Neuron-restrictive silencer factor–induced downregulation of μ-opioid receptor is involved in the reduction of morphine analgesia in sarcoma-induced bone cancer pain.
Keywords: Bone cancer pain, Neuron-restrictive silencer factor, μ-opioid receptor, Epigenetic modification
Abstract
Bone cancer pain has been reported to have unique mechanisms and is resistant to morphine treatment. Recent studies have indicated that neuron-restrictive silencer factor (NRSF) plays a crucial role in modulating the expression of the μ-opioid receptor (MOR) gene. The present study elucidates the regulatory mechanisms of MOR and its ability to affect bone cancer pain. Using a sarcoma-inoculated murine model, pain behaviors that represent continuous or breakthrough pain were evaluated. Expression of NRSF in the dorsal root ganglion (DRG) and spinal dorsal horn was quantified at the transcriptional and translational levels, respectively. Additionally, chromatin immunoprecipitation assays were used to detect NRSF binding to the promoter of MOR. Furthermore, NRSF was genetically knocked out by antisense oligodeoxynucleotide, and the expression of MOR and the effect of morphine were subsequently analyzed. Our results indicated that in a sarcoma murine model, NRSF expression is upregulated in dorsal root ganglion neurons, and the expression of NRSF mRNA is significantly negatively correlated with MOR mRNA expression. Additionally, chromatin immunoprecipitation analysis revealed that NRSF binding to the neuron-restrictive silencer element within the promoter area of the MOR gene is promoted with a hypoacetylation state of histone H3 and H4. Furthermore, genetically knocking down NRSF with antisense oligodeoxynucleotide rescued the expression of MOR and potentiated the systemic morphine analgesia. The present results suggest that in sarcoma-induced bone cancer pain, NRSF-induced downregulation of MOR is involved in the reduction of morphine analgesia. Epigenetically, up-regulation of MOR could substantially improve the effect of system delivery of morphine.
1. Introduction
Pain is one of the most severe and common cancer-related symptoms.30 Arising from primary skeletal malignancies or bone metastases from breast, prostate, and lung cancers, pain associated with bony neoplasms usually increases in magnitude with the progression of the skeletal sarcoma.10,27 Bone cancer pain is commonly divided into the following 2 categories: ongoing pain and breakthrough pain. Ongoing pain is often experienced as a dull, constant, and throbbing pain that is usually the first symptom of bone cancer and increases in intensity with time. Breakthrough pain refers to intermittent episodes of extreme pain usually occurring spontaneously or after using an affected limb.10,27,55 As bone cancer pain is highly disruptive to a patient's quality of life, there is an urgent need for discovering its potential mechanisms and developing new treatments.30,35
Opioids are the cornerstone of cancer-pain management.32 In patients with bone cancer pain, opioids are frequently prescribed to alleviate both ongoing and breakthrough pain. Animal models of bone cancer pain have shown that the opioid doses sufficient to inhibit pain behaviors were greater than those required to alleviate nociceptive behaviors of comparable magnitude caused by inflammatory pain.10,27,33 Furthermore, escalating doses of opioids may produce diverse and disabling adverse effects while providing poor pain control, a problem that frustrates pain physicians.16,34,36 Opioid agonists are effective in relieving pain in 70% to 90% of patients with cancer, but the remaining 10% to 30% patients with cancer still experience unresolved pain.11 Relatively little is known about the mechanisms underlying this phenomenon. Previous studies have confirmed that μ-opioid receptor (MOR) expression regulation is involved in the pathogenesis of neuropathic pain,22,39,47 and the modulation of MOR expression is also involved in the nociceptive behavior abnormalities associated with bone cancer pain.9,27,55 However, the modulation mechanisms of MOR expression remain uncertain.
Neuron-restrictive silencer factor (NRSF, also called repressor element silencing transcription factor, REST) can interact with the neuron-restrictive silencer element (NRSE, also called repressor element 1) found in the regulatory regions of neuron-specific target genes.1,5,42 As an originally recognized transcriptional repressor of neuronal genes, NRSF not only suppresses MOR transcription in cell models, but also epigenetically silences MOR in a variety of in vivo situations.8,18,19,47,53 However, whether NRSF is involved in the modulation of MOR expression induced by bone cancer pain has not been reported. Using a bone cancer pain animal model, the present study explores changes of NRSF expression and their potential effect on the transcription of MOR. The genetic knockdown of NRSF was performed by intrathecal delivery of antisense oligodeoxynucleotide (AS-ODN), and the pain behavior was examined after systemic morphine administration. The results indicate that NRSF plays an important role in the modulation of MOR transcription and may represent a novel analgesic target for bone cancer pain.
2. Materials and methods
2.1. Animals, cell culture, and implantation
Adult, male C3H/HeN mice (25-30 g; Weitong Lihua Laboratory Animal Technology Co, Ltd, Beijing, China) were used in the studies. The mice were housed in a vivarium with a 12-hour light/dark cycle and had access to food and water ad libitum. All experimental procedures were approved by the Animal Use and Care Committee for Research and Education of the Fourth Military Medical University (Xi'an, China), the National Institutes of Health's (NIH's) Guide for the Care and Use of Laboratory Animals and complied with the guidance outlined by the Ethical Issues of the IASP.60
We maintained 2472 NCTC murine sarcoma cells (American Type Culture Collection [ATCC], Rockville, MD) in NCTC 135 media (Sigma-Aldrich, St. Louis, MO) containing 10% horse sera (HyClone, Logan, UT) and passaged them weekly according to the recommendations of ATCC. The bone cancer pain model was induced as previously described.43 Briefly, mice were intraperitoneally (i.p.) anesthetized with sodium pentobarbital (50 mg/kg). After the hair had been shaved and skin had been disinfected, an incision was made in the skin overlying the left patella. The patellar ligament was then cut, and the condyle of the distal femur was exposed. Using a 0.5-mm half-round burr, a hole was drilled into the medullary cavity. Then, 20 μL of α-minimum essential medium (MEM) containing 1 × 105 sarcoma cells was injected using a 29 gauge needle and a 25-μL microsyringe (sarcoma-implanted mice). Afterwards, the injection hole was sealed with bone wax to prevent leakage of sarcoma cell-containing MEM outside the bone, followed by copious irrigation with sterile saline. Finally, the wound was closed. In the sham-implanted mice, only 25 μL of MEM was injected.
2.2. Behavioral analysis
Prior to inoculation, and again on days 3, 7, 10, 14, and 21 postoperation, naive, sham- or sarcoma-implanted mice were tested for pain-related behaviors, including ongoing pain (quantification of spontaneous flinches) and movement evoked breakthrough pain (limb use, mechanical allodynia, and weight bearing). Before each test was carried out, animals were placed in the testing environment and allowed to habituate for at least 30 minutes. Spontaneous flinching and limb use were assessed during normal ambulation in an open field. During a 2-minute observation period, the number of spontaneous flinches was recorded. Holding the left paw aloft unrelated to walking or grooming was defined as 1 flinch. Normal limb use was scored on a scale from 4 to 0 as follows: 4, normal use; 3, pronounced limp; 2, limp and guarding behavior; 1, partial nonuse of the limb in locomotor activity; and 0 = complete lack of limb use.27 Mechanical allodynia was measured by stimulation of the hind paw with von Frey monofilaments. Briefly, the mice were placed in transparent cages with a wire mesh floor, and a set of von Frey filaments (0.16, 0.4, 0.6, 1.0, 1.4, and 2.0 g; Stoelting, Wood Dale, Illinois) were applied perpendicular to the plantar surface of hind paws ipsilateral to the tumor-bearing legs in ascending order. Each mouse was tested 5 times per stimulus strength and the lowest von Frey filament that induced 3 or more positive responses in 5 applications was regarded as the paw withdrawal threshold (PWT). Weight-bearing was evaluated on a scale of 0 to 3 as follows: 0, the left hind paw was always lifted and there was no weight bearing at all; 1, the left hind paw was lifted from the floor but occasionally touched the floor; 2, the left hind limb was partially used to support body weight; and 3, the left hind limb was used to support full body weight with contralateral hind limb. All tests were performed by experimenters who were blinded to the treatment group. Data are presented as mean ± SD.
2.3. Assessment of bone destruction
To evaluate the extent of bone destruction induced by sarcoma cell inoculation, micro-computer tomography (micro-CT) scanning was performed. On days 14 or 21 postinjection, the femur of the left hind limb was sampled and scanned with a high-resolution x-ray micro-CT system for small animal imaging eXplore Locus SP (General Electric Company, Fairfield, CT). Standard reconstruction and a subsequent 2- and 3-dimensional morphometric analysis were processed. Quantification of bone destruction was rated similar to previously reported scales20,27 as follows: 0, normal bone; 1, medullary bone loss with no fracture; 2, loss of medullary bone and erosion of cortical bone; 3, full-thickness unicortical bone loss indicating unicortical bone fracture; and 4, full-thickness bicortical bone loss indicating bicortical bone fracture. Only animals with loss of medullary bone and erosion of cortical bone observed in the femur were included in the analysis. Mice with scores of 1 or 0 on femur micro-CT images were excluded from subsequent data analyses.
2.4. Immunofluorescence
The mice were re-anesthetized with an overdose of sodium pentobarbital (60 mg/kg, i.p.) and perfused transcardially with 20-mL phosphate-buffered saline (PBS, pH 7.4), followed by 50 mL of 4% (w/v) paraformaldehyde in 0.1-M phosphate buffer (pH 7.4). The L2-4 dorsal root ganglion (DRG) and the spinal cord were harvested (the contralateral side of the spinal cord was labeled by piercing a needle into the anterior horn) and postfixed in the same fixative for 2 hours (4°C), and then cryoprotected with 30% (w/v) sucrose in 0.1 M phosphate buffer (pH 7.4) at 4°C. Several days later, DRG were serially cut into 10 μm-thick sections on a frozen microtome (Kryostat 1720; Leitz, Mannheim, Germany) and mounted on gelatin-coated glass slides as 5 sets of every fifth serial sections. The spinal cord was also serially cut into 30 μm-thick sections and collected into 5 dishes containing 0.01 M PBS (pH 7.4). Each dish contained a one-fifth set of serial sections. One set of sections was used for immunostaining. Antigen retrieval was firstly performed by microwave treatment (10 minutes each for 3 times) in 10-mM citrate buffer (10-mM Citric Acid, 0.05% Tween 20, pH 6.0). Then the sections were washed 3 times with PBS and sequentially incubated in the following 2 steps: (1) rabbit antiserum against NRSF (1:200; Santa Cruz, TX) and goat antiserum against MOR (1:200; Santa Cruz; validated antibody in the antibody database of the Journal of Comparative Neurology website) diluted in 0.01 M PBS containing 1% bovine serum albumin and 0.3% Triton X-100, overnight at 4°C. (2) Alexa Fluor 594-conjugated donkey anti-rabbit IgG (1:200; Abcam, MA) and fluorescein isothiocyanate-conjugated donkey anti-goat IgG (1:200; Santa Cruz), for 2 hours at room temperature. Between the steps, the sections were washed with 0.01-M PBS (pH 7.4). Negative controls were performed on one set of sections by replacing primary antibodies with normal rabbit serum. Following immunostaining procedures, the sections were examined and analyzed using laser scanning confocal microscopy (FluoView FV1000; Olympus Co, Tokyo, Japan). Appropriate laser beams and filter settings for green-emitting fluorescein isothiocyanate (excitation 488 nm; emission 530 nm) or red-emitting Alexa 594 (excitation, 543 nm; emission, 590-615 nm) were applied to the sections. To determine the percentage of neurons immunoreactive for MOR and NRSF, MOR, or NRSF immunoreactive neurons and the total number of neurons with a distinct nucleus were counted in 5 randomly selected DRG sections per animal (n = 6 rats). This work was performed by experimenters who were blinded to the group assignment. The digital images were captured using FV10-ASW-1.6 software (Olympus, Tokyo, Japan), modified (15% to 20% contrast enhancement) in Photoshop CS5 (Adobe Systems, San Jose, CA) and then saved as TIFF files.
2.5. Western blot analysis
The mice were anesthetized with 1% sodium pentobarbital (60 mg/kg, i.p.) and sacrificed by decapitation. The left L2/3/4 DRG and the spinal cord innervated by the L2-4 dorsal roots were rapidly removed on ice. The NE-PER kit (ThermoFisher, Waltham, MA) was used to prepare cytosolic and nuclear cell fractions. Briefly, cytoplasmic contents and nuclear extract were separated according to the manufacture's protocol. Protease and phosphatase inhibitors (Sigma) were added to all buffers. Sample aliquots were stored at −70°C. The protein concentration was determined using the BCA method according to kit's instructions (ThermoFisher). Protein lysates (100 μg) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis on 7.5% gels and transferred (100 V for 2 hours) onto polyvinylidene difluoride membranes (Millipore, Billerica, MA) with a wet blotting system. The membranes were blocked with 5% nonfat milk at room temperature for 2 hours, followed by overnight incubation at 4°C with the following primary antibodies diluted in blocking buffer: goat antiserum against MOR (1:200; Santa Cruz, TX), rabbit antiserum against NRSF (1:200; Santa Cruz), or donkey antiserum against histone H3 (1:500; Millipore). Subsequently, the immunoblots were incubated with the following secondary antibodies for 2 hours at room temperature (1:5000; all from Santa Cruz): horseradish peroxidase (HRP)-conjugated donkey anti-goat IgG, HRP-conjugated donkey anti-rabbit IgG, or HRP-conjugated rabbit anti-donkey IgG. Between each step, the membranes were rinsed 3 times (10 minutes each) with Tris-buffered saline with Tween-20. Then, the chemiluminescence was detected using the enhanced chemiluminescence (ECL) system (Amersham Corporation, Arlington Heights, IL). Immunoreactivity was quantified by densitometric analysis using Image J software. Expression levels of MOR and NRSF were normalized against β-actin or histone H3, respectively. Data are presented as mean ± SD.
2.6. Quantitative real time reverse transcription-polymerase chain reaction
The left L2/3/4 DRG and the left hemi spinal cord innervated by the L2-4 dorsal roots were rapidly removed and frozen in liquid nitrogen and stored at −70°C until use. Total RNA was extracted using Trizol reagent (Thermo Fisher Scientific Inc, Waltham, MA) according to the manufacturer's instructions. The amount of RNA was measured using a spectrophotometer. A total of 500 ng of RNA was reversely transcribed into cDNA using the Reverse Transcription-Polymerase Chain Reaction Kit (Takara, Dalian, China), and quantitative real-time reverse transcription-polymerase chain reaction (quantitative reverse transcription-polymerase chain reaction [RT-PCR]) was performed with the SYBR PrimeScript Quantitative Real Time Reverse Transcription-Polymerase Chain Reaction Kit (Takara) according to the manufacturer's protocol, using the ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA). Expression of mMOR and mNRSF was normalized to RNA loading for each sample using the reference gene glyceraldehyde-3-phosphate dehydrogenase as an internal control. Oligonucleotide primers for RT-PCR reactions are shown below: MOR primers (upstream primer, ATCCTCTCTTCTGCCATTGGT and downstream primer, TGAAGGCGAAGATGAAGACA), NRSF primers (upstream primer, GTGCGAACTCACACAGGAGA, and downstream primer, AAGAGGTTTAGGCCCGTTGT),37 and glyceraldehyde-3-phosphate dehydrogenase primers (upstream primer, TATGACTCCACTCACGGCAAAT and downstream primer, GGGTCTCGCTCCTGGAAGAT). To confirm that a single band of the correct molecular weight was obtained, PCR products were analyzed on 2% agarose gels in all cases.
2.7. Chromatin immunoprecipitation assays
The levels of NRSF physically associated with the MOR promoter were assayed by the chromatin immunoprecipitation (ChIP) assay according to the protocols from Millipore and from previous reports.3,47 The L2-4 DRG of 3 mice were collected for each ChIP assay. After they had been homogenized in ice-cold cell lysis buffer (10 mM Tris-HCl, pH 8.0, 10 mM NaCl, 0.2% Nonidet P-40, 1 μM p-APMSF), DRG samples were transferred into PBS containing 1% formaldehyde at 37°C for 5 minutes to cross-link histone proteins to DNA, followed by the addition of 0.125-M glycine to stop the reaction. Subsequently, the samples were repeatedly washed with PBS and resuspended in SDS lysis buffer (50 mM Tris-HCl, pH 8.1, 10 mM EDTA, 1% SDS, and 1 μM p-APMSF). The chromatin was sonicated (Misonix 3000, Farmingdale, NY) to an average length of 200- to 500-bp fragments and then diluted 10-fold in ChIP dilution buffer (16.7 mM Tris-HCl, pH 8.1, 1.2 mM EDTA, 167 mM NaCl, 1.1% Triton X-100, 0.01% SDS, and 1 μM p-APMSF). After being precleared with normal-rabbit IgG (1:5000; Santa Cruz) and protein A-agarose beads (Cell Signaling Technology, Danvers, MA) for 1 hour at 4°C with rotation, the samples were centrifuged and the supernatant containing chromatin fragments were incubated overnight at 4°C with the rabbit anti-NRSF (1:200; Santa Cruz), rabbit anti-acetyl-H3 antibodies (1:500; Millipore), rabbit anti-acetyl-H4 antibodies (1:500, Millipore), or normal rabbit IgG (1:5000; Santa Cruz). Immunocomplexes were then collected with protein A-agarose beads. The histone complex was eluted from the antibody with elution buffer (1% SDS, 0.1 M NaHCO3) under rotation for 15 minutes at room temperature. The elutions were treated with 0.2 M NaCl at 65°C for 4 hours to reverse the cross-link reaction. Subsequently, the DNA was treated with proteinase K for 1 hour at 45°C and purified by phenol/chloroform extraction and ethanol precipitation. Then, the DNA was dissolved in TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0) and used for subsequent PCR analysis. PCR products were subjected to electrophoresis on a 2% agarose gel. Quantitative RT-PCR was performed according to the above-mentioned steps. The data were normalized to the corresponding input. Primers for MOR-NRSE (upstream primer, CTGTGAGAGGAAGAGGCTG and downstream primer, AAGTTGAGCCAGGAGCCAGGT) are published elsewhere.19,47 In addition, a control region (-1676/-1566) in the upstream area of MOR-NRSE was also analyzed (upstream primer, GCAGCAGTAAGCACCACAAG and downstream primer, TGGCTGAGCTGAAATCTGTG).
2.8. Oligonucleotide treatment
As previously reported, the AS-ODN was designed to target mice NRSF mRNA.47 The mice were randomly assigned to the following 3 groups: (1) AS-ODN group: mice received intrathecal injections of AS-ODN (5′-CGGAAGGGCTTGGCC-3′) at a dose of 10 μg in 5-μL aCSF; (2) Mismatch scrambled oligodeoxynucleotide (MS-ODN) group: mice received intrathecal injections of MS-ODN (5′-GTCGTCGGCGGAGCA-3′) at a dose of 10 μg in 5-μL aCSF; (3) Vehicle group: mice were intrathecally injected with 5-μL aCSF. The administrations were performed on days 15, 17, 19, and 20 post–sarcoma cell inoculation. The expression levels of NRSF or MOR and effect of morphine on pain behaviors were evaluated on postop day 21.
2.9. System morphine administration and behavior evaluation
On day 21 following the sarcoma cell inoculation, the AS-ODN, MS-ODN, or vehicle-treated animals were i.p. injected with saline or the following doses of morphine sulfate (MS; VA Medical Center, Minneapolis, MN): 0.3 mg/kg, 1 mg/kg, 3 mg/kg, and 10 mg/kg. Thirty minutes later, the spontaneous flinches, limb-use score and PWT were tested to evaluate the effect of AS-ODN administration with different morphine doses. Data are presented as percentages of the values of the saline-injected vehicle-treated sarcoma-bearing mice.
2.10. Rotarod test
Rotarod function was tested to exclude the effect of morphine-induced motor dysfunctions on pain behaviors, as has been performed elsewhere.4,45 Briefly, after saline or varying doses of morphine sulfate had been i.p. injected, sarcoma-bearing mice were placed onto the Ugo Basile 7650 Rotarod accelerator treadmill (Ugo Basile, Varese, Italy) at a constant speed of 4 rotations per minute. After 1 minute of training, the rotarod was linearly accelerated from 4 to 40 rpm over 5 minutes. The time before the mouse fell to the ground was recorded. Thirty minutes before morphine injection, animals were tested for baseline control. Data were expressed as percentages of the value of mean baseline control.
2.11. Statistical analyses
Statistical analyses were performed with SPSS software version 16.0 (SPSS Inc, Chicago, IL). Data were analyzed using repeated measures analysis of variance followed by Bonferroni correction for between-group comparisons. Changes in PWT were analyzed using the non-parametric Friedman test for repeated measures followed by q test for between-group comparisons as PWT results were nonlinearly dispersed. A P value less than 0.05 was considered statistically significant in all cases.
3. Results
3.1. Pain-related behaviors and bone destruction in sarcoma-inoculated mice
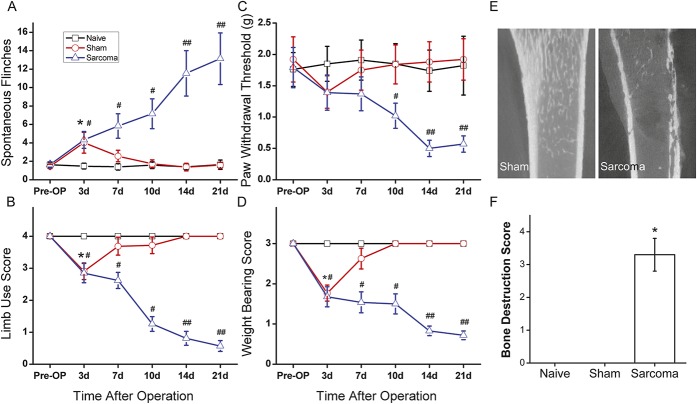
The bone cancer-induced pain behaviors were evaluated at different time points. On day 3 after surgery, signs of ongoing pain (spontaneous flinches) as well as movement-induced break-through pain (limb-use score and weight-bearing score) was detected in both sarcoma animals and sham-implanted animals (P < 0.05, respectively, as compared with naive mice, n = 8; Figs. 1A, B, D). On day 7 after surgery, pain-related behaviors in sham-implanted animals were alleviated, and there was no statistical difference between sham-implanted mice and naive mice. However, pain-related behaviors in sarcoma mice developed gradually over time. On day 10, sarcoma animals demonstrated a higher incidence of flinching, which was statistically significant (7.2 ± 1.6, n = 8; Fig. 1A). Additionally, significantly lower indices of limb use and lower PWT as well as impaired weight bearing were observed in the sarcoma animals when compared with naive animals (P < 0.05, respectively, n = 8; Figs. 1B, C, D). Evaluation on days 14 and 21 showed that the pain-related behaviors developed in sarcoma animals and that these behaviors occurred at a statistically significantly higher frequency than in naive mice (P < 0.01, respectively, n = 8; Figs. 1A–D).
Figure 1.
Evaluation of pain-related behaviors and bone destruction in sarcoma-inoculated mice. Spontaneous flinches (A), limb-use score (B), paw withdrawal threshold (C), and weight-bearing score (D) were evaluated before (preoperative[Pre-OP]) or 3, 7, 10, 14, 21 days after sarcoma cell inoculation. Micro-CT scanning (E) and quantification of bone destruction (F) showed various degrees of bone destruction were observed 21 days post–sarcoma cell inoculation. Data are expressed as mean ± SD (n = 6). #, *P < 0.05 in comparison with the naive group.
Micro-CT scanning was used to evaluate the extent of bone destruction. Micro-CT images showed that various degrees of bone destruction (eg, radiolucent lesion in the epiphysis and erosion of cortical bone 21 days post–sarcoma cell inoculation) that had been caused by tumor growth. The bone marrow was replaced by tumor tissue with nonhomogeneous signal density. In some severe cases, tumor growth extended outside the bone and periosteum with cortical bone discontinuity. No radiological changes were observed in the sham-operated and naive mice (Fig. 1E). Quantification of bone destruction showed that 21 days postoperation, the mean bone scores for tumor-bearing mice (3.3 ± 0.5) were significantly higher than those of the naive mice (0.0 ± 0.0; P < 0.05), while no significant differences were observed in sham-treated mice vs naive mice (0.0 ± 0.0, Fig. 1F).
3.2. μ-opioid receptor expression was reduced while neuron-restrictive silencer factor expression in the dorsal root ganglion of sarcoma bearing mice was significantly elevated
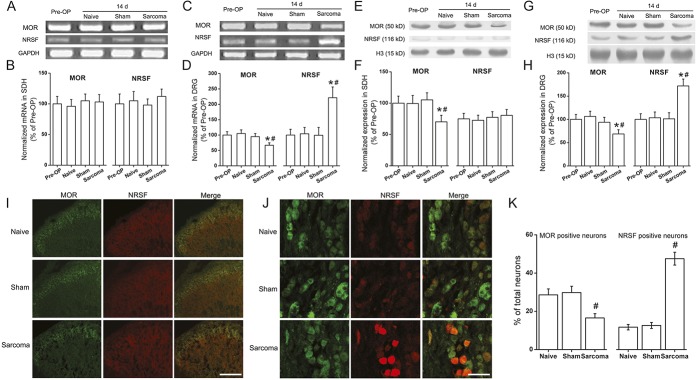
First, we investigated the MOR expression in the spinal cord and the DRG. As previously reported, quantitative RT-PCR revealed no apparent changes of MOR mRNA levels in the spinal dorsal horn55 (Figs. 2A, B). However, on day 14 after sarcoma cell implantation, MOR mRNA expression in the DRG was significantly downregulated compared with that in preoperative or naive animals (P < 0.05 respectively, n = 6; Figs. 2C, D). Western blot analysis indicated that MOR protein expression was significantly reduced in both spinal dorsal horn and DRG compared with preoperative or naive mice (P < 0.05, respectively, n = 6; Figs. 2E–H).
Figure 2.
Expression of μ-opioids receptor (MOR) and neuron-restrictive silencer factor (NRSF) in the spinal dorsal horn and dorsal root ganglion (DRG) of sarcoma bearing mice. On day 14 post–sarcoma cell inoculation, quantitative real time PCR indicated no apparent changes of MOR and NRSF mRNA levels in the spinal dorsal horn (A and B). However, MOR mRNA expression levels in the DRG were significantly downregulated compared to that in preoperative (Pre-OP) animals (*P < 0.05, n = 6) or naive animals (#P < 0.05, n = 6; C and D). Western blot analysis showed that in the spinal dorsal horn, MOR protein was significantly reduced compared with that of preoperative (*P < 0.05, n = 6) or naive mice (#P < 0.05, n = 6; E and F). No significant change of NRSF protein was observed in the spinal dorsal horn (E and F). In the DRG, a significant reduction of MOR expression was observed in sarcoma bearing mice compared with that of preoperative (*P < 0.05, n = 6) or naive mice (#P < 0.05, n = 6; G and H). Neuron-restrictive silencer factor protein was significantly increased (compared with that of preoperative mice, *P < 0.05, n = 6; or with naive mice, #P < 0.05, n = 6; G and H). Immunofluorescent staining showed that 14 days post–tumor cell inoculation, the MOR immunoreactivity was significantly reduced in the spinal dorsal horn (I). Neuron-restrictive silencer factor immunoreactivity in the spinal dorsal horn is low and is hardly detected (I). On day 14 in the DRG, the MOR-positive signal intensity was decreased while NRSF immunoreactivity was markedly upregulated compared with that in naive mice (J). Double-labeled immunofluorescence indicated that most NRSF immunopositive signals colocalized with MOR positive signals, suggesting that NRSF is widely expressed in the MOR-expressing DRG neurons (J). Analysis with the neuron counting results indicated that the percentage of MOR immunoreactive neurons in DRG was significantly reduced in sarcoma mice compared with that in naive animals, while the percentage of NRSF immunopositive neurons was obviously elevated (#P < 0.05 respectively, n = 6; K). Bar for (I), 200 μm; for (J), 50 μm. All data are expressed as mean ± SD (n = 6). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Neuron-restrictive silencer factor has been shown to bind to the NRSE sequence within the MOR gene and play important roles in modulating the expression of the MOR gene.8,18,19,47 Next, we investigated the expression of NRSF in the ipsilateral spinal dorsal horn or L2-4 DRG after sarcoma cell inoculation at both transcriptional and translational levels. On postoperative day 14, the expression of both NRSF mRNA and protein in DRG of sarcoma bearing animals were significantly upregulated compared to that in preoperative or naive mice (P < 0.05 respectively, n = 6; Figs. 2C, D, G, H), while no significant changes were observed in the naive or sham-operated mice. In addition, there were no obvious changes in the expression of NRSF mRNA or protein in the spinal dorsal horn of sarcoma mice based on quantitative RT-PCR or Western blot analysis (Figs. 2A, B, E, F).
The expression of MOR and NRSF in the spinal dorsal horn and DRG was further investigated with immunofluorescent staining. In the spinal dorsal horn, MOR immunoreactive fibers and neurons were mainly distributed in the superficial laminae. On day 14 after the surgery, MOR immunoreactivity was clearly reduced, which was in accordance with the quantification results of Western blot analysis. The immunostaining of NRSF in the spinal dorsal horn was so weak that immunofluorescence signal was almost not detected (Fig. 2I). In the DRG neurons, the MOR-positive signal intensity was decreased 14 days following tumor cell inoculation while NRSF immunoreactivity was markedly upregulated compared with that in naive mice. Double-labeled immunofluorescence indicated that most NRSF immunopositive signals colocalized with MOR positive signals, suggesting that NRSF is widely expressed in the MOR-expressing DRG neurons (Fig. 2J). Further analysis with the neuron counting results indicated that the percentage of MOR immunoreactive neurons in DRG was significantly reduced in sarcoma mice compared with that in naive animals, while the percentage of NRSF immunopositive neurons was obviously elevated (P < 0.05, respectively, n = 6; Fig. 2K). In the sections obtained from negative controls, immunoreactivity was completely eliminated through replacement of primary antibodies with normal rabbit serum (data not shown).
3.3. Expressions of μ-opioid receptor and neuron-restrictive silencer factor at different time points and the negative correlation between neuron-restrictive silencer factor mRNA and μ-opioid receptor mRNA
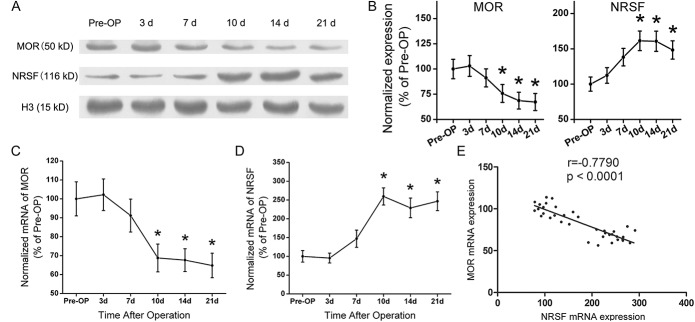
Observation at different time points with Western blot analysis indicated that the expression of MOR decreased while NRSF increased gradually over time. Ten days after sarcoma cell inoculation, the expression of MOR decreased significantly compared with that in preoperative mice. On day 14 postoperatively, the expression of MOR protein further decreased and this tendency continued to the end of the experiment. However, at day 10 postinoculation, the expression of NRSF in ipsilateral DRG was statistically elevated compared with that in preoperative mice. Sarcoma cell inoculation induced a long-lasting up-regulation of NRSF expression throughout the observation period (P < 0.05, respectively, n = 6; Figs. 3A, B).
Figure 3.
Expressions of μ-opioid receptor (MOR) and neuron-restrictive silencer factor (NRSF) at different time points and the negative correlation between NRSF mRNA and MOR mRNA. Western blot analysis showed that the expression of MOR decreased while the expression of NRSF increased gradually over time. Ten days postoperation, MOR expression was significantly reduced compared with that in preoperative mice. On postoperative day 14, expression of MOR protein further decreased and the tendency continued until postoperative day 21. Expression of NRSF in ipsilateral dorsal root ganglion was statistically different with that in preoperative mice 10 days after operation. The up-regulation of NRSF expression continued until the end of the observation period (P < 0.05, respectively, n = 6; A, B). Quantitative reverse transcription-polymerase chain reaction showed that 10 days after the operation, MOR mRNA reduced significantly while NRSF mRNA was significantly elevated compared with that of preoperative controls. The downregulations of MOR mRNA or upregulations of NRSF mRNA persisted at least 21 days post–sarcoma cell implantation (P < 0.05, respectively, n = 6; C, D). Analysis of linear correlation was used to evaluate the correlation between MOR and NRSF mRNA. A negative correlation exists between NRSF mRNA and MOR mRNA (E, r = −0.779, P < 0.0001). Data are expressed as mean ± SD. Pre-OP, preoperative.
Relative quantification of MOR and NRSF mRNA with quantitative RT-PCR demonstrated that 10 days after the operation, MOR mRNA were reduced while NRSF mRNA was significantly elevated compared to that of preoperative controls. The downregulation of MOR mRNA or up-regulations of NRSF mRNA persisted at least 21 days post–sarcoma cell implantation (P < 0.05, respectively, n = 6; Figs. 3C, D). The results of quantitative RT-PCR and Western blot analyses confirmed that altered transcription of both MOR and NRSF were reflected in their protein abundance. The relative relationship between MOR mRNA and NRSF mRNA was subsequently analyzed. Linear correlation analysis between MOR and NRSF mRNA indicated that expression of the MOR gene was negatively correlated with NRSF mRNA (r = −0.779, P < 0.0001; Fig. 3E).
3.4. The abundance of μ-opioid receptor-neuron-restrictive silencer element associated with neuron-restrictive silencer factor was significantly increased in dorsal root ganglion of sarcoma bearing mice with deacetylation of histone H3 and H4
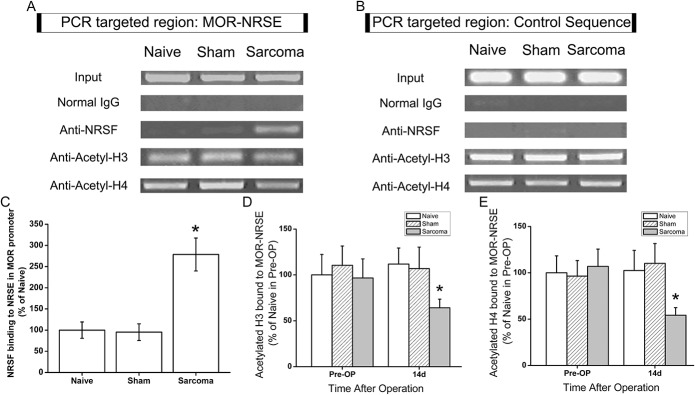
The above-mentioned results indicated that NRSF mRNA and protein expression in DRG neurons were dramatically upregulated in sarcoma-inoculated mice. We subsequently tried to clarify the functional activity of NRSF on MOR expression in bone cancer pain. Using ChIP analysis, we examined the expression of MOR-NRSE sequence binding with NRSF, as well as the acetylation state of histone H3 and H4 over MOR-NRSE. As the representative image in Figure 4A shows, 14 days after the surgery, MOR-NRSE sequence binding with NRSF was dramatically increased in preparation of DRG from tumor-bearing mice (P < 0.05, n = 6; Figs. 4A, C), whereas no detectable signal was observed in sham-operated mice. The elimination of positive binding by precipitation with normal IgG confirmed the specificity of the immunoprecipitation (Fig. 4A). We assumed that if NRSF mediated the repression of MOR transcription in DRG of sarcoma mice, an elevation of NRSF expression would be accompanied with deacetylation of histone proteins over the MOR-NRSE site. The abundance of acetylated H3 and H4 over MOR-NRSE sequence was then measured using the ChIP assay and quantitative RT-PCR. Fourteen days after sarcoma-cell implantation, the abundance of MOR-NRSE associated with acetylated H3 and H4 was dramatically reduced in ipsilateral DRG (P < 0.05 compared with naive animals, respectively, n = 6; Figs. 4A, D). A control region in the upstream area of MOR-NRSE was also targeted (Fig. 4B). Quantitative RT-PCR targeting the control sequence indicated that sarcoma inoculation did not change the binding of NRSF and the acetylation of H3 or H4 within the control genomic target (data not shown). These findings indicated that more NRSF was binding to the MOR-NRSE sequence in sarcoma-bearing mice and the acetylation of histone H3 and H4 around MOR-NRSE sequence was reduced.
Figure 4.
Neuron-restrictive silencer factor (NRSF) binding to the μ-opioid receptor (MOR) promoter in dorsal root ganglion of the sarcoma mice were greatly elevated with deacetylation of H3 and H4. On postoperative day 14, ChIP assays combined with quantitative reverse transcription-polymerase chain reaction (RT-PCR) were performed to quantify the NRSF-binding at MOR-neuron-restrictive silencer element (NRSE). Representative gel images of PCR results are shown in (A). Immunoprecipitation with normal IgG showed negligible PCR product, indicating specificity of the positive results. A control region in the upstream area of MOR-NRSE was also targeted and the representative PCR results are shown in (B). Quantitative RT-PCR targeting the control sequence indicated that sarcoma inoculation did not change the binding of NRSF and the acetylation of H3 or H4 within the control genomic target (data not shown). Further analysis with quantitative RT-PCR showed that NRSF binding to the MOR promoter in dorsal root ganglion of the sarcoma mice was significantly increased compared to that in naive mice (*P < 0.05, n = 6; C). ChIP analysis through immunoprecipitation with antibody to acetyl-H3 (D) or acetyl-H4 (E) showed that 14 days after sarcoma inoculation, acetylated H3 and H4 were significantly reduced compared with naive mice prior to surgery (*, P < 0.05, respectively, n = 6). All data were normalized to the corresponding input and are expressed as mean ± SD.
3.5. Downregulation of μ-opioid receptor induced by sarcoma implantation was blocked by neuron-restrictive silencer factor knockdown
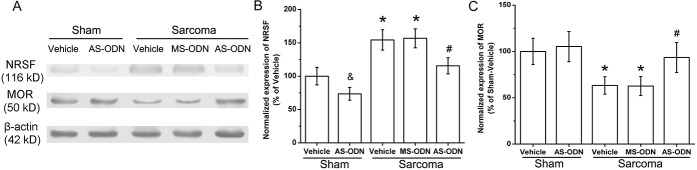
To determine the impact of NRSF on MOR expression at the DRG of L3-L5 in sarcoma-inoculated mice further, genetic knockdown of NRSF was performed by intrathecal delivery of AS-ODN. Western blot results showed that the expression of NRSF was significantly downregulated by intrathecal delivery of AS-ODN in the sarcoma bearing mice (compared with that of vehicle-treated sarcoma mice, P < 0.05, n = 4; Figs. 5A, B). However, intrathecal MS-ODN did not change NRSF expression. Additionally, after vehicle or MS-ODN intrathecal treatment in sarcoma-inoculated mice, MOR was still expressed at a low level (Figs. 5A, C). However, intrathecal delivery of AS-ODN significantly elevated the expression of MOR in DRG of L3-L5 (compared with that of vehicle-treated sarcoma bearing mice, P < 0.05, n = 4; Fig. 5C). The results indicate that NRSF antisense suppresses NRSF protein expression and rescues the expression of MOR.
Figure 5.
The impact of knocking down of neuron-restrictive silencer factor (NRSF) on μ-opioid receptor (MOR) expression in dorsal root ganglion of the sarcoma-inoculated mice. After intrathecal delivery of antisense oligodeoxynucleotide (AS-ODN) on days 15, 17, 19 and 20 post–sarcoma cell inoculation, Western blot was performed on postoperative day 21. In the sham operated animals, AS-ODN treatment significantly reduced the expression of NRSF. In the vehicle-treated sarcoma mice, expression of NRSF was remarkably elevated (compared with that of vehicle treated sham animals, &, *P < 0.05, n = 4 respectively; A, B). However, after AS-ODN administration, expression of NRSF in the sarcoma bearing mice was significantly downregulated (compared with vehicle treated sarcoma animals, #P < 0.05, n = 4; A, B). As speculated, after vehicle or Mismatch scrambled oligodeoxynucleotide (MS-ODN) treatment MOR was still expressed at a low level in sarcoma-inoculated mice (*P < 0.05 compared with vehicle treated sham mice, n = 4; C) However, intrathecal delivery of AS-ODN significantly elevated the expression of MOR in dorsal root ganglion (compared with that of vehicle treated sarcoma bearing mice, #P < 0.05, n = 4; C). Data are expressed as mean ± SD.
3.6. Neuron-restrictive silencer factor antisense knockdown potentiated morphine analgesia in sarcoma-bearing mice
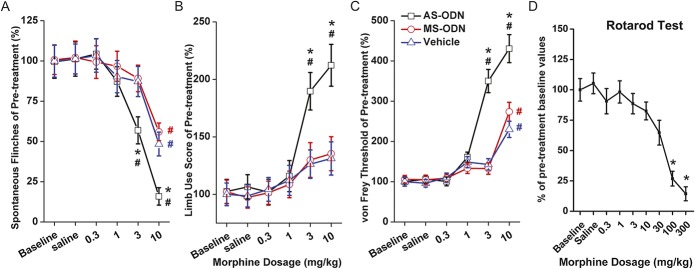
The effects of different doses of intraperitoneal morphine on pain-related behaviors after NRSF knockdown were evaluated. Antisense oligodeoxynucleotide treatment did not alter the baseline pain behavior in sarcoma-bearing mice. What was interesting is that in the spontaneous flinch test, only the 10-mg/kg dose caused significant alleviation in the vehicle-treated mice (compared with baseline values, P < 0.05, n = 6; Fig. 6A). However, reduction of spontaneous flinches was observed with the 3-mg/kg dose in the AS-ODN group (compared with values of baseline and Vehicle group, P < 0.05 respectively, n = 6; Fig. 6A). On the limb-use score test, a marker of clinical breakthrough pain in patients with bone cancer, there were no apparent changes observed after treatment with dosages of up to 10 mg/kg of morphine in the vehicle-treated mice, while in the AS-ODN treated mice, the limb-use score was significantly improved with a dosage of 3 and 10 mg/kg (compared with values of baseline and Vehicle group, P < 0.05, respectively, n = 6; Fig. 6B). A similar tendency was found on the PWT test (Fig. 6C). Taken together, these results indicate that the analgesic effects of i.p. administrated morphine were significantly potentiated by NRSF gene knockdown in sarcoma-bearing mice.
Figure 6.
Effects of different dosages of intraperitoneal morphine on pain-related behaviors after neuron-restrictive silencer factor knockdown. The effect of morphine on pain behaviors was evaluated on day 21 post–sarcoma cell inoculation. Antisense oligodeoxynucleotide (AS-ODN) treatment did not alter the baseline pain behaviors in sarcoma bearing mice. Remarkable reduction of spontaneous flinches was observed with 3-mg/kg dosage in the AS-ODN group (compared with values of baseline and Vehicle group, P < 0.05 respectively), while only 10 mg/kg caused significant alleviation in the vehicle treated mice (compared with values of baseline, P < 0.05, n = 6; A). Limb-use score was significantly improved with dosages of 3 mg/kg and 10 mg/kg (compared with values of baseline and Vehicle group, P < 0.05 respectively, n = 6; B). In the von Frey test, the mechanical threshold was significantly elevated with the dose of 10 mg/kg morphine in the vehicle-treated group; however, after AS-ODN administration, a lower dosage of morphine (3 mg/kg) resulted in significant elevation of mechanical threshold (compared with values of baseline and Vehicle group, P < 0.05 respectively, n = 6; C). No significant changes of rotarod function were observed after i.p. administration of morphine doses of up to 30 mg/kg (D). Values are expressed as a percent of pre-treatment baseline values ± SD (n = 6). In A, B, and C, #P < 0.05 in comparison with baseline value of the Vehicle group, *P < 0.05 in comparison with value of vehicle treated group with the same dosage of morphine injected. In (D), *P < 0.05 in comparison with the baseline value.
In addition, rotarod testing was performed to assess whether systemic administration of morphine influenced motor function. The results showed no significant changes in rotarod-related motor performance after doses of morphine up to 30-mg/kg i.p. administration. When the doses were escalated to 100 and 300 mg/kg, significant impairment on rotarod performance was detected as presumed (P < 0.05, n = 6; Fig. 6D), which were similar to observations reported previously.27
4. Discussion
The mechanisms of bone cancer pain are complex and may involve a combination of inflammatory and neuropathic pain with unique characteristics.10,25,33 During development of a sarcoma, soluble inflammatory and tumorigenic factors such as tumor necrosis factor-alpha are released while peripheral nerves are infiltrated and compressed.49,50 Primary nociceptive afferents may be sensitized by these soluble factors.7,11,13,43 In addition, astrocytosis and microglia activation, internalization of the substance P receptor, c-Fos expression and proinflammatory cytokine interleukin-1 (IL-1) beta, tumor necrosis factor-alpha,26 IL-18,56 IL-33,59 and IL-6 which are the cellular and neurochemical characteristics of chronic pain, can be detected in the spinal dorsal horn or DRG.7,10,26,43,56,59 Our previous report describes the effect of endogenous MOR ligands in the spinal cord and indicates that downregulated spinal endomorphin 2 is an important contributor to the neuropathological process of bone cancer pain.4 Neuroinflammation appears to be involved in the peripheral nerves, DRG and the spinal cord, all of which contribute to the complex properties of bone cancer pain.
Clinical observations and animal studies have both revealed that bone cancer pain is resistant to morphine when compared to inflammatory pain or other pain generators.27,55 In clinical practice, significantly higher doses of morphine are generally required to alleviate bone cancer pain compared to inflammatory pain. Using a preclinical model of bone cancer pain, Luger et al.27 indicated that the doses of morphine required to block bone cancer pain-related behaviors were 10 times higher than those required to block peak inflammatory pain behaviors of comparable magnitude. Other studies have also reported that bone cancer pain is difficult to manage and is relatively resistant to suppression by morphine.29,51,55 However, the reasons for this have remained unclear.
It has been shown in several animal pain models that the expression of MOR in the spinal dorsal horn and DRG is dramatically altered. For example, after peripheral nerve injury, MOR expression is markedly decreased in ipsilateral DRG and the inhibitory effect of MOR agonists was reduced following such injuries.22,39 In an inflammatory pain model, MOR expression was increased in the dorsal horn and DRG,15,28 and the inhibitory effect of MOR agonists was increased.44 Enhancement of MOR transgene expression in DRG neurons markedly potentiates morphine antinociceptive responses to thermal stimuli under both normal and inflamed conditions.54 These data indicate that the analgesic effect of MOR agonists may depend on the expression of MOR in the dorsal horn and DRG.55 There has been concern about the specificity of the antibody for opioid receptors; therefore, special attention was paid to the specificity of the antibody used in the present study.41 The validated MOR antibody was used and the results indicate that MOR expression in the DRG of the sarcoma-bearing mice was significantly reduced, paralleling observations reported elsewhere.55 However, whether reduction of MOR expression underlies pain behavioral changes and the resistance to morphine treatment in bone cancer pain and the potential regulation mechanisms remains unclear.
Transcriptional regulation of MOR expression has been recently examined, including studies of epigenetic mechanisms and transcription factors.52 Viet et al. evaluated the mechanisms of cancer pain and found that the MOR gene was methylated in cancer tissues. Furthermore, demethylating drugs reverse cancer pain thereby indicating that methylation contributes to the modulation of MOR gene in cancer pain.48 As a silencer factor that plays a critical role in elaboration of the neuronal phenotype, NRSF has been shown to bind to the neuron-restrictive silencer element (NRSE) of the MOR to function as a MOR gene expression repressor either in specific neuronal cell lines18,19 or in vivo animal studies.8,47 Kim et al.19 identified the conserved NRSE sequence in the promoter of the mouse MOR gene and demonstrated that NRSF specifically bound to NRSE to control MOR gene expression in specific neuronal cells. Recent in vivo studies confirmed that NRSF functions in the modulation of MOR gene expression either in the hippocampus or in the DRG.8,47 The present study demonstrated that NRSF protein was significantly upregulated in the DRG of sarcoma bearing mice, thereby causing epigenetic silencing of MOR. The negative correlation between NRSF mRNA and MOR mRNA revealed by linear correlation analysis indicated that NRSF might be responsible for the MOR downregulation after tumor inoculation. Further analysis by NRSF antisense knockdown provided direct evidence that NRSF is involved in the modulation of MOR transcription.
To test our hypothesis that regulation of MOR expression by NRSF was involved in the abnormality of pain behaviors in tumor-bearing mice, we subsequently performed gene knockdown of NRSF and investigated the effect of systemic morphine administration. Interestingly, although NRSF knockdown significantly rescued the expression of MOR, no significant changes were observed in the baseline pain behaviors of the AS-ODN-treated group. However, potentiation of morphine analgesia was observed in the AS-ODN group. Further analysis indicated that after AS-ODN treatment, spontaneous flinches were reduced to the same extent with lower doses of morphine. For the limb-use score–examination, which represents breakthrough pain that is clinically resistant to morphine,4 in the vehicle-treated group even higher doses of morphine (10 mg/kg) were not able to improve limb-use score significantly. Notably, even after AS-ODN administration, limb-use scores were markedly improved with lower doses of morphine (3 mg/kg). Considered overall, these findings strongly suggest that downregulation of MOR expression in the DRG contributes to the relative lack of efficacy of opioids in the treatment of bone cancer pain.55
The silencing activity of NRSF was correlated with its expression level5,42,47; however, the precise mechanisms underlying modulation of NRSF expression in the DRG remain elusive. Recent articles have indicated that HDAC-mediated epigenetic mechanisms are involved in the regulation of NRSF expression in the neuron.1,47 Within the 5′ end of the mouse NRSF gene, 3 promoters have been identified.21 Further analysis indicates that there are putative binding sites for AP-1 and Sp1 in the NRSF promoter II region.21,47 Previous studies have indicated that neuronal activity may regulate NRSF expression,40 and there were reports that neuronal activity may regulate gene expression through extracellular signal-regulated kinase (ERK) activation,14 a pathway that can lead to the activation of AP-1 and Sp-1.2,17 Considering this cumulative volume of evidence, we hypothesized that in the development of bone cancer, neuronal activity changes resulting in the activation of AP-1 and Sp-1 in the promoter region of NRSF gene through ERK activation. In addition, inflammatory responses may also involve in the activation of ERK and contribute to the regulation of NRSF expression. However, the exact mechanisms underlying the induction of NRSF require further study.
The modulation of tumor growth by local MOR agonists has raised concern in recent years. Currently, results are mixed and findings may depend on the model and the dose/concentration of MOR agonist used.20 An early study found that the antagonist of MOR inhibited the growth of metastatic murine neuroblastoma, resulting in increased survival times.57 Furthermore, both endogenous opioids (endomorphin-1, -2) and exogenous opioids can stimulate tumor growth and metastasis.6,12,24 Taken together, these results indicate that MOR is an important regulator of lung cancer progression.23,31 However, there are also reports that morphine inhibits cancer cell growth.46,58 The expression of MOR in bone sarcoma tissues and the effects of MOR on the growth of bone sarcoma have not been reported and investigations of that are beyond the aims of the present study. Another limitation of the present study is that the sham animals were injected with cell culture media, although it has been widely used elsewhere.9,34,43,55 As there were reports that benign keratinocytes placed adjacent to an injured nerve can induce neuronal hyperactivity,38 the injection of a benign counterpart to the malignant cell line may be a better sham control than the injection of cell culture media, to ensure that the malignant phenotype is responsible for the changes observed. Furthermore, we should be aware that there are limitations of the ChIP assay. The data obtained with the ChIP assay in the present study are correlational and we cannot infer cause and effect.
In conclusion, we have demonstrated that NRSF is significantly upregulated and bound to the promoter zone of MOR in the DRG of bone cancer mice. Genetic knockdown of NRSF rescues the expression of MOR and potentiates morphine analgesia. The expression of opioid receptors is not only regulated at the transcriptional level but also controlled by extensive post-transcriptional processing.52 Furthermore, the effect of a MOR agonist may be affected by the expression of MOR, the activation state, the development of tolerance, dependence, as well as hyperalgesia induced by long term use of agonist. All of these factors contribute to the complex property of the modulation of MOR expression and complicate the clinical utility of opioid pharmacotherapy. Further studies are needed at both the preclinical and clinical levels to develop pharmacological therapy, and to block/relieve bone cancer pain effectively with the goal of increasing the functional status and quality of life of humans with bone cancer pain.
Conflict of interest statement
The authors have no conflicts of interest to declare.
This work was supported by the National Natural Science Foundation of China (81300953, 81371947, 81171747, 31671087), Natural Science Foundation of JiangSu Province (BK20150555) and China Postdoctoral Science Foundation (2014T70982, 2012M521868, 2015M572814).
Acknowledgements
Author contributions: C. Zhu and J. Tang performed the animal surgery, carried out the immunofluorescence, Western blot study and drafted the manuscript. L. Chen and W. Wang performed the behavioral test. T. Ding participated in producing graphics and performed the statistical analysis. X.-P. Mei performed the Assessment of bone destruction. X.-T. He and W. Wang performed the Chromatin immunoprecipitation assays and Western blot analysis. L.-D. Zhang, Y.-L. Dong and Z.-J. Luo corresponded equally to this work in conceiving the study, and participating in its design and coordination. All authors read and approved the final manuscript. C. Zhu, J. Tang, and T. Ding have contributed equally to this work.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Ballas N, Mandel G. The many faces of REST oversee epigenetic programming of neuronal genes. Curr Opin Neurobiol 2005;15:500–6. [DOI] [PubMed] [Google Scholar]
- [2].Barré L, Venkatesan N, Magdalou J, Netter P, Fournel-Gigleux S, Ouzzine M. Evidence of calcium-dependent pathway in the regulation of human beta1,3-glucuronosyltransferase-1 (GlcAT-I) gene expression: a key enzyme in proteoglycan synthesis. FASEB J 2006;20:1692–4. [DOI] [PubMed] [Google Scholar]
- [3].Calderone A, Jover T, Noh KM, Tanaka H, Yokota H, Lin Y, Grooms SY, Regis R, Bennett MV, Zukin RS. Ischemic insults derepress the gene silencer REST in neurons destined to die. J Neurosci 2003;23:2112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chen L, Wang K, Yang T, Wang W, Mei XP, Zhu C, Wang W, Zhang FX, Li YQ. Downregulation of spinal endomorphin-2 correlates with mechanical allodynia in a rat model of tibia cancer. Neuroscience 2015;286:151–61. [DOI] [PubMed] [Google Scholar]
- [5].Chong JA, Tapia-Ramírez J, Kim S, Toledo-Aral JJ, Zheng Y, Boutros MC, Altshuller YM, Frohman MA, Kraner SD, Mandel G. REST: a mammalian silencer protein that restricts sodium channel gene expression to neurons. Cell 1995;80:949–57. [DOI] [PubMed] [Google Scholar]
- [6].Dai X, Song HJ, Cui SG, Wang T, Liu Q, Wang R. The stimulative effects of endogenous opioids on endothelial cell proliferation, migration and angiogenesis in vitro. Eur J Pharmacol 2010;628:42–50. [DOI] [PubMed] [Google Scholar]
- [7].Fang D, Kong LY, Cai J, Li S, Liu XD, Han JS, Xing GG. Interleukin-6-mediated functional upregulation of TRPV1 receptors in dorsal root ganglion neurons through the activation of JAK/PI3K signaling pathway: roles in the development of bone cancer pain in a rat model. PAIN 2015;156:1124–44. [DOI] [PubMed] [Google Scholar]
- [8].Formisano L, Noh KM, Miyawaki T, Mashiko T, Bennett MV, Zukin RS. Ischemic insults promote epigenetic reprogramming of mu opioid receptor expression in hippocampal neurons. Proc Natl Acad Sci U S A 2007;104:4170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Furuse S, Kawamata T, Yamamoto J, Niiyama Y, Omote K, Watanabe M, Namiki A. Reduction of bone cancer pain by activation of spinal cannabinoid receptor 1 and its expression in the superficial dorsal horn of the spinal cord in a murine model of bone cancer pain. Anesthesiology 2009;111:173–86. [DOI] [PubMed] [Google Scholar]
- [10].Goblirsch MJ, Zwolak PP, Clohisy DR. Biology of bone cancer pain. Clin Cancer Res 2006;12:6231s–5s. [DOI] [PubMed] [Google Scholar]
- [11].Goss JR, Harley CF, Mata M, O'Malley ME, Goins WF, Hu X, Glorioso JC, Fink DJ. Herpes vector-mediated expression of proenkephalin reduces bone cancer pain. Ann Neurol 2002;52:662–5. [DOI] [PubMed] [Google Scholar]
- [12].Gupta K, Kshirsagar S, Chang L, Schwartz R, Law PY, Yee D, Hebbel RP. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res 2002;62:4491–8. [PubMed] [Google Scholar]
- [13].Hua B, Gao Y, Kong X, Yang L, Hou W, Bao Y. New insights of nociceptor sensitization in bone cancer pain. Expert Opin Ther Targets 2015;19:227–43. [DOI] [PubMed] [Google Scholar]
- [14].Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol Dis 2001;8:1–10. [DOI] [PubMed] [Google Scholar]
- [15].Ji RR, Zhang Q, Law PY, Low HH, Elde R, Hökfelt T. Expression of mu-, delta-, and kappa-opioid receptor-like immunoreactivities in rat dorsal root ganglia after carrageenan-induced inflammation. J Neurosci 1995;15:8156–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jost L, Roila F. Management of cancer pain: ESMO clinical recommendations. Ann Oncol 2009;20(suppl 4):170–3. [DOI] [PubMed] [Google Scholar]
- [17].Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem 1995;270:16483–6. [DOI] [PubMed] [Google Scholar]
- [18].Kim CS, Choi HS, Hwang CK, Song KY, Lee BK, Law PY, Wei LN, Loh HH. Evidence of the neuron-restrictive silencer factor (NRSF) interaction with Sp3 and its synergic repression to the mu opioid receptor (MOR) gene. Nucleic Acids Res 2006;34:6392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kim CS, Hwang CK, Choi HS, Song KY, Law PY, Wei LN, Loh HH. Neuron-restrictive silencer factor (NRSF) functions as a repressor in neuronal cells to regulate the mu opioid receptor gene. J Biol Chem 2004;279:46464–73. [DOI] [PubMed] [Google Scholar]
- [20].King T, Vardanyan A, Majuta L, Melemedjian O, Nagle R, Cress AE, Vanderah TW, Lai J, Porreca F. Morphine treatment accelerates sarcoma-induced bone pain, bone loss, and spontaneous fracture in a murine model of bone cancer. PAIN 2007;132:154–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Koenigsberger C, Chicca JJ, II, Amoureux MC, Edelman GM, Jones FS. Differential regulation by multiple promoters of the gene encoding the neuron-restrictive silencer factor. Proc Natl Acad Sci U S A 2000;97:2291–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kohno T, Ji RR, Ito N, Allchorne AJ, Befort K, Karchewski LA, Woolf CJ. Peripheral axonal injury results in reduced mu opioid receptor pre- and post-synaptic action in the spinal cord. PAIN 2005;117:77–87. [DOI] [PubMed] [Google Scholar]
- [23].Lennon FE, Mirzapoiazova T, Mambetsariev B, Salgia R, Moss J, Singleton PA. Overexpression of the μ-opioid receptor in human non-small cell lung cancer promotes Akt and mTOR activation, tumor growth, and metastasis. Anesthesiology 2012;116:857–67. [DOI] [PubMed] [Google Scholar]
- [24].Leo S, Nuydens R, Meert TF. Opioid-induced proliferation of vascular endothelial cells. J Pain Res 2009;2:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liu S, Liu WT, Liu YP, Dong HL, Henkemeyer M, Xiong LZ, Song XJ. Blocking EphB1 receptor forward signaling in spinal cord relieves bone cancer pain and rescues analgesic effect of morphine treatment in rodents. Cancer Res 2011;71:4392–402. [DOI] [PubMed] [Google Scholar]
- [26].Liu S, Liu YP, Song WB, Song XJ. EphrinB-EphB receptor signaling contributes to bone cancer pain via Toll-like receptor and proinflammatory cytokines in rat spinal cord. PAIN 2013;154:2823–35. [DOI] [PubMed] [Google Scholar]
- [27].Luger NM, Sabino MA, Schwei MJ, Mach DB, Pomonis JD, Keyser CP, Rathbun M, Clohisy DR, Honore P, Yaksh TL, Mantyh PW. Efficacy of systemic morphine suggests a fundamental difference in the mechanisms that generate bone cancer vs inflammatory pain. PAIN 2002;99:397–406. [DOI] [PubMed] [Google Scholar]
- [28].Maekawa K, Minami M, Masuda T, Satoh M. Expression of mu- and kappa-, but not delta-, opioid receptor mRNAs is enhanced in the spinal dorsal horn of the arthritic rats. PAIN 1996;64:365–71. [DOI] [PubMed] [Google Scholar]
- [29].Mancini I, Dumon JC, Body JJ. Efficacy and safety of ibandronate in the treatment of opioid-resistant bone pain associated with metastatic bone disease: a pilot study. J Clin Oncol 2004;22:3587–92. [DOI] [PubMed] [Google Scholar]
- [30].Mantyh PW. Cancer pain and its impact on diagnosis, survival and quality of life. Nat Rev Neurosci 2006;7:797–809. [DOI] [PubMed] [Google Scholar]
- [31].Mathew B, Lennon FE, Siegler J, Mirzapoiazova T, Mambetsariev N, Sammani S, Gerhold LM, LaRiviere PJ, Chen CT, Garcia JG, Salgia R, Moss J, Singleton PA. The novel role of the mu opioid receptor in lung cancer progression: a laboratory investigation. Anesth Analg 2011;112:558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mercadante S. Intravenous morphine for management of cancer pain. Lancet Oncol 2010;11:484–9. [DOI] [PubMed] [Google Scholar]
- [33].Mercadante S, Fulfaro F. Management of painful bone metastases. Curr Opin Oncol 2007;19:308–14. [DOI] [PubMed] [Google Scholar]
- [34].Niiyama Y, Kawamata T, Yamamoto J, Furuse S, Namiki A. SB366791, a TRPV1 antagonist, potentiates analgesic effects of systemic morphine in a murine model of bone cancer pain. Br J Anaesth 2009;102:251–8. [DOI] [PubMed] [Google Scholar]
- [35].Pinski J, Dorff TB. Prostate cancer metastases to bone: pathophysiology, pain management, and the promise of targeted therapy. Eur J Cancer 2005;41:932–40. [DOI] [PubMed] [Google Scholar]
- [36].Portenoy RK, Lesage P. Management of cancer pain. Lancet 1999;353:1695–700. [DOI] [PubMed] [Google Scholar]
- [37].Qiang M, Rani CS, Ticku MK. Neuron-restrictive silencer factor regulates the N-methyl-D-aspartate receptor 2B subunit gene in basal and ethanol-induced gene expression in fetal cortical neurons. Mol Pharmacol 2005;67:2115–25. [DOI] [PubMed] [Google Scholar]
- [38].Radtke C, Vogt PM, Devor M, Kocsis JD. Keratinocytes acting on injured afferents induce extreme neuronal hyperexcitability and chronic pain. PAIN 2010;148:94–102. [DOI] [PubMed] [Google Scholar]
- [39].Rashid MH, Inoue M, Toda K, Ueda H. Loss of peripheral morphine analgesia contributes to the reduced effectiveness of systemic morphine in neuropathic pain. J Pharmacol Exp Ther 2004;309:380–7. [DOI] [PubMed] [Google Scholar]
- [40].Roopra A, Huang Y, Dingledine R. Neurological disease: listening to gene silencers. Mol Interv 2001;1:219–28. [PubMed] [Google Scholar]
- [41].Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O'Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell 2009;137:1148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Schoenherr CJ, Anderson DJ. The neuron-restrictive silencer factor (NRSF): a coordinate repressor of multiple neuron-specific genes. Science 1995;267:1360–3. [DOI] [PubMed] [Google Scholar]
- [43].Schwei MJ, Honore P, Rogers SD, Salak-Johnson JL, Finke MP, Ramnaraine ML, Clohisy DR, Mantyh PW. Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain. J Neurosci 1999;19:10886–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Stanfa LC, Dickenson AH. Cholecystokinin as a factor in the enhanced potency of spinal morphine following carrageenin inflammation. Br J Pharmacol 1993;108:967–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tang J, Zhu C, Li ZH, Liu XY, Sun SK, Zhang T, Luo ZJ, Zhang H, Li WY. Inhibition of the spinal astrocytic JNK/MCP-1 pathway activation correlates with the analgesic effects of tanshinone IIA sulfonate in neuropathic pain. J Neuroinflammation 2015;12:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Tegeder I, Grösch S, Schmidtko A, Häussler A, Schmidt H, Niederberger E, Scholich K, Geisslinger G. G protein-independent G1 cell cycle block and apoptosis with morphine in adenocarcinoma cells: involvement of p53 phosphorylation. Cancer Res 2003;63:1846–52. [PubMed] [Google Scholar]
- [47].Uchida H, Ma L, Ueda H. Epigenetic gene silencing underlies C-fiber dysfunctions in neuropathic pain. J Neurosci 2010;30:4806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Viet CT, Dang D, Ye Y, Ono K, Campbell RR, Schmidt BL. Demethylating drugs as novel analgesics for cancer pain. Clin Cancer Res 2014;20:4882–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wacnik PW, Eikmeier LJ, Ruggles TR, Ramnaraine ML, Walcheck BK, Beitz AJ, Wilcox GL. Functional interactions between tumor and peripheral nerve: morphology, algogen identification, and behavioral characterization of a new murine model of cancer pain. J Neurosci 2001;21:9355–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wacnik PW, Eikmeier LJ, Simone DA, Wilcox GL, Beitz AJ. Nociceptive characteristics of tumor necrosis factor-alpha in naive and tumor-bearing mice. Neuroscience 2005;132:479–91. [DOI] [PubMed] [Google Scholar]
- [51].Wacnik PW, Kehl LJ, Trempe TM, Ramnaraine ML, Beitz AJ, Wilcox GL. Tumor implantation in mouse humerus evokes movement-related hyperalgesia exceeding that evoked by intramuscular carrageenan. PAIN 2003;101:175–86. [DOI] [PubMed] [Google Scholar]
- [52].Wei LN, Loh HH. Transcriptional and epigenetic regulation of opioid receptor genes: present and future. Annu Rev Pharmacol Toxicol 2011;51:75–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Westbrook TF, Martin ES, Schlabach MR, Leng Y, Liang AC, Feng B, Zhao JJ, Roberts TM, Mandel G, Hannon GJ, Depinho RA, Chin L, Elledge SJ. A genetic screen for candidate tumor suppressors identifies REST. Cell 2005;121:837–48. [DOI] [PubMed] [Google Scholar]
- [54].Xu Y, Gu Y, Xu GY, Wu P, Li GW, Huang LY. Adeno-associated viral transfer of opioid receptor gene to primary sensory neurons: a strategy to increase opioid antinociception. Proc Natl Acad Sci U S A 2003;100:6204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Yamamoto J, Kawamata T, Niiyama Y, Omote K, Namiki A. Down-regulation of mu opioid receptor expression within distinct subpopulations of dorsal root ganglion neurons in a murine model of bone cancer pain. Neuroscience 2008;151:843–53. [DOI] [PubMed] [Google Scholar]
- [56].Yang Y, Li H, Li TT, Luo H, Gu XY, Lü N, Ji RR, Zhang YQ. Delayed activation of spinal microglia contributes to the maintenance of bone cancer pain in female Wistar rats via P2X7 receptor and IL-18. J Neurosci 2015;35:7950–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zagon IS, McLaughlin PJ. Opioid antagonists inhibit the growth of metastatic murine neuroblastoma. Cancer Lett 1983;21:89–94. [DOI] [PubMed] [Google Scholar]
- [58].Zagon IS, McLaughlin PJ. Opioids and differentiation in human cancer cells. Neuropeptides 2005;39:495–505. [DOI] [PubMed] [Google Scholar]
- [59].Zhao J, Zhang H, Liu SB, Han P, Hu S, Li Q, Wang ZF, Mao-Ying QL, Chen HM, Jiang JW, Wu GC, Mi WL, Wang YQ. Spinal interleukin-33 and its receptor ST2 contribute to bone cancer-induced pain in mice. Neuroscience 2013;253:172–82. [DOI] [PubMed] [Google Scholar]
- [60].Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. PAIN 1983;16:109–10. [DOI] [PubMed] [Google Scholar]