFC connectivity onto the dmPFC subsystem. Future research is warranted to further examine associations of peripheral markers of inflammation with patterns of activity within the DMN and to consider their functional relevance for present vs. prospective oriented cognitive functions that may guide self-relevant behaviors.
The coordinated activity of anterior midline regions of the DMN, including the ACC and networked regions of the adjacent mPFC, is proposed to play a role in evaluating the emotional significance of stimuli (Roy et al., 2012) and appraising threat, with ACC and mPFC regions comprising a larger hub that links higher order social cognitive and affective processes to subcortical brain regions that control autonomic and endocrine mechanisms known to modulate inflammation (Eisenberger & Cole, 2012). Neuroanatomical evidence supports this possibility, with afferent and efferent pathways connecting the mPFC, sgACC, and other ACC areas to subcortical nuclei involved in the experience of core affect and in related homeostatic regulation of peripheral physiology (Critchley & Harrison, 2013; Öngür & Price, 2000). Feedback from the periphery, including levels of systemic inflammation, may provide an important interoceptive drive in the assessment of biological threat and play a role in a circuit that fine tunes peripheral physiology in preparation for anticipated situations (Bechara, Tranel, Damasio, & Damasio, 1996). The current findings extend prior work to show an association of peripheral inflammation with tonic activity within anterior portions of the DMN, which in speculation may play a key role in the homeostatic control of physiological state (Critchley & Harrison, 2013).
Studies showing central effects of the experimental induction of peripheral immune activation provide support for afferent viscerosensory pathways that link peripheral inflammation to brain systems relevant to motivational and emotional behavior (Hannestad et al., 2012; Harrison, Brydon, Walker, Gray, Steptoe, & Critchley, 2009; Kullmann et al., 2013). By crossing the blood-brain barrier, binding to receptors on endothelial cells in brain microvasculature, and/or activating vagal nerve afferents, peripheral proinflammatory cytokines have been shown to stimulate the production of central proinflammatory cytokines by microglial cells (Banks & Kastin, 1991; Ek et al., 2001; Tracey, 2002; Yirmiya & Goshen, 2011). Converging evidence suggests that this afferent pathway communicates information regarding internal physiological states to areas of rostral ACC and other PFC areas that play a role in emotional regulation and motivation in response to threat appraisal (Critchley & Harrison, 2013; Eisenberger & Cole, 2012; Gianaros & Wager, 2015). The coordinated activity of ACC and PFC areas within the DMN may represent the top of a feedback circuit, receiving afferent viscerosensory representation, but also contributing to the “top-down” modulation of visceromotor pathways that control peripheral physiology, including the inflammatory response.
A network of regions within the ACC and PFC, including the dmPFC and sgACC identified here in association with IL-6, are also thought to play a role in the appraisal of threat and activate subcortical regions, including the amygdala, ventral pallidum, hypothalamus, hippocampus and insula, which contribute to emotional arousal and control peripheral autonomic and neuroendocrine mechanisms that regulate inflammatory gene expression (Eisenberger & Cole, 2012). The sgACC is proposed to be a key node in this visceromotor network (Drevets, Price, & Furey, 2008; Zhang et al., 2014), with sgACC activity relating to peripheral physiological indices of sympathetic and parasympathetic activity both at rest and in response to emotional, cognitive and motor tasks (Gianaros & Wager, 2015; Matthews, Paulus, Simmons, Nelesen, & Dimsdale, 2004; Thayer, Ahs, Fredrikson, Sollers, & Wager, 2012). Interestingly, the brain regions that show the greatest associations with tonic levels of physiologic arousal belong to the DMN (Nagai, Critchley, Featherstone, Trimble, & Dolan, 2004), and it is suggested that coordinated activity of anterior areas within the DMN during resting states may contribute to control of the autonomic nervous system (Wong, Masse, Kimmerly, Menon, & Shoemaker, 2007). Recent anatomical tracing findings in the nonhuman primate show that a major component of cortical influence over the adrenal medulla arises from sgACC (Dum, Levinthal, & Strick, 2016). Interestingly, we observed that DMN connectivity in the sgACC associated positively with IL-6 at rest. This raises the possibility that a sympathoadrenal pathway influenced by sgACC and networked areas contributes to tonic control of peripheral inflammation. In this regard, it is known that catecholamines act through beta-adrenergic receptors on immune cells to stimulate expression and release of proinflammatory mediators, such as IL-6 (Eisenberger & Cole, 2012).
Functional neural variation within the DMN is implicated in diverse social, cognitive, and affective processes, as well as psychiatric and neurological disorders that associate with systemic inflammation. For example, converging evidence suggests that bidirectional pathways link peripheral inflammation to neural activity within corticolimbic circuits that are believed to underlie affective symptoms of depression. Contrary to expectations, we did not observe an association of IL-6 or DMN connectivity with symptoms of depression. Although consistent evidence shows an association of major depressive disorder with elevated IL-6 (Haapakoski et al., 2015), findings are smaller and more variable when considering subclinical symptoms of depression in community samples (Howren, Lamkin, & Suls, 2009). In this regard, no participants in the current study were taking psychotropic medications or met criteria for current mood disorder, which may explain our null findings.
Our novel findings should be interpreted in the context of a number of limitations. First, the cross-sectional design precludes causal interpretations. As outlined above both afferent and efferent pathways link the central nervous and immune systems, with IL-6 serving as a primary messenger in a feedback loop. It is also possible that peripheral IL-6 and DMN connectivity independently relate to third factors, such as subclinical brain inflammation (Licastro et al., 2000) or genetic modification. Another limitation of the current study is the single assessment of IL-6 and CRP that occurred an average of 38 days before the MRI scan. Although evidence suggests both markers of inflammation are relatively stable over extended periods (Danesh et al., 2004; Glynn, MacFadyen, & Ridker, 2009; Hardikar et al., 2014; Rao, Pieper, Currie, & Cohen, 1994), a more reliable indicator of stable inter-individual variability would be derived from multiple assessments over time. In regard to time between the blood draw and scan, the current findings were retained in supplemental analyses that adjusted for this period. However, it will be important to include assessment of markers of inflammation on the day of brain imaging in future work. Future work should also consider the possibility that other peripheral markers of inflammation may associate differently with resting brain activity. In this regard, converging animal and human evidence suggests that IL-6 is one of a number of peripheral proinflammatory cytokines that stimulate central inflammatory processes and modulate brain function (Yirmiya & Goshen, 2011). In contrast, there is evidence that CRP does not permeate the blood-brain barrier under normal conditions (Hsuchou et al., 2012) and arguably reflects a downstream marker of the pro-inflammatory response, which may explain less consistent associations of CRP with resting-state functional connectivity. The current study was also limited to the examination of only one of several intrinsic brain networks. A number of resting networks have been identified with distinct patterns of connectivity and function (Yeo et al., 2011). We targeted the DMN because it is well characterized, has been implicated in the control of peripheral physiologic pathways known to modulate magnitude of inflammatory response, and has received the most attention in the context of conditions that associate with inflammation, e.g., depression (Kaiser, Andrews-Hanna, et al., 2015). In the future, an examination of the association of inflammation with functional connectivity of other resting networks is indicated. Finally, we observed that IL-6 was significantly associated with individual differences in FD, an index of fMRI signal motion. Recent evidence shows that motion parameters appear to track with individual difference factors that have been related to systemic inflammation (e.g., BMI (Hodgson et al., 2016)) and resting state connectivity (Siegel et al., 2016). To account for this, we adopted Power et al’s (Power et al., 2015) conservative approach and statistically adjusted for FD in all analyses. Our findings contribute to the literature by providing initial evidence that fMRI signal motion associates with peripheral levels of IL-6 and thus should be considered in studies examining the neurophysiological (e.g., resting state connectivity) correlates of individual differences in systemic inflammation.
To conclude, we provide initial evidence of an association between IL-6, a widely measured marker of systemic inflammation and risk for chronic disease, and resting-state DMN connectivity among a sample of relatively healthy, midlife adults. These findings are consistent with a brain system that connects higher order cognitive and emotional processes, e.g., appraisal processes that evaluate events and experiences for their personal significance, to subcortical systems that control peripheral physiological pathways that moderate systemic inflammation. IL-6, in turn, may participate in a feedback loop via viscerosensory pathways to influence coordinated resting activity of the DMN. At rest, this brain-body visceral control loop may contribute to the tonic regulation and representation of peripheral inflammation, coordinating inflammatory responses with other physiological processes that may possibly confer survival advantage.
Supplementary Material
Highlights.
The DMN is implicated in psychological processes that associate with inflammation.
Components of the DMN control peripheral pathways that moderate inflammation.
IL-6 found to covary with functional connectivity of the sgACC and dmPFC within the DMN.
Initial support for a brain-visceral control loop linking cognitive/emotional processes to IL-6.
Acknowledgments
This work was supported by National institutes of Health grants PO1 HL040962 (SBM) and R01 HL089850 (PJG).
Footnotes
Author contributions: A.L.M., P.J.G., and S.B.M. designed and performed research; A.L.M., P.J.G., K.K., L.K.S., T.E.K. and D. C-H.K. analyzed data; A.L.M., P.J.G., L.K.S., D. C-H. K., T.E.K. and S.B.M wrote the paper.
Conflict of interest statement: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen B, Jennings JR, Gianaros PJ, Thayer JF, Manuck SB. Resting high-frequency heart rate variability is related to resting brain perfusion. Psychophysiology. 2015;52(2):277–287. doi: 10.1111/psyp.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65(4):550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ. Blood to brain transport of interleukin links the immune and central nervous systems. Life Sci. 1991;48(25):PL117–121. doi: 10.1016/0024-3205(91)90385-o. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Damasio AR. Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cereb Cortex. 1996;6(2):215–225. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360(1457):1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23(2):137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. Tensorial extensions of independent component analysis for multisubject FMRI analysis. Neuroimage. 2005;25(1):294–311. doi: 10.1016/j.neuroimage.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Bennett CM, Miller MB. How reliable are the results from functional magnetic resonance imaging? Ann N Y Acad Sci. 2010;1191:133–155. doi: 10.1111/j.1749-6632.2010.05446.x. [DOI] [PubMed] [Google Scholar]
- Black PH. The inflammatory response is an integral part of the stress response: Implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain Behav Immun. 2003;17(5):350–364. doi: 10.1016/s0889-1591(03)00048-5. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili M, Woolwine BJ, Nemeroff CB, Berns GS, Miller AH. Anterior cingulate activation and error processing during interferon-alpha treatment. Biol Psychiatry. 2005;58(3):190–196. doi: 10.1016/j.biopsych.2005.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493(1):154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Harrison NA. Visceral influences on brain and behavior. Neuron. 2013;77(4):624–638. doi: 10.1016/j.neuron.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, … Gudnason V. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350(14):1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipasquale O, Cooper EA, Tibble J, Voon V, Baglio F, Baselli G, … Harrison NA. Interferon-alpha acutely impairs whole-brain functional connectivity network architecture - A preliminary study. Brain Behav Immun. 2015 doi: 10.1016/j.bbi.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213(1–2):93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Levinthal DJ, Strick PL. Motor, cognitive, and affective areas of the cerebral cortex influence the adrenal medulla. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1605044113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Cole SW. Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health. Nat Neurosci. 2012;15(5):669–674. doi: 10.1038/nn.3086. [DOI] [PubMed] [Google Scholar]
- Ek M, Engblom D, Saha S, Blomqvist A, Jakobsson PJ, Ericsson-Dahlstrand A. Inflammatory response: pathway across the blood-brain barrier. Nature. 2001;410(6827):430–431. doi: 10.1038/35068632. [DOI] [PubMed] [Google Scholar]
- Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, Miller AH. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, … Havlik RJ. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47(6):639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, … Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A. 2009;106(17):7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Marsland AL, Kuan DC, Schirda BL, Jennings JR, Sheu LK, … Manuck SB. An inflammatory pathway links atherosclerotic cardiovascular disease risk to neural activity evoked by the cognitive regulation of emotion. Biol Psychiatry. 2014;75(9):738–745. doi: 10.1016/j.biopsych.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Wager TD. Brain-Body Pathways Linking Psychological Stress and Physical Health. Curr Dir Psychol Sci. 2015;24(4):313–321. doi: 10.1177/0963721415581476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn RJ, MacFadyen JG, Ridker PM. Tracking of high-sensitivity C-reactive protein after an initially elevated concentration: the JUPITER Study. Clin Chem. 2009;55(2):305–312. doi: 10.1373/clinchem.2008.120642. [DOI] [PubMed] [Google Scholar]
- Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimaki M. Cumulative meta-analysis of interleukins 6 and 1beta, tumour necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain Behav Immun. 2015;49:206–215. doi: 10.1016/j.bbi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamani C, Mayberg H, Stone S, Laxton A, Haber S, Lozano AM. The subcallosal cingulate gyrus in the context of major depression. Biol Psychiatry. 2011;69(4):301–308. doi: 10.1016/j.biopsych.2010.09.034. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Farmer M, Fogelman P, Gotlib IH. Depressive Rumination, the Default- Mode Network, and the Dark Matter of Clinical Neuroscience. Biol Psychiatry. 2015;78(4):224–230. doi: 10.1016/j.biopsych.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad J, Subramanyam K, Dellagioia N, Planeta-Wilson B, Weinzimmer D, Pittman B, Carson RE. Glucose metabolism in the insula and cingulate is affected by systemic inflammation in humans. J Nucl Med. 2012;53(4):601–607. doi: 10.2967/jnumed.111.097014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardikar S, Song X, Kratz M, Anderson GL, Blount PL, Reid BJ, … White E. Intraindividual variability over time in plasma biomarkers of inflammation and effects of long-term storage. Cancer Causes Control. 2014;25(8):969–976. doi: 10.1007/s10552-014-0396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 2009;66(5):407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Dolan RJ, Critchley HD. Neural origins of human sickness in interoceptive responses to inflammation. Biol Psychiatry. 2009;66(5):415–422. doi: 10.1016/j.biopsych.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson K, Poldrack RA, Curran JE, Knowles EE, Mathias S, Goring HH, … Glahn DC. Shared Genetic Factors Influence Head Motion During MRI and Body Mass Index. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71(2):171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Hsuchou H, Kastin AJ, Mishra PK, Pan W. C-reactive protein increases BBB permeability: implications for obesity and neuroinflammation. Cell Physiol Biochem. 2012;30(5):1109–1119. doi: 10.1159/000343302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyvarinen A, Oja E. Independent component analysis: algorithms and applications. Neural Netw. 2000;13(4–5):411–430. doi: 10.1016/s0893-6080(00)00026-5. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11(9):625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;21(4):374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Allen B, Gianaros PJ, Thayer JF, Manuck SB. Focusing neurovisceral integration: cognition, heart rate variability, and cerebral blood flow. Psychophysiology. 2015;52(2):214–224. doi: 10.1111/psyp.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JR, Sheu LK, Kuan DC, Manuck SB, Gianaros PJ. Resting state connectivity of the medial prefrontal cortex covaries with individual differences in high-frequency heart rate variability. Psychophysiology. 2016;53(4):444–454. doi: 10.1111/psyp.12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph NT, Muldoon MF, Manuck SB, Matthews KA, MacDonald LA, Grosch J, Kamarck TW. The Role of Occupational Status in the Association Between Job Strain and Ambulatory Blood Pressure During Working and Nonworking Days. Psychosom Med. 2016;78(8):940–949. doi: 10.1097/PSY.0000000000000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry. 2015;72(6):603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Whitfield-Gabrieli S, Dillon DG, Goer F, Beltzer M, Minkel J, … Pizzagalli DA. Dynamic Resting-State Functional Connectivity in Major Depression. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172(11):1075–1091. doi: 10.1176/appi.ajp.2015.15020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann JS, Grigoleit JS, Lichte P, Kobbe P, Rosenberger C, Banner C, … Schedlowski M. Neural response to emotional stimuli during experimental human endotoxemia. Hum Brain Mapp. 2013;34(9):2217–2227. doi: 10.1002/hbm.22063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrenz F, Wrede K, Forsting M, Engler H, Schedlowski M, Elsenbruch S, Benson S. Alterations in functional connectivity of resting state networks during experimental endotoxemia - An exploratory study in healthy men. Brain Behav Immun. 2016;54:17–26. doi: 10.1016/j.bbi.2015.11.010. [DOI] [PubMed] [Google Scholar]
- Licastro F, Pedrini S, Caputo L, Annoni G, Davis LJ, Ferri C, … Grimaldi LM. Increased plasma levels of interleukin-1, interleukin-6 and alpha-1-antichymotrypsin in patients with Alzheimer’s disease: peripheral inflammation or signals from the brain? J Neuroimmunol. 2000;103(1):97–102. doi: 10.1016/s0165-5728(99)00226-x. [DOI] [PubMed] [Google Scholar]
- Luc G, Bard JM, Juhan-Vague I, Ferrieres J, Evans A, Amouyel P, … Ducimetiere P. C-reactive protein, interleukin-6, and fibrinogen as predictors of coronary heart disease: the PRIME Study. Arterioscler Thromb Vasc Biol. 2003;23(7):1255–1261. doi: 10.1161/01.ATV.0000079512.66448.1D. [DOI] [PubMed] [Google Scholar]
- Marin IA, Kipnis J. Central Nervous System: (Immunological) Ivory Tower or Not? Neuropsychopharmacology. 2017;42(1):28–35. doi: 10.1038/npp.2016.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Gianaros PJ, Kuan DC, Sheu LK, Krajina K, Manuck SB. Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain Behav Immun. 2015;48:195–204. doi: 10.1016/j.bbi.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews SC, Paulus MP, Simmons AN, Nelesen RA, Dimsdale JE. Functional subdivisions within anterior cingulate cortex and their relationship to autonomic nervous system function. Neuroimage. 2004;22(3):1151–1156. doi: 10.1016/j.neuroimage.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, … Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Menard C, Pfau ML, Hodes GE, Russo SJ. Immune and Neuroendocrine Mechanisms of Stress Vulnerability and Resilience. Neuropsychopharmacology. 2017;42(1):62–80. doi: 10.1038/npp.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Cole SW. Health psychology: developing biologically plausible models linking the social world and physical health. Annual Review of Psychology. 2009;60:501–524. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- Muldoon MF, Laderian B, Kuan DC, Sereika SM, Marsland AL, Manuck SB. Fish oil supplementation does not lower C-reactive protein or interleukin-6 levels in healthy adults. J Intern Med. 2016;279(1):98–109. doi: 10.1111/joim.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Critchley HD, Featherstone E, Trimble MR, Dolan RJ. Activity in ventromedial prefrontal cortex covaries with sympathetic skin conductance level: a physiological account of a “default mode” of brain function. Neuroimage. 2004;22(1):243–251. doi: 10.1016/j.neuroimage.2004.01.019. [DOI] [PubMed] [Google Scholar]
- O’Connor MF, Irwin MR, Wellisch DK. When grief heats up: pro-inflammatory cytokines predict regional brain activation. Neuroimage. 2009;47(3):891–896. doi: 10.1016/j.neuroimage.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öngür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10(3):206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Petersen SE. Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage. 2015;105:536–551. doi: 10.1016/j.neuroimage.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH. Is depression an inflammatory disorder? Curr Psychiatry Rep. 2011;13(6):467–475. doi: 10.1007/s11920-011-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao KM, Pieper CS, Currie MS, Cohen HJ. Variability of plasma IL-6 and crosslinked fibrin dimers over time in community dwelling elderly subjects. Am J Clin Pathol. 1994;102(6):802–805. doi: 10.1093/ajcp/102.6.802. [DOI] [PubMed] [Google Scholar]
- Roriz-Filho JS, Sa-Roriz TM, Rosset I, Camozzato AL, Santos AC, Chaves ML, … Roriz-Cruz M. (Pre)diabetes, brain aging, and cognition. Biochim Biophys Acta. 2009;1792(5):432–443. doi: 10.1016/j.bbadis.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci. 2012;16(3):147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, … Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34-57. [PubMed] [Google Scholar]
- Siegel JS, Mitra A, Laumann TO, Seitzman BA, Raichle M, Corbetta M, Snyder AZ. Data Quality Influences Observed Links Between Functional Connectivity and Behavior. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. 2014;140(3):774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Way BM, Eisenberger NI, Taylor SE. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proc Natl Acad Sci U S A. 2010;107(33):14817–14822. doi: 10.1073/pnas.1009164107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Ahs F, Fredrikson M, Sollers JJ, 3rd, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev. 2012;36(2):747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. The inflammatory reflex. Nature. 2002;420(6917):853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51(1):32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;106(3):203–214. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- Wong SW, Masse N, Kimmerly DS, Menon RS, Shoemaker JK. Ventral medial prefrontal cortex and cardiovagal control in conscious humans. Neuroimage. 2007;35(2):698–708. doi: 10.1016/j.neuroimage.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, … Newman AB. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292(18):2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, … Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25(2):181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Zhang S, Hu S, Chao HH, Ide JS, Luo X, Farr OM, Li CS. Ventromedial prefrontal cortex and the regulation of physiological arousal. Soc Cogn Affect Neurosci. 2014;9(7):900–908. doi: 10.1093/scan/nst064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.