Abstract
Shift work (SW) is viewed as a risk factor for the development of many serious health conditions, yet prospective studies that document such risks are rare. The current study addressed this void by testing the hypothesis that long-term exposure to repeated diurnal phase shifts, mimicking SW, will accelerate disease onset or death in inbred mice with genetic risk of developing cancer, diabetes, or autoimmune disease. The data indicate that 1) life-long exposure to simulated SW accelerates death in female cancer-prone AKR/J mice; 2) a significant proportion of male NON/ShiLtJ mice, which have impaired glucose tolerance but do not normally progress to type 2 diabetes, develop hyperglycemia, consistent with diabetes (that is, blood glucose 250 mg/dL or greater) after exposure to simulated SW for 8 wk; and 3) MRL/MpJ mice, which are prone to develop autoimmune disease, showed sex-related acceleration of disease development when exposed to SW as compared with mice maintained on a stable photocycle. Thus, long-term exposure to diurnal phase shifts that mimic SW reduces health or longevity in a wide variety of disease models. Our approach provides a simple way to assess the effect of chronic diurnal disruption in disease development in at-risk genotypes.
Abbreviations: DPS, diurnal phase shift; LD, light:dark; SW, shift work
People and other animals are evolutionarily adapted to live in the context of a 24-h solar day that is organized into relatively stable periods of light and darkness. However, beginning with electrification and bolstered by the information–internet age, deviations from the inherent daily pattern of nocturnal sleep and daytime activity have become increasingly common, and daily periods of rest and activity have become increasingly unstable. Many people commonly experience day–night disorganization in association with work requirements, care-giving, lifestyle choice, and health conditions.
Shift work (SW) generally includes any job that requires work outside of standard daytime hours. According to the Bureau of Labor Statistics, roughly 15 to 18% of wage and salary earners in the United States work full-time on evening shift, night shift, rotating shifts, or other nontraditional schedules (for example, on-call, split shifts).36 In theory, if a night-shift worker adapts completely to an alternative 24 h rest-activity schedule by maintaining the same schedule on days off and limiting light exposure to the hours of wakefulness, then circadian disruption and its consequences might be minimal. However, because of social factors, such complete adaptation is rare; shift workers often maintain a night-active schedule during the work week and switch to a day-active schedule on weekends. Furthermore, SW is not optional or short-term for many people, and in our increasingly ‘24/7’ society, the elimination of SW is not a realistic goal.24,53,54
SW is now viewed as a risk factor for many disease conditions, including cancer and neurologic, gastrointestinal, metabolic, autoimmune, and cardiovascular disease.6,14,20,35,37,41,45,59 If such associations are confirmed and reflect causality, then even a modest reduction in the detrimental impact of SW could have significant public health benefit.3,24 Identifying the long-term health effects of chronic perturbation of the natural diurnal integration of behavior and physiology is crucial to developing sound recommendations and preventive strategies that will protect public health and mitigate disease risk.
Although epidemiologic evidence indicates that long-term diurnal disruption increases disease risk, validating the effects of long-term or repeated exposure to diurnal disruption on such risks is difficult, if not impossible, to achieve in a prospective human study. In addition to the fundamental problems of the necessary duration and cost of such a study, reliance on self-report of behavioral habits or disease incidence introduces considerable uncertainty into the data, limiting confidence in both the conclusions that can be drawn and the applicability of those conclusions to other populations or situations. Furthermore, many people with various ailments are not aware of their condition, and undiagnosed disease could differ between shift workers and nonshift workers due to factors such as access to medical care.24 In addition, some people may have personal characteristics or situational life factors that enhance or mitigate the adverse effect of SW or influence the person's ability to maintain a healthy lifestyle despite engaging in SW (for example, need to provide child care, availability of a supportive partner). Finally, some people may abandon jobs requiring SW because of health problems or coping issues.2,24 Thus, although SW is viewed as a risk factor for the development of many serious health conditions in humans, conducting prospective studies to document such risks is difficult.
In animal studies, environment, diet, age, genetics, and dependent measures can be rigidly controlled, and studies can be extended for a sufficient duration to evaluate long term age-related outcomes. Therefore, we used animal models to test the hypothesis that long-term exposure to repeated diurnal phase shifts (DPS), mimicking SW, accelerates disease onset or death in inbred mice with genetic risk of developing cancer (AKR/J), diabetes (NON/ShiLtJ), or autoimmune disease (MRL/MpJ). We targeted these conditions on the basis of a literature review indicating that humans who experience frequent jet lag or prolonged exposure to SW are epidemiologically at an increased risk of developing a variety of cancers, including lymphoma.13,25,27,28,42 Animal studies also support the hypothesis that exposure to light during the night increases cancer risk.4,15,20 Based on the preponderance of evidence in both humans and animals, the World Health Organization has classified SW as a probable carcinogen.53 Considerable data also indicate that exposure to SW promotes the development of autoimmune diseases, including systemic lupus erythematosus, autoimmune thyroiditis, multiple sclerosis, and rheumatoid arthritis.10,22,32,44 A large literature in humans links sleep and circadian disruption to increased risk of metabolic syndrome or diabetes (for example, see references 1, 16, 19, and 51).
In this study, we used a novel perturbation that captures the intermittent phase-delay and phase-advance experiences of people who engage in SW.
Materials and Methods
General housing conditions.
Unless otherwise specified below, mice were housed in same-sex groups of 5 in standard filter-topped cages (12 in. × 7.5 in. × 5 in.; 90 in.2 of floor space) on woodchip bedding (Beta-Chips, Gateway Supply Company, St Louis, MO) beginning at 4 wk of age. Ambient temperature was maintained at 25 ± 1 °C. Food (Laboratory Rodent Diet 5001, LabDiet, St Louis, MO) and tap water (City Light, Water, and Power, Springfield, IL) were available without restriction. Mice were assumed to be free of standard adventitious pathogens in light of procurement from a reputable vendor and maintenance of the mice in areas free of such organisms, as documented by scheduled testing of sentinel animals maintained in the same room. Euthanasia was performed by exsanguination under isoflurane anesthesia. All procedures involving animals were approved by the Laboratory Animal Care and Use Committee of the Southern Illinois University School of Medicine.
Validation of the DPS/SW schedule.
C57BL/6J mice (age, 4 wk) were housed individually upon receipt in an environmentally controlled chamber that was maintained at 25 ± 1 °C on a 12:12-h light:dark (LD) cycle. In all experiments included in this report and as previously reported,58 environmental chambers were not light-tight due to a 2-in. opening that permitted cables to exit the chamber. To avoid an effect of changes in room lighting on lighting conditions within the chambers, room lights remained on at all times by design, and chamber doors were opened only during the chamber light phase. Room light intensity was approximately 175 lx. Within chambers at cage level with the chamber door closed, light intensity was approximately 5 to 10 lx during the dark phase (chamber internal lighting off) and 125 to 145 lx during the light phase (chamber internal lighting on).
At 5 to 6 wk of age, mice underwent surgery for implantation of an abdominal transmitter (model TA-F10 [1.6 g], Data Sciences, St Paul, MN) that allowed telemetric measurement of locomotor activity and core temperature, as previously described.55,57 Transmitters were gas-sterilized prior to implantation. Mice were anesthetized by subcutaneous injection of a mixture of ketamine (50 mg/kg) and xylazine (50 mg/kg) and were supplemented with additional anesthetic during surgery when needed. All surgery was conducted by using standard aseptic techniques. The abdomen was shaved with a no. 40 clipper blade, and the skin was disinfected with alternating scrubs of povidone–iodine and alcohol. A midline incision of approximately 3 cm was made in the abdominal skin. The linea alba was identified and incised, exposing the abdominal cavity. The transmitter was implanted with the rounded end directed cranially. Sterile saline (1 mL) was added to the abdominal cavity to lubricate the transmitter and support hydration in the mouse. The abdominal muscles were closed in a simple continuous pattern with 4-0 polyglycolic acid suture. The skin was closed with no fewer than 3 simple interrupted sutures with 4-0 polyglycolic acid suture. The mice recovered from anesthesia in a cage placed on a heating pad. When still present, sutures were removed at 10 d after surgery. Mice received the analgesic ibuprofen in the drinking water (1 mg/mL) beginning on the day before surgery and continuing for 5 d after surgery.
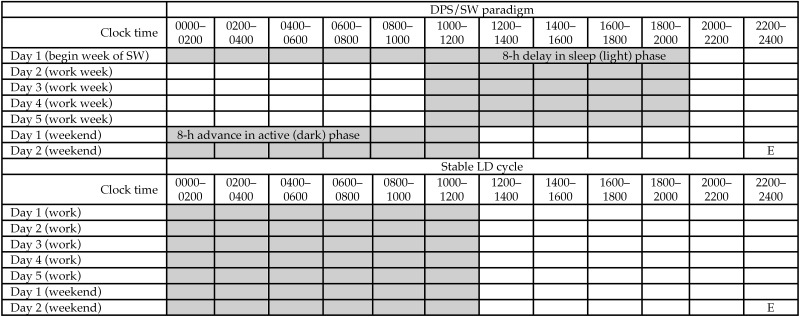
After surgery, mice were housed individually in cages that contained a running wheel. Mice were permitted approximately 3 wk for stabilization on a stable 12:12-h light:dark (LD) cycle prior to the beginning of data collection. Core temperature, locomotor activity, and running-wheel activity were the monitored under the stable 12:12-h LD cycle for 1 wk. All mice were then switched to a schedule of repeated DPS that was designed to mimic SW (DPS/SW paradigm, Figure 1),58 with continued monitoring of core temperature and locomotor and running-wheel activity. On this schedule, the dark (active) phase was extended by 8 h on the first day of the simulated work week. This ‘shifted’ LD cycle was maintained for 5 d. On day 6 (the beginning of the simulated weekend), the onset of the dark (active) phase was advanced by 8 h, thus returning to the control schedule. After 2 weekend-schedule days, the DPS/SW cycle began again. Mice remained on the DPS/SW schedule for 4 wk and were then returned to a stable 12:12-h LD schedule for an additional 2 wk. At the end of the study, mice underwent euthanasia by isoflurane overdose for recovery of the abdominal transmitters.
Figure 1.
Diurnal phase shift/shift work (DPS/SW) paradigm. Gray boxes show the dark phase. Whenever possible, consistent with indices of imminent death, mice will undergo euthanasia (E) immediately before dark onset at the end of the simulated weekend.
Longevity in AKR/J mice exposed to DPS/SW.
AKR/J mice are notable as the inbred mouse strain found to have the shortest natural lifespan among the 32 inbred strains evaluated.61 AKR/J mice express the ecotropic retrovirus AKV in all tissues and die at a relatively young age (median age: female, 36 wk [252 d]; male, 41 wk [287 d]) due to the development of leukemia and lymphoma.9,17
Male and female AKR/J mice (Jackson Laboratory, Bar Harbor, ME) were received at 4 wk of age. At 5 wk of age, isoflurane-anesthetized mice were subcutaneously implanted with microchips that allow remote mouse identification and measurement of body temperature by using a wand-type reader (model IPTT300, BioMedic Data Systems, Seaford, DE). The microchip was implanted without incision or wound closure by using a 12-gauge delivery device. Individual chips weigh approximately 0.125 g.
Four experimental groups were established as follows: female-LD, n = 40; female- SW, n = 38; male-LD, n = 38; and male-SW, n = 39 (numbers differed due to availability of animals from the vendor). Power analysis based on our previous longevity data from AKR/J mice56 indicated that group sizes of 40 would likely allow detection of a 15% reduction in mean life span with an α of 0.05 and power of 0.8. In human terms, a 15% reduction in mean lifespan would be equivalent to having the 2011 national mean human longevity fall from 78.7 y23 to approximately 66.9 y.
Beginning at 6 wk of age, mice either remained on the standard 12:12-h LD cycle or were transferred to the DPS schedule (Figure 1). The body weight and temperature of each mouse were measured weekly at the time of cage change. Average daily food consumption per mouse was determined by subtracting the weight of food remaining in the cage from the weight of food placed in the cage at the time of weekly cage change, divided by the number of mice in the cage and by 7 d. No attempt was made to correct for spillage. The temperature of each mouse was measured by using the wand chip reader. Mice in a given experimental group were recombined as deaths occurred to maintain 5 mice per cage to the extent possible, based on data showing that cage conditions change as the number of mice per cage changes.55 No fighting was observed as a result of these recombinations.
Using a rolling 4-wk average of the product of weekly body weights and temperatures (BW×T), we established a 90% threshold for careful daily individual monitoring for each mouse according to deviation from individual baseline norms. Mice whose values fell below the 90% threshold for 2 consecutive weeks were euthanized. However, some mice died spontaneously before euthanasia without showing signs of severe illness.
Hyperglycemia in NON/Shi/LtJ mice exposed to DPS/SW.
NON/ShiLtJ mice, which are a recommended control strain for NZO/HlLtJ mice (http://www.jax.org/strain/002105), have both genetic risk and resistance factors for the development of type 2 diabetes; they typically develop impaired glucose tolerance but do not normally progress to type 2 diabetes.7,29,30,34,48
NON/ShiLtJ breeder mice were fed a 4.5% fat diet (Laboratory Rodent Diet 5001, LabDiet). At 3 wk of age, offspring were weaned onto either the 4.5% fat diet or an 11% fat diet (Mouse Diet 5105, LabDiet), which is more similar to the human diet than the 60% fat diet used in many mouse models of diabetes. At 8 wk of age, mice either remained on a stable 12:12-h LD cycle or were switched to a DPS/SW schedule (Figure 1) for the duration of the study (8 additional weeks). On the day of euthanasia, mice were fasted for the last 5 h of the light phase, weighed, and killed by exsanguination under isoflurane anesthesia immediately before dark onset at the end of day 2 of the weekend schedule (Figure 1). Serum was prepared for measurement of glucose, triglycerides, and cholesterol (IDEXX BioResearch, Columbia, MO). Insulin was measured using an ELISA kit (Ultra Sensitive Mouse Insulin ELISA Kit, Crystal Chem, Downer's Grove, IL) according to manufacturer's instructions.
Autoimmune disease in MRL/MpJ mice exposed to DPS/SW.
MRL/MpJ mice were developed as a strain that exhibits lymphoproliferation. At generation 12 during development, the spontaneous mutation Faslpr was detected, which accelerates expression of the autoimmune phenotype. Both the wildtype MRL/MpJ and the congenic mutant (MRL-Faslpr) strains are maintained at the Jackson Laboratory. Beginning at about 3 mo of age, MRL-Faslpr mice develop high levels of circulating immune complexes and severe proliferative glomerulonephritis, and females and males typically die at 17 and 22 wk of age, respectively. Because the rapid onset of disease and death of MRL-Faslpr mice would complicate detection of SW-related acceleration of morbidity and mortality, we elected to use MRL/MpJ mice, which develop similar but milder disease with more gradual onset and remain healthy for more than a year.26 Female and male MRL/MpJ mice normally die at median ages of 554 and 639 d (79 and 91 wk) of age, respectively.60
Male and female MRL/MpJ mice (Jackson Laboratory) were received at 4 wk of age. At 5 wk of age, isoflurane-anesthetized mice were subcutaneously implanted with microchips that allow remote measurement of body temperature by using a wand-type reader (model IPTT300, BioMedic Data Systems). The microchip was implanted without incision or wound closure by using a 12-gauge delivery device. Individual chips weigh approximately 0.125 g.
Eight experimental groups were established as follows: female-LD-12wk, n = 14; female-LD-24wk, n = 30; female-SW-12wk, n = 24; female-SW-24wk, n = 25; male-LD-12wk, n = 24; male-LD-24wk, n = 27; male-SW-12wk, n = 36; and male-SW-24wk, n = 21. Beginning at 6 wk of age, mice either remained on the standard 12:12-h LD cycle or were transferred to the DPS/SW schedule (Figure 1). The body weight and temperature of each mouse were measured weekly at the time of cage change. Average daily food consumption per mouse was determined by weighing food placed in and remaining in the cage at the time of the weekly cage change, divided by 7 (to account for the number of days) and by the number of mice in the cage. No attempt was made to correct for spillage. The temperature of each mouse was measured by using the wand chip reader. Mice remained on the assigned LD or SW schedule for 12 or 24 wk prior to euthanasia. Body weight and temperature were measured immediately before euthanasia. Euthanasia was performed by exsanguination under isoflurane anesthesia. Blood glucose was measured immediately by using a glucometer (One Touch Ultra Glucometer, LifeScan, Milpitus, CA). Urine was collected by free catch at the time of euthanasia and immediately assayed for protein by using a Chemstrip (Roche Diagnostics, Indianapolis, IN). Dermatitis was evaluated in an unblinded manner as a score of 0 (none), 1 (mild), or 2 (marked). Kidneys were removed, weighed, and fixed in formalin for subsequent histologic analysis.
Histopathology.
Histopathologic assessment was conducted on kidney collected from MRL/MpJ mice that underwent euthanasia after either 12 or 24 wk on study. Tissue were fixed in formalin and stained with hematoxylin and eosin. Tissue evaluation was performed in a blinded manner. Kidneys (2 sections per mouse) were assessed for the number of foci of nephrocalcinosis and the number of observed instances of tubular degeneration or regeneration, glomerular parietal cell hyperplasia and glomerular basement membrane thickening. Inflammation was described and scored in terms of degree (0, none; 1, very mild; 2, mild; or 3, moderate) and extent (1, focal; 2, multifocal). Within each assessment (renal pathology and renal inflammation), scores for each mouse were summed and then used for statistical analysis.
Statistical analysis.
All descriptive statistics are expressed as mean ± SEM. A P value of less than 0.05 was used throughout to indicate statistically significant effects, whereas P values from 0.05 to 0.10 are reported as statistical trends.
For the AKR/J study, survival data were analyzed by using the Wilcoxon test (SAS version 9.4, SAS Institute, Cary, NC). AKR/J repeated-measures data were analyzed by using mixed-model ANOVA (SAS 9.4).
Data from the NON/ShiLtJ study were analyzed using a Z-test (http://www.socscistatistics.com/tests/ztest/).
Repeated-measures data for MRL/MpJ mice were analyzed by using a mixed-model ANOVA (SAS version 9.4). A one-way ANOVA calculator with posthoc Tukey for multiple comparisons (statistica.mooo.com/OneWay_Anova_with_TukeyHSD) was used to analyze data collected at the 12- or 24-wk time points.
Results
SW model.
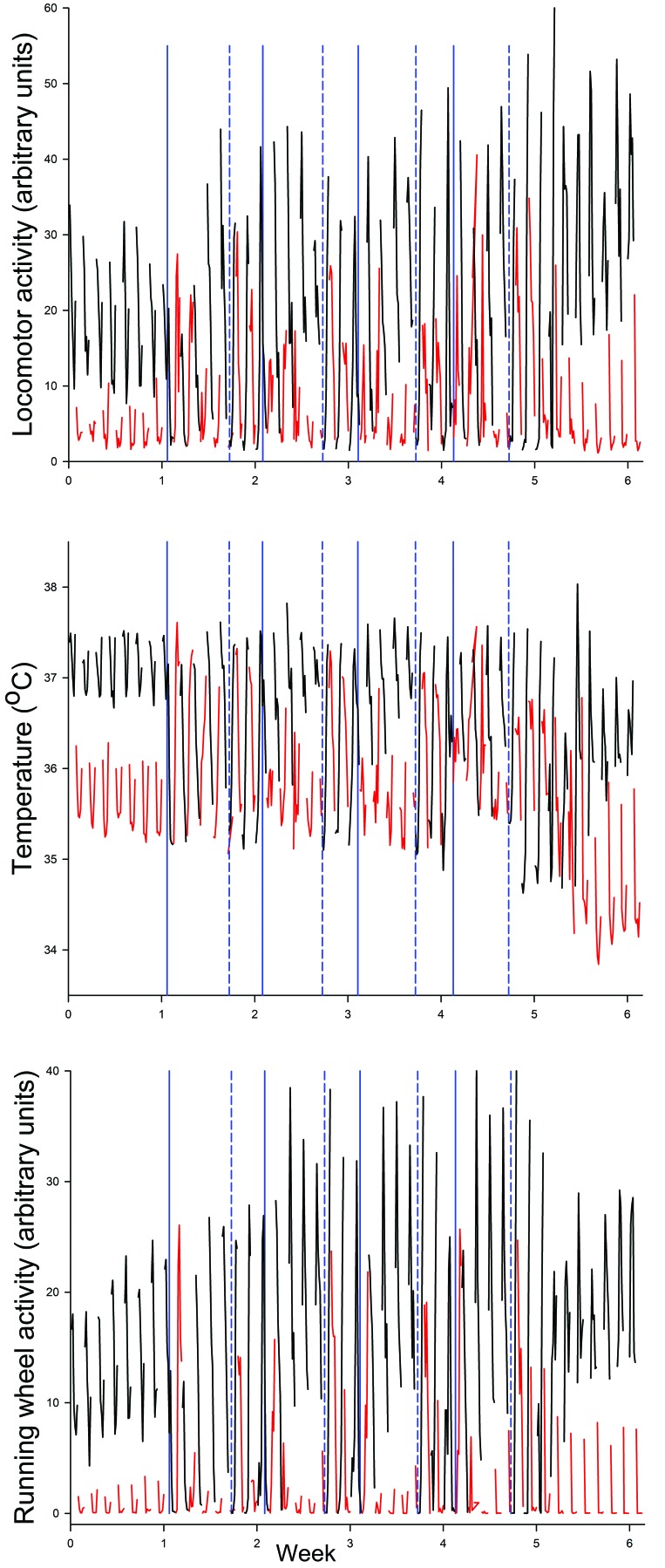
To verify that the simulated SW schedule disrupted rhythms of body temperature, locomotor activity, and running-wheel activity, we evaluated individually housed male C57BL/6J mice (n = 5) before and after 4 sequential weeks of exposure to the SW schedule (Figures 1 and 2). The overlapping traces of values measured for each variable during dark (black lines) and light (red lines) periods illustrate the resulting severe and repeated behavioral and physiologic disorganization of normal LD differentiation. Dark- and light-phase values for all 3 measures were visually distinct during the baseline week and became severely disrupted during the first week of SW and on subsequent weekend schedules. After release from SW, distinctly separate patterns were reestablished within a week of exposure to a stable LD cycle.
Figure 2.
Disruptive effects of repeated diurnal phase shifts. C57BL/6J mice (n = 5) were exposed to 1 wk of a normal 12:12-h LD cycle and then to 4 sequential weeks of diurnal phase shifts (DPS), simulating shift work (SW; see Figure 1 for details). Vertical blue lines denote times of phase advances (solid) and phase delays (dashed). Mice then resumed a stable 12:12-h LD cycle. Core temperature, locomotor activity, and running-wheel activity were recorded continuously for the duration of the study. Values were summarized over 2-h periods and are displayed as mean values obtained when the lights were on (red) and off (black). The data show marked and repeated disruption of normal diurnal patterns of all 3 measures. As compared with the distinct LD separations seen during the baseline and postDPS periods, mice showed disorganized LD differentiation in association with phase advances and delays.
AKR/J mice exposed to DPS/SW.
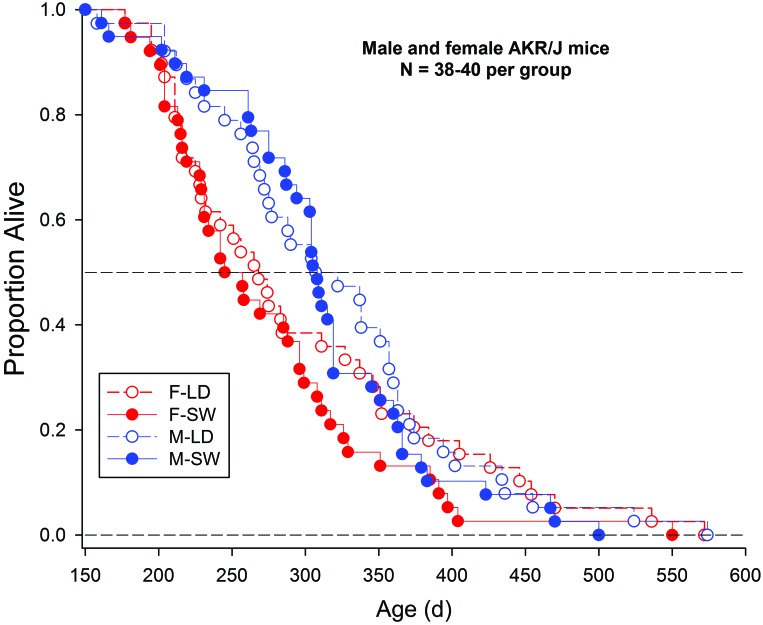
In this study, male and female AKR/J mice were exposed to either a stable 12:12-h LD cycle or simulated SW throughout their life span (n = 38 to 40 per group, with groups designated as male-LD, male-SW, female-LD, and female-SW). Survival data are shown in Figure 3. The combined analysis of all 4 groups revealed significant effects of sex (P = 0.031), with male mice having a longer life span than female mice, as previously reported.56 Median survival times for female-LD, female -SW, male -LD, and male-SW mice were 268, 251, 314.5, and 308 d, respectively. The data were further analyzed in subsets composed of animals that died before or after the median for each group. These analyses revealed significant differences among mice that died before the overall median (median values for female-LD, female-SW, male-LD and male-SW were 216, 216, 264, and 275 d, respectively; P = 0.0004) and after the overall median (median values for F-LD, F-SW, M-LD and M-SW were 351.5, 311, 363, and 355.5 d, respectively; P = 0.0118). Among mice that died before the group median, the difference was attributable to sex, whereas among those that died after the overall median, the difference was attributable to the shorter lifespan in the female-SW mice.
Figure 3.
Kaplan–Meier plot of survival curves of AKR/J mice exposed to stable or shifted LD cycles. Male (M) and female (F) AKR/J mice were exposed to either a stable 12:12-h LD cycle or to a repeated DPS cycle simulating SW (n = 38 to 40 per group) beginning at 5 wk of age and continuing until death.
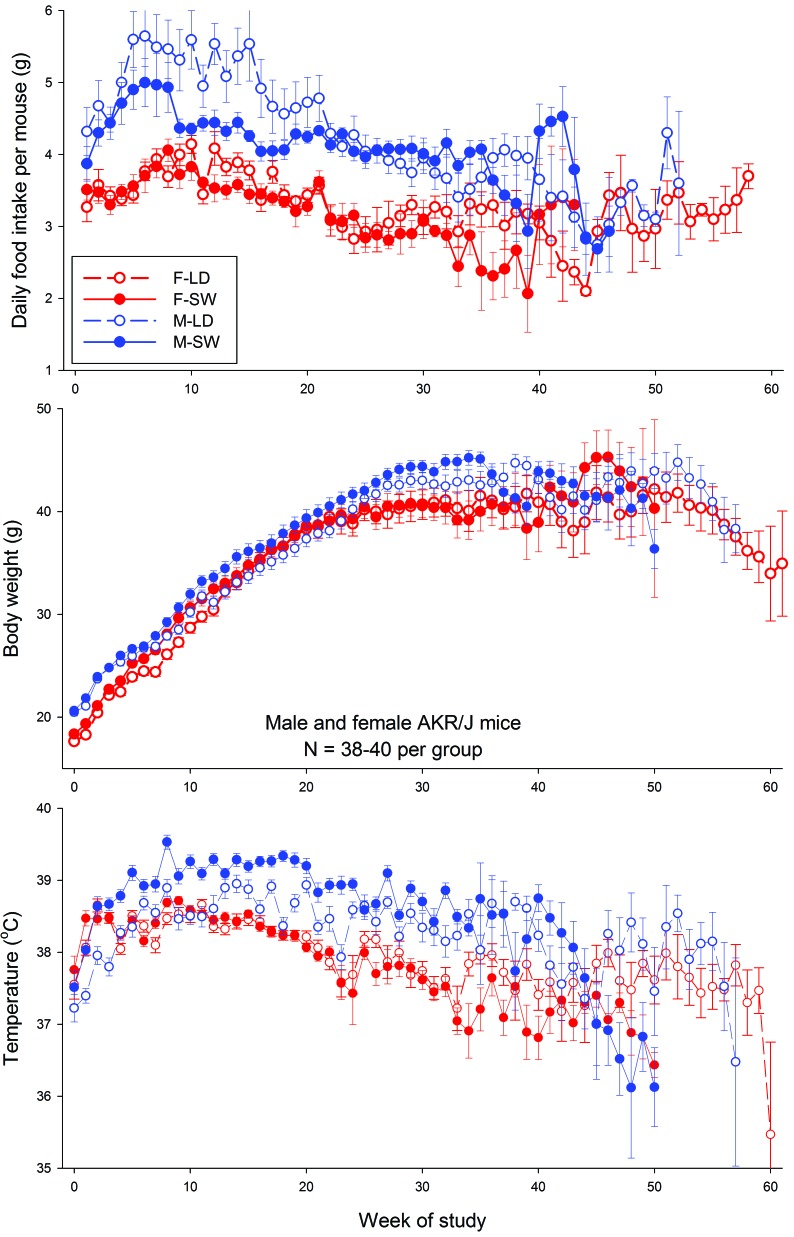
Figure 4 shows the average values for food intake, body weight, and core temperature over the duration of the study. Analysis of data collected through week 44 of the study (that is, at times when 3 or more mice remained alive in all groups) revealed significant time-by-treatment interactions for all 3 measures in both male and female mice (food intake, P = 0.0015; body weight, P < 0.001; temperature, P < 0.0003).
Figure 4.
Food intake, body weight, and temperature of male (M) and female (F) AKR/J mice maintained on a stable LD cycle or on a simulated SW schedule throughout their lifespan.
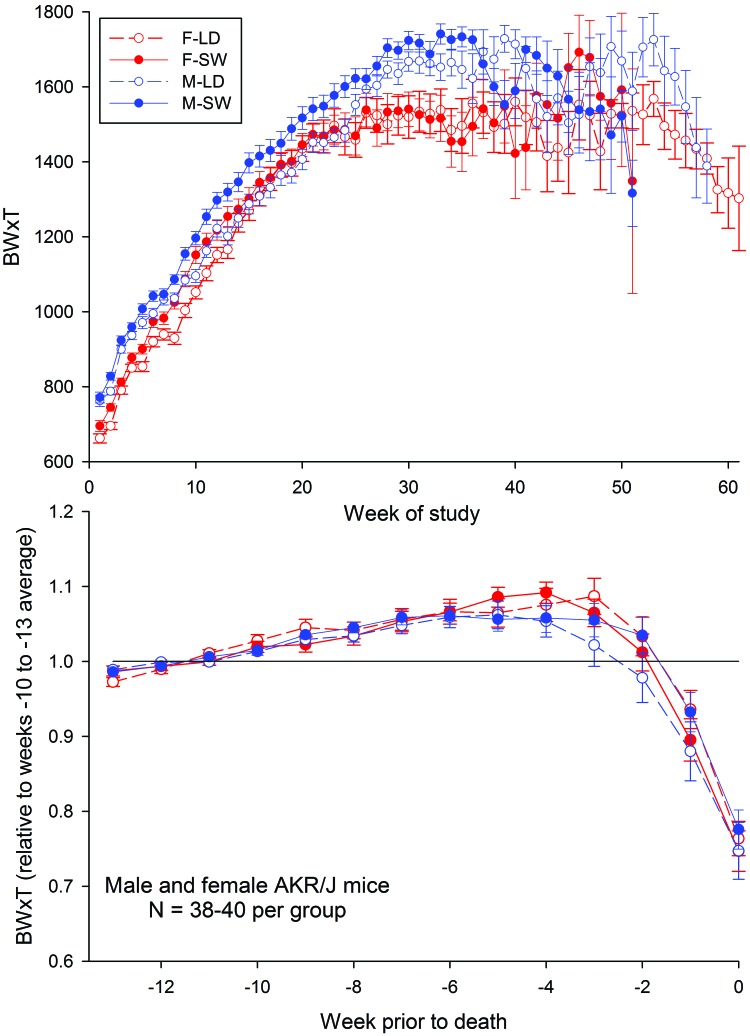
Figure 5 shows the effects of exposure to SW on BW×T, which has previously been validated as a marker of imminent death in AKR/J mice.47 Data in the top panel were analyzed in distinct temporal segments that began at week 5 and were repeated at 5-wk intervals through week 50. For female mice, significant treatment effects (that is, higher values for female-SW mice than female-LD mice) were detected during intervals spanning weeks 0 to 5 (P = 0.0147), 0 to 10 (P = 0.0018), and 15 (P = 0.011). For male mice, significant treatment effects (that is, higher values of BW×T in male-SW mice than male-LD mice) were detected during intervals spanning weeks 0 to 10 (P = 0.0145) through 0 to 35 (P = 0.0107), with trends apparent at weeks 5, 40, and 45 (P = 0.0773, 0.0703 and 0.0743, respectively). This pattern indicates that during adulthood, male mice exposed to SW had higher BW×T scores, driven primarily by higher body weights, as compared with LD mice. However, as aging progressed, these effects dissipated. For female mice, a similar effect occurred earlier in life but had abated after 20 wk of exposure to SW.
Figure 5.
Composite weight×temperature values (BW×T) for male (M) and female (F) AKR/J mice maintained on a stable LD cycle or on a simulated SW schedule throughout their lifespan Top, mean values of body weight × temperature collected across time. Bottom, mean values normalized against the average of values measured during weeks 10 through 13 prior to death for each mouse.
To evaluate the terminal decline in health as a function of treatment, BW×T values for each mouse were normalized against mean values within a fixed time interval (weeks 10 through 13 prior to death). Analysis of these normalized data revealed a significant (P < 0.05) effect of time but no significant effect of sex or SW exposure (Figure 5, lower panel). Mice showed gradual increases in the BW×T measure until 2 wk prior to death. After this time, the values fell precipitously. The finding that treatment (LD or SW) did not differentially affect BW×T measures either shortly before or after mice began their terminal decline further validates this measure as a marker of imminent death.
Hyperglycemia in NON/ShiLtJ mice exposed to DPS/SW.
Male NON/ShiLtJ mice develop impaired glucose tolerance but do not normally progress to type 2 diabetes.7,29,30,34,48 We hypothesized that repeated exposure to DPS/SW would precipitate type 2 diabetes, as indicated by fasting hyperglycemia greater than 250 mg/dL in these at-risk mice. At 8 wk of age, male NON/ShiLtJ mice either remained on a stable 12:12-h LD cycle (n = 15) or were switched to DPS/SW (n = 15; Figure 1) for the duration of the study (8 additional weeks). Mice were maintained on either a 4.5% or 11% fat diet during that time. On the day of euthanasia, mice were fasted for the last 5 h of the light phase, weighed, and killed by exsanguination under isoflurane anesthesia immediately before dark onset at the end of day 2 of the weekend schedule (Figure 1).
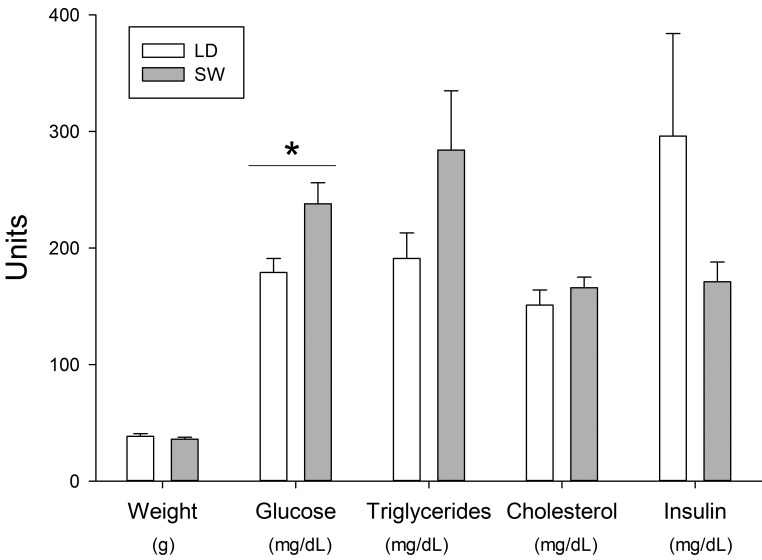
Diet did not significantly affect any of the measured variables, and therefore data from both diet groups were combined for analysis with regard to the effects of LD or SW. Among the mice that had experienced DPS/SW, 40% (6 of 15) had fasting hyperglycemia greater than 250 mg/dL, independent of diet and without weight gain, whereas none of the 15 LD mice had hyperglycemia greater than 250 mg/dL (P < 0.05, Z-test; Figure 6).
Figure 6.
Effects of 8 wk of exposure to DPS/SW on body weight and serum metabolites Male NON/ShiLtJ mice were exposed to a DPS/SW schedule or to a stable LD cycle for 8 sequential weeks (n = 15 per group). Body weights were measured immediately before euthanasia, which was performed at equivalent diurnal time for all mice, which had been food-fasted for 5 h previously. *, P < 0.05, student's t test, comparing LD and SW mice.
Autoimmune disease in MRL/MpJ mice exposed to DPS/SW.
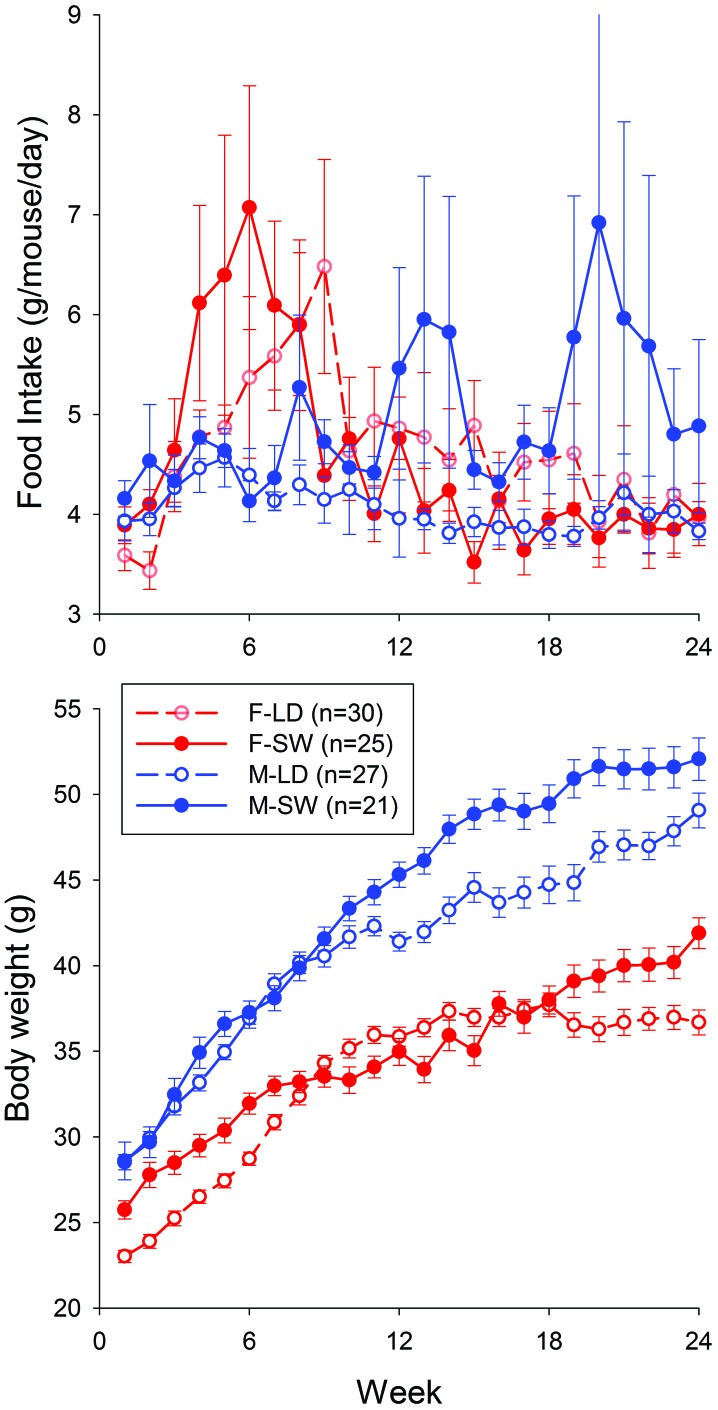
Half of the mice in this experiment were maintained on a stable LD cycle or a SW schedule for 24 wk. Food intakes and body weight were measured weekly. Despite high variability, the food intakes of female mice showed a significant time-by-treatment interaction (P = 0.0282); the food intakes of male mice showed no significant time, treatment, or interaction effects (Figure 7). Male and female mice both showed significant time-by-treatment interactions with regard to body weight (P < 0.001; Figure 7). Male mice developed higher body weights than did LD mice during the latter weeks of the 24-wk experiment (Figure 7).
Figure 7.
Food intake and body weight values for MRL/MpJ mice maintained on a stable LD cycle or on a simulated SW schedule for 24 wk. Top, mean values of food intake (g/mouse/day, determined weekly) across time. Bottom, mean values of body weight (g, measured weekly) across time. Male (M) and female (F) mice both showed significant (P < 0.001) time-by-treatment interactions with regard to body weight.
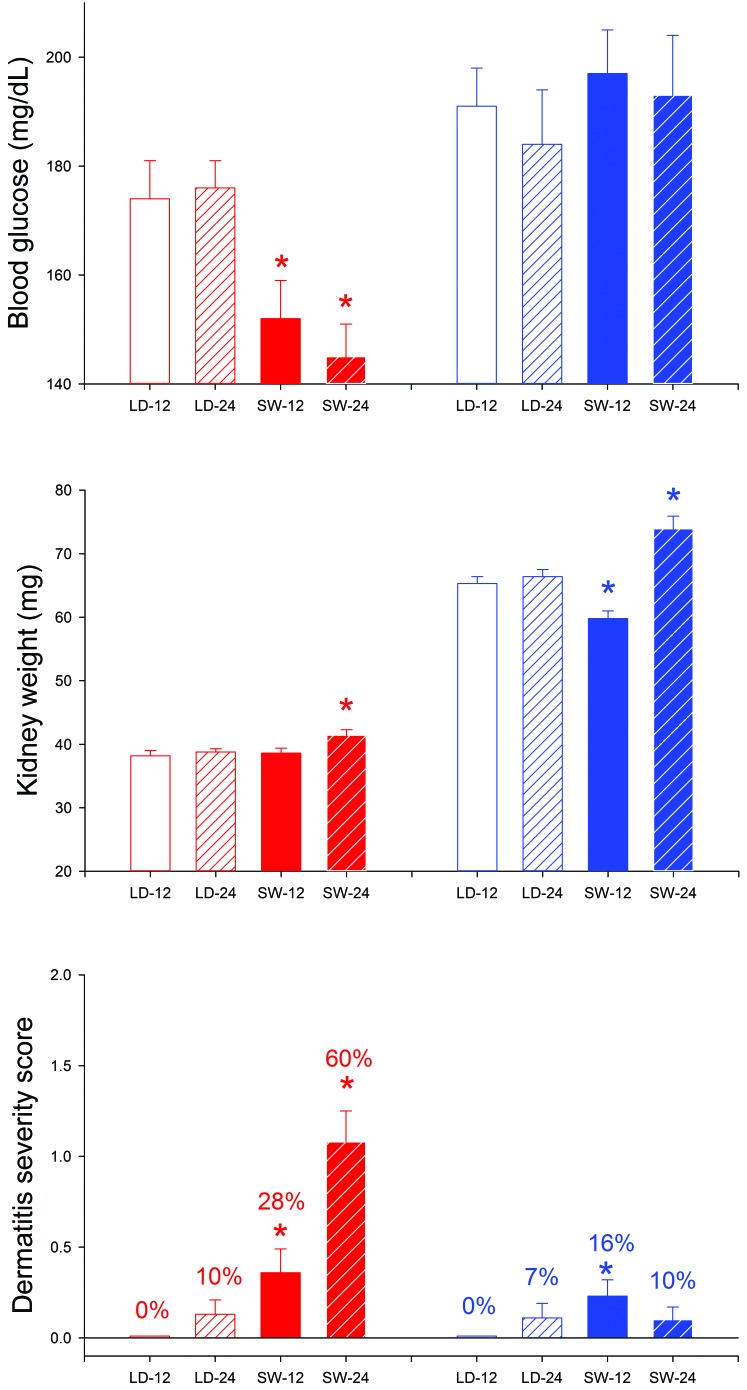
Figure 8 shows data collected at euthanasia from mice that were killed after either 12 or 24 wk on study. The mice killed at 24 wk were those reported in the previous paragraph. Among mice killed at the 24-wk time point, male-SW mice had significantly (P < 0.05) higher body weights and temperatures than did male-LD mice. Female-SW mice had significantly (P < 0.05) lower body temperatures than did F-LD mice at both the 12- and 24-wk time points and developed significant hypoglycemia and severe dermatitis. M-SW mice did not develop significant hypoglycemia or comparable dermatitis. However, male-SW mice had enlarged kidneys and elevated urine protein (P < 0.005 for both) at the 24-wk time point, as compared with male-LD mice. Similar effects did not develop in female mice.
Figure 8.
Pathophysiologic measures from MRL/MpJ mice maintained on a stable LD cycle or on a simulated SW schedule for 12 or 24 wk. Red and blue bars denote female and male mice, respectively. Clear and filled bars denote LD and SW, respectively. Solid and striped bars denote 12- and 24-wk time points, respectively. On the dermatitis panel, percentages indicate the proportion of mice that showed dermatitis. *, P < 0.05 between LD and SW mice that were euthanized at the same time point.
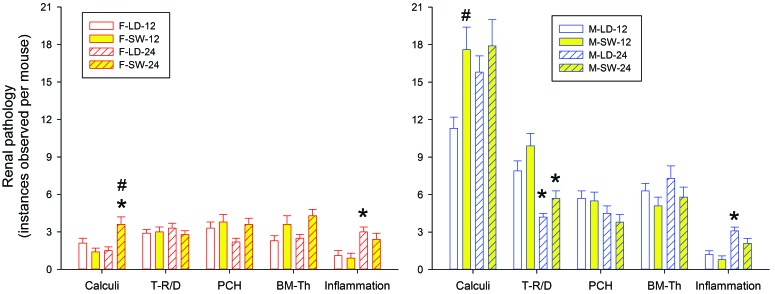
Figure 9 summarizes histopathologic findings from mice killed after either 12 or 24 wk on study. The number of renal calculi detected at 24 wk was significantly higher in F-SW mice as compared with F-LD mice at 24 wk and F-SW mice at 12 wk. The number of renal calculi was also significantly higher in M-SW mice at 24 wk as compared with both M-LD mice at 24 wk and M-SW mice at 12 wk (P < 0.01). M-SW mice also had more renal calculi than did M-LD mice at the 12 wk time point (P < 0.01). Thus, SW appeared to promote formation of renal calculi in both sexes in a time-dependent manner.
Figure 9.
Histopathology findings from MRL/MpJ mice maintained on a stable LD cycle or on a simulated SW schedule for 12 or 24 wk. BM-T, basement membrane thickening; PCH, parietal cell hyperplasia; T-RD, tubular regeneration–degeneration. #, P < 0.05 between LD and SW mice at the same time point; *, P < 0.05 between 12 wk and 24 wk for mice in the same treatment group.
Numerous sex-associated differences were noted in renal pathology, with more changes noted in male mice than in female. Under both LD and SW conditions and at both time points, male mice had significantly more instances of calculi and tubular regeneration-degeneration than did female mice (P < 0.01 for all comparisons). Under LD conditions and at both time points, male mice also showed more instances of parietal cell hyperplasia, basement membrane thickening, and inflammation (P < 0.05 for all comparisons). However, these measures were not significantly affected by exposure to SW.
Discussion
Growing awareness of the importance of circadian coordination in maintaining health is fostering increased consideration of diurnal timing in the pathogenesis and treatment of various disease conditions. However, because diurnal tailoring of both lifestyles and clinical therapies is logistically challenging, clear and unambiguous demonstration of effect will be important in 1) promoting awareness and acceptance of the risks of diurnal asynchrony and 2) developing and implementing strategies to mitigate risk. The prevalence of chronic diurnal disturbance in our society and its associated health risks underscore the need to define the influence of circadian disorganization on the development and severity of chronic diseases. The experiments we report here provide a powerful platform for further investigation of these important issues.
With regard to cancer, a significant body of evidence links exposure to SW with greater cancer risk and increased cancer mortality (for recent examples, see references 12, 18, 21, 31, 43, 46, and 52). The AKR/J mouse model we used focused our study on death in association with genetic risk for lymphoma. Our data indicate that among mice that succumbed to lymphoma at a relatively early age (that is, those that died prior to the median life span), exposure to SW did not contribute significantly to the likelihood of death. In contrast, female mice that did not succumb to an early cancer death (that is, those died after the median lifespan) showed significantly shorter life spans after lifelong exposure to SW. These finding suggest that 1) death associated with aggressive disease or low resistance is not promoted by SW and 2) age, SW, and sex may interact to shorten life in older female mice after long-term exposure to SW. Our study in AKR/J mice provides a useful platform for further studying these relationships.
The SW model that we used could also be applied to other models of inherent cancer risk in mice and to implanted tumor models, as well as to consideration of metastasis and treatment. Human and animal studies of SW and cancer risk have primarily focused on breast, lung, and prostate cancer risk and mortality. For example, a prospective cohort study of 74,862 registered US nurses over a 22-y follow-up period found that lung cancer mortality was significantly higher in women with more than 5 y of rotating night SW as compared with women who never worked night shifts.18 A meta-analysis of 16 studies incorporating 2,020,641 participants, 10,004 incident breast cancer cases, and 7185 cancer-related deaths found that SW carried a breast cancer risk ratio of 1.09 as compared with daytime work.31 Furthermore, the effect was dose-related; the duration of SW increased the risk of breast cancer morbidity by 1.9% for 5 y, 2.5% for 5 to 10 y, 7.4% for 10 to 20 y, and 8.8% for more than 20 y of SW.31 Another meta-analysis studied the association of prostate cancer and night SW in a systematic review that comprised 2,459,845 individuals from 8 published studies.46 The overall analysis indicated that night SW was associated with a significantly elevated risk ratio of 1.24 for prostate cancer.46 In addition, increments of SW of 5 y duration were associated with a 2.8% increase in the risk of prostate cancer.46 A number of mechanisms have been proposed to contribute to the negative effect of SW on cancer and cancer risk (for example, see references 5, 11, and 33). These possibilities might be investigated by using the model system we present here.
We incidentally noted that the median life span of ‘control’ AKR/J mice in our current survival study was somewhat longer than was reported in earlier studies by our lab and others.56,61 We speculate that this longer median lifespan might reflect loss of the causative oncogenic virus or other risk factors in this strain as a result of genetic drift in the context of prolonged breeding and natural selection for longer reproductive life spans. This possibility warrants further investigation.
The hyperglycemia we observed in male NON/ShiLtJ mice exposed to simulated SW, coupled with a trend toward lower mean insulin levels in those mice (Figure 6), suggests the possibilities of early stages of pancreatic β-cell failure, SW-related disruption of normal patterns of metabolic interactions, or both. In similar studies, C57BL/6J and diabetes-prone TALLYHO/JngJ mice that were subjected to 6-h phase advances every 4 to 6 wk had lower glucose than did mice on stable LD cycles at the 20- and 30-wk time points, respectively; however, in comparing the LD and phase-advanced mice, fasting insulin concentrations were elevated in the C57BL/6J strain yet reduced in TALLYHO/JngJ strain.39,40 The reasons for the differences between those studies and ours are unknown but perhaps are related to either the different mouse strains used or the different phase disruptions that were imposed. As in our study, one of these studies found no differences in body weight of C57BL/6J mice maintained on stable compared with disrupted LD cycles, whereas TALLYHO/JngJ mice showed a significant weight gain.39,40
We speculate that extending the duration of SW exposure would precipitate hyperglycemia greater than 250 mg/dL in a greater proportion of mice in association with significant reductions in insulin and elevated triglycerides. A large literature in humans links sleep and circadian disruption to increased risk of metabolic syndrome or diabetes. For example, a study of 28,731 Danish female nurses who were followed for as long as 20 y found that, as compared with nurses who worked day shifts, those who worked night or evening shifts had a significantly higher risk of diabetes (odds ratios of 1.58 and 1.29, respectively) in a fully adjusted model that included BMI.19 Similarly, a meta-analysis of 12 observational studies involving 226,652 participants found an odds ratio of 1.09 for risk of diabetes in association with shift work, with a stronger association in men as compared with women (odds ratios of 1.37 and 1.09, respectively).16 Differences between men and women also were reported in a study of data from 3918 and 4935 nondiabetic men and women, with approximately 8% of both men and women working at night.51 Among the men, exposure to night work was associated with increased BMI and waist circumference, whereas women showed higher fasting plasma glucose, glycated hemoglobin, and 2-h plasma glucose.51 We did not test female NON/ShiLtJ mice in our study, because they are reported to be resistant to the development of diabetes, whereas male mice become glucose intolerant.7,29,30,34,48 We therefore focused on male mice, given that our goal was to evaluate at-risk animals to detect acceleration of onset or exacerbation of abnormalities. A meta-analysis of 36 studies that included 1,061,555 participants reported that pooled relative risks of sleeping less than 5 h, 6 h, and more than 9 h per day were respectively 1.48, 1.18 and 1.36.1 Poor sleep quality, obstructive sleep apnea, and shift work were associated with pooled diabetes risk ratios of 1.40, 2.02 and 1.40, respectively.1 Finally, the pooled relative risks of being overweight, having a family history of diabetes, and being physically inactive were 2.99, 2.33, and 1.20, respectively.1 Thus, the risk of developing diabetes in association with sleep disturbances is comparable to that of traditional risk factors, which led those authors to suggest that sleep disturbances should be considered in clinical guidelines for screening for type 2 diabetes.1
The well-known effects of sleep and sleep disruption on immune function have suggested that inadequate, poor quality, or disturbed sleep could disrupt immune function and trigger immune system abnormalities.49 Our data support this idea. According to the Jackson Laboratory, male and female MRL/MpJ mice typically die at average ages of 84 and 78 wk, respectively. Therefore, our imposed exposures of a maximum of 24 wk of simulated SW did not even reach the expected median age of death, yet disease was far more pronounced in mice exposed to SW as compared with those maintained on a stable LD cycle. Our study further revealed a prominent difference in the key target organ of damage in male and female lupus-prone MRL/MpJ mice (kidneys and skin, respectively) after exposure to simulated SW. Similarly, in human patients with lupus, mucocutaneous symptoms are more common in women than in men, whereas nephritis and renal failure are more frequent in men than in women.38,50
As in our study, human studies also support an effect of sleep and circadian disruption on the development of autoimmune diseases, including systemic lupus erythematosus. For example, a large population-based cohort study of 144,396 subjects (48,132 patients with sleep disorders and 96,264 controls) who were followed for 1,477,055 person–years found that those with sleep disorders had a higher incidence density rate and a 2.2-fold higher adjusted hazard ratio of developing systemic lupus erythematosus than did the controls.8 The increased risk was particularly apparent in patients age 49 y and younger (adjusted risk ratio of 2.3 compared with controls).8 A similar cohort study of 84,996 adult patients with nonsleep apnea sleep disorders and a matched control population found that patients had a significantly greater overall risk for incident autoimmune diseases as compared with controls (adjusted hazard ratio, 1.47), including higher risks for the specific conditions of systemic lupus erythematosus, rheumatoid arthritis, ankylosing spondylitis, and Sjogren syndrome (adjusted hazard ratios of 1.81, 1.45, 1.53, and 1.51, respectively). A study of 265 patients with recently diagnosed systemic lupus erythematosus at 4 clinics compared with 355 matched controls who were identified through driver's license records also found an association between systemic lupus erythematosus and shift work (odds ratio, 1.6).10 With regard to immunologic consequences of long-term diurnal disruption, our previous report documented simulated-SW–related changes in the responses of mice with latent herpesvirus infections to secondary inflammatory challenge.58
The mounting evidence of adverse repercussions of long-term diurnal disruption, together with the large number of affected people, indicates that even seemingly minor improvements in recommendations for managing such disruption could result in significant improvements in public health. In addition to the data presented, we here provide a simple approach that enables such disruptions to be studied with regard to a wide variety of disease types.
Acknowledgments
This work was support in part by NIH grant 5R03AG047117, by a grant from the McElroy Foundation, and by the Southern Illinois University School of Medicine. We thank Megan Ilsley-Woods for valuable technical assistance.
References
- 1.Anothaisintawee T, Reutrakul S, Van Cauter E, Thakkinstian A. 2016. Sleep disturbances compared to traditional risk factors for diabetes development: systematic review and meta-analysis. Sleep Med Rev 30:11–24. [DOI] [PubMed] [Google Scholar]
- 2.Axelsson J, Puttonen S. 2012. Night shift work increases the risk for type 2 diabetes. Evid Based Med 17:193–194. [DOI] [PubMed] [Google Scholar]
- 3.Bajraktarov S, Novotni A, Manusheva N, Nikovska DG, Miceva-Velichovska E, Zdraveska N, Samardjska VC, Richter KS. 2011. Main effects of sleep disorders related to shift work-opportunities for preventive programs. EPMA J 2:365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blask DE, Dauchy RT, Sauer LA. 2005. Putting cancer to sleep at night: the neuroendocrine/circadian melatonin signal. Endocrine 27:179.–. [DOI] [PubMed] [Google Scholar]
- 5.Blask DE, Hill SM, Dauchy RT, Xiang S, Yuan L, Dupleissis T, Mao L, Dauchy E, Sauer LA. 2011. Circadian regulation of molecular, dietary, and metabolic signaling mechanisms of human breast cancer growth by the nocturnal melatonin signal and the consequences of its disruption by light at night. J Pineal Res 51:259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen-Goodspeed M, Lee CC. 2007. Tumor suppression and circadian function. J Biol Rhythms 22:291–298. [DOI] [PubMed] [Google Scholar]
- 7.Cho YR, Kim HJ, Park SY, Ko HJ, Hong EG, Higashimori T, Zhang Z, Jung DY, Ola MS, Lanoue KF, Leiter EH, Kim JK. 2007. Hyperglycemia, maturity-onset obesity, and insulin resistance in NONcNZO10/LtJ males, a new mouse model of type 2 diabetes. Am J Physiol Endocrinol Metab 293:E327–E336. [DOI] [PubMed] [Google Scholar]
- 8.Chung WS, Lin CL, Kao CH. 2015. Association of systemic lupus erythematosus and sleep disorders: a nationwide population-based cohort study. Lupus 25:382–388. [DOI] [PubMed] [Google Scholar]
- 9.Coffin JM, Stoye JP, Frankel WN. 1989. Genetics of endogenous murine leukemia viruses. Ann N Y Acad Sci 567:39–49. [DOI] [PubMed] [Google Scholar]
- 10.Cooper GS, Parks CG, Treadwell EL, St Clair EW, Gilkenson GS, Dooley MA. 2004. Occupational risk factors for the development of systemic lupus erythematosus. J Rheumatol 31:1928–1933. [PubMed] [Google Scholar]
- 11.Feillet C, van der Horst GT, Levi F, Rand DA, Delauney F. 2015. Coupling between the circadian clock and cell cycle oscillators: implication for healthy cells and malignant growth. Front Neurol 6:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fenga C. 2016. Occupational exposure and risk of breast cancer. Biomed Rep 4:282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filipski E, Li XM, Levi F. 2006. Disruption of circadian coordination and malignant growth. Cancer Causes Control 17:509–514. [DOI] [PubMed] [Google Scholar]
- 14.Fonken LK, Nelson RJ. 2014. The effects of light at night on circadian clocks and metabolism. Endocr Rev 35:648–670. [DOI] [PubMed] [Google Scholar]
- 15.Fu L, Pelicano H, Liu J, Huang P, Lee C. 2002. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 111:41–50. [DOI] [PubMed] [Google Scholar]
- 16.Gan Y, Yang C, Tong X, Sun H, Cong Y, Yin X, Li L, Cao S, Dong X, Gong Y, Shi O, Deng J, Bi H, Lu Z. 2014. Shift work and diabetes mellitus: a meta-analysis of observational studies. Occup Environ Med 72:72–78. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert DJ, Neumann PE, Taylor BA, Jenkins NA, Copeland NG. 1993. Susceptibility of AKXD recombinant inbred mouse strains to lymphomas. J Virol 67:2083–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu F, Han J, Laden F, Pan A, Caporaso NE, Stampfer MJ, Kawachi I, Rexrode KM, Willett WC, Hankinson SE, Speizer FE, Schemhammer ES. 2015. Total and cause-specific mortality of U.S. nurses working rotating night shifts. Am J Prev Med 48:241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen AB, Stayner L, Hansen J, Andersen ZJ. 2016. Night shift work and incidence of diabetes in the Danish Nurse Cohort. Occup Environ Med 73:262–268. [DOI] [PubMed] [Google Scholar]
- 20.Haus EL, Smolenskyy MH. 2013. Shift work and cancer risk: Potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med Rev 17:273–284. [DOI] [PubMed] [Google Scholar]
- 21.He C, Anand ST, Ebell MH, Vena JE, Robb SW. 2014. Circadian disrupting exposures and breast cancer risk: a meta-analysis. Int Arch Occup Environ Health 88:533–547. [DOI] [PubMed] [Google Scholar]
- 22.Hedström AK, Akerstedt T, Hillert J, Olsson T, Alfredsson L. 2011. Shift work at young age is associated with increased risk for multiple sclerosis. Ann Neurol 70:733–741. [DOI] [PubMed] [Google Scholar]
- 23.Hoyert DL, Xu J. 2012. Deaths: preliminary data for 2011. Natl Vital Stat Rep 61:1–51. [PubMed] [Google Scholar]
- 24.Kivimäki M, Batty GD, Hublin C. 2011. Shift work as a risk factor for future type 2 diabetes: evidence, mechanisms, implications, and future research directions. PLoS Med 8:e1001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kojo K, Pukkala E, Auvinen A. 2005. Breast cancer risk among Finnish cabin attendants: a nested case-control study. Occup Environ Med 62:488–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolaja GJ, Fast PE. 1982. Renal lesions in MRL mice. Vet Pathol 19:663–668. [DOI] [PubMed] [Google Scholar]
- 27.Kubo T, Ozasa K, Mikami K, Wakai K, Fujino Y, Watanabe Y, Miki T, Nakao M, Hayashi K, Suzuki K, Mori M, Washio M, Sakauchi F, Ito Y, Yoshimura T, Tamakoshi A. 2006. Prospective cohort study of the risk of prostate cancer among rotating-shift workers: findings from the Japan collaborative cohort study. Am J Epidemiol 164:549–555. [DOI] [PubMed] [Google Scholar]
- 28.Lahti TA, Partonen T, Kyyronen P, Kauppinen T, Pukkala E. 2008. Night-time work predisposes to non-Hodgkin lymphoma. Int J Cancer 123:2148–2151. [DOI] [PubMed] [Google Scholar]
- 29.Leiter EH. 2009. Selecting the “right” mouse model for metabolic syndrome and type 2 diabetes research. Methods Mol Biol 560:1–17. [DOI] [PubMed] [Google Scholar]
- 30.Leiter EH, Reifsnyder PC. 2004. Differential levels of diabetogenic stress in 2 new mouse models of obesity and type 2 diabetes. Diabetes 53 Suppl. 1:S4–S11. [DOI] [PubMed] [Google Scholar]
- 31.Lin X, Chen W, Wei F, Ying M, Wei W, Xie X. 2015. Night-shift work increases morbidity of breast cancer and all-cause mortality: a meta-analysis of 16 prospective cohort studies. Sleep Med 16: 1381–1387. [DOI] [PubMed] [Google Scholar]
- 32.Magrini A, Pietroiusti A, Coppeta L, Babbucci A, Barnaba E, Papadia C, Iannaccone U, Boscolo P, Bergamaschi E, Bergamaschi A. 2006. Shift work and autoimmune thyroid disorders. Int J Immunopathol Pharmacol 19 Suppl 4:31–36. [PubMed] [Google Scholar]
- 33.Masri S, Kinouchi K, Sassone-Corsi P. 2015. Circadian clocks, epigenetics, and cancer. Curr Opin Oncol 27:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathews CE, Bagley R, Leiter EH. 2004. ALS/Lt: a new type 2 diabetes mouse model associated with low free radical scavenging potential. Diabetes 53 Suppl.1:S125–S129. [DOI] [PubMed] [Google Scholar]
- 35.Maywood ES, O'Neill J, Wong GK, Reddy AB, Hastings MH. 2006. Circadian timing in health and disease. Prog Brain Res 153:253–269. [DOI] [PubMed] [Google Scholar]
- 36.McMenamin TM. 2007. A time to work: recent trends in shift work and flexible schedules. Mon Labor Rev December:3–15. [Google Scholar]
- 37.Morris CJ, Yang JN, Scheer FA. 2012. The impact of the circadian timing system on cardiovascular and metabolic function. Prog Brain Res 199:337–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy G, Isenberg D. 2013. Effect of gender on clinical presentation in systemic lupus erythematosus. Rheumatology(Oxford) 52:2108–2115. [DOI] [PubMed] [Google Scholar]
- 39.Nascimento NF, Hicks JA, Carlson KN, Hatzidis A, Amaral DN, Seggio JA. 2016. 6-h advances alter circadian activity patterns, fasting glucose, and insulin levels in C57BL6/J mice. Biol Rhythm Res 47:133–143. [Google Scholar]
- 40.Nascimento NF, Hicks JA, Carlson KN, Hatzidis A, Logan RW, Seggio JA. 2015. Long-term wheel-running and acute 6-h advances alter glucose tolerance and insulin levels in TALLYHO/JngJ mice. Chronobiol Int 33:108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan A, Schemhammer ES, Sun Q, Hu FB. 2011. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med 8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parent ME, El-Zein M, Rousseau MC, Pintos J, Siemiatycki J. 2012. Night work and the risk of cancer among men. Am J Epidemiol 176:751–759. [DOI] [PubMed] [Google Scholar]
- 43.Purdue MP, Hutchings SJ, Rushton L, Silverman DT. 2015. The proportion of cancer attributable to occupational exposures. Ann Epidemiol 25:188–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puttonen S, Oksanen T, Vahtera J, Pentti J, Virtanen M, Salo P, Kivimaki M. 2010. Is shift work a risk factor for rheumatoid arthritis? The Finnish public sector study. Ann Rheum Dis 69:679–680. [DOI] [PubMed] [Google Scholar]
- 45.Rajaratnam SM, Arendt J. 2001. Health in a 24-h society. Lancet 358:999–1005. [DOI] [PubMed] [Google Scholar]
- 46.Rao D, Yu H, Bai Y, Zheng X, Xie L. 2015. Does night-shift work increase the risk of prostate cancer? A systematic review and meta-analysis. Onco Targets Ther 8:2817–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ray MA, Johnston NA, Verhulst SJ, Trammell RA, Toth LA. 2010. Identification of markers for imminent death in mice used in longevity and aging research. J Am Assoc Lab Anim Sci 49:282–288. [PMC free article] [PubMed] [Google Scholar]
- 48.Reifsnyder PC, Leiter EH. 2002. Deconstructing and reconstructing obesity-induced diabetes (diabesity) in mice. Diabetes 51:825–832. [DOI] [PubMed] [Google Scholar]
- 49.Sangle SR, Tench CM, D'Cruz DP. 2015. Autoimmune rheumatic disease and sleep: a review. Curr Opin Pulm Med 21:553–556. [DOI] [PubMed] [Google Scholar]
- 50.Schwartzman-Morris J, Putterman C. 2012. Gender differences in the pathogenesis and outcome of lupus and of lupus nephritis. Clin Dev Immunol 2012:604892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silva-Costa A, Rotenberg L, Coeli CM, Nobre AA, Harter-Griep R. 2016. Night work is associated with glycemic levels and anthropometric alterations preceding diabetes: Baseline results from ELSA-Brazil. Chronobiol Int 33:64–72. [DOI] [PubMed] [Google Scholar]
- 52.Stevens RG, Brainard GC, Blask DE, Lockley SW, Motta ME. 2014. Breast cancer and circadian disruption from electric lighting in the modern world. CA Cancer J Clin 64:207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, Altieri A, Benbrahim-Tallaa L, Cogliano VJ, WHO International Agency For Research on Cancer Monograph Working Group 2007. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol 8:1065–1066. [DOI] [PubMed] [Google Scholar]
- 54.Szosland D. 2010. Shift work and metabolic syndrome, diabetes mellitus and ischaemic heart disease. Int J Occup Med Environ Health 23:287–291. [DOI] [PubMed] [Google Scholar]
- 55.Toth LA, Trammell RA, Ilsley-Woods M. 2015. Interactions between housing density and ambient temperature in the cage environment: effects on mouse physiology and behavior. J Am Assoc Lab Anim Sci 54:708–717. [PMC free article] [PubMed] [Google Scholar]
- 56.Trammell RA, Cox L, Toth LA. 2012. Markers for heightened monitoring, imminent death, and euthanasia in aged inbred mice. Comp Med 62:172–178. [PMC free article] [PubMed] [Google Scholar]
- 57.Trammell RA, Verhulst S, Toth LA. 2013. Environmental perturbation, inflammation and behavior in healthy and virus-infected inbred mice. Brain Behav Immun 33:139–152. [DOI] [PubMed] [Google Scholar]
- 58.Trammell RA, Toth LA. 2016. Effects of chronic diurnal disruption and acute inflammatory challenge on mice with latent murine gammaherpesvirus infection. Comp Med 66: 445–454. [PMC free article] [PubMed] [Google Scholar]
- 59.Vyas MV, Garg AX, Iansavichus AV, Costella J, Donner A, Laugsand LE, Janszky I, Mrkobrada M, Parranga G, Hackam DG. 2012. Shift work and vascular events: systematic review and meta-analysis. BMJ 345:e4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yuan R, Meng Q, Nautyal J, Flurkey K, Tsaih SW, Krier R, Parker MG, Harrison DE, Paigen B. 2012. Genetic coregulation of age of female sexual maturation and lifespan through circulating IGF1 among inbred mouse strains. Proc Natl Acad Sci USA 109:8224–8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yuan R, Tsaih SW, Petkova SB, Marin de Evsikova C, Xing S, Marion MA, Bogue MA, Mills KD, Peters LL, Bult CJ, Rosen CJ, Sundberg JP, Harrison DE, Churchill GA, Paigen B. 2009. Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell 8:277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]