Supplemental Digital Content is Available in the Text.
A novel and potent protein kinase G-1α (PKG-1α) inhibitor is used to demonstrate the important roles of PKG in capsaicin-induced acute pain and in persistent inflammatory pain.
Key words: Protein kinase G, Inflammatory pain, TRPV1, CFA, Osteoarthritic pain, Long-term hyperexcitability, Chronic pain
Abstract
Activating PKG-1α induces a long-term hyperexcitability (LTH) in nociceptive neurons. Since the LTH correlates directly with chronic pain in many animal models, we tested the hypothesis that inhibiting PKG-1α would attenuate LTH-mediated pain. We first synthesized and characterized compound N46 (N-((3R,4R)-4-(4-(2-fluoro-3-methoxy-6-propoxybenzoyl)benzamido)pyrrolidin-3-yl)-1H-indazole-5-carboxamide). N46 inhibits PKG-1α with an IC50 of 7.5 nmol, was highly selective when tested against a panel of 274 kinases, and tissue distribution studies indicate that it does not enter the CNS. To evaluate its antinociceptive potential, we used 2 animal models in which the pain involves both activated PKG-1α and LTH. Injecting complete Freund's adjuvant (CFA) into the rat hind paw causes a thermal hyperalgesia that was significantly attenuated 24 hours after a single intravenous injection of N46. Next, we used a rat model of osteoarthritic knee joint pain and found that a single intra-articular injection of N46 alleviated the pain 14 days after the pain was established and the relief lasted for 7 days. Thermal hyperalgesia and osteoarthritic pain are also associated with the activation of the capsaicin-activated transient receptor protein vanilloid-1 (TRPV1) channel. We show that capsaicin activates PKG-1α in nerves and that a subcutaneous delivery of N46 attenuated the mechanical and thermal hypersensitivity elicited by exposure to capsaicin. Thus, PKG-1α appears to be downstream of the transient receptor protein vanilloid-1. Our studies provide proof of concept in animal models that a PKG-1α antagonist has a powerful antinociceptive effect on persistent, already existing inflammatory pain. They further suggest that N46 is a valid chemotype for the further development of such antagonists.
1. Introduction
Chronic pain afflicts millions in the United States and is especially serious because the most effective treatments contain opiates that can lead to addiction. There is strong evidence that many types of chronic pain, including that from neuritis,11 osteoarthritis (OA),30 colitis,4 cystitis,38 ischemia,7 and metastatic bone cancer34 are sustained by a long-term hyperexcitability (LTH) in the cell bodies of first-order neurons in the nociceptive pathway. The LTH, which initially appears in response to an injury or inflammation, results in persistent inputs to the CNS that are manifest as allodynia and hyperalgesia. The induction of the LTH depends on the activation of protein kinase G-1α (PKG-1α) in nociceptive neurons26,36,39,42,43,50 whose cell bodies reside in peripheral sensory ganglia and, since the LTH is an alteration in the phenotype, it can theoretically last indefinitely. Protein kinase G-1α is not present in motor neurons or glia,42 and studies of PKG null mice indicate that it is not involved in acute pain.46 The importance of PKG-1α in mediating persistent pain has been well documented by studies of hyperalgesia and allodynia in rodents.46 In particular, a direct link between PKG-1α and LTH was demonstrated using a nerve constriction model in the rat.39 The constriction induced LTH in dorsal root ganglion (DRG) neurons and thermal hyperalgesia 1 day later. Notable was that both the LTH and the hyperalgesia were blocked by the PKG-1 inhibitor Rp-8-pCPT-cGMPS (RPG). Furthermore, Liu et al.25 showed that RPG effectively reduced the hyperexcitability and significantly suppressed the hyperalgesia and allodynia that accompanies bone cancer in a rat model.
These findings strongly support the idea that an inhibitor of PKG-1α can alleviate pain associated with the LTH. In addition, since the neurons that contain the PKG-1α are in peripheral ganglia, the inhibitor need not enter the CNS. At present, there is no effective inhibitor of PKG-1α for drug development. Rp-8-pCPT-cGMPS and a similarly acting compound, KT5823, do not have high potency (Ki = 0.5 μM and Ki = 0.234 μM, respectively),8,18 are accompanied by nonspecific effects, and have very poor pharmacokinetic profiles.2,12 The oligopeptide inhibitor DT-2 and its D version (D)-DT2 are highly specific PKG inhibitors in vitro, but lose specificity for PKG in cell homogenates.13 We now describe the synthesis and characterization of N46, a member of a promising new class of potent, selective PKG-1α inhibitors and show that systemic and local delivery N46 alleviates 2 types of LTH-PKG-1α-mediated pain in rat models: inflammation-induced thermal hyperalgesia and knee joint pain from OA.
2. Materials and methods
2.1. Animals
Adult male Sprague-Dawley rats (250-300 g), obtained from Harlan Laboratories (Indianapolis, IN), were used for all experiments. Animals were housed 3 per cage and given water and food ad libitum. The room was kept at a constant temperature with a 12 hours alternating light/dark cycle. All animal handling and experimental procedures were approved by Institutional Animal Care And Use Committees of Geisinger Commonwealth School of Medicine and Columbia University.
2.2. Pain models
Inflammatory pain was induced by injecting 100 μL of complete Freund's adjuvant (CFA) (Sigma-Aldrich, St. Louis, MO) into the plantar surface of the hind paw to induce acute and persistent (chronic) inflammatory pain. Osteoarthritic pain was induced by injecting 50 μL of saline containing 3 mg MIA (Sigma-Aldrich) into the joint cavity as described.47 Capsaicin-associated pain was induced by intraplantar injection of 10 μg capsaicin.14,29
2.3. Compound administration
Rp-8-pCPT-cGMPS was obtained from EMD Chemicals Inc (Gibbstown, NJ) and suspended in saline for injection. N46, at >95% purity, was synthesized at Columbia University and by Schering-Plough Corp (Kenilworth, NJ).
For intrathecal administration, the compounds were suspended in a 1% DMSO/saline mix and injected in a volume of 15 μL at the L4/L5 levels using a disposable 30-gauge needle mated to a Hamilton microliter syringe (Hamilton, Reno, NV) followed by a saline flush. The injections were performed under isoflurane anesthesia and a tail flick indicated that the tip of the needle was inserted into the subarachnoid space. In other applications, N46 was dissolved in a quarter normal saline solution and used 1 mL for intravenous (iv), 20 μL for subcutaneous, and 50 μL for intra-articular injections.
2.4. Behavioral analysis
All data were collected using a double-blind protocol. To quantitatively assess the thermal sensitivity of the hind paw, rats were placed on the glass surface of a plantar testing apparatus (Hargreaves test, model 336; IITC Inc/Life Science Instruments, Woodland Hills, CA). The rats were allowed to acclimate for 30 minutes before testing. The temperature of the glass surface was maintained constant at 30°C. A mobile radiant heat source located under the glass was then focused onto the hind paw of each rat. The apparatus was adjusted at the beginning of the study, so that the baseline paw withdrawal latency in normal rats was approximately 10 seconds. This beam intensity was then kept unchanged throughout the study. A cutoff of 20 seconds was used to prevent potential tissue damage.
Mechanical sensitivity was tested by placing animals in plexiglass cages with a wire grid bottom and then stimulating their hind paws with a series of von Frey hairs of logarithmically increasing stiffness (0.4-15 g; Stoelting, Wood Dale, IL); each hair was presented perpendicularly to the plantar surface for approximately 6 seconds in either ascending or descending strength. Each trial started with a von Frey force of 2 g. If there was no withdrawal response, the next higher force was delivered. If there was a response, the next lower force was delivered. Based on the response pattern and the force of the final filament, the 50% paw withdrawal threshold was calculated according to a method described by Chaplan et al.9
An incapacitance tester (Harvard Apparatus, MA) was used to assess changes in weight distribution between the hind paws as a behavior measurement of osteoarthritic pain. Each animal was restrained in the testing chamber and each hind paw rested on a force plate. The force exerted by each paw is averaged over a 5-second period and each data point represents 3 trials. Results are presented as the percent difference between weight of treated hind limb and weight of vehicle-treated limb.
Following injection of small compounds, and prior to behavioral testing, all animals were weighed as a general measure of health and observed for abnormalities or deficits, such as reduced motion and sedation that might be caused by nonspecific drug effects.
2.4.1. In vitro kinase assays
Protein kinase G assays were performed in Tris buffer by quantitating the incorporation of 32P from [γ-32P] ATP. The reaction mixture (100 μL) contained 100 ng of purified bovine PKG-1α (Promega, Madison, WI), 5 μg of BPDE peptides, 25 mM Tris–HCl (pH 7.5), 5 mM glycerol phosphate, 2 mM DTT, 100 μM Na3VO4,10 mM MgCl2, and the tested compound at the indicated concentrations. The reaction, initiated by 10 μM [32P]-ATP, was incubated at 37°C for 20 minutes and terminated with 50 mM EDTA. The labeled peptides were captured on cellulose phosphate filters and quantified by counting in a β scintillation counter. For cAMP-dependent kinase (PKA) assays, the recombinant catalytic domain of human PKA and substrate kemptide were used. The reported IC50s and percentage inhibitions are single determinations in duplicates. All IC50s were determined by testing the compounds at 10 different concentrations ranging from 1 nM to 100 μM in duplicate.
2.5. Kinase panel screening
N46 was examined at 10 μM for their ability to inhibit 274 kinases using the kinaseProfiler enzyme profiling service (Millipore, Billerica, MA). Each kinase was assayed at its optimal ATP concentration according to the manufacturer's standard protocol.
2.6. Molecular modeling
A homology model of PKG-1α was prepared using Prime (Prime, version 1.6; Schrödinger, Inc, New York, NY, 2007) on the crystal structure of PKA (PDB code: 1BX6) using default settings. The virtual screen was performed using Glide (Glide, version 4.5; Schrödinger, Inc, New York, NY, 2007).
2.7. Analysis of plasma and tissue concentrations of N46
Concentrations of N46 were determined by liquid chromatography coupled with mass spectrometry (LC/MS) (2100A; Shimadzu Scientific Instruments, Kyoto, Japan). A C18 column (2.1 × 50 mm; SymmetryShield, Waters, Milford, MA) was used. The mobile phase composition was a gradient from acetonitrile-water (0:100, vol/vol) to acetonitrile-water (100:0, vol/vol) in 20 minutes. An aliquot of 10 μL was injected into the high-performance liquid chromatography (HPLC) system; N46 was eluted at 10.99-minute and detected at the wavelength of 220 nm.
The concentration of N46 in plasma after iv injection was determined by adding150 μL of acetonitrile to 50 μL of plasma. After centrifugation, the supernatant was used for injection onto the LC/MS system. Tissues were homogenized in 0.1N sodium carbonate (2 mL), followed by addition of methanol (6 mL). The mixture was mixed and centrifuged, and the supernatant was collected for LC/MS analysis. All results were corrected for % recovery using standard curves obtained by adding acetonitrile to extracts containing increasing amounts of N46.
2.8. Statistical analyses
Data were expressed as means ± SEM. A 2-tailed unpaired t test was used with a significance level set at 0.05 to compare 2 groups. A 1-way ANOVA was used to assess multiple changes between groups followed by Dunnett's post hoc test. A 2-way ANOVA followed by Bonferroni posttest was used to assess the effect of N46 over time in the mechanical sensitivity test.
3. Results
3.1. Rp-8-pCPT-cGMPS inhibits inflammatory thermal hyperalgesia
To determine a contribution for PKG-1α in thermal hyperalgesia, we injected CFA into the plantar surface of the hind paw in rats. Complete Freund's adjuvant causes intense and persistent pain in humans,15 and this well-defined protocol results in a thermal hyperalgesia that is easily quantified by measuring a paw withdrawal latency (PWL) in response to heat.31 The next day all of the injected rats exhibited swelling and redness associated with inflammation and the thermal test showed profound thermal hyperalgesia. The rats were then given a single injection of either vehicle or 100, 125, or 250 nmol RPG into the intrathecal space adjacent to the L4/L5 DRG that house the cell bodies of the neurons that innervate the paw (Fig. 1). Using a double-blind protocol, we measured the PWL the following day and found that the thermal hyperalgesia had been alleviated significantly by the 250 nmol RPG compared to the vehicle control (Fig. 1A). There were no significant changes in the thermal threshold on the contralateral noninflamed paw (Fig. 1B). These results are consistent with the idea that PKG-1α is a significant component of an inflammation-induced thermal hyperalgesia. As reported previously,39,45 there were no differences between the rats injected with RPG and the controls with regard to eating, drinking, digestion or control of motor movements and exploring.
Figure 1.
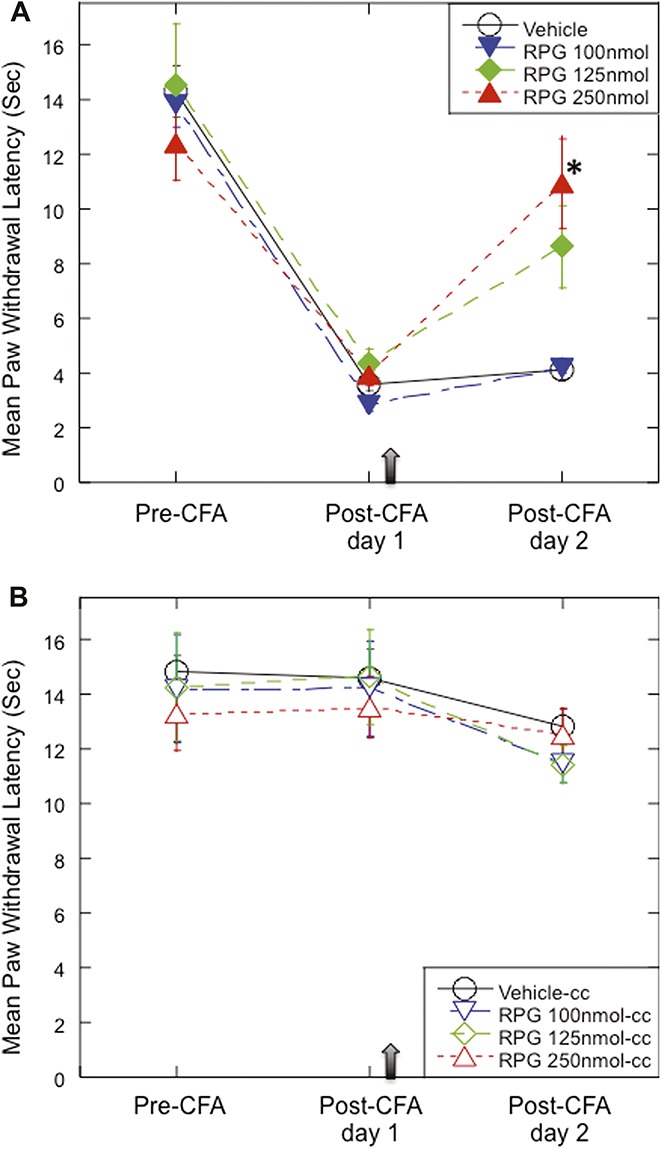
Inhibiting protein kinase G-1 (PKG-1) reduces complete Freund's adjuvant (CFA)-induced thermal hyperalgesia. Paw withdrawal latency (PWL) to heat stimuli was assessed (pre-CFA) and then 100 ul CFA was injected into the plantar surface of the right hind paw. One day later (post-CFA day 1), when the rats exhibited thermal hyperalgesia, Rp-8-pCPT-cGMPS (RPG) (100, 125, and 250 nmol, n = 6, 6, and 10, respectively) or the vehicle control (saline, n = 10) was administered intrathecally as indicated by arrow. Paw withdrawal latency was measured again the following day (post-CFA day 2). (A) Data (mean ± SEM) indicated that injection of 250 nmol RPG resulted in a significant improvement in mean PWL compared to the vehicle control (P = 0.001). (B) Mean PWL in the contralateral hind paw.
3.2. Synthesis of a novel, selective, and stable protein kinase G-1α inhibitor
To firmly establish a role for PKG-1α in chronic pain required the development of a potent and selective inhibitor of this kinase. What follows is a brief description of how this was accomplished. The details of the chemical syntheses have been submitted elsewhere.
Protein kinase G is a member of the Ser/Thr AGC family of kinases and (−)-balanol (Fig. 2A), a product of the fungus Verticillium balanoides, is a highly potent, but nonselective inhibitor of these kinases23 with a very short half-life in serum due to the presence of ester linkages. Balanol acts by binding to the ATP site and a comparison of amino acid sequences at the ATP domain of several kinases showed that the sequence in the α and β type-1 PKG isoforms is the same, but that it differs from that in PKA, PKB, and PKC. We therefore compared the crystal structure of balanol bound to the ATP pocket in PKA (PDB code: 1BX632) to a homology model of balanol bound to PKG-1α (Fig. 2A). Significantly, several of the amino acids juxtaposed to balanol in PKG-1α were different in PKA (see legend to Fig. 2A) and these differences are conserved in PKB-β, PKC-theta, and many other kinases.
Figure 2.
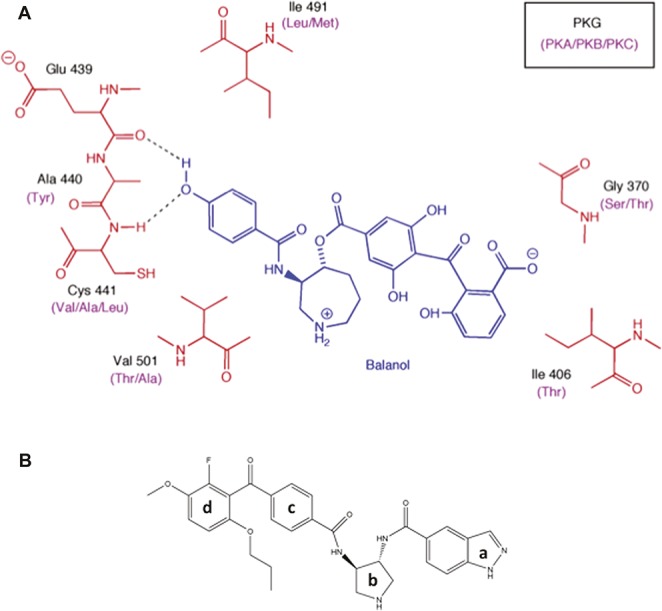
Structural modeling of the PKG kinase domain with balanol. (A) 2D projection of balanol (blue) bound to PKG and PKA/B/C. The black labels indicate the amino acids in the binding site for PKG which differ from the corresponding amino acids (purple labels) in PKA, PKB, and PKC. (B) N46 consists of an indazole group (a) connected to a pyrrolidine ring (b) via an amide linkage. The pyrrolidine is linked to a benzophone ring (c) by an amide and the latter is attached via a ketone to a terminal benzophone ring (d).
Guided by these observations, we systematically modified balanol and each derivative was assayed against recombinant active PKG-1α and PKA. We used PKA in the initial assays as a convenient surrogate for the other Ser/Thr kinases. In parallel, we performed a virtual screen of a library consisting of 1.3 million commercially available, drug-like compounds. After many rounds of structural activity relationship studies (detailed data to be published elsewhere), we synthesized a lead compound, N46 (Fig. 2B), which is a hybrid of a balanol and a small compound that was discovered from the virtual screen. Comparing Figures 2A and B, the phenol in balanol was replaced with an indazole group and the ester linkages between the rings in balanol were replaced by amides to protect the compound from degradation by serum esterases (see below). Additionally, Ile406 in PKG-1α is replaced by a polar residue (Thr88) in PKA and, based on our docking models, this group should have favorable hydrophobic interactions with Ile406 in PKG-1α and unfavorable interactions with Thr88 in PKA. We therefore added a propoxy group to the 6-position of the external phenyl ring of the benzophenone moiety (Ring d, Fig. 2B) to improve potency and selectivity. Thus, compound N46 has an IC50 for PKG-1α and PKA of 7 nM and 5 μM, respectively, a 714-fold difference. Kinetic studies showed that N46 is a noncompetitive inhibitor of PKG-1α (data not shown).
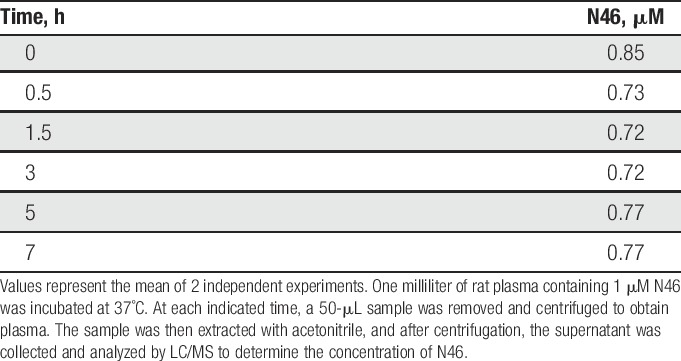
To further assess its selectivity, N46 was tested at 10 μM against a panel of 274 kinases that included representatives of all of the kinome branches. Each kinase was assayed at its optimal ATP concentration (Supplementary Table 1, available online at http://links.lww.com/PAIN/A379). The α and β isoforms of PKG-1 have the same sequence at their ATP binding site and N46 was equally effective against both. Protein kinase G-1β is not present in nerves.42 In contrast, N46 was ineffective against the vast majority of the other kinases. We then extended these studies by assaying at 4 lower concentrations the human kinase isoforms most affected by N46 (Table 1). As predicted from the models, the Ser/Thr kinases PKB and PKC were not inhibited to anywhere near the extent as PKG-1α. In addition, at a concentration of 750 nM, where PKG-1α activity is completely blocked, only 12 kinases were inhibited more than 50% by N46 (Table 1) and only one, AMPK, has been linked to nociception (see discussion). Replacing the ester linkages in balanol with amides in N46 had the anticipated effect in that N46 is stable in plasma for at least 7 hours (Table 2).
Table 1.
Kinases inhibited by N46.
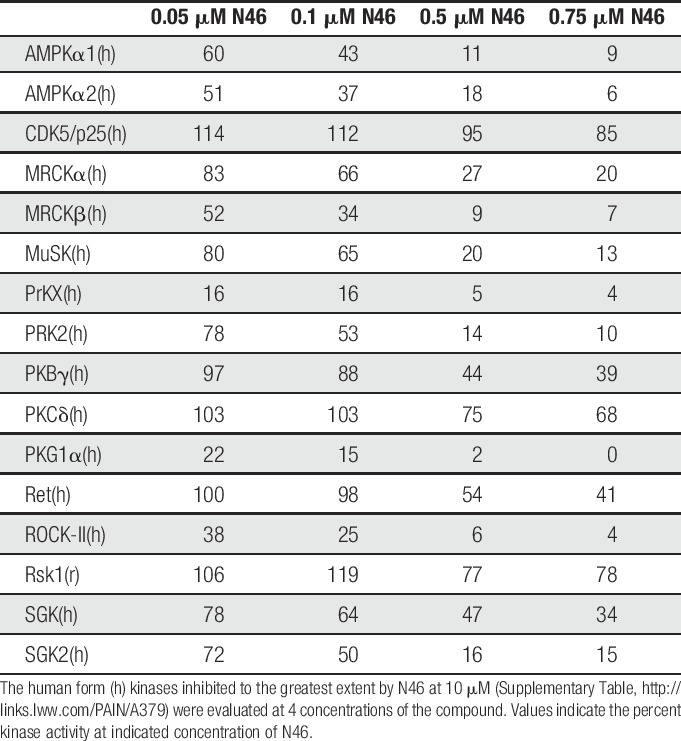
Table 2.
Stability of N46 in plasma in vitro.
3.3. N46 blocks inflammatory thermal hyperalgesia
To assess the effectiveness of N46 in alleviating inflammation-induced thermal hyperalgesia, we repeated the CFA experiment described above. Intrathecal injection of 0.25 nmol N46 resulted in a marked reduction in the inflammation-induced thermal hyperalgesia compared to the vehicle (Fig. 3A). There were no significant changes in the thermal threshold on the contralateral noninflamed paw (Fig. 3B). In addition, N46 was effective at a much lower dosage than the RPG (Fig. 1), which correlates directly with their ability to inhibit PKG-1α. Since intrathecal injection is not a preferred method of drug delivery, we tested the efficacy of N46 following intravenous injection. The day after CFA injection, when PWL measurements indicated the presence of thermal hyperalgesia, we injected either 1 nmol N46 or vehicle into the tail vein. Twenty-four hours later, the N46 had significantly increased the PWL, but the vehicle did not (Fig. 3C). The N46 did not alter baseline nociception since there were no changes in the thermal responsiveness of the contralateral hind paw (Fig. 3B and D). Significantly, a single injection of N46 provided relief for at least 24 hours, whereas many analgesics that have been tested in this model are short lasting28,31
Figure 3.
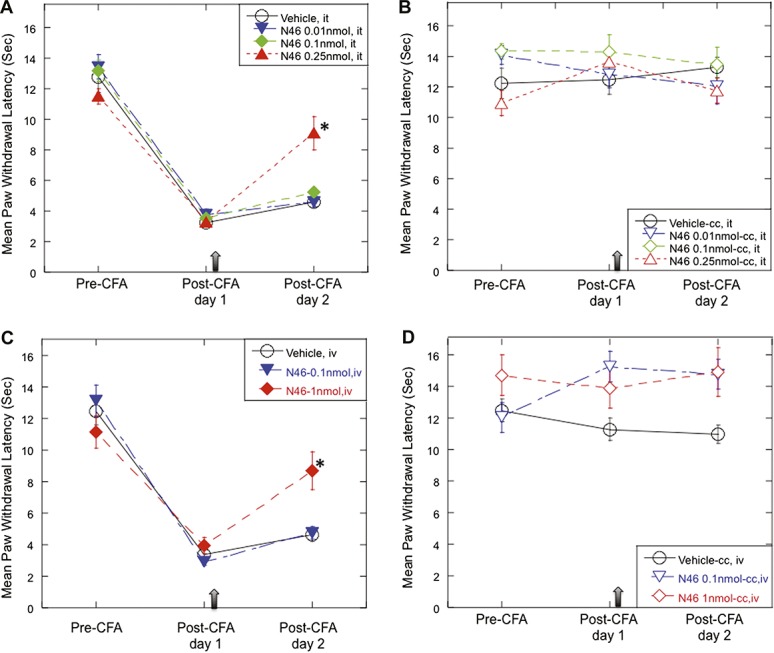
N46 attenuates inflammation-induced thermal hyperalgesia. (A) One day after CFA injection, N46 (0.01, 0.1, and 0.25 nmol; n = 6, 6, and 11, respectively) was administered intrathecally (arrow). One day later (post-CFA day 2), the rats injected with 0.25 nmol N46 exhibited a significant decrease in heat hyperalgesia compared to the vehicle control (0.1% DMSO in quarter saline, n = 11); P = 0.0007. (B) The mean PWL in the contralateral hind paw. (C) One day after administering CFA, N46 (0.1, and 1 nmol; n = 12 and 7, respectively) was injected into the tail vein (arrow). The next day PWL measurements showed that 0.25 nmol N46 had significantly reduced the heat hyperalgesia compared to the vehicle control (1% DMSO/quarter saline, n = 9); P = 0.0033. (D) The mean withdrawal latency in the contralateral hind paw. Data represent mean ± SEM.
3.4. N46 attenuates pain in a rat model of chronic osteoarthritis
Studies have shown that OA pain correlates with the presence of LTH.30 To determine whether N46 can alleviate the pain of OA, we used a rat model in which the OA is induced by injecting monosodium iodoacetate (MIA) into the knee joint.5,17,24 The pain that develops at the joint causes impaired weight bearing on the inflamed limb that can last for more than 28 days.5,17,24 Monosodium iodoacetate was injected and 14 days later, when the rats clearly showed evidence of pain at the injected joint, we injected 5 nmol N46 or the vehicle control into the intra-articular space. Subsequent testing showed a significant improvement in weight bearing distribution on the 15th and 21nd days relative to the controls (Fig. 4). These results were promising because N46 acted 14 days after the pain was established and the single injection provided relief for 7 days.
Figure 4.
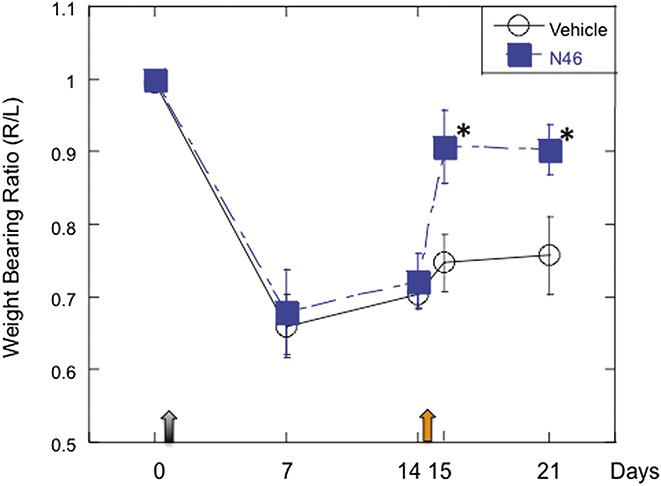
Local injection of N46 reduces osteoarthritic pain. Baseline measurements of weight bearing on the right and left hind limbs were assessed (day 0) and then the rats were injected with 3 mg of monosodium iodoacetate (MIA) into the right knee and saline in the left knee (grey arrow). Fourteen days later, a significant weight-bearing difference was observed between the right and left hind limbs P < 0.0001, indicating osteoarthritis (OA) pain. N46 (5 nmol) or vehicle control (quarter saline) was then administrated into the intra-articular space of the MIA-injected joints (orange arrow). Subsequent evaluation showed that the N46 significantly reversed the MIA-induced shift in weight bearing both 1 day (day 15) and 7 days (day 21) after N46 injection (n = 15) compared to corresponding vehicle treatment (n = 6), P = 0.022 and 0.038, respectively. Data represent mean ± SEM.
3.5. Distribution of N46 in vivo
N46 did not affect the contralateral leg in these experiments and there was nothing to indicate problems with digestion or bladder function and no evidence of sedation. Nevertheless, the isoforms of PKG are present in many tissues. To begin to determine the fate of N46 in vivo, we injected N46 into the tail vein of rats and then collected tissue samples for analysis (Table 3). Most of the N46 was rapidly excreted and by 20 hours the initial amount in the major organs was markedly reduced. Very important was the finding of stable levels of N46 in the DRG because they contain the neuronal cell bodies that exhibit the LTH and the activated PKG-1α. N46 was present in the heart 10 minutes after injection, but was not detected after 20 hours (Table 3). Nevertheless, before further developing the N46 chemotype, we wanted a preliminary early assessment of potential problems with heart function.16 We therefore obtained baseline measurements of the heart rate and systolic and diastolic blood pressure for 30 minutes, injected 1uM N46 and then monitored these functions over the subsequent 30 minutes. There was no significant effect on any of these parameters (data not shown). We also examined the potential effects on the cardiac hERG potassium channel. Interference with this channel can cause type-1 Long QT syndrome.40 hERG tail currents were measured at 6 concentrations of N46 and dose-dependent inhibition of the hERG current yielded a calculated IC50 of 4.47 μM (Fig. 5), which is more than 2 orders of magnitude greater than the IC50 for PKG-1α. These studies suggest that a therapeutic dose of N46 does not affect the heart rhythm in the short term.
Table 3.
Tissue distribution of N46 in vivo.
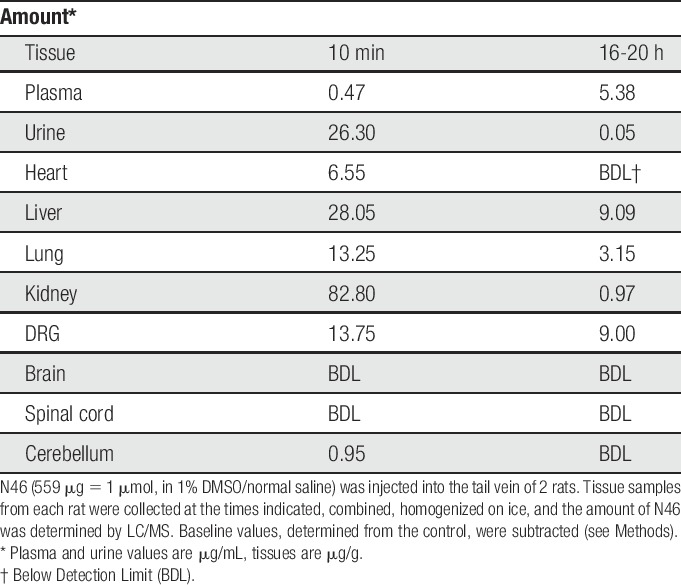
Figure 5.
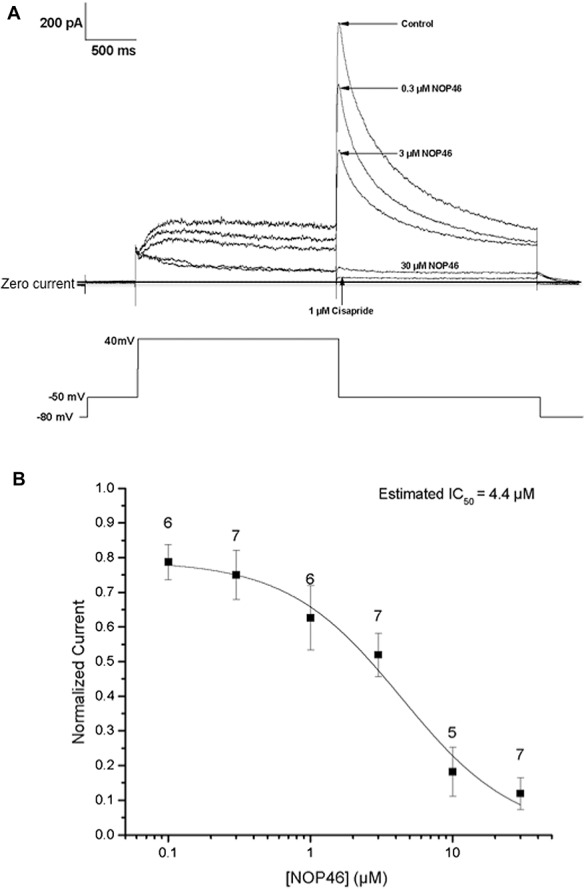
The effect of N46 on the amplitude of hERG tail currents. (A) The voltage protocol (lower panel) was applied to the cell once every 10 seconds. Recordings were obtained in the presence of control solution and after the addition of 0.1, 0.3, 1.0, 3, 10 and 30 μM N46 and the positive control, 1 μM cisapride-evoked hERG tail currents were continuously monitored throughout the experiment. Each concentration of N46 was added to each cell for 5 minutes and the next dose was cumulatively applied and sample responses to 3 of the concentrations and the control are shown. (B) A 6-point dose response was collated from 13 individual cells. Each point represents the mean percent inhibition at each concentration. Error bars indicate the mean ± SEM.
3.6. N46 alleviates pain elicited by capsaicin
There is evidence that OA pain and the inflammation-induced thermal hyperalgesia involve the activation of the cation channel transient receptor protein vanilloid-1 (TRPV1).21 Since N46 blocks these pains, there might be a connection between PKG-1α and the TRPV1. Capsaicin, a selective activator of the TRPV1, causes an acute thermal hyperalgesia and allodynia when injected into the hind paw of the rat.14 To investigate whether inhibiting PKG-1α attenuates capsaicin-induced hypersensitivity, we injected the hind paw with capsaicin alone (10 μg), N46 alone (10 nmol), or N46 (10 nmol), followed immediately by capsaicin (Fig. 6). The capsaicin alone dramatically decreased the mechanical threshold, as a measure of allodynia, whereas N46 alone had no effect (Fig. 6A). In contrast, injection of N46 and capsaicin significantly attenuated the allodynia, as indicated by the increased mechanical threshold measured 5, 10, 30, and 60 minutes later relative to capsaicin alone (Fig. 6A). Additionally, co-injecting N46 effectively reduced the capsaicin induced thermal sensitivity, as shown by the increase of withdrawal latency to heat at 5-minute post-injection compared to capsaicin alone (Fig. 6B).
Figure 6.
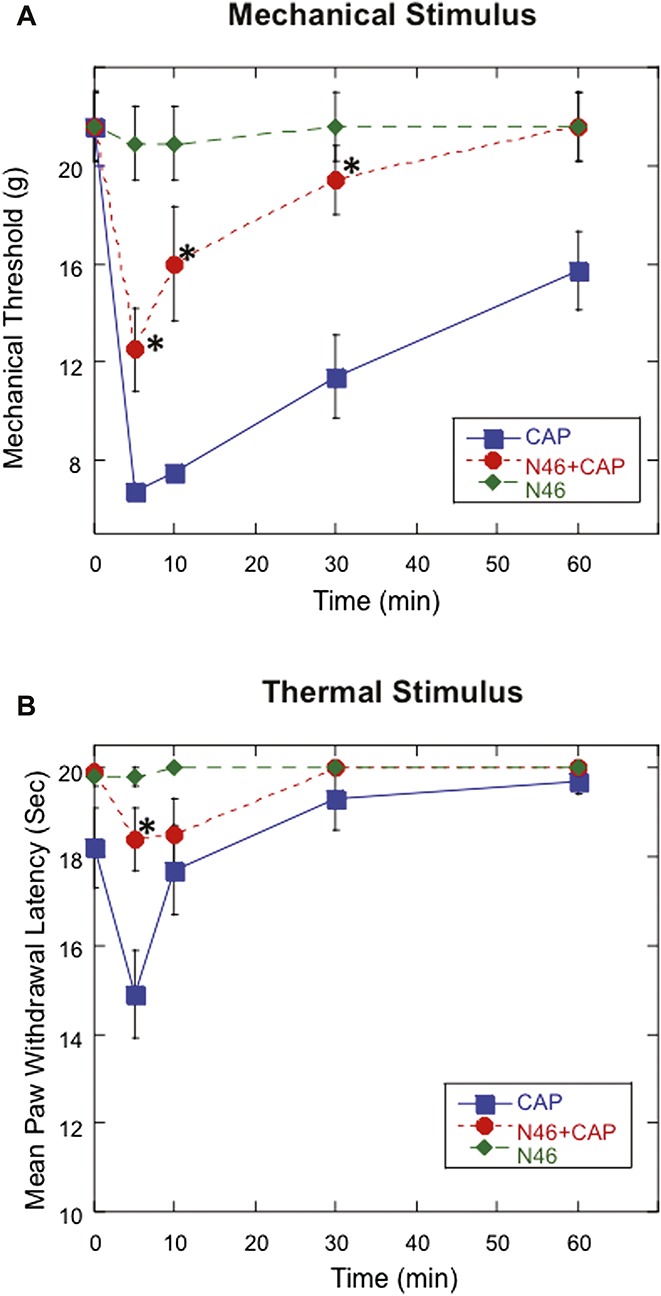
Local treatment of N46 inhibits acute nociceptive response elicited by intraplantar injection of capsaicin. Mechanical thresholds for paw withdrawal were determined at designated time points after injecting either 10 μg capsaicin, 10 nmol N46, or 10 nmol N46 immediately followed by 10 μg capsaicin. (A) Injecting both N46 and capsaicin significantly increased mechanical threshold measured at 5, 10, 30, and 60 minutes later when compared to capsaicin treatment only (*P = 0.0059, <0.0001, 0.0002 and 0.0049, respectively; n = 15 in each group). (B) Injection of both N46 and capsaicin also produced a significant increase in withdrawal latency 5 minutes later at postinjection 5 minutes compared to the capsaicin alone (*P < 0.0001). Data represent mean ± SEM.
We wanted to see whether the activation of the TRPV1 by capsaicin also activates PKG-1α, but could not do this directly because the nerves in the hind paw are minor constituents among heterogeneous tissues. Instead, we took advantage of studies showing that receptors for agents that elicit inflammatory pain at terminals are functional in axons.48 We injected capsaicin (0.1 μM) directly under the perineurium of the sciatic nerve and an equal volume of vehicle into the contra-lateral nerve as a control. Thirty minutes later the 1 cm nerve segment containing the injection site from each nerve was collected, homogenized, and samples containing an equal amount of protein were assayed for PKG-1α activity. We found that 40 ± 15% of the PKG-1α in the nerve segment injected with capsaicin was active vs only 1.3 ± 0.5% in the control (Fig. 7). The minimal PKG-1α activation in the control indicated that there was little or no damage to axons by the injection procedure. Since PKG-1α is not present in glial cells or motor axons,42 these data indicate that capsaicin binding to TRPV1 in axons activates PKG-1α.
Figure 7.
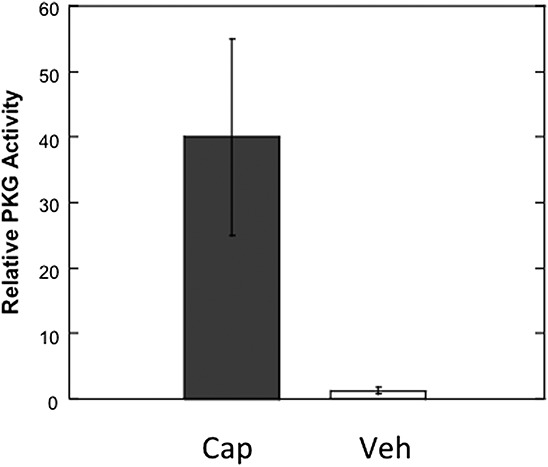
The activation of axonal PKG by capsaicin. Capsaicin (0.1 μM) was locally injected under the perineurium of the sciatic nerve. An equal volume of vehicle was administered into the contralateral nerve. Thirty minutes later, the 1 cm nerve segment containing the injection site from each nerve was harvested, homogenized, and assayed for PKG-1α activity. PKG activity appeared in the capsaicin-treated nerve segment (dark grey bar), but not in the vehicle treated nerve (open bar), (P < 0.05; N = 4). Data represent mean ± SEM.
4. Discussion
4.1. Protein kinase G-1α is an important target for chronic pain
Most efforts to alleviate chronic pain have focused on the long-term potentiation (LTP) that appears at the synapse between the first and second order neurons.20,35 However, the LTP is maintained by action potentials from the site of the lesion and typically diminishes within a day or 2 as the lesion heals.37,49 Consequently, LTP appears to mediate acute rather than chronic pain. In contrast, long lasting pain arises in response to lesions that activate molecular signals.1,41,42 These signals alter the phenotype of the affected neurons to promote repair and maintain an awareness of the injured site during the long recuperation. Among these is the nociceptive signal PKG-1α. Protein kinase G-1α has low basal activity in axons, but when activated at the site of an injury or inflammation is transported retrogradely to the cell bodies of the neurons where it initiates changes in gene expression that result in LTH.42,43 Because the LTH arises from a phenotypic change in the electrophysiological properties of the neurons, it can persist for a very long time. The finding that the PKG-1α inhibitor, RPG, effectively attenuated radicular pain,39 bone cancer pain,25 and inflammatory thermal hyperalgesia (Fig. 1) indicates that a clinically relevant inhibitor of PKG-1α would be beneficial in alleviating the many types of persistent pain associated with LTH. Moreover, since PKG-1α is located in ganglia in the periphery, the inhibitor need not enter the CNS and thereby avoids potential side effects such as drug tolerance and sedation.
4.2. Synthesis of N46, a potent and selective inhibitor of Protein kinase G-1
N46 represents a new class of PKG inhibitors that was obtained by modifying (−)-balanol, a potent, but non-selective inhibitor of Ser/Thr kinases. The ATP pocket in PKG differs from that of other members of this kinase family and our computer-based models allowed us to design derivatives of (−)-balanol that would fit into the ATP site of PKG, but not that of other kinases. N46, differs from (−)-balanol in being much more selective and more stable in serum (Tables 1 and 2). Selectivity is a significant issue because kinases have essential roles in many cell types. We therefore evaluated the selectivity of N46, first against a panel of 274 kinases (Supplemental Table 1, available online at http://links.lww.com/PAIN/A379). and then by examining in greater detail the 16 human kinases that were most affected by N46 (Table 1). Only 12 of the latter, which included isoforms, were inhibited by more than 50% at a dose of N46 where PKG-1 was inhibited 100%. An off-target hit rate of 4.4% (12 out of 274) makes N46 a highly selective PKG-1 inhibitor.
The finding that N46 was effective against AMPK was interesting because this kinase contributes to nociception and the development of hyperexcitability after nerve injury.27 However, the response to nerve injury is far more global than that to inflammation, due to the need to promote repair. Whether AMPK is also activated by an inflammation warrants investigation. As to why N46 recognizes AMPK, our homology models show that N46 selectivity for PKG-1 can be attributed to the hydrophobicity around the terminal phenone distal to the hinge region and it turns out that AMPK has very similar residues in that region. There is, however, an alanine in the hinge region in PKG-1 that is not present in AMPK and it should be possible to take advantage of this difference to design a derivative of N46 that will discriminate between the 2 kinases.
N46 is an effective analgesic for inflammatory pain associated with activated PKG-1α and the presence of LTH.
Both N46 and RPG alleviated the thermal hyperalgesia that appears after injecting CFA into the hind paw (Figs. 1 and 3A), thereby validating a connection between PKG-1α and thermal hyperalgesia. However, N46 was effective when delivered via injection into the intrathecal space or the tail vein and also blocked pain that was already present for 2 days, indicating that it was acting on the mechanism responsible for persistent pain. We also showed that N46 was effective in attenuating the pain associated with OA. Osteoarthritis was selected because of its association with LTH.30 An intra-articular injection of monosodium iodoacetate in rats produces joint pathology that resembles human OA, including ulceration, fibrillation, loss of proteoglycan in cartilage tissue, exposure of subchondral bone17,22 as well as a persistent pain that lasts for weeks.24,47 Fourteen days after confirming a significant shift of weight bearing from the monosodium iodoacetate-injected limb to the contralateral limb, we injected 5 nmol N46 into the intra-articular space. One day later, there was a significant improvement in weight bearing asymmetry and the effect lasted for 7 days (Fig. 4). These results are promising because they show that a single dose of N46 not only attenuated the pain, but it did so well after the pain was established and that a single injection was effective for 7 days.
N46 was effective in both models, but we do not know in either whether PKG-1α was chronically active in response to the lesion, or whether it was being continuously activated due to the persistent presence of inflammatory agents.42–44 Regardless, N46 will be effective in either circumstance because it both blocks the activation of PKG-1α and inhibits the already activated kinase.
4.3. Studies in vivo
N46 did not affect the contralateral leg in our experiments (Fig. 3C and D), indicating that its effects are not global, nor did it affect motor control, which is consistent with the fact that PKG-1α is absent from motor neurons.42 To get an idea of how N46 is distributed among the tissues in the rat, N46 was injected and tissues were removed and assayed at 10 minutes and 20 hours later (Table 3). N46 is widely distributed by 10 minutes, but is largely excreted and its level in most tissues has declined by 20 hours (Table 3). N46 was not detected in the CNS, which would explain the absence of sedation in the rats injected with N46, but was present in the DRG in our experiments. The latter is significant because the DRG house the neuronal cell bodies that contain the activated PKG-1α and exhibit the LTH. The finding that N46 was present in the heart within 10 minutes prompted us to follow a recent recommendation to evaluate potential heart problems early, rather than later in drug development.16 Consequently, we tested several parameters of heart function from the time of N46 injection and continuously for 30 minutes and found no evidence of short-term affects. These findings are encouraging, but preliminary and more extensive and longer-term assessments will be necessary as the development of N46 as an antinociceptive agent progresses.
4.4. Protein kinase G and the transient receptor protein vanilloid-1 channel
Studies of OA in humans showed an increase in TRPV1 immunoreactivity in synovium21 and studies in rats indicated that TRPV1 antagonists can reduce the pain of OA.10,19 Unfortunately, these antagonists have limited use because they induce hyperthermia.6,28 It is significant, therefore, that several lines of evidence support the idea that the activation of the TRPV1 is mediated by PKG-1α: First, both the TRPV1 and PKG-1α are co-localized in lightly myelinated C-type nociceptive neurons.42 Second, the levels of TRPV1 protein increase in bone cancer,33 as does the activity and level of PKG-1α. Third, capsaicin binds selectively to the TRPV1 and we found, consistent with work by others,14,29 that injecting capsaicin into the hind paw elicits an acute allodynia (Fig. 6A) and a transient thermal hypersensitivity (Fig. 6B) that are both effectively attenuated by N46 (Fig. 6). Fourth, capsaicin stimulates cyclic GMP (cGMP) production via nitric oxide (NO) in DRG neurons,3 which is the cascade that activates PKG-1α. Lastly, when we injected capsaicin under the perineurium of the sciatic nerve, there was a significant activation of PKG-1α relative to controls (Fig. 7). Consequently, the ability of N46 to alleviate capsaicin-induced mechanical and thermal hypersensitivities suggests that inhibiting PKG-1α could extend beyond pain associated with LTH and might alleviate inflammatory pain that arises in response to the TRPV1.
5. Conclusion
We have demonstrated that N46 is an effective antinociceptive agent in rat models of inflammatory chronic pain. Two of the results are particularly promising: N46 attenuated pain that was already well established, and, as shown in the OA model, a single dose of N46 could alleviate pain for a long period, suggesting that long-term exposure to N46 might not be necessary. Therefore, we have provided proof of principle that a clinically acceptable form of N46 has the potential to be an important treatment for some types of chronic inflammatory pain.
Conflict of interest statement
The authors have no conflict of interests to declare.
Supported by the Arlene & Arnold Goldstein Family Foundation, and the Commonwealth Medical College.
Supplementary Material
Acknowledgements
The authors would like to thank Drs. Zhengxiang Zhu, Serge Cremers, and Tiffany Thomas for their help in acquiring the LC/MS/MS data for Tables 2 and 3. Y.-J. Sung, S. Deng, Y. Xie, J. Greenwood, R. Farid, D. W. Landry, and R. T. Ambon are listed as coinventors on a patent, US8846742B, assigned to The Trustees of Columbia University in the City of New York. The patent is not licensed and the authors have no conflict of interests to declare.
Appendix A. Supplemental Digital Content
Supplemental Digital Content associated with this article can be found online at http://links.lww.com/PAIN/A379.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
References
- [1].Ambron RT, Zhang XP, Gunstream JD, Povelones M, Walters ET. Intrinsic injury signals enhance growth, survival, and excitability of Aplysia neurons. J Neurosci 1996;16:7469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J 2003;371(pt 1):199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bauer MB, Murphy S, Gebhart GF. Stimulation of cyclic GMP production via a nitrosyl factor in sensory neuronal cultures by algesic or inflammatory agents. J Neurochem 1995;65:363–72. [DOI] [PubMed] [Google Scholar]
- [4].Beyak MJ, Ramji N, Krol KM, Kawaja MD, Vanner SJ. Two TTX-resistant Na+ currents in mouse colonic dorsal root ganglia neurons and their role in colitis-induced hyperexcitability. Am J Physiol Gastrointest Liver Physiol 2004;287:G845–855. [DOI] [PubMed] [Google Scholar]
- [5].Bove SE, Calcaterra SL, Brooker RM, Huber CM, Guzman RE, Juneau PL, Schrier DJ, Kilgore KS. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthritis Cartilage 2003;11:821–30. [DOI] [PubMed] [Google Scholar]
- [6].Brederson JD, Kym PR, Szallasi A. Targeting TRP channels for pain relief. Eur J Pharmacol 2013;716:61–76. [DOI] [PubMed] [Google Scholar]
- [7].Bulka A, Hao JX, Wiesenfeld-Hallin Z. Response characteristics of cutaneous mechanoreceptors in neuropathic rats. Neurosci Lett 2002;317:89–92. [DOI] [PubMed] [Google Scholar]
- [8].Butt E, Eigenthaler M, Genieser HG. (Rp)-8-pCPT-cGMPS, a novel cGMP-dependent protein kinase inhibitor. Eur J Pharmacol 1994;269:265–8. [DOI] [PubMed] [Google Scholar]
- [9].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53:55–63. [DOI] [PubMed] [Google Scholar]
- [10].Chu KL, Chandran P, Joshi SK, Jarvis MF, Kym PR, McGaraughty S. TRPV1-related modulation of spinal neuronal activity and behavior in a rat model of osteoarthritic pain. Brain Res 2011;1369:158–66. [DOI] [PubMed] [Google Scholar]
- [11].Eliav E, Herzberg U, Ruda MA, Bennett GJ. Neuropathic pain from an experimental neuritis of the rat sciatic nerve. PAIN 1999;83:169–82. [DOI] [PubMed] [Google Scholar]
- [12].Fouty B, Komalavilas P, Muramatsu M, Cohen A, McMurtry IF, Lincoln TM, Rodman DM. Protein kinase G is not essential to NO-cGMP modulation of basal tone in rat pulmonary circulation. Am J Physiol 1998;274(2 pt 2):H672–678. [DOI] [PubMed] [Google Scholar]
- [13].Gambaryan S, Butt E, Kobsar A, Geiger J, Rukoyatkina N, Parnova R, Nikolaev VO, Walter U. The oligopeptide DT-2 is a specific PKG I inhibitor only in vitro, not in living cells. Br J Pharmacol 2012;167:826–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gilchrist HD, Allard BL, Simone DA. Enhanced withdrawal responses to heat and mechanical stimuli following intraplantar injection of capsaicin in rats. PAIN 1996;67:179–88. [DOI] [PubMed] [Google Scholar]
- [15].Gould HJ., III Complete Freund's adjuvant-induced hyperalgesia: a human perception. PAIN 2000;85:301–3. [DOI] [PubMed] [Google Scholar]
- [16].Guth BD. Preclinical cardiovascular risk assessment in modern drug development. Toxicol Sci 2007;97:4–20. [DOI] [PubMed] [Google Scholar]
- [17].Guzman RE, Evans MG, Bove S, Morenko B, Kilgore K. Mono-iodoacetate-induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: an animal model of osteoarthritis. Toxicol Pathol 2003;31:619–24. [DOI] [PubMed] [Google Scholar]
- [18].Hidaka H, Kobayashi R. Pharmacology of protein kinase inhibitors. Annu Rev Pharmacol Toxicol 1992;32:377–97. [DOI] [PubMed] [Google Scholar]
- [19].Honore P, Chandran P, Hernandez G, Gauvin DM, Mikusa JP, Zhong C, Joshi SK, Ghilardi JR, Sevcik MA, Fryer RM, Segreti JA, Banfor PN, Marsh K, Neelands T, Bayburt E, Daanen JF, Gomtsyan A, Lee CH, Kort ME, Reilly RM, Surowy CS, Kym PR, Mantyh PW, Sullivan JP, Jarvis MF, Faltynek CR. Repeated dosing of ABT-102, a potent and selective TRPV1 antagonist, enhances TRPV1-mediated analgesic activity in rodents, but attenuates antagonist-induced hyperthermia. PAIN 2009;142:27–35. [DOI] [PubMed] [Google Scholar]
- [20].Ikeda H, Stark J, Fischer H, Wagner M, Drdla R, Jager T, Sandkuhler J. Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science 2006;312:1659–62. [DOI] [PubMed] [Google Scholar]
- [21].Kelly S, Chapman RJ, Woodhams S, Sagar DR, Turner J, Burston JJ, Bullock C, Paton K, Huang J, Wong A, McWilliams DF, Okine BN, Barrett DA, Hathway GJ, Walsh DA, Chapman V. Increased function of pronociceptive TRPV1 at the level of the joint in a rat model of osteoarthritis pain. Ann Rheum Dis 2015;74:252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kobayashi K, Imaizumi R, Sumichika H, Tanaka H, Goda M, Fukunari A, Komatsu H. Sodium iodoacetate-induced experimental osteoarthritis and associated pain model in rats. J Vet Med Sci 2003;65:1195–9. [DOI] [PubMed] [Google Scholar]
- [23].Koide K, Bunnage ME, Gomez Paloma L, Kanter JR, Taylor SS, Brunton LL, Nicolaou KC. Molecular design and biological activity of potent and selective protein kinase inhibitors related to balanol. Chem Biol 1995;2:601–8. [DOI] [PubMed] [Google Scholar]
- [24].Liu P, Okun A, Ren J, Guo RC, Ossipov MH, Xie J, King T, Porreca F. Ongoing pain in the MIA model of osteoarthritis. Neurosci Lett 2011;493:72–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liu S, Zhang MY, Chen LP, Liu YP, Liu GJ. cGMP and cGMP-dependent protein kinase I pathway in dorsal root ganglia contributes to bone cancer pain in rats. Spine (Phila Pa 1976) 2014;39:1533–41. [DOI] [PubMed] [Google Scholar]
- [26].Luo C, Gangadharan V, Bali KK, Xie RG, Agarwal N, Kurejova M, Tappe-Theodor A, Tegeder I, Feil S, Lewin G, Polgar E, Todd AJ, Schlossmann J, Hofmann F, Liu DL, Hu SJ, Feil R, Kuner T, Kuner R. Presynaptically localized cyclic GMP-dependent protein kinase 1 is a key determinant of spinal synaptic potentiation and pain hypersensitivity. Plos Biol 2012;10:e1001283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Melemedjian OK, Asiedu MN, Tillu DV, Sanoja R, Yan J, Lark A, Khoutorsky A, Johnson J, Peebles KA, Lepow T, Sonenberg N, Dussor G, Price TJ. Targeting adenosine monophosphate-activated protein kinase (AMPK) in preclinical models reveals a potential mechanism for the treatment of neuropathic pain. Mol Pain 2011;7:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mills C, McMackin M, Jaffe R, Yu J, Zininberg E, Slee D, Gogas K, Bradbury M. Effects of the transient receptor potential vanilloid 1 antagonist A-425619 on body temperature and thermoregulation in the rat. Neuroscience 2008;156:165–74. [DOI] [PubMed] [Google Scholar]
- [29].Mitchell K, Bates BD, Keller JM, Lopez M, Scholl L, Navarro J, Madian N, Haspel G, Nemenov MI, Iadarola MJ. Ablation of rat TRPV1-expressing Adelta/C-fibers with resiniferatoxin: analysis of withdrawal behaviors, recovery of function and molecular correlates. Mol Pain 2010;6:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Morales-Aza BM, Chillingworth NL, Payne JA, Donaldson LF. Inflammation alters cation chloride cotransporter expression in sensory neurons. Neurobiol Dis 2004;17:62–9. [DOI] [PubMed] [Google Scholar]
- [31].Nagakura Y, Okada M, Kohara A, Kiso T, Toya T, Iwai A, Wanibuchi F, Yamaguchi T. Allodynia and hyperalgesia in adjuvant-induced arthritic rats: time course of progression and efficacy of analgesics. J Pharmacol Exp Ther 2003;306:490–7. [DOI] [PubMed] [Google Scholar]
- [32].Narayana N, Diller TC, Koide K, Bunnage ME, Nicolaou KC, Brunton LL, Xuong NH, Ten Eyck LF, Taylor SS. Crystal structure of the potent natural product inhibitor balanol in complex with the catalytic subunit of cAMP-dependent protein kinase. Biochemistry 1999;38:2367–76. [DOI] [PubMed] [Google Scholar]
- [33].Niiyama Y, Kawamata T, Yamamoto J, Omote K, Namiki A. Bone cancer increases transient receptor potential vanilloid subfamily 1 expression within distinct subpopulations of dorsal root ganglion neurons. Neuroscience 2007;148:560–72. [DOI] [PubMed] [Google Scholar]
- [34].Peters CM, Ghilardi JR, Keyser CP, Kubota K, Lindsay TH, Luger NM, Mach DB, Schwei MJ, Sevcik MA, Mantyh PW. Tumor-induced injury of primary afferent sensory nerve fibers in bone cancer pain. Exp Neurol 2005;193:85–100. [DOI] [PubMed] [Google Scholar]
- [35].Ruscheweyh R, Wilder-Smith O, Drdla R, Liu XG, Sandkuhler J. Long-term potentiation in spinal nociceptive pathways as a novel target for pain therapy. Mol Pain 2011;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Saade NE, Farhat O, Rahal O, Safieh-Garabedian B, Le Bars D, Jabbur SJ. Ultra violet-induced localized inflammatory hyperalgesia in awake rats and the role of sensory and sympathetic innervation of the skin. Brain Behav Immun 2008;22:245–56. [DOI] [PubMed] [Google Scholar]
- [37].Sandkuhler J, Liu X. Induction of long-term potentiation at spinal synapses by noxious stimulation or nerve injury. Eur J Neurosci 1998;10:2476–80. [DOI] [PubMed] [Google Scholar]
- [38].Sculptoreanu A, de Groat WC, Buffington CA, Birder LA. Abnormal excitability in capsaicin-responsive DRG neurons from cats with feline interstitial cystitis. Exp Neurol 2005;193:437–43. [DOI] [PubMed] [Google Scholar]
- [39].Song XJ, Wang ZB, Gan Q, Walters ET. cAMP and cGMP contribute to sensory neuron hyperexcitability and hyperalgesia in rats with dorsal root ganglia compression. J Neurophysiol 2006;95:479–92. [DOI] [PubMed] [Google Scholar]
- [40].Staudacher I, Schweizer PA, Katus HA, Thomas D. hERG: protein trafficking and potential for therapy and drug side effects. Curr Opin Drug Discov Devel 2010;13:23–30. [PubMed] [Google Scholar]
- [41].Sung YJ, Ambron RT. Pathways that elicit long-term changes in gene expression in nociceptive neurons following nerve injury: contributions to neuropathic pain. Neurol Res 2004;26:195–203. [DOI] [PubMed] [Google Scholar]
- [42].Sung YJ, Chiu DT, Ambron RT. Activation and retrograde transport of protein kinase G in rat nociceptive neurons after nerve injury and inflammation. Neuroscience 2006;141:697–709. [DOI] [PubMed] [Google Scholar]
- [43].Sung YJ, Walters ET, Ambron RT. A neuronal isoform of protein kinase G couples mitogen-activated protein kinase nuclear import to axotomy-induced long-term hyperexcitability in Aplysia sensory neurons. J Neurosci 2004;24:7583–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sung YJ, Wu F, Schacher S, Ambron RT. Synaptogenesis regulates axotomy-induced activation of c-Jun-activator protein-1 transcription. J Neurosci 2006;26:6439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tao YX, Johns RA. Activation of cGMP-dependent protein kinase Ialpha is required for N-methyl-D-aspartate- or nitric oxide-produced spinal thermal hyperalgesia. Eur J Pharmacol 2000;392:141–5. [DOI] [PubMed] [Google Scholar]
- [46].Tegeder I, Del Turco D, Schmidtko A, Sausbier M, Feil R, Hofmann F, Deller T, Ruth P, Geisslinger G. Reduced inflammatory hyperalgesia with preservation of acute thermal nociception in mice lacking cGMP-dependent protein kinase I. Proc Natl Acad Sci U S A 2004;101:3253–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wu C, Gavva NR, Brennan TJ. Effect of AMG0347, a transient receptor potential type V1 receptor antagonist, and morphine on pain behavior after plantar incision. Anesthesiology 2008;108:1100–8. [DOI] [PubMed] [Google Scholar]
- [48].Zelenka M, Schafers M, Sommer C. Intraneural injection of interleukin-1beta and tumor necrosis factor-alpha into rat sciatic nerve at physiological doses induces signs of neuropathic pain. PAIN 2005;116:257–63. [DOI] [PubMed] [Google Scholar]
- [49].Zhang HM, Zhou LJ, Hu XD, Hu NW, Zhang T, Liu XG. Acute nerve injury induces long-term potentiation of C-fiber evoked field potentials in spinal dorsal horn of intact rat. Sheng Li Xue Bao 2004;56:591–6. [PubMed] [Google Scholar]
- [50].Zhang X, Wu J, Fang L, Willis WD. The effects of protein phosphatase inhibitors on the duration of central sensitization of rat dorsal horn neurons following injection of capsaicin. Mol Pain 2006;2:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


