ABSTRACT
Nerve dependence is a phenomenon observed across a stunning array of species and tissues. From zebrafish to fetal mice to humans, research across various animal models has shown that nerves are critical for the support of tissue repair and regeneration. Although the study of this phenomenon has persisted for centuries, largely through research conducted in salamanders, the cellular and molecular mechanisms of nerve dependence remain poorly-understood. Here we highlight the near-ubiquity and clinical relevance of vertebrate nerve dependence while providing a timeline of its study and an overview of recent advancements toward understanding the mechanisms behind this process. In presenting a brief history of the research of nerve dependence, we provide both historical and modern context to our recent work on nerve dependent limb regeneration in the Mexican axolotl.
Introduction
Tissue regeneration has been a source of scientific study and fascination for nearly five hundred years, but the mystery of how and why certain organisms regenerate lost tissue remains as enigmatic and compelling as ever. One major question which has emerged throughout the past centuries of research is that of nerve dependent regeneration. Nearly every known example of vertebrate tissue regeneration requires the presence of intact peripheral nerves, a striking commonality given the vast diversity of regenerating organisms and tissues. Although nerve dependence was first discovered in the salamander nearly two hundred years ago, the molecular mechanisms underpinning this phenomenon have only recently begun to come to light.
Nerve dependence across models of vertebrate tissue regeneration
Vertebrates display a vast range of regenerative abilities, and nerve dependence has been observed in regenerative species ranging from fish to mammals. Zebrafish cannot regenerate amputated fins if they have undergone denervation,1 and nerve dependence has also been observed in the regenerating barbels of catfish.2 African clawed froglets (Xenopus laevis) generate a nerve dependent hypomorphic spike after amputation, whereas hindlimb regeneration in larval frogs becomes progressively more nerve dependent as the animal develops.3,4 This progression is likely due to the abundance of mitogens during early limb development.5
Anuran amphibians appear to forego their ability to regenerate complete appendages after metamorphosis, but urodele amphibians demonstrate nerve dependent limb regeneration throughout adulthood. A variety of newt and ambystomatid salamander species, among which the Mexican axolotl (Ambystoma mexicanum) is the most widely studied, have been used as models for regeneration research. Urodele limbs are anatomically similar to human limbs and are generally capable of regenerating after amputation regardless of the age of the animal. This regeneration normally proceeds via the formation and subsequent growth of a hyperinnervated proliferative mass called the blastema. However, denervation of the salamander limb completely prevents the formation of this blastema and halts regeneration early in the process. The salamander is thus a robust and reliable model for the study of nerve dependence, and it will therefore be the main focus of this review. For further review of known examples of nerve dependence in both vertebrates and invertebrates, see ref. 6
Mammals are not generally known for their regenerative prowess, but nerves do appear to play an important role during both early and adult mammalian tissue regeneration. Fetal mammals are capable of scarless epidermal wound regeneration, but denervation disrupts this regenerative program in both mice7 and lambs.8 Prenatal nerve dependence is not limited to mammals, as wound healing in the developing chick embryo is also dependent on cutaneous innervation.9 Furthermore, denervation of the vagus nerve inhibits cardiac tissue regeneration in the neonatal mouse and can be rescued via supplementation with nerve growth factor and Neuregulin-1.10 Although adult mammals are far more limited in their regenerative abilities, mice are capable of regenerating their digit tips so long as the amputation plane does not extend past the nail bed. Few studies have looked at innervation during this process, and thus the role and necessity of nerves for the support of mammalian digit regeneration remains unclear. In 2014, Rinkevich et al. found that denervation of the amputated digit tip led to defects in digit patterning, although cell turnover was unaffected.11 In contrast, a 2016 study by Johnston et al. indicated that Schwann cells dedifferentiate after amputation and secrete essential paracrine factors, including oncostatin M and PDGF-AA, that act to stimulate mesenchymal cell proliferation.12 Study of the nerve dependency of ear hole-punch regeneration also remains in its early stages. Denervation leads to tissue regression and necrosis in mouse strains which are capable of healing ear wounds.13 Ear tissue regeneration in the African spiny mouse proceeds from the innervated (proximal) side of the wound, and the regenerating tissue is densely innervated by invading peripheral nerves.14,15 While much remains to be learned about the role of nerves during mammalian tissue regeneration, it is clear that nerve dependence represents an exciting avenue for further research in both fetal and adult animal models.
Although humans display relatively little capacity for tissue regeneration, nerves nevertheless play an important role in human wound healing, and loss of innervation is a leading cause of chronic wound formation. Paraplegic and quadriplegic patients experience more complications and develop more chronic wounds than nonparalegic patients during wound healing in their denervated regions.16 Moreover, peripheral neuropathy in diabetic patients can result in impaired wound healing and the formation of diabetic ulcers on the extremities, a condition which has become one of the leading causes of non-traumatic lower limb amputations in developed countries.17,18 This pathology does not appear to be a direct result of insulin resistance or depletion, but is instead partially caused by aberrant apoptosis and inflammation in denervated skin wounds.19 For further review of the role of nerves during mammalian wound healing, see ref. 20 Elucidating the underlying mechanisms of nerve dependency thus has wide-ranging implications for the study of human health and medicine.
History of nerve dependence research in the salamander
The study of nerve dependency began nearly two centuries ago, and salamanders have been the subject of this research from the very beginning. For a brief timeline of this history of study, see Fig. 1. Salamanders have proven an ideal model for the study of nerve dependency because their brachial nerves are easy to surgically access and denervation of the amputated salamander limb reliably inhibits regeneration. An anonymous aquatic salamander featured in what is credited to be the first study demonstrating the requirement of nerve axons for regeneration. In 1823, Tweedy John Todd submitted a comprehensive overview of salamander regenerative capacity to the Quarterly Journal of Science, Literatures and the Arts. In it, he noted for the first time that:
“if the sciatic nerve be intersected at the time of amputation, that part of the stump below the section of the nerve mortifies… If the division of the nerve be made after the healing of the stump, [regeneration] is either retarded or entirely prevented. And if the nerve be divided after [regeneration] has commenced, or considerably advanced, the new growth either remains stationary, or it wastes…” (p.91,21).
Figure 1.
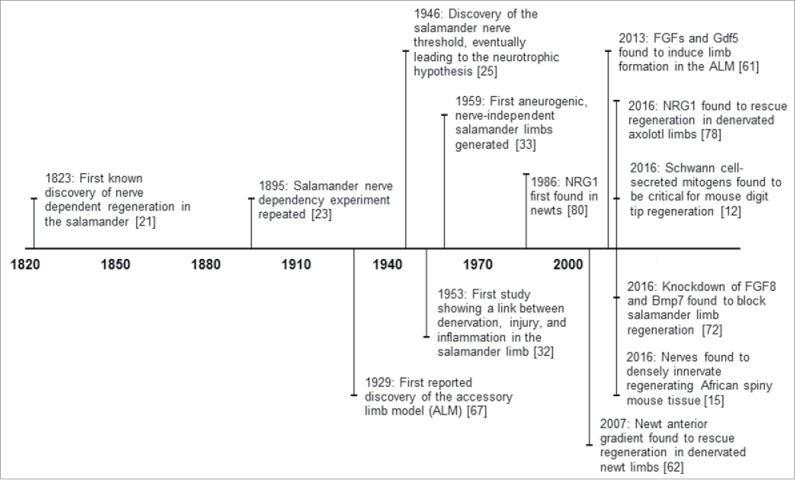
Timeline of landmark nerve dependency studies throughout history, along with a selection of the most recent findings in the field.
Despite the novelty of these findings, which accurately depicted the results of denervation at various stages during regeneration, Todd's remarkable observations were left largely untouched for more than seventy years.
As the 19th century came to a close, a number of researchers independently attempted to repeat Todd's experiments, and in doing so led to a renewed interest in nerve dependent limb regeneration. Because the regenerating salamander provided an accessible and re-usable model for the study of tissue development, the basis for many of these early studies arose from the desire to study the role of nerves on tissue growth, rather than direct interest in the phenomenon of regeneration. In 1913, the Scottish physician Diarmid Paton wrote an extensive contemporary overview of turn-of-the-century nerve dependence studies.22 In his series of lectures, entitled “The Nervous and Chemical Regulators of Metabolism,” Paton describes a research environment characterized by equal parts enthusiasm and controversy. Although several scientists, led by German researchers Wolff23 and Walter,24 reported that they had reproduced Todd's experiment, the wide variety of salamander species available to study led to a heated debate on the ubiquity of amphibian nerve dependency. Paton, himself convinced by Wolff and Walter's work, rather scathingly writes that one scientist, attempting to reject the nerve dependence hypothesis, decided to study the newt Triturus viridescens “after rejecting various amphibia because the results were not satisfactory” (p.13). Nevertheless, it appears that the scientific community was united enough at this point in time for Paton to confidently declare “with the large series of experimental investigations upon [nerve dependence], we need not concern ourselves further than we have already done” (p.14). His question for salamander researchers going forward, “to what extent does the nervous system dominate the metabolism?” (p.14) has resonated throughout the twentieth century and up to the modern day.
This increased focus on the presence and necessity of nerves led salamander researchers to concentrate on parsing out the contributions of various types of nerves. While debate over the contributions of different types of nerves persisted for decades, the lab of Marcus Singer put many of these arguments to rest throughout the middle part of the 20th century. In a series of seminal works published during the 1940's and throughout the following decades, Singer found that the type of nerve present has no effect: instead, all nerve fibers contribute roughly equally to the process and nerves must be present in sufficient quantity to trigger regeneration ([25–27], for review of Singer's work see refs. 28,29). While the precise cause of this “nerve threshold” remains unknown, Singer's ultimate hypothesis- that peripheral nerves produce one or more factors which are essential for blastema formation and growth- laid the groundwork for future studies of what is now known as the “neurotrophic” model of nerve support (Fig. 2A). This neurotrophic hypothesis has since its inception served as the basis for studies of nerve dependence in the regenerating salamander limb.
Figure 2.
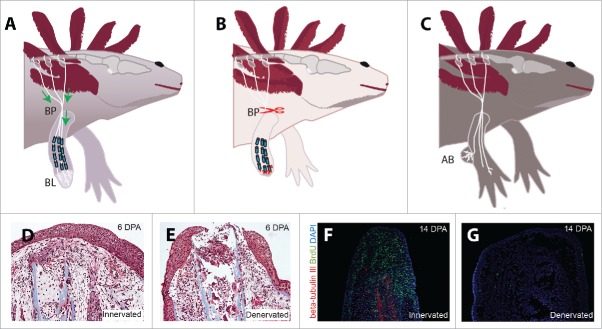
(A) Diagram of the neurotrophic hypothesis, which states that nerve-secreted mitogens are transported from the dorsal root ganglia through the brachial plexus (BP) to support the proliferating blastema (BL). (B) Illustration of the inhibition hypothesis, in which denervation at the brachial plexus induces the release of inhibitory factors from Schwann Cells at the wound site. (C) Diagram of the accessory limb model, in which a peripheral nerve is deviated to a wound site to induce the formation of an accessory blastema (AB). (D) A control limb at 6 days post amputation (DPA). (E) A denervated limb at 6 DPA demonstrating extreme histolysis and inflammation. (F) The hyperinnervated regenerating blastema is highly proliferative at 14 DPA, as demonstrated by BrdU incorporation and staining. (G) Denervation eliminates beta-tubulin III staining of axons and substantially reduces the proliferative index of limbs at 14 DPA.
With one major mystery solved, focus shifted to the elucidation of other aspects of nerve dependence. A series of studies conducted by a number of researchers in the 1940's and 50's focused primarily on limb regression, a phenomenon first observed by Todd more than a century prior. If the salamander limb is denervated at the time of amputation, it typically undergoes extensive histolysis and tissue regression (Fig. 2D, E). In certain cases, most often in larval animals, the denervated limb may even fall off completely.30,31 In 1953, Charles Thornton found a link between histolysis, denervation, and tissue injury when he conducted a study in which he crushed the radius and ulna of otherwise-intact limbs with a pair of watchmaker's forceps. When the limb was fully innervated, this crush injury resulted in complete regeneration. However, if the limb was denervated at the time of injury, it underwent histolysis, inflammation, and tissue degradation so severe that the limb sometimes fell off at the site of injury. Only after complete regression to the shoulder and consequent reinnervation did the limbs finally regrow. Denervation of an intact limb, meanwhile, did not cause any appreciable change in its tissue architecture.32 This study uncovered a link between denervation, injury, and inflammation, and few studies have attempted to follow up on it, indicating that this relationship may be an intriguing avenue for future research.
The discovery of nerve-independent salamander limb regeneration in the late 1950's added an intriguing new dimension to the mystery of nerve dependence. In 1959, C.L. Yntema generated aneurogenic animals by removing the neural tube from embryos and found that the limbs of these animals regenerated despite their lack of innervation.33 This phenomenon was quickly confirmed in the following years, and in 1953 Steen & Thornton grafted nerveless limbs onto innervated host animals and examined their regenerative ability over time. Strikingly, the grafted aneurogenic limbs retained their ability to regenerate in the absence of nerves for several days after the surgery. However, the limbs lost their nerve-independence upon complete innervation from the host, and thereafter were incapable of overcoming denervation.34 It has since been hypothesized that limbs become “addicted” to one or more regeneration-critical factors which are produced in the developing limb but later supplied only by the nerves upon innervation. One candidate factor is anterior gradient, which is expressed in the developing aneurogenic limb but decreased after grafting and innervation.35
A series of grafting and in vitro studies in the latter half of the 20th century implicated proliferating blastemal cells as a major target of the nerves. In 1977, Liversage & Globus showed that blastemas cultured in vitro proliferated only if they remained innervated by spinal cord implants,36 while Goldhamer et al. found that implantation of dorsal root ganglia into the blastemas of denervated limbs rescued cell cycling, although it did not increase cell cycling in fully-innervated blastemas.37 Further characterization of cell cycling in the blastema showed that denervation effectively halts mitosis in blastemal cells,38,39 and thus the modern study of nerve dependence has largely focused on elucidating the links between peripheral nerves and blastemal growth (Fig. 2F, G).
While the blastema is widely-accepted as a probable target of peripheral nerves, other tissues remain more controversial. Because the epidermis is extensively invaded by nerves after amputation,40-42 a number of researchers have suggested that the apical epidermal cap (AEC) formed after amputation is dependent on innervation. Indeed, studies have found that the expression of Dlx-3 and Sp9, both of which are expressed in the regenerating wound epidermis, is nerve-dependent.43,44However, during his groundbreaking early neurotrophic studies, Singer ablated sensory nerves from the wound epidermis and showed that limbs can regenerate with motor nerves alone.25 Later on, Sidmer and Singer showed that removing the sensory supply and preventing innervation of the epidermis still resulted in regeneration,45 a finding that was supported by a concurrent Thornton study which demonstrated that epidermal innervation is not essential for the formation of the AEC.46 Finally, Singer and Inoue found that denervation does not significantly alter the morphology of the AEC, and they also managed to generate animals which exhibited a heavily-innervated wound epidermis but could not regenerate.47 While these results do not preclude the possibility of paracrine signaling from mesenchymal nerves to the epidermis, they do call into question whether the direct innervation of the epidermis is critical for regeneration. For further review of the neurotrophic hypothesis and the possible targets of the nerve, see ref. 94. A 1975 study also suggested that one target of the nerve may be the vasculature, as denervation was found to block angiogenesis in the regenerating newt limb.48 Therefore, although it is clear that peripheral nerves are critical for cell cycling in the blastema, the relation of nerves to tissues beyond the blastema remains unclear.
Molecular mechanisms of nerve dependence
Despite this long history of the study of nerve dependence, many of the mechanisms underlying this process remain poorly-understood, largely because many modern techniques have not been optimized for use with salamanders. Nevertheless, more recent approaches have strongly supported the notion that nerves supply one or more growth factors which are crucial for blastemal proliferation. Implicated factors include substance P,49,50 insulin,51-53 transferrin,54,55 nerve growth factor,56,57 and fibroblast growth factors with BMPs.-61 Much of this work has been conducted on cultured blastemal cells, and together these findings suggest that there is a constellation of factors working in conjunction to communicate proliferative signals from the nerve to the regenerating blastema. For a more detailed overview of these studies and their findings, see Table 1.
Table 1.
A brief description of the evidence for various candidate factors which have been suggested as essential nerve-derived mitogens during limb regeneration.
| Factor | Relevant findings |
|---|---|
| Substance P | Increase in substance P-liked immunoreactivity in peripheral nerves after amputation.50 Immunohistochemically found in the blastema and reduced after denervation; has a mitogenic effect on cultured blastema cells which can be blocked by adding substance P antiserum to nerve co-cultures.49 |
| Insulin | A blastema does not form if the limb is amputated following pancreatectomy.53 Insulin increases DNA and protein synthesis of blastemal cells in vitro; long-term exposure to insulin decreases the amount of time spent in G1.52 For review see.51 |
| Transferrin | Immunohistochemically found in both Schwann cells and axons; expression increases in nerves during limb regeneration and is decreased upon denervation; appears to be secreted by the ends of nerves after axotomy.54 Blocking transferrin with antiserum blocks the growth-promoting effects of nerve extracts on cultured blastemal cells; treatment with transferrin maintained DNA synthesis in denervated blastemas in vivo.55 |
| Nerve growth factor | Injections increased the length of the regenerate and the speed of digit formation.56 Treatment with NGF increases the labeling index of dorsal root ganglia, as does amputation of the limb, although the effects are not combinatory.57 |
| Newt anterior gradient | Expressed in the blastema and lost upon denervation; stimulates blastemal cell proliferation in culture; electroporation into denervated newt limbs rescues regeneration.62 Stimulation of blastemal cells can be blocked with a mutation to the anterior gradient active site or the addition of antibody to the receptor Prod1.93 |
| Fibroblast growth factors and BMPs | Stimulates the proliferation of cultured blastemal cells in a dose-dependent manner.58 Upregulated during regeneration and downregulated after denervation.59 Injection into denervated blastemas shows dose-dependent stimulation of blastemal cell mitotic index.60 Supplementation with FGFs and Gdf5 induces nerve-independent accessory limb formation.61 FGF8 and Bmp7 electroporated into dorsal root ganglia are expressed at the ends of peripheral nerves; knockdown of Fgf8 and Bmp7 blocks blastema formation72 |
| Neuregulin-1 | Present in the newt nervous system and the regenerating blastema; lost upon denervation; increases proliferation in cultured blastemal cells.80 Expressed in the dorsal root ganglia and peripheral nerves of newts; injections into denervated newt blastemas induced regenerative growth.81 Found along with its active receptor ErbB2 in the regenerative blastema and lost upon denervation; supplementation rescues regeneration to digits in denervated limbs; inhibition of ErbB2 signaling blocks regeneration.78 |
| Oncostatin M and PDGF-AA | Expressed in the regenerating mouse digit tip; supplementation rescues regeneration after denervation.12 |
In 2007, Kumar et al. rescued regeneration in denervated newt limbs via electroporation of newt anterior gradient.62 However, it is not known whether anterior gradient plays a similar role in the commonly studied axolotl because there are surprising differences in the regenerative capabilities of axolotls (members of the family Ambystomatidae) and newts (of the family Salamandridae), which may have diverged more than 145 million years ago.63 Most notably, newts appear to be incapable of recovering from denervation and are permanently unable to regenerate after a single denervation surgery.64 This stands in contrast with the axolotl, which is capable of re-innervating the limb and regenerating even after repeated denervations.30 Moreover, adult newts appear to demonstrate a divergent method of muscle regeneration as compared to axolotls. Larval newts, like both larval and artificially metamorphosed axolotls, regenerate muscle via the recruitment of stem cells.65 However, adult newts switch to muscle fiber dedifferentiation after reaching adulthood,66 whereas axolotls are neotenic and do not undergo metamorphosis unless forced to do so via treatment with thyroid hormone. To our knowledge, there have not been any studies of nerve dependence in artificially metamorphosed axolotls or in closely related tiger salamanders (Ambystoma tigrinum), raising the possibility that newts and axolotls demonstrate contrasting methods of limb regeneration because of the divergent evolution of molecular regenerative mechanisms, differences in developmental maturation programs, or a combination of both factors.
The nerve-secreted mitogen hypothesis has received further support in the form of a technique now called the accessory limb model (ALM, Fig. 2C). First described by Locatelli in 1929 67 and first thoroughly characterized with modern techniques by Endo, Bryant and Gardiner in 2004,68 the ALM elegantly demonstrates the necessity of peripheral nerves for blastema formation. Inducing a wound in the epidermis and then deviating a peripheral nerve beneath this wound results in the formation of a proliferating bump. Satoh et al.'s thorough characterization of the bump formed by this surgery concluded that it expresses blastema-specific markers such as prx-1 and msx-2, thus indicating that it is analogous to the blastema formed after limb amputation.69 In keeping with prior research, which has shown that denervation of the limb during the late blastema stage does not impair limb patterning and instead results in the formation of a miniaturized limb,26,70,71 the presence of a nerve is sufficient to promote proliferation but does not contribute to limb patterning or differentiation in this model. Instead, a fully-patterned limb forms only if posterior epidermal tissue from the contralateral limb is grafted onto the anterior wound site. Nevertheless, the formation of this “accessory blastema” provides a compelling model for precisely examining the targets and molecular mechanisms of innervation during the process of blastema formation. A 2013 study by Makanae et al. showed that Gdf5 and FGFs are together capable of inducing blastema growth and limb formation in this model even in the absence of a deviated nerve.61 Even more recently, Satoh et al. have shown that FGF8 and Bmp7 are expressed at the ends of peripheral nerves, and knockdown of these factors inhibits blastema formation in regenerating limbs.72 These findings reinforce the validity of the AL model as an analog to the amputated limb while demonstrating the necessity of FGFs and BMPs for nerve dependent limb regeneration.
Although there is strong evidence supporting the mitogenic hypothesis of axolotl nerve dependence, there are some indications that denervation results in more than just the loss of a critical proliferative signal. In 1998, Irvin and Tassava showed that implantation of axotomized peripheral nerves into amputated limbs slowed blastema formation, suggesting that denervated nerves secrete inhibitory factors which may block proliferation.73 Tassava also showed that implantation of axotomized peripheral nerves into aneurogenic limbs inhibited regeneration, further indicating the possibility of inhibitory factors secreted by peripheral nerves after denervation.74 These findings are not unprecedented, as studies in various animal models have shown that peripheral nerve injury- whether from crush, axotomy, or disease- induces significant inflammation via Schwann cell activation (for review see refs. 75-77). While more study in this area is needed, it is possible that denervation of the amputated salamander limb inhibits amputation in two ways: 1) it results in a loss of nerve-derived mitogens that are essential for blastema formation and 2) it induces inflammatory signals from injured nerves which then inhibit the formation of a regeneration-permissive cellular environment (Fig. 2C).
Neuregulin-1 as a nerve-derived blastemal mitogen
Our recently published work78 builds on this long history and illuminates some previously unexplored molecular mechanisms of regeneration by examining the role of Neuregulin-1 (NRG1), a nerve-derived mitogen, during axolotl limb regeneration. NRG1, which is known to have a role in both cardiac and peripheral nerve development (for review see ref. 79), was first implicated in newt limb regeneration by Brockes and Kintner in 1986,80 and again by Wang et al. in 2000.81 Via immunohistochemical and in situ analysis, we found that NRG1 and its active receptor ErbB2 are expressed in the regenerating axolotl blastema. While NRG1 protein was expressed in dorsal root ganlia and peripheral nerves as well as the mesenchyme and wound epithelium in the preblastema stage of regeneration, it was particularly highly expressed in the proliferating blastema and in fact colocalized with a majority of proliferating blastema cells, as assessed by BrdU incorporation. Denervation of the limb resulted in a significant decrease in NRG1 and ErbB2-positive cells, suggesting that NRG1 signaling is dependent on the presence of nerves.
In order to determine whether NRG1 is capable of bypassing the presence of nerves to induce regeneration, we implanted NRG1-soaked beads underneath the wound epithelium of denervated limbs at 6 days post amputation (DPA). It must be noted that denervation and bead implantation occurred on day 6, after the initial inflammatory processes had concluded. This mirrors the 1987 results from Tomlinson and Tassava, which showed that denervation followed by dorsal root ganglia implantation rescued regeneration at 10 and 14 DPA, but never at 1 DPA.82 It is therefore possible that NRG1 supplementation is insufficient to fully rescue denervation starting from day 0, as it is apparently crucial for blastema formation and growth but may not be involved in earliest steps of the regenerative process such as dedifferentiation and wound healing.
In addition to rescuing limb regeneration, we also inhibited regeneration in fully innervated limbs via the specific ErbB2 inhibitor Mubritinib. Treatment with 500 nMol Mubritinib substantially reduced proliferation at the amputation site and blocked regeneration at both 0 and 16 DPA. Inhibition of EGFR, which can heterodimerize with ErbB2 and signal through EGF, reduced epidermal proliferation and produced a markedly different phenotype from ErbB2 inhibition. We therefore concluded that NRG1 signaling through ErbB2 is a crucial upstream nerve-derived proliferative signal during initial blastema formation as well as blastema proliferation. Further research will be needed in order to determine NRG1's role among the vast array of candidate factors discovered throughout the past decades of research.
Our findings have broad implications that extend beyond the field of salamander research. While it has long been known that nerve dependency is a phenomenon which extends across phylogeny, it has recently become increasingly clear that NRG1 itself plays a crucial role in regeneration across species and tissues as well. Although our study was the first to examine the role of NRG1 in axolotl limb regeneration, recent studies have implicated it in the regeneration of both peripheral nerves83-85 and the mammalian heart, with the latter topic gaining considerable recent interest. Bersell et al. found in 2009 that injection of NRG1 induced cardiomyocyte proliferation and injury repair in adult mice.86 A subsequent study by Gemberling et al. in 2015 found that overexpression of NRG1 in zebrafish cardiomyocytes promoted cardiac regeneration and induced substantial cardiac proliferation and growth in uninjured animals,87 while another recent study found that administration of NRG1 rescued regeneration in denervated neonatal mouse hearts.10 Research into the role of NRG1 for heart regeneration continues, and it is increasingly clear that NRG1 signaling is crucial for a number of regenerative processes in animals ranging from fish to mammals.
NRG1 has also been implicated for its neuroprotective and anti-inflammatory effects in the central and peripheral nervous systems. NRG1 treatment attenuated neuroinflammation after stroke induction in rats,88 and administration of NRG1 has also been shown to protect dopaminergic neurons in a mouse model of Parkinson's disease.89 Furthermore, NRG1 signaling is altered in patients with Alzheimer's disease,90 and a recent 2016 study suggested that it is crucial for protecting cortical neurons against oxidative stress and damage.91 This neuroprotective role seemingly applies to the PNS as well, as overexpression of NRG1 is sufficient to alleviate peripheral nerve demyelination in a mouse model of Charcot-Marie-Tooth disease.92 It remains unknown whether NRG1 plays a role in attenuating the inflammatory response after axolotl limb amputation, and in fact the potential inhibitory effects of limb denervation are still largely unexplored in salamanders. NRG1 thus may play a variety of crucial roles during the process, and it stands as a promising candidate for the study of neuroprotective therapy in mammals.
Our findings are thus both a continuation of a long history of study and a new avenue for further research in the axolotl and beyond. As a novel upstream candidate factor for neurotrophic regeneration, NRG1 represents a substantial step toward solving the centuries-old mystery of nerve-dependent axolotl regeneration, and it may also represent a promising target candidate for the future study of appendage regeneration and neuroprotection in non-regenerating animals.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Simões M, Bensimon-Brito A, Fonseca M, Farinho A, Valério F, Sousa S, Afonso N, Kumar A, Jacinto A. Denervation impairs regeneration of amputated zebrafish fins. BMC Dev Biol 2014; 14(1):49; PMID:25551555; http://dx.doi.org/ 10.1186/s12861-014-0049-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Goss R. An experimental analysis of taste barbel regeneration in the catfish. J Exp Zool 1956; 131(1):27-49; http://dx.doi.org/ 10.1002/jez.1401310103 [DOI] [Google Scholar]
- [3].Suzuki M, Satoh A, Ide H, Tamura K. Nerve-dependent and -independent events in blastema formation during Xenopus froglet limb regeneration. Dev Biol 2005; 286(1):361-375; PMID:16154125; http://dx.doi.org/ 10.1016/j.ydbio.2005.08.021 [DOI] [PubMed] [Google Scholar]
- [4].Filoni S, Velloso CP, Bernardini S, Cannata SM. Acquisition of nerve dependence for the formation of a regeneration blastema in amputated hindlimbs of larval Xenopus laevis: The role of limb innervation and that of limb differentiation. J Exp Zool 1995; 273(4):327-341; PMID:8530914; http://dx.doi.org/ 10.1002/jez.1402730407 [DOI] [PubMed] [Google Scholar]
- [5].Filoni S, Bernardini S, Cannata SM, Ghittoni R. Nerve-independence of limb regeneration in larval Xenopus laevis is related to the presence of mitogenic factors in early limb tissues. J Exp Zool 1999; 284(2):188-96; PMID:10404647; http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- [6].Kumar A, Brockes J. Nerve dependence in tissue, organ, and appendage regeneration. Trend Neurosci 2012; 35(11):691-699; PMID:22989534; http://dx.doi.org/ 10.1016/j.tins.2012.08.003 [DOI] [PubMed] [Google Scholar]
- [7].Kishi K, Ohyama K, Satoh H, Kubota Y, Tanaka T, Imanishi N, Nakajima H, Kawamura K, Nakajima T. Mutual dependence of murine fetal cutaneous regeneration and peripheral nerve regeneration. Wound Repair Regen 2006; 14(1):91-99; PMID:16476077; http://dx.doi.org/ 10.1111/j.1524-475X.2005.00093.x [DOI] [PubMed] [Google Scholar]
- [8].Stelnicki E, Doolabh V, Lee S, Levis C, Baumann FG, Longaker MT, Mackinnon S. Nerve dependency in scarless fetal wound healing. Plastic Reconst Surg 2000; 105(1):140-147; http://dx.doi.org/ 10.1097/00006534-200001000-00024 [DOI] [PubMed] [Google Scholar]
- [9].Harsum S, Clarke J, Martin P. A reciprocal relationship between cutaneous nerves and repairing skin wounds in the developing chick embryo. Dev Biol 2001; 238(1):27-39; PMID:11783991; http://dx.doi.org/ 10.1006/dbio.2001.0395 [DOI] [PubMed] [Google Scholar]
- [10].Mahmoud A I, O'Meara CC, Gemberling M, Zhao L, Bryant DM, Zheng R, Gannon JB, Cai L, Choi WY, Egnaczyk GF, et al.. Nerves regulate cardiomyocyte proliferation and heart regeneration. Dev Cell 2015; 34(4):387-399; PMID:26256209; http://dx.doi.org/ 10.1016/j.devcel.2015.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rinkevich Y, Montoro DT, Muhonen E, Walmsley GG, Lo D, Hasegawa M, Januszyk M, Connolly AJ, Weissman IL, Longaker MT. Clonal analysis reveals nerve-dependent and independent roles on mammalian hind limb tissue maintenance and regeneration. Proc Natl Acad Sci U S A 2014; 111(27):9846-51; PMID:24958860; http://dx.doi.org/ 10.1073/pnas.1410097111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Johnston A, Yuzwa SA, Carr MJ, Mahmud N, Storer MA, Krause MP, Jones K, Paul S, Kaplan DR, Miller FD. Dedifferentiated schwann cell precursors secreting paracrine factors are required for regeneration of the mammalian digit tip. Cell Stem Cell 2016; 19(4):433-448; PMID:27376984; http://dx.doi.org/ 10.1016/j.stem.2016.06.002 [DOI] [PubMed] [Google Scholar]
- [13].Buckley G, Metcalfe A, Ferguson M. Peripheral nerve regeneration in the MRL/MpJ ear wound model. J Anat 2011; 218(2):163-72; PMID:20950365; http://dx.doi.org/ 10.1111/j.1469-7580.2010.01313.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Seifert A, Kiama SG, Seifert MG, Goheen JR, Palmer TM, Maden M. Skin shedding and tissue regeneration in African spiny mice (Acomys). Nature 2012; 489(7417):561-5; PMID:23018966; http://dx.doi.org/ 10.1038/nature11499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gawriluk T, Simkin J, Thompson KL, Biswas SK, Clare-Salzler Z, Kimani JM, Kiama SG, Smith JJ, Ezenwa VO, Seifert AW. Comparative analysis of ear-hole closure identifies epimorphic regeneration as a discrete trait in mammals. Nat Commun 2016; 7:11164; PMID:27109826; http://dx.doi.org/ 10.1038/ncomms11164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Basson M, Burney R. Defective wound healing in patients with paraplegia and quadriplegia. Surg Gynecol Obstet 1982; 155(1):9-12; PMID:7089844 [PubMed] [Google Scholar]
- [17].World Health Organization Global Report on Diseases. 2016, Geneva. [Google Scholar]
- [18].Johannesson A, Larsson GU, Ramstrand N, Turkiewicz A, Wiréhn AB, Atroshi I. Incidence of lower-limb amputation in the diabetic and nondiabetic general population. A 10-year population-based cohort study of initial unilateral and contralateral amputations and reamputations. Diabetes Care 2009; 32(2):275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Brown D, Kao W, Greenhalgh D. Apoptosis down-regulates inflammation under the advancing epithelial wound edge: Delayed patterns in diabetes and improvement with topical growth factors. Surgery 1997; 121(4):372-380; PMID:9122866; http://dx.doi.org/ 10.1016/S0039-6060(97)90306-8 [DOI] [PubMed] [Google Scholar]
- [20].Barker A, Rosson G, Dellon A. Wound healing in denervated tissue. Ann Plast Surg 2006; 57(3):339-42; PMID:16929207; http://dx.doi.org/ 10.1097/01.sap.0000221465.69826.b7 [DOI] [PubMed] [Google Scholar]
- [21].Todd T. On the process of reproduction of the members of the aquatic salamander. Q J Sci Lit Arts 1823; 16:84-96. [Google Scholar]
- [22].Noël Paton D, The nervous and chemical regulators of metabolism : lectures. 1913, London: Macmillan. [Google Scholar]
- [23].Wolff G, Studien I. Die regeneration der urodelenlinse. Entwicklungsphysiologische 1895; 1:380-390. [Google Scholar]
- [24].Walter F. Welche bedeutung hat das nervensystem für die regeneration der triton extremitäten? Willhem Roux Archiv für Entwicklungsmechanik der Organismen 1911; 104:317-358. [Google Scholar]
- [25].Singer M. The nervous system and regeneration of the forelimb of adult Triturus. V. The influence of number of nerve fibers, including a quantitative study of limb innervation. J Exp Zool 1946; 101(3):299-337; PMID:21027532; http://dx.doi.org/ 10.1002/jez.1401010303 [DOI] [PubMed] [Google Scholar]
- [26].Singer M, Craven L. The growth and morphogenesis of the regenerating forelimb of adult Triturus following denervation at various stages of development. J Exp Zool 1948; 108(2):279-308; PMID:18873226; http://dx.doi.org/ 10.1002/jez.1401080207 [DOI] [PubMed] [Google Scholar]
- [27].Singer M. The influence of the nerve in regeneration of the amphibian extremity. Quarter Rev Biol 1952; 27(2):169-200; http://dx.doi.org/ 10.1086/398873 [DOI] [PubMed] [Google Scholar]
- [28].Singer M. On the nature of the neurotrophic phenomenon in urodele limb regeneration. Am Zoolog 1978; 18(4):829-841; http://dx.doi.org/ 10.1093/icb/18.4.829 [DOI] [Google Scholar]
- [29].Singer M. The trophic quality of the neuron: some theoretical considerationS. Prog Brain Res 1964; 13:228-32; PMID:14302961 [DOI] [PubMed] [Google Scholar]
- [30].Schotte O, Butler E. Morphological effects of denervation and amputation of limbs in urodele larvae. J Exp Zool 1941; 97:95-121; http://dx.doi.org/ 10.1002/jez.1400970202 [DOI] [Google Scholar]
- [31].Butler E, Schotté O. Effects of delayed denervation on regenerative activity in limbs of urodele larvae. J Exp Zool 1949; 112(3):361-392; PMID:15404782; http://dx.doi.org/ 10.1002/jez.1401120302 [DOI] [PubMed] [Google Scholar]
- [32].hornton C. Histological modifications in denervated injured fore limbs of Amblystoma larvae. J Exp Zool 1953; 122(1):119-149; http://dx.doi.org/ 10.1002/jez.1401220108 [DOI] [Google Scholar]
- [33].Yntema C. Regeneration in sparsely innervated and aneurogenic forelimbs of Amblystoma larvae. J Exp Zool 1959; 140(1):101-123; PMID:13846546; http://dx.doi.org/ 10.1002/jez.1401400106 [DOI] [PubMed] [Google Scholar]
- [34].Steen T, Thornton C. Tissue interaction in amputated aneurogenic limbs of Ambystoma larvae. J Exp Zool 1963; 154(2):207-221; PMID:14085418; http://dx.doi.org/ 10.1002/jez.1401540208 [DOI] [PubMed] [Google Scholar]
- [35].Kumar A, Delgado JP, Gates PB, Neville G, Forge A, Brockes JP. The aneurogenic limb identifies developmental cell interactions underlying vertebrate limb regeneration. Proc Natl Acad Sci 2011; 108(33):13588-13593; PMID:21825124; http://dx.doi.org/ 10.1073/pnas.1108472108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Liversage R, Globus M. In vitro regeneration of innervated forelimb deplants of Ambystoma larvae. Cana J Zool 1977; 55(7):1195-1199; http://dx.doi.org/ 10.1139/z77-155 [DOI] [PubMed] [Google Scholar]
- [37].Goldhamer D, Tomlinson B, Tassava R. Ganglia implantation as a means of supplying neurotrophic stimulation to the newt regeneration blastema: cell-cycle effects in innervated and denervated limbs. J Exp Zool 1992; 262(1):71-80; PMID:1583454; http://dx.doi.org/ 10.1002/jez.1402620110 [DOI] [PubMed] [Google Scholar]
- [38].Maden M. Neurotrophic control of the cell cycle during amphibian limb regeneration. J Embryol Exp Morphol 1978; 48:169-75; PMID:744947 [PubMed] [Google Scholar]
- [39].Goldhamer D, Tassava R. An analysis of proliferative activity in innervated and denervated forelimb regenerates of the newt, notophthalmus viridescens. Development 1987; 100(4):619-628. [Google Scholar]
- [40].Singer M. The invasion of the epidermis of the regenerating forelimb of the urodele, Triturus, by nerve fibers. J Exp Zool 1949; 111(2):189-209; PMID:18141498; http://dx.doi.org/ 10.1002/jez.1401110204 [DOI] [PubMed] [Google Scholar]
- [41].Taban C. les fibres nerveuses et l‘épithélium dans l’édification des regenerats de pattes ( in situ ou induites) chez le triton. Arch Sci 1949; 2(148):553-561. [Google Scholar]
- [42].Thornton C. The relation of epidermal innervation to limb regeneration in Amblystoma larvae. J Exp Zool 1954; 127(3):577-601; http://dx.doi.org/ 10.1002/jez.1401270307 [DOI] [Google Scholar]
- [43].Mullen L, Bryant SV, Torok MA, Blumberg B, Gardiner DM. Nerve dependency of regeneration: the role of Distal-less and FGF signaling in amphibian limb regeneration. Dev (Cambridge, England) 1996; 122(11):3487-3497. [DOI] [PubMed] [Google Scholar]
- [44].Satoh A, Graham GM, Bryant SV, Gardiner DM. Neurotrophic regulation of epidermal dedifferentiation during wound healing and limb regeneration in the axolotl (Ambystoma mexicanum). Dev Biol 2008; 319(2):321-335; PMID:18533144; http://dx.doi.org/ 10.1016/j.ydbio.2008.04.030 [DOI] [PubMed] [Google Scholar]
- [45].Sidman R, Singer M. Limb regeneration without innervation of the apical epidermis in the adult newt, Triturus. J Exp Zool 1960; 144(2):105-109; http://dx.doi.org/ 10.1002/jez.1401440202 [DOI] [Google Scholar]
- [46].Thornton C. Regeneration of asensory limbs of ambystoma larvae. Copeia 1960; 1960(4):371-373; http://dx.doi.org/ 10.2307/1439783 [DOI] [Google Scholar]
- [47].Singer M, Inoue S. The nerve and the epidermal apical cap in regeneration of the forelimb of adult Triturus. J Exp Zool 1964; 155(1):105-115; PMID:14118835; http://dx.doi.org/ 10.1002/jez.1401550108 [DOI] [PubMed] [Google Scholar]
- [48].Smith A, Wolpert L. Nerves and angiogenesis in amphibian limb regeneration. Nature 1975; 257(5523):224-225; PMID:1161022; http://dx.doi.org/ 10.1038/257224a0 [DOI] [PubMed] [Google Scholar]
- [49].Globus M, Smith M, Vethamany-Globus S. Evidence supporting a mitogenic role for substance P in amphibian limb regeneration. Ann N Y Acad Sci 1991; 632(1):396-399; PMID:1719886; http://dx.doi.org/ 10.1111/j.1749-6632.1991.tb33135.x [DOI] [PubMed] [Google Scholar]
- [50].Anand P, McGregor GP, Gibson SJ, Maden M, Polak JM, Bloom SR. Increase of substance P-like immunoreactivity in the peripheral nerve of the axolotl after injury. Neurosci Lett 1987; 82(3):241-245; PMID:2447537; http://dx.doi.org/ 10.1016/0304-3940(87)90263-1 [DOI] [PubMed] [Google Scholar]
- [51].Globus M. Neurotrophic contribution to a proposed tripartite control of the mitotic cycle in the regeneration blastema of the newt, notophthalmus (Triturus) viridescens. Am Zool 1978; 18(4):855-868; http://dx.doi.org/ 10.1093/icb/18.4.855 [DOI] [Google Scholar]
- [52].Vethamany-Globus S, Liversage R. In vitro studies of the influence of hormones on tail regeneration in adult <em>Diemictylus viridescens</em>. J Embryol Exp Morphol 1973; 30(2):397-413; PMID:4761673 [PubMed] [Google Scholar]
- [53].Vethamany-Globus S, Liversage R. Effects of insulin insufficiency on forelimb and tail regeneration in adult <em>Diemictylus viridescens</em>. J Embryol Exp Morphol 1973; 30(2):427-447; PMID:4586766 [PubMed] [Google Scholar]
- [54].Kiffmeyer W, Tomusk E, Mescher A. Axonal transport and release of transferrin in nerves of regenerating amphibian limbs. Dev Biol 1991; 147(2):392-402; PMID:1916015; http://dx.doi.org/ 10.1016/0012-1606(91)90297-G [DOI] [PubMed] [Google Scholar]
- [55].Mescher A, Connell E, Hsu C, Patel C, Overton B. Transferrin is necessary and sufficient for the neural effect on growth in amphibian limb regeneration blastemas. Dev Growth Different 1997; 39(6):677-684; http://dx.doi.org/ 10.1046/j.1440-169X.1997.t01-5-00003.x [DOI] [PubMed] [Google Scholar]
- [56].Weis J, Weis P. The effect of nerve growth factor on limb regeneration in Ambystoma. J Exp Zool 1970; 174(1):73-78; PMID:5444567; http://dx.doi.org/ 10.1002/jez.1401740108 [DOI] [PubMed] [Google Scholar]
- [57].Grillo R, Detmar C, Mitchell O. The effect of nerve growth factor and limb regeneration on the spinal and sympathetic ganglia of Triturus. J Exp Zool 1977; 202(2):259-265; PMID:925672; http://dx.doi.org/ 10.1002/jez.1402020216 [DOI] [PubMed] [Google Scholar]
- [58].Albert P, Boilly B, Courty J, Barritault D. Stimulation in cell culture of mesenchymal cells of newt limb blastemas by EDGF I or II (basic or acidic FGF). Cell Different 1987; 21(1):63-68; http://dx.doi.org/ 10.1016/0045-6039(87)90449-0 [DOI] [PubMed] [Google Scholar]
- [59].Christensen R, Weinstein M, Tassava R. Fibroblast growth factors in regenerating limbs of Ambystoma: Cloning and semi-quantitative RT-PCR expression studies. J Exp Zool 2001; 290(5):529-540; PMID:11555861; http://dx.doi.org/ 10.1002/jez.1097 [DOI] [PubMed] [Google Scholar]
- [60].Mescher A, Gospodarowicz D. Mitogenic effect of a growth factor derived from myelin on denervated regenerates of newt forelimbs. J Exp Zool 1979; 207(3):497-504; http://dx.doi.org/ 10.1002/jez.1402070318 [DOI] [Google Scholar]
- [61].Makanae A, Hirata A, Honjo Y, Mitogawa K, Satoh A. Nerve independent limb induction in axolotls. Dev Biol 2013; 381(1):213-226; http://dx.doi.org/ 10.1016/j.ydbio.2013.05.010 [DOI] [PubMed] [Google Scholar]
- [62].Kumar A, Godwin JW, Gates PB, Garza-Garcia AA, Brockes JP. Molecular basis for the nerve dependence of limb regeneration in an adult vertebrate. Science (New York, N.Y.) 2007; 318(5851):772-777; http://dx.doi.org/ 10.1126/science.1147710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zhang P, Wake D. Higher-level salamander relationships and divergence dates inferred from complete mitochondrial genomes. Mol Phylogenet Evol 2009; 53(2):492-508; PMID:19595776; http://dx.doi.org/ 10.1016/j.ympev.2009.07.010 [DOI] [PubMed] [Google Scholar]
- [64].Liversage R, McLaughlin D. Effects of delayed amputation on denervated forelimbs of adult newt. J Embryol Exp Morphol 1983; 75:1-10; PMID:6886605 [PubMed] [Google Scholar]
- [65].Sandoval-Guzman T, Wang H, Khattak S, Schuez M, Roensch K, Nacu E, Tazaki A, Joven A, Tanaka EM, Simon A. Fundamental differences in dedifferentiation and stem cell recruitment during skeletal muscle regeneration in two salamander species. Cell Stem Cell 2014; 14(2):174-87; PMID:24268695; http://dx.doi.org/ 10.1016/j.stem.2013.11.007 [DOI] [PubMed] [Google Scholar]
- [66].Tanaka H, Ng NC, Yang Yu Z, Casco-Robles MM, Maruo F, Tsonis PA, Chiba C. A developmentally regulated switch from stem cells to dedifferentiation for limb muscle regeneration in newts. Nat Commun 2016; 7:11069; http://dx.doi.org/ 10.1038/ncomms11069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Locatelli P. Der Einfluss des Nervensystems auf die Regeneration. Wilhelm Roux' Archiv für Entwicklungsmechanik der Organismen 1929; 114(4):686-770; http://dx.doi.org/ 10.1007/BF02078924 [DOI] [PubMed] [Google Scholar]
- [68].Endo T, Bryant S, Gardiner D. A stepwise model system for limb regeneration. Dev Biol 2004; 270(1):135-145; PMID:15136146; http://dx.doi.org/ 10.1016/j.ydbio.2004.02.016 [DOI] [PubMed] [Google Scholar]
- [69].Satoh A, Gardiner DM, Bryant SV, Endo T. Nerve-induced ectopic limb blastemas in the axolotl are equivalent to amputation-induced blastemas. Dev Biol 2007; 312(1):231-244; PMID:17959163; http://dx.doi.org/ 10.1016/j.ydbio.2007.09.021 [DOI] [PubMed] [Google Scholar]
- [70].Schotté O, Butler E. Phases in regeneration of the urodele limb and their dependence upon the nervous system. J Exp Zool 1944; 97(2):95-121; http://dx.doi.org/ 10.1002/jez.1400970202 [DOI] [Google Scholar]
- [71].Powell J. Analysis of histogenesis and regenerative ability of denervated forelimb regenerates of Triturus viridescens. J Exp Zool 1969; 170(2):125-147; PMID:5804911; http://dx.doi.org/ 10.1002/jez.1401700202 [DOI] [PubMed] [Google Scholar]
- [72].Satoh A, Makanae A, Nishimoto Y, Mitogawa K. FGF and BMP derived from dorsal root ganglia regulate blastema induction in limb regeneration in Ambystoma mexicanum. Dev Biol 2016; 417(1):114-125; PMID:27432514; http://dx.doi.org/ 10.1016/j.ydbio.2016.07.005 [DOI] [PubMed] [Google Scholar]
- [73].Irvin B, Tassava R. Effects of peripheral nerve implants on the regeneration of partially and fully innervated urodele forelimbs. Wound Repair Regen 1998; 6(4):382-387; PMID:9824557; http://dx.doi.org/ 10.1046/j.1524-475X.1998.60414.x [DOI] [PubMed] [Google Scholar]
- [74].Tassava R, Olsen-Winner C. Responses to amputation of denervated ambystoma limbs containing aneurogenic limb grafts. J Exp Zool A Comp Exp Biol 2003; 297(1):64-79; PMID:12911114; http://dx.doi.org/ 10.1002/jez.a.10263 [DOI] [PubMed] [Google Scholar]
- [75].Dubový P, Jančálek R, Kubek T. Role of inflammation and cytokines in peripheral nerve regeneration. Int Rev Neurobiol 2013; 108:173-206; http://dx.doi.org/ 10.1016/B978-0-12-410499-0.00007-1 [DOI] [PubMed] [Google Scholar]
- [76].Ydens E, Lornet G, Smits V, Goethals S, Timmerman V, Janssens S. The neuroinflammatory role of Schwann cells in disease. Neurobiol Dis 2013; 55:95-103; PMID:23523637; http://dx.doi.org/ 10.1016/j.nbd.2013.03.005 [DOI] [PubMed] [Google Scholar]
- [77].Cámara-Lemarroy C, Guzmán-de la Garza F, Fernández-Garza N. Molecular inflammatory mediators in peripheral nerve degeneration and regeneration. Neuroimmunomodulation 2010; 17(5):314-324; PMID:20407283; http://dx.doi.org/ 10.1159/000292020 [DOI] [PubMed] [Google Scholar]
- [78].Farkas J, Freitas P, Bryant DM, Whited JL, Monaghan JR.. Neuregulin-1 signaling is essential for nerve-dependent axolotl limb regeneration; Development 2016. [DOI] [PubMed] [Google Scholar]
- [79].Falls D. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res 2003; 284(1):14-30; PMID:12648463; http://dx.doi.org/ 10.1016/S0014-4827(02)00102-7 [DOI] [PubMed] [Google Scholar]
- [80].Brockes J, Kintner C. Glial growth factor and nerve-dependent proliferation in the regeneration blastema of urodele amphibians. Cell 1986; 45(2):301-306; PMID:3698099; http://dx.doi.org/ 10.1016/0092-8674(86)90394-6 [DOI] [PubMed] [Google Scholar]
- [81].Wang L. Marchionni M, Tassava R. Cloning and neuronal expression of a type III newt neuregulin and rescue of denervated, nerve-dependent newt limb blastemas by rhGGF2. J Neurobiol 2000; 43(2):150-158; PMID:10770844; http://dx.doi.org/ 10.1002/(SICI)1097-4695(200005)43:2%3c150::AID-NEU5%3e3.0.CO;2-G [DOI] [PubMed] [Google Scholar]
- [82].Tomlinson B, Tassava R. Dorsal root ganglia grafts stimulate regeneration of denervated urodele forelimbs: timing of graft implantation with respect to denervation. Development 1987; 99(2):173-86; PMID:3652994 [DOI] [PubMed] [Google Scholar]
- [83].Fricker F, Lago N, Balarajah S, Tsantoulas C, Tanna S, Zhu N, Fageiry SK, Jenkins M, Garratt AN, Birchmeier C, et al.. Axonally derived neuregulin-1 is required for remyelination and regeneration after nerve injury in adulthood. J Neurosci 2011; 31(9):3225-33; PMID:21368034; http://dx.doi.org/ 10.1523/JNEUROSCI.2568-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Ronchi G, Gambarotta G, Di Scipio F, Salamone P, Sprio AE, Cavallo F, Perroteau I, Berta GN, Geuna S. ErbB2 receptor over-expression improves post-traumatic peripheral nerve regeneration in adult mice. PLoS One 2013; 8(2):e56282; PMID:23437108; http://dx.doi.org/ 10.1371/journal.pone.0056282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Ronchi G, Haastert-Talini K, Fornasari BE, Perroteau I, Geuna S, Gambarotta G.. The Neuregulin1/ErbB system is selectively regulated during peripheral nerve degeneration and regeneration. Eur J Neurosci 2015; 43(3). [DOI] [PubMed] [Google Scholar]
- [86].Bersell K, Arab S, Haring B, Kühn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell 2009; 138(2):257-70; PMID:19632177; http://dx.doi.org/ 10.1016/j.cell.2009.04.060 [DOI] [PubMed] [Google Scholar]
- [87].Gemberling M, Karra R, Dickson AL, Poss KD. Nrg1 is an injury-induced cardiomyocyte mitogen for the endogenous heart regeneration program in zebrafish. Elife 2015; 4; PMID:25830562; http://dx.doi.org/ 10.7554/eLife.05871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Xu Z, Jiang J, Ford G, Ford BD. Neuregulin-1 is neuroprotective and attenuates inflammatory responses induced by ischemic stroke. Biochem Biophy Res Commun 2004; 322(2):440-446; PMID:15325249; http://dx.doi.org/ 10.1016/j.bbrc.2004.07.149 [DOI] [PubMed] [Google Scholar]
- [89].Carlsson T, Schindler FR, Höllerhage M, Depboylu C, Arias-Carrión O, Schnurrbusch S, Rösler TW, Wozny W, Schwall GP, Groebe K, et al.. Systemic administration of neuregulin-1β1 protects dopaminergic neurons in a mouse model of Parkinson's disease. J Neurochem 2011; 117(6):1066-1074; PMID:21517849; http://dx.doi.org/ 10.1111/j.1471-4159.2011.07284.x [DOI] [PubMed] [Google Scholar]
- [90].Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, et al.. Neuregulin 1 and Susceptibility to Schizophrenia. Am J Hum Genet 2002; 71(4):877-892; PMID:12145742; http://dx.doi.org/ 10.1086/342734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Jiang Q, Chen S, Hu C, Huang P, Shen H, Zhao W. Neuregulin-1 (Nrg1) signaling has a preventive role and is altered in the frontal cortex under the pathological conditions of Alzheimer's disease. Mol Med Rep 2016; 14(3):2614-2624; PMID:27486021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Fledrich R, Stassart RM, Klink A, Rasch LM, Prukop T, Haag L, Czesnik D, Kungl T, Abdelaal TA, Keric N, et al.. Soluble neuregulin-1 modulates disease pathogenesis in rodent models of Charcot-Marie-Tooth disease 1A. Nat Med 2014; 20(9):1055-1061; PMID:25150498; http://dx.doi.org/ 10.1038/nm.3664 [DOI] [PubMed] [Google Scholar]
- [93].Grassme K, Garza-Garcia A, Delgado JP, Godwin JW, Kumar A, Gates PB, Driscoll PC, Brockes JP. Mechanism of action of secreted newt anterior gradient protein. PLoS One 2016; 11(4):e0154176; PMID:27100463; http://dx.doi.org/ 10.1371/journal.pone.0154176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Stocum D. The role of peripheral nerves in urodele limb regeneration. Eur J Neurosci 2011; 34:908-916. [DOI] [PubMed] [Google Scholar]