Abstract
The role of host type I IFN signaling and its interaction with other immune pathways during bacterial infections is incompletely understood. Type II IFN signaling plays a key role during numerous bacterial infections including granulocytic anaplasmosis (GA) caused by Anaplasma phagocytophilum infection. The function of combined type I and type II IFN signaling and their potential synergism during GA and similar tick-borne diseases is a topic of current research investigation. The goal of this study was to evaluate 2 mouse models of absent type I/type II IFN signaling in experimental A. phagocytophilum infection to determine the effects of background strain. Mice lacking both type I and type II IFN receptor signaling (IFNAR−/−/IFNGR–/–) on either the 129/SvEv or C57BL/6J genetic background were evaluated at days 0, 6, 8, and 12 of infection. Pathogen burden in multiple organs was largely similar between strains of infected mice, with few significant differences. Background strain influenced the immune response to infection. Mice of the 129/SvEv strain developed more severe hematologic abnormalities, particularly more severe leukocytosis with marked neutrophilia and lymphocytosis, throughout acute infection. Histopathologic changes occurred in infected mice of both strains and varied in severity by organ. 129/SvEv mice developed more severe pathologic changes in spleen and bone marrow, whereas C57BL/6J mice developed more severe renal pathology. This work highlights the importance of mouse background strain in dictating pathophysiologic response to infection and informs future work regarding the loss of type I and type II IFN signaling on the immune response during GA.
Abbreviations: AG129, IFNAR−/−/IFNGR–/– mice on 129/SvEv background strain; AGB6, IFNAR−/−/IFNGR–/– mice on C57BL/6 (B6) background strain; dpi, days postinfection; GA, granulocytic anaplasmosis
Granulocytic anaplasmosis (GA) caused by the tick-borne bacterial pathogen Anaplasma phagocytophilum occurs in humans and numerous animal species including dogs, horses, and ruminants.51 GA is characterized by fever, joint pain, and multiple peripheral cytopenias as well as clinical chemistry alterations including increased hepatic transaminases.4,13,22
Clinical outcomes of human infection are quite diverse, with minimal or no symptoms occurring in a majority of infected humans. In the United States, a hospitalization rate of 36% was reported, with 7% requiring intensive care and a case fatality rate of less than 1%.3,15,20 In contrast, a cohort study in China reported a mortality rate of 26.5% (22 of 83) in hospitalized patients.34 A. phagocytophilum is endemic or potentially endemic in many countries. Seroprevalence varies greatly by geographic region and correlates with prevalence of its appropriate Ixodes spp. tick vector as well as the bacterium itself. A recent survey of Ixodes scapularis ticks in Pennsylvania reported an infection rate of 3.3% for A. phagocytophilum.27
Domestic ruminants are susceptible to GA, which is currently the most widespread tick-borne infection of livestock in Europe.50 Infection rates determined by PCR analysis in European livestock are as high as 20% in cattle, 37% in sheep, and 5.6% in goats.51 Clinical disease can be accompanied by decreased milk production, abortion, decreased fertility, and poor growth in juveniles; economic losses due to these perturbations can be substantial.24,50,56 As with human infection, GA in horses is associated with a diverse range of outcomes, from mild disease with nonspecific clinical signs to rare acute mortality despite appropriate treatment.22 Histologic evidence of vasculitis and multiple organ failure is documented in fatal cases of equine GA.22,23,33,36
A. phagocytophilum infection of laboratory mice recapitulates many of the clinicopathologic abnormalities of human infections.26,52 A number of inbred mouse strains are susceptible to infection but severity of disease and pathogen burden vary by strain, and strain-specific genetic abnormalities can greatly affect immune responses to infection in wild-type mice.9 In addition, research on GA frequently uses genetically altered mice, commonly on the C57BL/6 (B6) or 129/SvEv (129) genetic background, including various immunodeficient mouse models.9 Infection outcomes are thereby influenced by combined effects of genetic manipulation and inbred mouse strain.
The role of type I interferon (IFN) signaling in tick-borne bacterial infections is a subject of current scientific interest. Beneficial effects of host type I IFN signaling in viral infections are well established, but the role of host type I IFN signaling in bacterial infections is unclear and likely complex, given that both beneficial and deleterious effects are described.14,53 The specific role of type I IFN signaling in GA and related rickettsial infections is starting to undergo scientific investigation. Type I IFN signaling promoted pathogenic effects of infection and increased pathogen burden in a mouse model of severe monocytic ehrlichiosis; one relevant mechanism was altered effects of signaling by IFNγ, the sole type II IFN, due to absent type I IFN signaling.57 IFNγ signaling mediates host protective responses in monocytic ehrlichiosis and is a key controller of pathogen burden in early GA.1,8,55 However, the absence of IFNγ signaling lessens inflammatory pathology in GA independent of pathogen burden.1,37 The combined effects of type I and II IFN signaling during GA are poorly understood, and infection outcomes in the complete absence of both signaling pathways have not been described previously.
A recent study examining several outcomes of GA in mouse models lacking Stat1 signaling found that infected Stat1-deficient mice exhibited greatly increased pathogen burden and levels of inflammatory indicators compared with infected wildtype 129Sv mice.18 Stat1 deletion represses type II IFN signaling and partially reduces type I IFN signaling, as type I IFN signaling induces formation of the ISG factor 3 complex composed of the phosphorylated forms of STAT1 and STAT2 along with IRF9, as well as numerous STAT complexes that lack STAT1.42 As such, Stat1 deletion represents loss of most biologic activities of type I and II IFN signaling, but is not a complete abolishment of IFN signaling. Development of a mouse model entirely lacking type I and II IFN signaling is indicated for further investigation. Refinement of mouse models requires consideration of several key factors, particularly background strain. Strain has been an important variable in prior GA studies using genetically altered mice, including an investigation of GA in IFNγ-deficient mice of 2 background strains that found increased susceptibility to infection in IFNγ-deficient mice on the 129Sv genetic background compared with those on the C57BL/6 genetic background.55
The goal of this study was to determine whether mouse genetic background influenced pathogen burden and hematologic and histopathologic abnormalities in experimental A. phagocytophilum infection of mice lacking both type I and type II IFN signaling. Hematologic parameters have not been reported previously during infection of mice lacking type I IFN, Stat1, or type II IFN signaling. This information is necessary to determine the model's usefulness in future research, because hematopathology is a key readout of disease due to natural or experimental infection. Mice of C57BL/6 and 129/SvEv background strains deficient in both type I and type II IFN receptors (IFNAR−/−/IFNGR–/–) were used in an acute A. phagocytophilum infection model. The results of the study proved that background strain influences several key hematopathologic outcomes in response to infection and therefore may dictate the severity of disease and interpretation of generated data.
Materials and Methods
Mice and in vivo experimental infection.
Female Beige SCID (C.B-17/IcrHsd-Prkdcscid Lystbg-J) mice (age, 6 to 7 wk) were purchased from Envigo (Indianapolis, IN). AG129 mice (129/SvEv-Ifnar1tm1Agt Ifngr1tm1Agt), a 129/SvEv strain deficient for the receptors for both type I and type II IFN, were a gift from Dr HB Greenberg (Stanford University).54 The C57BL/6 congenic strain, AB6, was generated by backcrossing C57BL/6.129S2-Ifnar1tm1Agt/Mmjax (N5) to C57BL/6J (Jackson Laboratory, Bar Harbor, ME) for an additional 5 generations. The AGB6 (C57BL/6.129 -Ifnar1tm1Agt Ifngr1tm1Agt) strain was generated by crossing AB6 (N10) and GB6 (N11) (C57BL/6.129S7-Ifngr1tm1Agt/J).
The Stanford vivaria are AAALAC-accredited. Husbandry is performed in accordance with the Guide for the Care and Use of Laboratory Animals28 and the Public Health Service Policy on Humane Care and Use of Laboratory Animals38. Room conditions included a temperature of 23 °C ± 2 °C, relative humidity of 30% to 40%, and a 12:12-h light:dark cycle (lights on, 0700 h). All mouse colonies are maintained under SPF conditions in irradiated disposable IVC (Innocage, Innovive, San Diego, CA) with irradiated, corncob bedding, irradiated food (Teklad 2918 Global 18% Protein Rodent Diet, Envigo) and UV-irradiated, acidified (pH, 2.5 to 3.0), reverse-osmosis–purified bottled water (Aquavive, Innovive). The colonies are monitored for adventitious viral, bacterial, and fungal pathogens by a dirty-bedding sentinel program. The sentinels are tested every 4 mo and were found free of mouse parvovirus, minute virus of mice, mouse hepatitis virus, mouse rotavirus, Theiler murine encephalomyelitis virus, murine norovirus, Sendai virus, mouse adenovirus types 1 and 2, ectromelia (mousepox), lymphocytic choriomeningitis virus, pneumonia virus of mice, respiratory enterovirus III (reovirus 3), Mycoplasma pulmonis, Helicobacter spp., Pasteurella pneumotropica, and ecto- and endoparasites. Mice in this study were housed in filter-topped, static cages with nonsterile hardwood chip bedding, fed commercial rodent chow (Teklad 2018 Global 18% Protein Rodent Diet, Envigo Laboratories) without restriction and provided with reverse-osmosis–purified water. Mice were euthanized by CO2 asphyxiation according to the 2013 AVMA guidelines on euthanasia.
A. phagocytophilum strain NCH1 was used for all infections and was maintained through 2 consecutive intraperitoneal passages of infected blood in Beige SCID mice, as previously described.26 Blood from infected Beige SCID mice was collected approximately 10 d after inoculation and pooled, and a 25-µL aliquot was tested by quantitative PCR analysis to detect A. phagocytophilum p44 DNA and to quantify and standardize the initial infectious dose. Experimental mice between 6 and 8 wk of age were inoculated intraperitoneally with 100 µL of pooled infected anticoagulated blood that contained an infectious dose of 234,924 p44 copy number (see PCR section following). Mice were weighed and monitored daily during infection for development of clinical signs including body condition scoring, assessment for alert mentation and responsiveness to stimuli, hydration evaluation via skin tenting, and evaluation for fur ruffling, postural abnormalities (for example, hunching) and facial grimace scale. Mice were euthanized at 0, 6, 8, and 12 d postinfection (dpi). Total animal numbers for the experiments were 10 dpi 0 (control) B6 IFNAR−/−/IFNGR–/– (AGB6) mice, 10 dpi 0 (control) 129 IFNAR−/−/IFNGR–/– (AG129) mice, 18 infected AGB6 mice, and 18 infected AG129 mice. For the infected mice, 6 mice of each background (AGB6 or AG129) were euthanized at each time point (6, 8, and 12 dpi) of infection. Male and female mice were evenly distributed within each group.
Tissue collection and processing.
Mice were weighed after euthanasia. Blood, spleen, liver, kidneys, lung, brain, sternum, inguinal lymph nodes, and bone marrow were collected at necropsy. All animals were carefully examined at gross necropsy by a board-certified veterinary pathologist (JJ). Blood was collected by cardiocentesis and placed into EDTA microtainers (Becton Dickinson, Rutherford, NJ). Whole blood was used for quantitative PCR analysis and to perform CBC counts and differential leukocyte counts through review of blood smears. The spleen was removed and weighed. Sections of spleen, liver, kidneys, lung, and brain were placed in 10% buffered neutral formalin solution (Fisher Scientific, Pittsburgh, PA) for histopathology. Sternums were placed in Cal-Ex decalcifying solution (Fisher Scientific) for 24 h and then into 10% buffered neutral formalin for histopathology. Portions (25 mg each) of liver, kidney (comprising pooled sections from both kidneys), lung, and brain along with pooled bilateral inguinal lymph nodes were removed and banked at –80 °C for quantitative PCR evaluation. A single-cell suspension was made from the remaining spleen. In brief, the spleen was gently crushed through a 70-µm nylon sterile strainer (BD Biosciences, San Jose, CA) and suspended in staining buffer (Dulbecco modified PBS containing 0.5% bovine serum albumin and 5 mM EDTA). Bone marrow was harvested from both femurs and both tibias by removing musculature and flushing by using staining buffer. A single-cell suspension was made by passing bone marrow though a 25-gauge needle. Bone marrow and spleen cell suspensions were counted on an automated hematology analyzer (model XT-2000iV, Sysmex, Kobe, Japan), after which aliquots of 0.5 × 106 cells were pelleted and banked at –80 °C for quantitative PCR evaluation.
DNA extraction and quantitative PCR analysis.
DNA was extracted from 25 μL of peripheral blood, from banked spleen and bone marrow cell pellets, from banked 25-mg portions of liver, kidney (comprising pooled sections from both kidneys), lung, and brain and from banked pooled lymph nodes by using the DNeasy Tissue Kit (QIAGEN, Valencia, CA). Forward and reverse primers and probe were synthesized by using p44 target gene sequences (GenBank accession no., AF037599) exactly as previously described.10 The probe was labeled at the 5′ end with the reporter dye FAM (6-carboxyfluorescein) and at the 3′ end with the quencher dye TAMRA (6-carboxy-tetramethyl-rhodamine). TaqMan Universal PCR Master Mix (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA) was used, and thermal cycling DNA amplification, data acquisition, and initial data analysis were performed in a StepOne Plus system (Applied Biosystems). A standard curve was created by using p44 plasmid DNA as template. A log dilution series was used as previously described25 with analytical sensitivity of 3 to 109 copies.
Hematologic and histopathologic evaluation.
CBC analysis was performed on the Sysmex XT-2000iV analyzer within 4 h of blood collection. Blood smears were prepared and stained with Wright–Giemsa stain (Camco Quik Stain II, Cambridge Diagnostic Products, Fort Lauderdale, FL). Tissues in 10% buffered neutral formalin were embedded in paraffin, sectioned to 5 µm thickness, and stained with hematoxylin and eosin (Richard-Allan Scientific, Kalamazoo, MI). All blood smears and histopathology slides were evaluated in a blinded manner by board-certified veterinary clinical and anatomic pathologists (JJ and AK). Leukocyte differentials (200 cells) were performed manually and blood cell morphology assessed. Histopathologic abnormalities were graded on a semiquantitative scale: 0, no significant lesions; 1+, mild abnormality; 2+, moderate abnormality; 3+, marked abnormality. Extramedullary hematopoiesis was present in spleens of all mice and was not a scored parameter, because it is an expected finding in normal mouse spleen and because significant increases can be challenging to confirm morphologically.
Statistical analyses.
Data were initially exported into Excel 2013 (Office Professional Plus, Microsoft, Redmond, WA). Subsequent analysis was performed in Prism 6 for Windows (GraphPad Software, La Jolla, CA). A P value of less than 0.05 was considered significant. Normal distribution was evaluated by using the D'Agostino and Pearson omnibus normality test. Outlier data were identified by using the ROUT method with Q = 1% and removed from subsequent analysis. Serial mouse weight measurements were analyzed by using one-way repeated-measures ANOVA. Pathogen burden in peripheral blood was normalized to the absolute neutrophil count, similar to prior studies.11 All data were normally distributed either in raw form or after log-normal transformation, with the exception of data from brain tissue. Data from brain tissue were analyzed by using the Kruskal–Wallis nonparametric test with Dunn posthoc testing. Data from all other tissues were analyzed by using one-way ANOVA with Holm–Sidak posthoc testing (α = 5%) to generate multiplicity-adjusted P values. All hematology parameters were normally distributed either in raw form or after log-normal transformation and were analyzed by using one-way ANOVA with Holm–Sidak post hoc testing (α = 5%). Spleen weight data were nonnormally distributed and were analyzed by using Mann–Whitney nonparametric rank comparison.
Results
Clinical signs of illness were rarely observed. Rare mice of both strains displayed mild ruffling of fur periodically throughout infection, but other clinical signs were not noted. Serial weight measurements did not differ significantly over time (data not shown).
Pathogen burden.
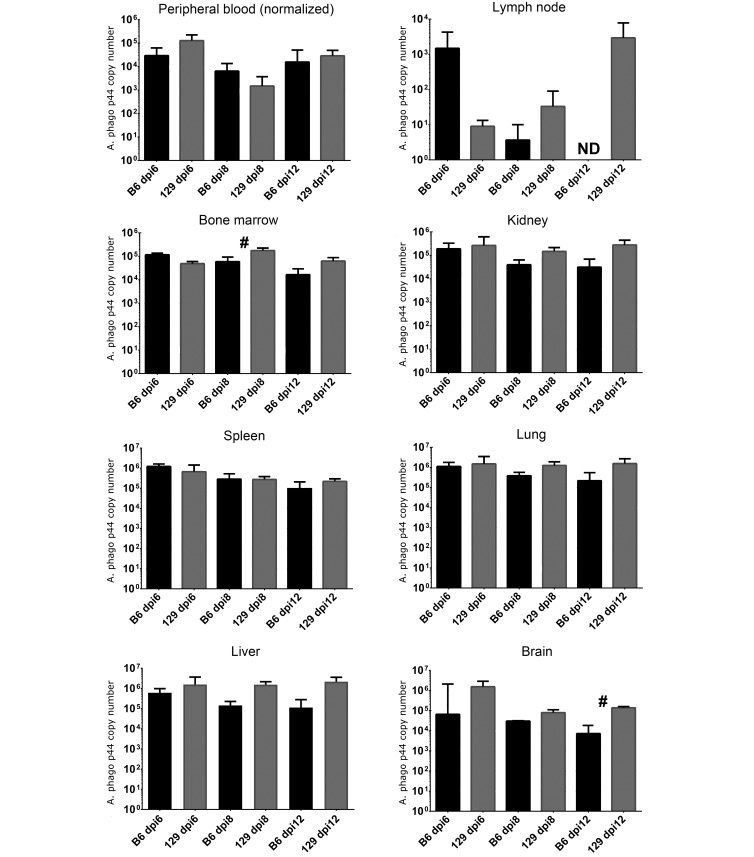
Data were analyzed for differences in pathogen burden between mice of the 2 strains in each organ or tissue and at each time point of infection. Pathogen burden was significantly (P < 0.05) higher in AG129 mice than AGB6 mice at 8 dpi in bone marrow and at 12 dpi in brain (Figure 1). At 12 dpi, pathogen burden in inguinal lymph node was detectable in AG129 mice, with a mean value of 2938 p44 copies per 25 µL of whole blood, but was not detectable in AGB6 mice. Other differences between strains were not statistically significant. One AG129 mouse and three AGB6 mice were negative for pathogen DNA in peripheral blood yet had high levels in other organs or tissues at the same time point (data from individual mice not shown).
Figure 1.
Mean (error bar, 1 SD) pathogen burden quantified by quantitative PCR analysis as bacterial copy number for all tissues except brain (median [bars, interquartile range]) in C57BL/6J (B6) and 129/SvEv (129) IFNAR−/−/IFNGR–/– mice at 6, 8, and 12 days postinfection (dpi). A total of 18 infected mice are represented. Pathogen burden from 25 µL of peripheral blood was normalized to the absolute neutrophil count per mL for each mouse prior to statistical analysis. Pathogen burden in spleen and bone marrow represents 0.5 × 106 cells. Pathogen burden in kidney, liver, lung, and brain represents 25 mg tissue. Pathogen burden in lymph node represents both inguinal nodes. #, Value significantly different (P < 0.05) between mice of the 2 backgrounds at the same time point; ND, not detected. Black bars = AGB6 mice; grey bars = AG129 mice.
Hematologic findings.
Data were analyzed for differences in each analyte between mice of the 2 backgrounds at each time point of infection. In addition, data were analyzed for differences within mice of the same background strain between dpi 0 (uninfected controls) and at each time point.
RBC parameters.
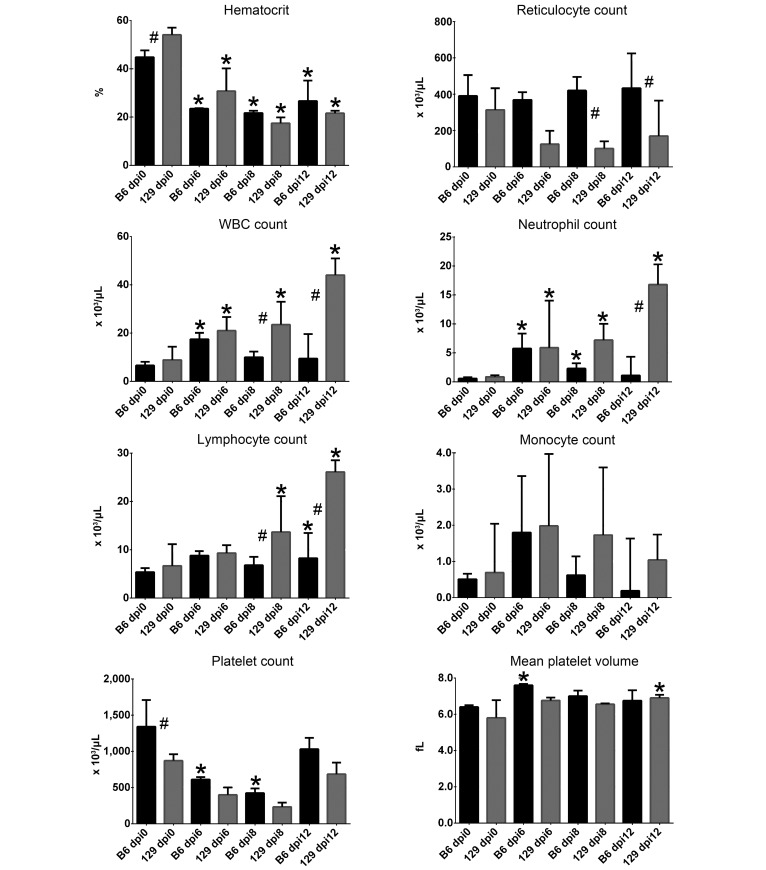
Hct at dpi 0 differed significantly between strains, with values in AG129 mice higher than those in AGB6 mice (Figure 2). This strain-associated difference was reduced by infection. Hct in infected AGB6 and AG129 mice was significantly (P < 0.05) lower than that in the strain-matched dpi 0 controls at all time points. RBC regenerative response, as measured by reticulocyte count, significantly differed due to background strain at 8 and 12 dpi, with values in AG129 mice lower at both time points; reticulocyte count did not differ between strain-matched dpi 0 mice and infected mice of each background at any point. Infection-induced nonregenerative anemia (defined as a reticulocyte response that is inadequate, given the degree of anemia) was therefore present in mice of both backgrounds, with a lack of appropriate regeneration that was more pronounced in AG129 mice. At 12 dpi, anemia was resolving faster in AGB6 mice, consistent with the higher degree of reticulocytosis, although values were still significantly lower than those in matched dpi 0 mice.
Figure 2.
Median (bars, interquartile range) values for key hematologic parameters in C57BL/6J (B6) and 129/SvEv (129) IFNAR−/−/IFNGR–/– mice at 0, 6, 8, and 12 days postinfection (dpi). *, Value significantly different (P < 0.05) within strain and relative to dpi 0; #, value significantly different (P < 0.05) between mice of the 2 background strains at the same time point. Black bars = AGB6 mice; grey bars = AG129 mice.
WBC parameters.
Total WBC count was higher in AG129 mice than AGB6 mice at 8 and 12 dpi. In AG129 mice, WBC was higher at all time points of infection compared with strain-matched dpi 0 control mice. WBC count in infected AGB6 mice was higher than that in matched dpi 0 control mice at 6 dpi. Increased total WBC count in infected AG129 mice was due to increases in neutrophils and lymphocytes, because neutrophils were significantly increased at 6 and 8 dpi, and lymphocytes were increased at 12 dpi. Although monocyte counts did not differ significantly, both AGB6 and AG129 mice tended to develop monocytosis compared with matched dpi 0 mice starting at 6 dpi, with resolution (that is, mean value returning to dpi 0 baseline) by 12 dpi in AGB6 but not AG129 mice.
Platelet parameters.
Baseline platelet count at dpi 0 was significantly (P < 0.05) higher in AGB6 mice than AG129 mice. Platelet count at 6 and 8 dpi in infected AGB6 mice was lower (P < 0.05) than that in strain-matched dpi 0 controls. At 6 dpi, MPV was higher (P < 0.05) in AGB6 mice than in matched controls, indicating a platelet regenerative response that began soon after infection in this strain. By dpi 12, platelet counts in AGB6 mice did not differ from baseline values, compatible with regeneration and recovery from acute thrombocytopenia. Platelet counts did not differ significantly in infected AG129 mice, although mean values at 6 and 8 dpi were decreased compared with matched controls (Figure 2). MPV was significantly increased at 12 dpi in infected AG129 mice, consistent with a platelet regenerative response to thrombocytopenia that was substantially delayed in this strain when compared with that in AGB6 mice, which began at 6 dpi.
Gross pathologic and histopathologic findings.
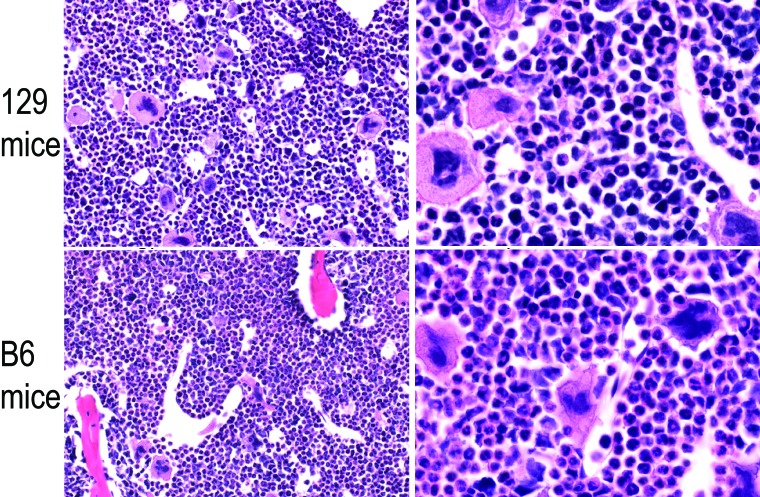
Splenomegaly was noted grossly in all infected mice. Spleen weights were significantly (P < 0.05) increased in mice of both backgrounds at each time point of infection compared with strain-matched dpi 0 control mice (Table 1). In addition, spleens at 12 dpi were larger (P < 0.05) in AG129 mice than AGB6 mice. No other gross lesions were observed at necropsy of any animal. Significant findings from histopathologic evaluation of routinely prepared sections are listed in Table 2. No significant lesions were noted in cerebrum at any time point. Numerous histopathologic alterations, including bone marrow myeloid hyperplasia, were noted in the organs examined. Histopathologic changes, including neutrophilic interstitial pneumonia, were often similar in infected mice of both backgrounds. More severe splenic lymphoid hyperplasia and reactive plasmacytosis were documented in AG129 mice. Splenomegaly was attributed to the lymphoid hyperplasia noted on histopathology, with potentially an additional contribution from increased extramedullary hematopoiesis that was not scored. AGB6 mice had slightly more severe changes in liver and kidney, including neutrophilic multifocal hepatitis and suspected membranoproliferative glomerulonephritis. Representative histopathologic images of each tissue or organ with histopathologic abnormalities in AG129 and AGB6 mice at 12 dpi are shown in Figures 3 and 4. We chose the 12-dpi time point because it represented the point of greatest severity for many histopathologic changes.
Table 1.
Descriptive statistics and Pvalues for spleen weights (mg) from all C57BL/6J (B6) and 129/SvEv (129) IFNAR−/−–IFNGR–/–mice at 0, 6, 8, and 12 dpi
| 0 dpi |
6 dpi |
8 dpi |
12 dpi |
|||||
| B6 | 129 | B6 | 129 | B6 | 129 | B6 | 129 | |
| Minimum | 60 | 40 | 183 | 135 | 174 | 171 | 136 | 254 |
| 25% Percentile | 63 | 45 | 188.3 | 139.3 | 174.3 | 187.5 | 141 | 269.3 |
| Median | 68.5 | 50.5 | 237.5 | 163.5 | 239.5 | 310 | 162 | 315 |
| 75% Percentile | 75.5 | 61 | 274.8 | 217.8 | 340.8 | 329 | 224.8 | 321.8 |
| Maximum | 80 | 67 | 276 | 232 | 353 | 337 | 280 | 324 |
| 95% CI of median, lower bound | 60 | 42 | 183 | 135 | 174 | 171 | 136 | 254 |
| 95% CI of median, upper bound | 80 | 61 | 276 | 232 | 353 | 337 | 280 | 324 |
| P value (compared with dpi 0 value) | ns | ns | <0.01 | <0.01 | <0.001 | <0.01 | <0.001 | <0.01 |
| P value (between strains) | ns | ns | ns | ns | ns | ns | <0.01 | <0.01 |
ns, not statistically significant
Data from most groups were nonparametrically distributed. Mann–Whitney testing was used to compare spleen weights from infected mice with either control (0 dpi) mice of the same strain or with mice of the other strain at the same day of infection.
Table 2.
Summarized histopathologic findings from representative 129/SvEv (129) and C57BL/6J (B6) IFNAR−/−/IFNGR–/–mice at 0, 6, 8, and 12 dpi
| 129 | B6 | |
| Spleen | ||
| 0 dpi | no significant lesions | no significant lesions |
| 6 dpi | 1+ lymphoid hyperplasia (2/2) | 1+ lymphoid hyperplasia (1/2) |
| 8 dpi | 3+ lymphoid hyperplasia, reactive plasmacytosis (2/2) | 1+ lymphoid hyperplasia, reactive plasmacytosis (2/2) |
| 12 dpi | 2+ and 3+ lymphoid hyperplasia, reactive plasmacytosis | 1+ lymphoid hyperplasia, reactive plasmacytosis (2/2) |
| Liver | ||
| 0 dpi | no significant lesions | 2+ glycogenosis (1/2) |
| 6 dpi | 1+ extramedullary hematopoiesis (2/2) | 1+ lymphoplasmacytic and neutrophilic portal hepatitis (2/2); 1+ extramedullary hematopoiesis (2/2) |
| 8 dpi | 2+ lymphoplasmacytic portal hepatitis (2/2); 1+/2+ extramedullary hematopoiesis | 1+ multifocal random neutrophilic hepatitis (1/2); 1+ extramedullary hematopoiesis (2/2) |
| 12 dpi | 2+ lymphoplasmacytic portal hepatitis (2/2); 1+ extramedullary hematopoiesis (2/2) | 2+ lymphoplasmacytic and neutrophilic portal hepatitis (1/2); 1+ multifocal random neutrophilic hepatitis (1/2); 1+ extramedullary hematopoiesis (2/2) |
| Lung | ||
| 0 dpi | no significant lesions | no significant lesions |
| 6 dpi | 1+ neutrophilic interstitial pneumonia (2/2); circulating neutrophilia (2/2) | 2+ neutrophilic interstitial pneumonia with segmental pleuritis and mesothelial hyperplasia (2/2); circulating neutrophilia (2/2) |
| 8 dpi | 2+ neutrophilic interstitial pneumonia (2/2); circulating neutrophilia (2/2) | 2+ neutrophilic interstitial pneumonia with segmental pleuritis and mesothelial hyperplasia (2/2); circulating neutrophilia (2/2) |
| 12 dpi | 2+ neutrophilic interstitial pneumonia with segmental pleuritis and mesothelial hyperplasia (2/2); circulating neutrophilia (2/2) | 1+ and 3+ neutrophilic interstitial pneumonia (2/2); segmental pleuritis and mesothelial hyperplasia (1/2); circulating neutrophilia (2/2) |
| Kidney | ||
| 0 dpi | no significant lesions | 1+ focal tubular proteinosis (1/2) |
| 6 dpi | 1+ focal tubular proteinosis (1/2) | 1+ global neutrophilic membranoproliferative glomerulonephritis (2/2); 1+ focal tubular proteinosis (2/2) |
| 8 dpi | no significant lesions | 1+ global neutrophilic membranoproliferative glomerulonephritis (2/2); 1+ focal tubular proteinosis (1/2) |
| 12 dpi | 1+ global neutrophilic membranoproliferative glomerulonephritis (2/2) | 2+ global neutrophilic membranoproliferative glomerulonephritis (2/2); 1+ focal tubular proteinosis (2/2) |
| Bone marrow | ||
| 0 dpi | no significant lesions | no significant lesions |
| 6 dpi | 3+ increase in myeloid:erythroid ratio (2/2) | 2+/3+ increase in myeloid:erythroid ratio |
| 8 dpi | 3+ increase in myeloid:erythroid ratio (2/2) | 2+/3+ increase in myeloid:erythroid ratio |
| 12 dpi | 3+ increase in myeloid:erythroid ratio (2/2) | 2+ and 3+ increase in myeloid:erythroid ratio |
Two mice from each background time point of infection were evaluated; data are reported in parentheses as ‘no. affected/no. evaluated,’ except where multiple degrees of the same abnormality were noted in each mouse.
Figure 3.
Histopathology image comparison between 129/SvEv (129) and C57BL/6J (B6) IFNAR−/−/IFNGR-/- mice at 12 d postinfection. Paired images for (A, B) spleen, (C, D) liver, (E,F) lung, and (G, H) kidney. Hematoxylin and eosin stain; magnification: 10× (A, C, E, G), 40× (B, D, F, H).
Figure 4.
Bone marrow image comparison between 129/SvEv (129) and C57BL/6J (B6) IFNAR−/−/IFNGR-/- mice at 12 d postinfection. Tissue sections were routinely stained with hematoxylin and eosin. Paired histopathologic images shown at 40× (left panels) and 100× (right panels) objective magnification.
Discussion
The findings in this study highlight the importance of mouse background strain in the hematologic and pathologic alterations during infection with A. phagocytophilum. Mice entirely deficient in both type I and type II IFN signaling have not previously been used for experimental A. phagocytophilum infection, and there is minimal to no information regarding infection-induced hematologic and histopathologic changes in mice that lack either type of IFN signaling. The absence of type I and type II IFN signaling potentially uncovered differences in infection-induced outcomes between mice of different genetic backgrounds that may be lessened or absent in wildtype mice of the same backgrounds. Future work comparing these differences with those in wildtype mice and mice deficient in either type I or type II IFN will be necessary to determine the relative roles of IFN signaling deficiency compared with strain in these infection-induced outcomes. The most common mouse background strains used in GA research are C3H/HeN mice and B6 mice, with B6 mice often used due to availability of mutant mice on this background.9 IFNγ is well established as a key effector of the immune response to infection and is markedly upregulated by A. phagocytophilum infection in humans, horses, and mice.1,19,21,39 Absence or reduction of IFNγ signaling results in increased pathogen burden in early infection, but pathogen elimination still occurs.1,7,39 A comparison of type II IFN signaling deficiency in mice of the 129 and B6 background strains found that, compared with B6 mice deficient in IFNγ, 129 mice deficient in either IFNγ or its receptor had higher pathogen burdens through the first 3 wk of infection.55 Histopathologic changes in some organs and tissues during experimental GA are lessened when IFNγ signaling is absent or reduced, even though pathogen burdens may be significantly higher compared with those in infected wild-type mice.1,37,55
The outcome of A. phagocytophilum infection in mice lacking type I IFN signaling is incompletely understood. A recent study evaluating infection in 129Sv mice lacking Stat1 reported increased infection-induced pathology, including development of clinical signs of illness, in the infected Stat1-deficient mice compared with wildtype mice.18 A. phagocytophilum infection of C57BL/6 mice deficient in the receptor for IFNα resulted in significantly higher pathogen burden in peripheral blood, spleen and lung, suggesting an important role of type I IFN in inducing an IFNγ response.7 A marked increase in splenic cell Stat1 phosphorylation was previously demonstrated during acute A. phagocytophilum infection in B6 mice, with a corresponding increase in plasma IFNγ concentration.17 In human neutrophils, A. phagocytophilum infection decreased the expression of IFNγ receptor 1 (CD119) and diminished the IFNγ-induced phosphorylation of STAT1, a likely mechanism of impaired antibacterial effects.12
Recent work with related bacterial pathogens revealed a critical role for type I IFN during mouse infection with Ixodes ovatus Ehrlichia, a highly virulent bacterium transmitted by Ixodes ovatus.57 In wildtype mice, type I IFN was induced by infection and promoted increased pathogen burden and decreased survival, compared with infected mice that either lacked type I IFN receptor (that is, Ifnar-deficient) or were administered type I IFN-neutralizing agents. Induction of IFNγ during infection with Ixodes ovatus Ehrlichia was minimal. In contrast, induction of type I IFN was not seen in the same study57 during infection with the less-virulent pathogen Ehrlichia muris, whereas IFNγ induction was marked. In the present study, mouse strain was a significant factor regarding differences in pathogen burden in a few but not most time points. For example, pathogen burden trended higher in AG129 mice than in AGB6 at later time points of infection. At 12 dpi, bacteria were still present in all mice at all sites, with the exception of lymph nodes in AGB6 mice.
The RBC and platelet responses in our study were generally consistent with natural disease of humans and animals and experimental infection in mice, given that nonregenerative anemia and thrombocytopenia occurred by 6 dpi in mice of both strains. Mean reticulocyte counts were substantially lower in infected AG129 mice compared with infected AGB6 mice, indicating greater deficiency in appropriate RBC regeneration in response to anemia. MPV increased significantly in B6 mice, and platelet count rebounded by 12 dpi; 129 mice had a smaller increase in MPV, but platelet count also rebounded toward baseline by 12 dpi. WBC count increased with infection in both strains, in contrast to the leukopenia that generally occurs in natural and experimental infections.9,26,35 Leukocytosis in the current study was due to neutrophilia and lymphocytosis, both uncommon findings during infection; neutropenia and lymphopenia are reported frequently.6,9,35 Leukocytosis resolved quickly in infected AGB6 mice but worsened over time in infected AG129 mice. Specifically, neutrophilia in AG129 mice at 12 dpi was severe, particularly in contrast to mild neutrophilia of AGB6 mice, which had resolved by this point. Bone marrow myeloid hyperplasia was found in infected mice of both backgrounds but was more severe in AG129 mice; myeloid hyperplasia always correlated with neutrophilia and monocytosis in AG129 mice—but not AGB6 mice—at matched time points. Prior work revealed that myeloid hyperplasia also occurs in bone marrow of infected wild-type C3H/HeN mice, a model that typically develops peripheral leukopenia during GA.9,26,30 Discrepancies between bone marrow myeloid hyperplasia and peripheral leukocyte counts in the current study may be due to differences in magnitude of hyperplasia or in leukocyte trafficking and turnover and presumably relate to high pathogen burdens and perturbed immune signaling in IFN-deficient mice. Future work is needed to dissect the specific effects of each IFN-signaling pathway on the hematopathology of infection.
The generally marked hematologic differences between these 2 genetic backgrounds during infection, with more severe abnormalities in AG129 mice in most cases, are in contrast to the less prominent differences found in pathogen burden. These findings suggest that a more severe and prolonged host immune response to infection occurs in the AG129 mice; in other words, disease tolerance is generally lower in the 129 strain, whereas resistance to infection is similar between the 2 strains.2 Histopathologic changes, including neutrophilic interstitial pneumonia, were often similar in infected mice of both backgrounds. AGB6 mice had slightly more severe changes in liver and kidney, including neutrophilic multifocal hepatitis and suspected membranoproliferative glomerulonephritis. Spleen weight was significantly different between mouse backgrounds only at 12 dpi; histologically, splenic lymphoid hyperplasia and reactivity were also more severe in infected AG129 mice than infected AGB6 mice. Spleen weight generally reflects the contribution of lymphoid hyperplasia and extramedullary hematopoiesis in GA, although other cellular responses (for example, neutrophil infiltration due to inflammation and/or increased splenic homing) contribute.16,30,40 Increased extramedullary hematopoiesis in the AGB6 mice is a plausible explanation for the similar splenic weights during infection despite the greater lymphoid hyperplasia in AG129 mice. The current study evaluated infection through day 12; more pronounced strain-based histopathologic differences may occur later in infection. In addition, we fully evaluated only 2 mice from each group by histopathology, and inadvertent bias (that is, other mice might have had differing lesions) cannot be excluded and must be considered in future work.
Several hematologic and histopathologic changes found in the current study differ from those reported after experimental and natural infection, and the absence of IFN signaling-induced mechanisms can be posited as a likely mechanism. The hematopoietic role of IFNγ signaling is complex, given that IFNγ has been linked to hematopoietic suppression and aplastic bone marrow failure syndromes, but IFNγ-stimulated proliferation of hematopoietic stem and progenitor cells is reported also.5 The exact level of IFNγ and the specific maturation state of the stem and progenitor cells are likely factors in determining the particular outcome of IFNγ-based hematopoietic effects.31 In a study of mycobacterial infection, hematopoietic stem cell mobilization required IFNγ–STAT1 signaling, whereas type I IFN signaling played a minor role.44 The absence of IFNγ signaling in the mice we used may permit the development of leukocytosis through one or more mechanisms, including reduction of myelosuppressive effects of infection, altered leukocyte trafficking and sequestration, and decreased leukocyte destruction. Increased proliferation and mobilization of hematopoietic stem and progenitor cells occurs in wildtype mice infected with A. phagocytophilum.29 Reduced IFNγ production due to IL12/23p40 deficiency in B6 mice accelerated both the development of and subsequent recovery from thrombocytopenia and increased splenic neutrophilia.40 Further evaluation of the effects of a lack of type I and type II IFN signaling on hematopoiesis during GA is indicated.
The results presented here support the evaluation of background strain effects when developing and refining mouse models of GA and other infectious diseases. The use of IFN-deficient 129 mice may strongly influence results, given that leukocytosis and other abnormalities were profound compared with those in B6 mice. Strain differences in experimental mouse models can have a major effect on study results; differences in mouse models used to study a single disorder can greatly complicate the meta-analysis of findings.45,46,48,49 The relevance of mice for modeling the human immune system is subject to critical debate, and alternatives proposed include the use of ‘humanized mice.’32,41,43,47 Natural infections of domestic animals may be provide more relevant modeling of human infection, but such natural models are frequently unavailable. Ultimately, rational selection of a mouse experimental model relies on available evidence of how pathophysiologic alterations of interest occur in a particular genetic background, and data interpretation should proceed with an understanding of potential strain-specific effects.
Acknowledgments
This study could not have been completed without the assistance of Elias Godoy, Roberta Moorhead, the Animal Diagnostic Lab, and the PAN Core Facility in Stanford University School of Medicine. Research reported in this publication was supported by the Office of the Director, NIH, under award no. K01OD011150 and by Stanford University School of Medicine Discovery Innovation Fund award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Research also was supported by the Department of Comparative Medicine, Stanford University School of Medicine. The corresponding author can provide supporting research data on request.
References
- 1.Akkoyunlu M, Fikrig E. 2000. γ interferon dominates the murine cytokine response to the agent of human granulocytic ehrlichiosis and helps to control the degree of early rickettsemia. Infect Immun 68:1827–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayres JS, Schneider DS. 2012. Tolerance of infections. Annu Rev Immunol 30:271–294. [DOI] [PubMed] [Google Scholar]
- 3.Bakken JS, Dumler JS. 2000. Human granulocytic ehrlichiosis. Clin Infect Dis 31:554–560. [DOI] [PubMed] [Google Scholar]
- 4.Bakken JS, Dumler JS. 2015. Human granulocytic anaplasmosis. Infect Dis Clin North Am 29:341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldridge MT, King KY, Goodell MA. 2011. Inflammatory signals regulate hematopoietic stem cells. Trends Immunol 32:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batungbacal MR, Scott GR, Burrells C. 1982. The lymphocytopaenia in tickborne fever. J Comp Pathol 92:403–407. [DOI] [PubMed] [Google Scholar]
- 7.Birkner K, Steiner B, Rinkler C, Kern Y, Aichele P, Bogdan C, von Loewenich FD. 2008. The elimination of Anaplasma phagocytophilum requires CD4+ T cells but is independent of Th1 cytokines and a wide spectrum of effector mechanisms. Eur J Immunol 38:3395–3410. [DOI] [PubMed] [Google Scholar]
- 8.Bitsaktsis C, Huntington J, Winslow G. 2004. Production of IFNγ by CD4 T cells is essential for resolving ehrlichia infection. J Immunol 172:6894–6901. [DOI] [PubMed] [Google Scholar]
- 9.Borjesson DL, Barthold SW. 2002. The mouse as a model for investigation of human granulocytic ehrlichiosis: current knowledge and future directions. Comp Med 52:403–413. [PubMed] [Google Scholar]
- 10.Borjesson DL, Simon SI, Hodzic E, Ballantyne CM, Barthold SW. 2002. Kinetics of CD11b/CD18 up-regulation during infection with the agent of human granulocytic ehrlichiosis in mice. Lab Invest 82:303–311. [DOI] [PubMed] [Google Scholar]
- 11.Borjesson DL, Simon SI, Hodzic E, DeCock HE, Ballantyne CM, Barthold SW. 2003. Roles of neutrophil β2 integrins in kinetics of bacteremia, extravasation, and tick acquisition of Anaplasma phagocytophila in mice. Blood 101:3257–3264. [DOI] [PubMed] [Google Scholar]
- 12.Bussmeyer U, Sarkar A, Broszat K, Ludemann T, Moller S, van Zandbergen G, Bogdan C, Behnen M, Dumler JS, von Loewenich FD, Solbach W, Laskay T. 2009. Impairment of γ-interferon signaling in human neutrophils infected with Anaplasma phagocytophilum. Infect Immun 78:358–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrade DD, Foley JE, Borjesson DL, Sykes JE. 2009. Canine granulocytic anaplasmosis: a review. J Vet Intern Med 23:1129–1141. [DOI] [PubMed] [Google Scholar]
- 14.Carrero JA. 2013. Confounding roles for type I interferons during bacterial and viral pathogenesis. Int Immunol 25:663–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapman AS, Bakken JS, Folk SM, Paddock CD, Bloch KC, Krusell A, Sexton DJ, Buckingham SC, Marshall GS, Storch GA, Dasch GA, McQuiston JH, Swerdlow DL, Dumler SJ, Nicholson WL, Walker DH, Eremeeva ME, Ohl CA, Tickborne Rickettsial Diseases Working G, Cdc 2006. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis—United States: a practical guide for physicians and other healthcare and public health professionals. MMWR Recomm Rep 55:1 –27. [PubMed] [Google Scholar]
- 16.Chen G, Severo MS, Sakhon OS, Choy A, Herron MJ, Felsheim RF, Wiryawan H, Liao J, Johns JL, Munderloh UG, Sutterwala FS, Kotsyfakis M, Pedra JH. 2012. Anaplasma phagocytophilum dihydrolipoamide dehydrogenase 1 affects host-derived immunopathology during microbial colonization. Infect Immun 80:3194–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi KS, Dumler JS. 2013. Anaplasma phagocytophilum, interferon γ production and Stat1 signaling. Microbiol Immunol 57:207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi KS, Scorpio DG, Dumler JS. 2014. Stat1 negatively regulates immune-mediated injury with Anaplasma phagocytophilum infection. J Immunol 193:5088–5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies RS, Madigan JE, Hodzic E, Borjesson DL, Dumler JS. 2011. Dexamethasone-induced cytokine changes associated with diminished disease severity in horses infected with Anaplasma phagocytophilum. Clin Vaccine Immunol 18:1962–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dumler JS. 2012. The biologic basis of severe outcomes in Anaplasma phagocytophilum infection. FEMS Immunol Med Microbiol 64:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dumler JS, Trigiani ER, Bakken JS, Aguero-Rosenfeld ME, Wormser GP. 2000. Serum cytokine responses during acute human granulocytic ehrlichiosis. Clin Diagn Lab Immunol 7:6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dzięgiel B, Adaszek Ł, Kalinowski M, Winiarczyk S. 2013. Equine granulocytic anaplasmosis. Res Vet Sci 95:316–320. [DOI] [PubMed] [Google Scholar]
- 23.Franzén P, Berg AL, Aspan A, Gunnarsson A, Pringle J. 2007. Death of a horse infected experimentally with Anaplasma phagocytophilum. Vet Rec 160:122–125. [DOI] [PubMed] [Google Scholar]
- 24.Grøva L, Olesen I, Steinshamn H, Stuen S. 2011. Prevalence of Anaplasma phagocytophilum infection and effect on lamb growth. Acta Vet Scand 53:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hodzic E, Feng S, Fish D, Leutenegger CM, Freet KJ, Barthold SW. 2001. Infection of mice with the agent of human granulocytic ehrlichiosis after different routes of inoculation. J Infect Dis 183:1781–1786. [DOI] [PubMed] [Google Scholar]
- 26.Hodzic E, Ijdo JW, Feng S, Katavolos P, Sun W, Maretzki CH, Fish D, Fikrig E, Telford SR, 3rd, Barthold SW. 1998. Granulocytic ehrlichiosis in the laboratory mouse. J Infect Dis 177:737–745. [DOI] [PubMed] [Google Scholar]
- 27.Hutchinson ML, Strohecker MD, Simmons TW, Kyle AD, Helwig MW. 2015. Prevalence rates of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae), Anaplasma phagocytophilum (Rickettsiales: Anaplasmataceae), and Babesia microti (Piroplasmida: Babesiidae) in host-seeking Ixodes scapularis (Acari: Ixodidae) from Pennsylvania. J Med Entomol 52:693–698. [DOI] [PubMed] [Google Scholar]
- 28.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 29.Johns JL, Borjesson DL. 2011. Downregulation of CXCL12 signaling and altered hematopoietic stem and progenitor cell trafficking in a murine model of acute Anaplasma phagocytophilum infection. Innate Immun 18:418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johns JL, Macnamara KC, Walker NJ, Winslow GM, Borjesson DL. 2009. Infection with Anaplasma phagocytophilum induces multilineage alterations in hematopoietic progenitor cells and peripheral blood cells. Infect Immun 77:4070–4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King KY, Goodell MA. 2011. Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat Rev Immunol 11:685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Legrand N, Weijer K, Spits H. 2006. Experimental models to study development and function of the human immune system in vivo. J Immunol 176:2053–2058. [DOI] [PubMed] [Google Scholar]
- 33.Lepidi H, Bunnell JE, Martin ME, Madigan JE, Stuen S, Dumler JS. 2000. Comparative pathology, and immunohistology associated with clinical illness after Ehrlichia phagocytophila-group infections. Am J Trop Med Hyg 62:29–37. [DOI] [PubMed] [Google Scholar]
- 34.Li H, Zhou Y, Wang W, Guo D, Huang S, Jie S. 2011. The clinical characteristics and outcomes of patients with human granulocytic anaplasmosis in China. Int J Infect Dis 15: e859–e896 [DOI] [PubMed] [Google Scholar]
- 35.Lotrič-Furlan S, Rojko T, Jelovsek M, Petrovec M, Avsic-Zupanc T, Lusa L, Strle F. 2015. Comparison of clinical and laboratory characteristics of patients fulfilling criteria for proven and probable human granulocytic anaplasmosis . Microbes Infect 17: 829 –833. [DOI] [PubMed] [Google Scholar]
- 36.Madigan JE, Gribble D. 1987. Equine ehrlichiosis in northern California: 49 cases (1968 to 1981). J Am Vet Med Assoc 190:445–448. [PubMed] [Google Scholar]
- 37.Martin ME, Caspersen K, Dumler JS. 2001. Immunopathology and ehrlichial propagation are regulated by interferon γ and interleukin 10 in a murine model of human granulocytic ehrlichiosis. Am J Pathol 158:1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Office of Laboratory Animal Welfare, NIH, US Department of Health and Human Services 2015. Policy on humane care and use of laboratory animals. Bethesda (MD): NIH. [Google Scholar]
- 39.Pedra JH, Sutterwala FS, Sukumaran B, Ogura Y, Qian F, Montgomery RR, Flavell RA, Fikrig E. 2007. ASC/PYCARD and caspase 1 regulate the IL18–IFNγ axis during Anaplasma phagocytophilum infection. J Immunol 179:4783–4791. [DOI] [PubMed] [Google Scholar]
- 40.Pedra JH, Tao J, Sutterwala FS, Sukumaran B, Berliner N, Bockenstedt LK, Flavell RA, Yin Z, Fikrig E. 2007. IL12/23p40-dependent clearance of Anaplasma phagocytophilum in the murine model of human anaplasmosis. FEMS Immunol Med Microbiol 50:401–410. [DOI] [PubMed] [Google Scholar]
- 41.Perlman H, Budinger GR, Ward PA. 2013. Humanizing the mouse: in defense of murine models of critical illness. Am J Respir Crit Care Med 187:898–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Platanias LC. 2005. Mechanisms of type I and type II interferon-mediated signalling. Nat Rev Immunol 5:375–386. [DOI] [PubMed] [Google Scholar]
- 43.Rämer PC, Chijioke O, Meixlsperger S, Leung CS, Munz C. 2011. Mice with human immune system components as in vivo models for infections with human pathogens. Immunol Cell Biol 89:408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rauch I, Muller M, Decker T. 2013. The regulation of inflammation by interferons and their STATs. JAKSTAT 2:e23820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rivera J, Tessarollo L. 2008. Genetic background and the dilemma of translating mouse studies to humans. Immunity 28:1–4. [DOI] [PubMed] [Google Scholar]
- 46.Sabroe I, Dockrell DH, Vogel SN, Renshaw SA, Whyte MK, Dower SK. 2007. Identifying and hurdling obstacles to translational research. Nat Rev Immunol 7:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG, Inflammation and Host Response to Injury Large Scale Collaborative Research Program 2013. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA 110:3507–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shinagawa K, Kojima M. 2003. Mouse model of airway remodeling: strain differences. Am J Respir Crit Care Med 168:959–967. [DOI] [PubMed] [Google Scholar]
- 49.Smith LM, Bonafonte MT, Mead JR. 2000. Cytokine expression and specific lymphocyte proliferation in 2 strains of Cryptosporidium parvum-infected γ-interferon knockout mice. J Parasitol 86:300–307. [DOI] [PubMed] [Google Scholar]
- 50.Stuen S. 2007. Anaplasma phagocytophilum—the most widespread tickborne infection in animals in Europe. Vet Res Commun 31 Suppl 1:79–84. [DOI] [PubMed] [Google Scholar]
- 51.Stuen S, Granquist EG, Silaghi C. 2013. Anaplasma phagocytophilum—a widespread multi-host pathogen with highly adaptive strategies. Front Cell Infect Microbiol 3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Telford SR, 3rd, Dawson JE, Katavolos P, Warner CK, Kolbert CP, Persing DH. 1996. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci USA 93:6209–6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trinchieri G. 2010. Type I interferon: friend or foe? J Exp Med 207:2053–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van den Broek MF, Muller U, Huang S, Aguet M, Zinkernagel RM. 1995. Antiviral defense in mice lacking both α/β and γ interferon receptors. J Virol 69:4792–4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang T, Akkoyunlu M, Banerjee R, Fikrig E. 2004. Interferon γ deficiency reveals that 129Sv mice are inherently more susceptible to Anaplasma phagocytophilum than C57BL/6 mice. FEMS Immunol Med Microbiol 42:299–305. [DOI] [PubMed] [Google Scholar]
- 56.Woldehiwet Z. 2008. Immune evasion and immunosuppression by Anaplasma phagocytophilum, the causative agent of tickborne fever of ruminants and human granulocytic anaplasmosis. Vet J 175:37–44. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y, Thai V, McCabe A, Jones M, MacNamara KC. 2014. Type I interferons promote severe disease in a mouse model of lethal ehrlichiosis. Infect Immun 82:1698–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]