Abstract
Isoflurane anesthesia alters the blood levels of several neuroendocrine hormones associated with normal metabolism and physiology and increases stress, but the effect of brief CO2 anesthesia on these parameters is unknown. In this study, we examined the effects of isoflurane (4%) compared with brief CO2 (70% CO2, 30% air) anesthesia on circadian rhythms of plasma measures of physiology and metabolism. Adult male Sprague–Dawley rats (Crl:SD; n = 6 per group) were maintained on a 12:12-h light:dark (300 lx; lights on, 0600) photoperiod. After 1 wk of acclimation, a series of 6 low-volume blood draws were collected by cardiocentesis under anesthesia using isoflurane (10 min or less) compared with CO2 (1 min or less) at a single circadian time point every 4 d (0400, 0800, 1200, 1600, 2000, or 2400) over 3 wk to assess arterial blood glucose, lactic acid, and potassium and plasma melatonin, leptin, insulin, total fatty acids, and corticosterone concentrations. Results revealed that plasma levels (mean ± SEM) of melatonin were low (11 ± 1 pg/mL) during the light phase in both groups but were significantly lower during the dark phase in the isoflurane group (48 ± 6 pg/mL) compared with the CO2 group (162 ± 18 pg/mL). In addition, prominent circadian rhythms of arterial plasma levels of corticosterone, glucose, total fatty acids, lactic acid, and potassium were altered in the isoflurane group compared with the CO2 group. These findings demonstrate that the normal circadian rhythms of endocrine physiology and metabolism observed during brief CO2 anesthesia in rats are markedly disrupted by isoflurane anesthesia.
Abbreviation: TFA, total fatty acids
In laboratory animal research, investigators and veterinarians use various anesthetics to prevent unnecessary stress, pain, and discomfort.42 When determining the best anesthetic for use during a procedure, several factors regarding the anesthetic agent are evaluated. These include ease of administration and monitoring, ability to produce rapid unconsciousness and recovery,40 appropriate duration for the proposed procedure, elimination or minimization of pain and stress, no or minimal side effects on normal metabolism and physiology, and no occupational health risks.14,26 Species differences are often dramatic,26,44 and can be an overlooked factor when considering anesthetic agents. It is imperative, however, to know the effects of anesthetics commonly approved by IACUC on research outcomes.
Melatonin, a neurohormone that is released from the pineal gland primarily at night, is a key player in animal health maintenance and wellbeing.15-17,23,30,31,41,45 Through the nighttime melatonin signal, the daily light–dark cycle resets the circadian clock located in the suprachiasmatic nuclei of the hypothalamus, thereby entraining peripheral clocks in all body tissues, so that core body processes are regulated.11 The nocturnal circadian melatonin signal informs all the cells, tissues, and organs of the body of the time of day, so that they can adjust their functions for optimal homeostasis. Circadian disruption constitutes a significant change in phasing, periodicity, amplitude, or duration of a circadian rhythm from its normal pattern under 12:12-h light:dark conditions, resulting in the interference of the body's normal homeostasis of virtually all biochemical, neurobehavioral, and physiologic events.11 Specifically, melatonin levels have been reported to alter regulation of metabolic hormones, including insulin, leptin, glucose, cholesterol, triglycerides, and corticosterone, among others.47 Many pathologic conditions, such as metabolic syndrome and obesity, are known to involve disturbances of this system.11,46,47 Therefore, protecting this normal biologic timing, particularly the nocturnal melatonin surge, is of paramount importance in mammals used for research.
There is still much to learn about the modes of action of some commonly used anesthetics;42 specifically their effect on animals and humans studied without the confounder of preanesthetics and surgical procedures,19,32,34,42,55 varying exposure durations, and method of induction and maintenance.17,42 The mechanism of action and target sites of isoflurane and CO2 are still largely unknown,1 however, recent reports implicate activation or inhibition of background K+ channels, respectively. Previous studies revealed the adverse effects of isoflurane and CO2 on animal physiology8,42,48 and, in turn, the potential effect of their use in investigations using animal models. In contrast, both isoflurane and CO2 are considered to be superior anesthetics under certain conditions.38
The effects of isoflurane and CO2 vary depending on the species. Potency (measured as minimum alveolar concentration), anesthetic-sparing effect, and ED50 values10,26 are all dependent on the species studied. In addition, aspects of normal physiology, including hypoxic ventilatory response, sensitivity of the central respiratory center, and bicarbonate buffering capacity, vary depending on the species (for example, rats, dogs, humans),44 as evidenced through increased resting pH and PaCO2. Genetic adaptions required for burrowing in rodents have been proposed44 as an explanation for some of the physiologic differences. Therefore, the effects of anesthesia and analgesia in humans should not be assumed to be similar in other species.14,42 Species-specific differences are often overlooked in regard to anesthetic mechanisms of action and the resulting effects on normal physiology44 and, consequently, scientific outcomes.
In this study we hypothesized that, compared with brief exposure to CO2 (70% CO2, 30% air), isoflurane (4%) anesthesia would have detrimental effects on circadian rhythms of physiology and metabolism in male Sprague–Dawley rats, similar to those reported in dogs and humans.5,30,49 Parameters measured included glucose, lactic acid, blood gases, potassium, total fatty acids (TFA), melatonin, leptin, insulin, and corticosterone, because we routinely measure these markers for our circadian studies. We assessed 6 equispaced circadian time points encompassing the 24-h day over a 3-wk period. In addition, we compared the duration of induction, procedure, and recovery time and noted any behavioral signs exhibited during induction, because all of these factors are important to consider when determining the optimal anesthetic for research studies.
Materials and Methods
Reagents.
HPLC-grade chloroform, ethyl ether, methanol, glacial acetic acid, heptane, and hexane were purchased from Fisher Scientific (Pittsburg, PA). Free fatty acid, rapeseed oil methyl ester standards, boron–trifluoride–methanol, potassium chloride, sodium chloride, and perchloric and trichloroacetic acids were purchased from Sigma Scientific (St Louis, MO). Ultrapure water (catalog no. 400000) was purchased from Cayman Chemical (Ann Arbor, MI).
Animals, housing conditions, and diet.
Male Sprague–Dawley rats (weight, 350 to 500 g) used in this study were purchased from Charles River (Crl:CD), Kingston, NY) and were certified by the vendor to be pathogen-free for all known rodent bacterial, viral, and parasitic pathogens. Rats were maintained in the Tulane University School of Medicine facility, which has been AAALAC-accredited since 1962. All animal use and accompanying procedures were in accordance with the Guide for the Care and Use of Laboratory Animals34 and approved by the IACUC.
Rats were housed in IVC containing hardwood maple bedding (catalog no. 7090, Sanichips, Harlan Teklad, Madison, WI; 2 bedding changes weekly). Serum samples from sentinel animals on combined soiled bedding from project rats were tested quarterly (multiplex fluorescent immunoassays for rat corona virus, Sendai virus, pneumonia virus of mice, sialodacryoadentis virus, Kilham rat virus, Toolan H1 virus, reovirus type 3, Mycoplasma pulmonis, lymphocytic choriomeningitis virus, mouse adenovirus 1 and 2, Hantaan virus, Encephalitozoon cuniculi, cilia-associated respiratory bacillus, parvovirus NS1, rat parvoviruses, rat murine virus, and rat theilovirus (Idexx Research Animal Diagnostic Laboratory, Colombia, MO) and for external and internal parasites to ensure that rats remained infection-free; all test results were negative during the course of the experiment. Rats had free access to a commercial diet (no. 5053 Irradiated Laboratory Rodent Diet, Purina, Richmond, IN) and acidified water. Quadruplicate determinations of this diet contained 3.9 g TFA per 100 g of diet, composed of 1.06% myristic (C14:0), 15.94% palmitic (C16:0), 1.47% palmitoleic (C16:1n7), 3.97% stearic (C18:0), 22.22% oleic (C18:1n9), 54.54% linoleic (C18:2n6), and 0.26% arachidonic (C20:4n6) acids; minor amounts of other fatty acids comprised 0.54%. Food and water intake was measured every 3 to 4 d; premeasured amount of waters (500 mL) and food (500 g) were provided in the cage on cage change day. On subsequent cage change days, the remaining food and water was removed and measured by using a digital scale or graduated cylinder, respectively, and recorded. These measurements were then divided by the product of the number of days since change and the number of rats per cage (that is, 2) to give the amount of food and water consumed per animal daily. Individual rats were marked with a sharpie for identification, and body weights were measured weekly. The overall body weight of all rats at the start of experiments after acclimation to the facility and room lighting was 432 ± 30 g.
Caging, lighting regimens, and spectral transmittance measurements.
On arrival, a Department of Comparative Medicine laboratory technician randomly assigned each rat to a standard ventilated polysulfone laboratory rat cage (n = 6 per group, 2 per cage; 10.5 in. × 19 in. × 8 in.; wall thickness, 0.1 in., catalog no. 10198, Allentown Caging, Allentown, PA) for a 1-wk acclimation period. All cages had identical stainless steel lids (catalog no. 10SS, Ancare, Bellmore, NY) for cradling food and the water bottle and were covered with polysulfone translucent microfilter tops (catalog no. N10MBT, Ancare). The rats were maintained in climate- and light-controlled rooms (21 to 25 °C; 50% to 55% relative humidity), with half of the animals receiving diurnal lighting of 12 h light followed by 12 h dark (lights on, 0600), and the other half set on reverse-phase lighting of 12 h dark followed by 12 h light (lights on, 1800 h) so that 2 circadian time point blood collections could be done in the same day from different animal groups. Animal rooms were lighted with overhead white fluorescent lamps as described previously18 and were completely devoid of light contamination during the dark phase. Daily (0800) during the course of this experiment, the lighting intensity in the animal room was measured at 1 m above the floor in the center of the room by using a radiometer–photometer and radiance detector with filter and diffuser, as previously reported,18 which were calibrated regularly. All cages were rotated daily on the room shelf in 8 preestablished, specific positions to ensure that there were no significant differences between cages in lighting intensity inside the cages (at rodent eye level). To this end, lighting intensity was measured inside each cage (with no animals inside) while it was on the shelf rack (model no. RS10198U30MVFLG, Allentown). Once all intensities at each of the 8 positions were approximately equal, cage positions on the shelf were labeled 1 through 8. During the experiment, cages were rotated one position to the right on the shelf daily; the cage in position 8 was rotated to position 1. Cage cleaning procedures, as previously reported,18 did not cause any variations in light intensity measurements throughout the course of the study.
Blood collection.
After the 1-wk acclimation to the facility and then a 2-wk exposure under the described lighting conditions, blood was collected from rats from each group at one circadian time point (0400, 0800, 1200, 1600, 2000, or 2400) every 4 d by cardiocentesis under either isoflurane or CO2 gas anesthesia. These low-volume collections (0.5 to 1.0 mL, depending on body weight) were spaced 4 d apart to minimize adverse effects of serial blood collection, as previously reported.18 Briefly, 4% isoflurane (rate, 2 L per minute) was passed into the bottom of a acrylic gas anesthesia chamber (10 in. × 8 in. × 8 in.), displacing air at a rate of 20% volume per minute. On loss of righting reflex, each rat was removed from the chamber and placed in a nose cone connected by a T-piece to the same isoflurane supply line while blood (0.5 to 1 mL; less than 5% total blood volume) was withdrawn by using a 3-mL luer-lock syringe (Tyco Healthcare, Mansfield, MA) and 25-gauge, 3/8-in. needle (Tyco) moistened with sodium heparin (5000 USP units/mL, Sagent Pharmaceuticals, Schaumburg, IL). Over the last decade in our laboratory, mortality and morbidity of rats due to cardiocentesis has been less than 5%.21
For CO2 anesthesia, 100% CO2 (rate, 2 L per minute) was passed into the same acrylic gas anesthesia chamber, displacing air at a rate of 20% volume per minute, to keep concentrations constant between animals. On loss of righting reflex, each rat was removed and allowed to breathe room air while blood was withdrawn, as opposed to requiring additional anesthesic via a nosecone as in the isoflurane anesthesia group. CO2 and O2 concentrations inside the anesthesia chamber when no animals were present were monitored (model no. 8762, serial no. 01120587, IAQ-Calc Indoor Air Quality Meter, TSI, Shoreview, MN, and Type 401, serial no. E065052 Eagle Series Portable Multigas Detector, RKI Instruments, Union City, CA).
Dark-phase sampling was performed in less than 2 min for CO2 anesthesia and in less than 10 min for isoflurane anesthesia under 1 or 2 safelight red lamps (Kodak 1A, model B, catalog no. 152 1517, 120 V, 15 W, Kodak, Rochester, NY) to preserve the normal nocturnal melatonin surge. Exposure at rodent eye level was 45 to 179.5 µW/cm2 (18.4 to 73.3 lx), depending on the use of 1 or 2 lights and their position during sampling.
Induction, procedure, and recovery.
For both CO2 and isoflurane anesthesia protocols, the induction, procedure, and recovery times were recorded for each rat by using benchtop timers (catalog no. 14-648-34, Traceable Benchtop Timer, Fisher Scientific). Induction time was defined as the interval from placement of the rat into the anesthesia chamber to the onset of unconsciousness, defined as the absence of the righting reflex and the presence of relaxed body condition. Toe pinch (pedal withdrawal reflex) was used to evaluate anesthetic depth (surgical plane), along with recumbency and muscle relaxation on loss of consciousness and decreased respiratory rate. In addition, the absence of purposeful movement during the procedure indicated appropriate anesthetic depth for animal wellbeing.26 Recovery time was defined as the time interval from the end of the procedure to sternal recumbency or spontaneous return to righting. Observation notes were used to record behavior during these phases.
Melatonin analysis.
Plasma melatonin levels were measured by using a melatonin 125I-radioimmunoassay kit (Bühlmann, Switzerland) and analyzed in an automated gamma counter (Cobra 5005, Packard, Palo Alto, CA) as previously described.18 Briefly, C18 reverse-phase extraction columns included in the kit were used to extract the melatonin from the samples by using 0.125 mL of plasma for 2400 and 0400 time points. For the 0800, 1200, 1600, and 2000 time points, 0.25 mL of plasma within the same group were pooled to make a total of 0.5 mL, because of the low levels of melatonin in these samples (that is, prior to the normal nighttime surge). The functional sensitivity for the assay was 0.9 pg/mL. Intraassay precision of the column extraction and radioimmunoassay procedures combined was 8.2%.
ELISA analysis of corticosterone, insulin, and leptin.
Plasma samples were prepared in duplicate for measurement of corticosterone (catalog no. 55-CORMS-E01), insulin (catalog no. 80-INSRTH-E01, rat high range), and leptin (catalog no. 22-LEPMS-E01, mouse or rat) levels by using chemiluminescent ELISA diagnostic kits (ALPCO, Salem, NH) and read on a microplate reader (450 nM; VersaMax Microplate Reader, Molecular Devices, Sunnyvale, CA) as previously described.18 Detection sensitivity for corticosterone, insulin, and leptin were 4.5 ng/mL, 0.124 ng/mL, and 10 pg/mL, respectively. Lower limits of detection of corticosterone, inulin, and leptin were 15 ng/mL, 0.15 ng/mL, and 10 pg/mL, respectively, and the coefficient of variation of all assays was less than 4.0%.
Fatty acid extraction and analysis.
Plasma free fatty acids were extracted as previously described18 from 0.05-mL samples in duplicate by using an internal standard consisting of heptadecanoic acid (100 µg) dissolved in chloroform. A gas chromatograph (model 58990A, Hewlett Packard, Palo Alto, CA) fitted with a flame ionization detector, auto injector (both adjusted to 220 °C), and integrator was used to analyze the methyl esters of fatty acids according to their retention time compared with known standards. A 0.25 mm × 30 cm capillary column using helium as the carrier gas was used for separations. The minimal detection level for the assay was 0.05 µg/mL.
Glucose, lactate, blood gases, and potassium measurements.
After each blood collection, a portion (less than 95 µL) of the total sample was used to measure pH, pO2, pCO2, glucose, and lactic acid (iSTAT1 Analyzer with CG4+ and CG8+ cartridges, Abbott Laboratories, East Windsor, NJ). The remaining blood sample was centrifuged at 9660 × g for 10 min at 4 °C (accuSpin Micro17R centrifuge, Fisher Scientific). Plasma was transferred to a new microtube (Sigma-Aldrich) and stored at –20 °C until assayed for melatonin, TFA, corticosterone, insulin, and leptin.
Statistical analysis.
All data are presented as mean ± SEM unless otherwise noted and were compared by using one-way ANOVA followed by the Bonferroni multiple-comparison test or 2-tailed t test (Prism, GraphPad Software, La Jolla, CA). Due to assay errors, protocol sample requirements (noted earlier for melatonin analysis), or sample volume limitations, sample numbers vary but are 6 or greater, unless otherwise noted. This experiment was repeated once. Differences between the group means were considered statistically significant at a P value of less than 0.05.
Results
Measurement of caging illumination.
Light illumination measurements were 75.6 ± 9.8 µW/cm2 (184.4 ± 67.9 lx; mean ± SEM, n = 8) inside the cage at rat eye level. As mentioned previously, cage positions were rotated daily among presetablished positions to ensure equal luminance between groups.
Dietary and water intakes and body growth rate.
Food consumption, water intake, and body weight did not differ between groups; therefore, these data were combined. Food consumption (mean ± 1 SD) was 6.7 ± 0.5 g per 100 g body weight, and water intake among subjects was 10.6 ± 1.4 mL per 100 g body weight. Body growth rate averaged 24.3 ± 12.9 g weekly. These values are comparable to those reported by the stock producer.30
Anesthesia chamber measurements.
Atmospheric CO2 and O2 gases inside the anesthesia chamber were measured while no animals were present and validated in a subsequent session by simulating the CO2 anesthesia methodology. Baseline atmospheric O2 measured inside the chamber with the lid fully open prior to any gas introduction was 19.5% ± 0.1% (n = 6). Within 1 min of turning on the CO2 gas, atmospheric CO2 exceeded 6000 ppm, the monitors’ upper limit. To simulate removal and replacement of successive rats for anesthesia, the top was opened for 5 s and then closed, and O2 and CO2 again were measured at the front of the chamber, 2 in. from the bottom and 2 in. from the top of the chamber. This process was repeated 23 times. Because no significant difference was found between trials, data were averaged (top CO2, 17.8% ± 0.4%; bottom CO2, 4.4% ± 0.26%, and greater than 6000 ppm, 0.6%).
Induction, procedure, and recovery.
The animal anesthesia induction period for CO2 was 16.4 ± 3.1 s (mean ± 1 SD, n = 12) compared with isoflurane anesthesia induction time of 222 ± 42 s (n = 38). The cardiocentesis procedure duration for both groups was 19.3 ± 4.0 s (n = 50). Recovery time for the CO2 anesthesia group was 42.8 ± 33.8 s (n = 12) compared with the isoflurane group recovery time of 294 ± 18 s (n = 38). Behaviors noted during isoflurane induction included nose twitching, raising, paddling after loss of righting reflex, and a period of excitement or staggering for several minutes. Behaviors noted during CO2 induction included nose twitching, raising, nose touching, and a few seconds of staggering before loss of righting reflex.
Plasma melatonin.
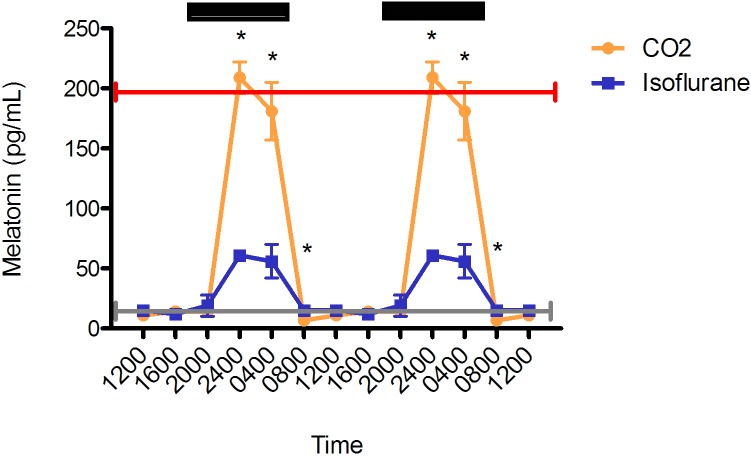
Both the isoflurane and CO2 anesthesia groups demonstrated prominent circadian rhythms (Figure 1). As expected, daytime melatonin concentrations for both groups were low (isoflurane, 12.0 ± 1.0 pg/mL; CO2, 10.4 ± 1.4 pg/mL). However, during the dark phase, plasma melatonin was lower in rats exposed to isoflurane anesthesia (48.0 ± 6.5 pg/mL) compared with those exposed to CO2 anesthesia (162.4 ± 18.5 pg/mL). In addition, the isoflurane group's melatonin concentrations was significantly (P < 0.05) lower than the CO2 group's at 2400 (isoflurane, 60.9 ± 3.2 pg/mL [n = 6]; CO2, 208.5 ± 13.2 pg/mL [n = 10]) and 0400 (isoflurane, 55.6 pg/mL ± 13.5 [n = 5]; CO2, 181.1 ± 24.2 [n = 7] pg/mL). At 0800, plasma melatonin differed significantly (P < 0.05) between the CO2 (6.7 pg/mL ± 1.4 [n = 6]) and isoflurane (14.5 ± 1.5 pg/mL [n = 3]) groups, but both values were within expected physiologic daytime ranges. No significant differences between groups were found at 1200, 1600, and 2000. At 2400, plasma melatonin concentrations reached their peak for both groups of rats.
Figure 1.
Circadian rhythm of arterial plasma melatonin (pg/mL) concentrations in rats that underwent isoflurane anesthesia (blue) compared with brief CO2 anesthesia (amber). Black bars represent the dark phase; *, significant (P < 0.05) difference between groups. Values are double plotted. The total numbers of samples after pooling (see methods) at time points 0400, 0800, 1200, 1600, 2000, and 2400 were 5, 3, 6, 2, 4, and 6 for the isoflurane group and 7, 6, 6, 6, 4, and 10 for the CO2 group, respectively. For reference, the peak nighttime (red line) and daytime (gray line) values in decapitated Wistar rats are shown.9
Plasma corticosterone.
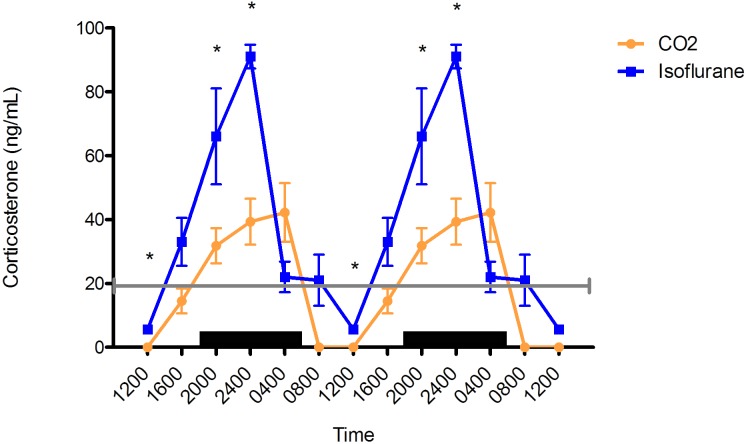
In both groups, plasma corticosterone concentrations had a clear diurnal rhythm, peaking during the dark phase and reaching the nadir during the light phase (Figure 2). In the isoflurane group, peak concentrations were phase-advanced by 4 h, occurring at 2400 (91 ± 3.7 ng/mL), whereas plasma corticosterone in the CO2 group (42.2 ± 9.2 ng/mL) peaked at 0400 and was significantly lower than the isoflurane level at 1200, 2000, and 2400 (P < 0.05) and tended to be lower at 0400 (P < 0.07) and 0800 (P < 0.06).
Figure 2.
Circadian rhythm of arterial plasma corticosterone (ng/mL) concentrations in rats that underwent isoflurane anesthesia (blue) compared with brief CO2 anesthesia (amber). Black bars represent the dark phase; *, significant (P < 0.05) difference between groups. Values are double plotted. The total numbers of samples at time points 0400, 0800, 1200, 1600, 2000, and 2400 were 5, 3, 3, 6, 4, and 5 for the isoflurane group and 3, 3, 3, 6, 9, and 3 for the CO2 group, respectively. For reference, corticosterone in trunk blood taken at decapitation of male Sprague–Dawley rats has been reported to be low at the onset of the light phase5,16 (20 ng/mL; gray line), with a peak (approximately) during the dark phase.16
Plasma TFA.
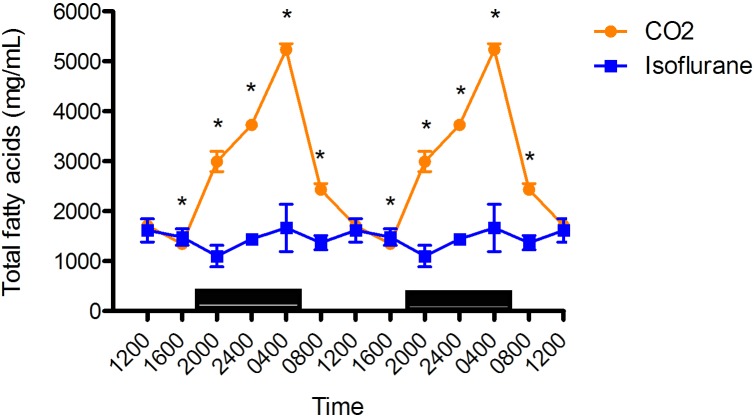
Diurnal rhythms of arterial plasma TFA in male rats with free access to food were present for both anesthesia groups (Figure 3), but were lower (P < 0.05) in the isoflurane group than the CO2 anesthesia group at 0400 (isoflurane, 1666 ± 136 µg/mL [n = 12]; CO2, 5236 µg/mL ± 37 [n = 11]), 0800 (isoflurane,1369 ± 45 µg/mL [n = 12]; CO2, 2435 ± 34 µg/mL [n = 12]), 2000 (isoflurane,1102 ± 62 µg/mL [n = 12]; CO2, 2995 ± 58 µg/mL [n = 12]), and 2400 (isoflurane,1441 ± 6 µg/mL [n = 12]; CO2, 3730 µg/mL ± 32.2 [n = 12]). The isoflurane anesthesia group had significantly (P < 0.05) higher plasma TFA levels at 1600 (isoflurane,1484 ± 52.0 µg/mL [n = 10]; CO2, 1346 ± 23 µg/mL [n = 12]). No significant difference was found at 1200. In both the isoflurane and CO2 anesthesia groups, plasma TFA peaked at 0400.
Figure 3.
Circadian rhythm of arterial plasma total fatty acids (µg/mL) concentrations in rats that underwent isoflurane anesthesia (blue) compared with brief CO2 anesthesia (amber). Black bars represent the dark phase; *, significant (P < 0.05) difference between groups. Black bars represent the dark phase; *, significant (P < 0.05) difference between groups. Values are double plotted. The total numbers of samples at time points 0400, 0800, 1200, 1600, 2000, and 2400 were 6 in all cases for the isoflurane group and 11, 12, 12, 12, 12, and 12 for the CO2 group, respectively. The current report is the first description of circadian rhythmicity in plasma TFA concentrations of Sprague–Dawley rats; however, the circadian rhythmicity of plasma fatty acids is well known.1
Blood glucose.
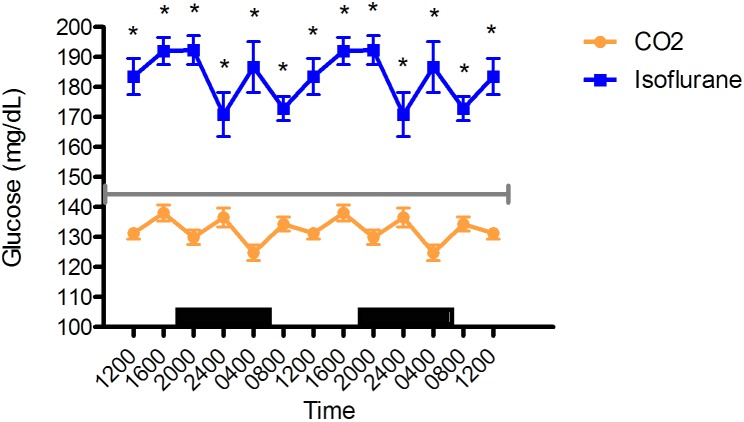
Blood glucose concentrations in the isoflurane group were significantly (P < 0.05) higher than those for the CO2 group and were at hyperglycemic levels at all time points (Figure 4). Overall blood glucose values (mean ± SEM) for the isoflurane and CO2 anesthesia groups were 183 ± 3 mg/dL (n = 36) and 132 ± 2 mg/dL (n = 72), respectively.
Figure 4.
Circadian rhythm of arterial blood glucose (mg/mL) concentrations in rats that underwent isoflurane anesthesia (blue) compared with brief CO2 anesthesia (amber). Black bars represent the dark phase; all values differed significantly (P < 0.05) between groups at each time point. Values are double plotted. The total numbers of samples at time points 0400, 0800, 1200, 1600, 2000, and 2400 were 6 in all cases for the isoflurane group and 11, 12, 12, 12, 12, and 12 for the CO2 group, respectively. For reference, the gray line represents the vendor-reported mean values in Sprague–Dawley rats.30
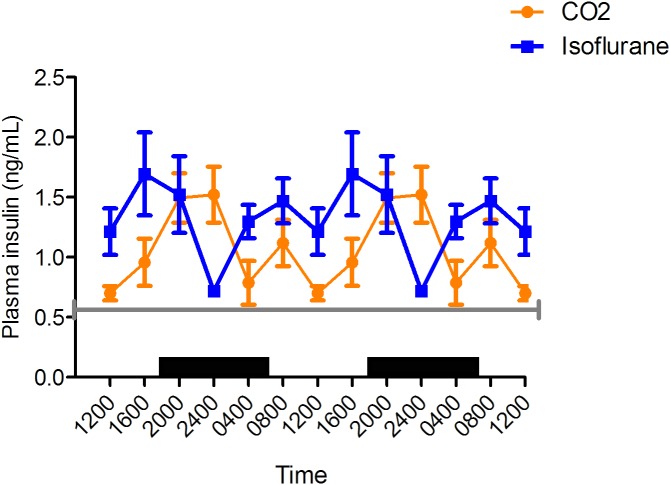
Plasma insulin.
Plasma insulin concentrations in the isoflurane anesthesia group peaked at 1600, compared with 2400 for the CO2 group (Figure 5), and reached a nadir at 2400 for the isoflurane group compared with 1200 for the CO2. Circadian cycling was evident in both groups, but plasma insulin was significantly (P < 0.05) higher in the isoflurane group than the CO2 group at 0400 (isoflurane, 1.3 ± 0.1 ng/mL [n = 6]; CO2, 0.8 ± 0.2 ng/mL [n = 7]) and 1200 (isoflurane, 1.2 ng/mL ± 0.2 [n = 5]; CO2, 0.7 ng/mL ± 0.1 [n = 8]) but lower at 2400 (isoflurane, 0.7 ± 0.0 ng/mL [n = 6]; CO2, 1.5 ± 0.2 ng/mL [n = 6]). Mean insulin concentration over the 24-h period was higher for the isoflurane group (1.5 ± 0.5 ng/mL) than the CO2 group (1.1 ± 0.3 ng/mL).
Figure 5.
Circadian rhythm of arterial plasma insulin (ng/mL) concentrations in rats that underwent isoflurane anesthesia (blue) compared with brief CO2 anesthesia (amber). Black bars represent the dark phase; *, significant (P < 0.05) difference between groups. Values are double plotted to show rhythmicity. The total numbers of samples at time points 0400, 0800, 1200, 1600, 2000, and 2400 were 6, 6, 5, 5, 6, and 6 for the isoflurane group and 7, 7, 8, 5, 5, and 6 for the CO2 group, respectively. For reference, the gray line represents fasting plasma insulin concentrations reported from tail vein samples in Sprague–Dawley rats.54
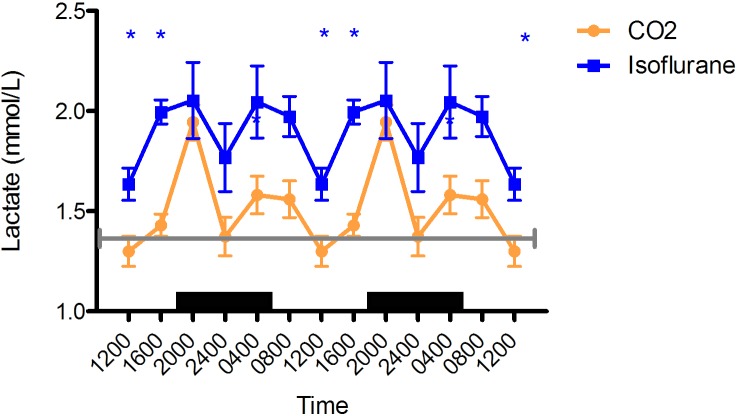
Blood lactate.
For both isoflurane and CO2 anesthesia groups, blood lactate concentrations peaked at 2000 (isoflurane, 2.0 ± 0.2 mmol/L [n = 6]; CO2, 1.9 ± 0.1 mmol/L [n = 12]; P < 0.05) and reached nadir at 1200 (isoflurane, 1.6 ± 0.1 mmol/L [n = 6]; CO2, 1.2 ± 0.1 mmol/L [n = 12]; P < 0.05), with the circadian rhythms following each other closely (Figure 6). In addition, blood lactate was higher in the isoflurane group than the CO2 group at 1600 (isoflurane, 2.0 ± 0.1 mmol/L [n = 6]; CO2, 1.5 ± 0.1 mmol/L [n = 12]; P < 0.05) and 0800 (isoflurane, 2.0 ± 0.1 mmol/L [n = 6]; CO2, 1.5 ± 0.1 mmol/L [n = 12]; P = 0.064).
Figure 6.
Circadian rhythm of arterial blood lactate (mmol/L) concentrations in rats that underwent isoflurane anesthesia (blue) compared with brief CO2 anesthesia (amber). Black bars represent the dark phase; *, significant (P < 0.05) difference between groups. Values are double plotted to show rhythmicity. The total numbers of samples at time points 0400, 0800, 1200, 1600, 2000, and 2400 were 4, 6, 6, 6, 6, and 6 for the isoflurane group and 11, 12, 12, 12, 12, 12 for the CO2 group, respectively. For reference, the gray line represents the mean value reported in Sprague–Dawley rats.34
Blood gases.
At the time points of 0400, 0800, 1200, 1600, 2000, and 2400, rats anesthetized with isoflurane had a mean PO2 of 139.8 ± 41.7, 179.0 ± 48.2, 221.2 ± 31.6, 260.5 ± 25.8, 239.0 ± 52.2, and 203.0 ± 11.5 mm Hg, respectively, and a PCO2 of 55.7 ± 2.3, 54.4 ± 4.1, 48.9 ± 1.2, 54.4 ± 1.4, 50.7 ± 1.4, and 40.7 ± 0.6 mm Hg, respectively. These measurements are comparable to the control values determined for brief CO2 anesthesia in previous studies.18,20
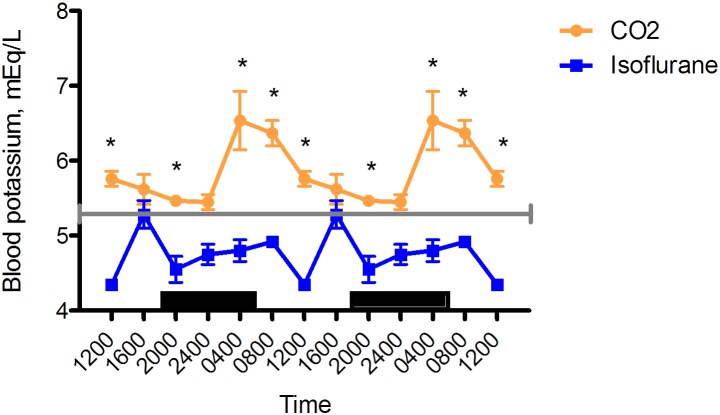
Blood potassium.
Blood potassium concentrations were lower in the isoflurane group than the CO2 group at 0400 (isoflurane, 4.80 ± 0.16 mEq/L [n = 6]; CO2, 6.54 ± 0.31 mEq/L [n = 11]; P < 0.05), 0800 (isoflurane, 4.92 ± 0.08 mEq/L [n = 6]; CO2, 6.37 ± 0.18 mEq/L [n = 13]; P < 0.05), 1200 (isoflurane, 4.35 ± 0.07 mEq/L [n = 6]; CO2, 5.76 ± 0.14 mEq/L [n = 12]; P < 0.05), and 2000 (isoflurane, 4.55 ± 0.19 mEq/L [n = 6], CO2 5.46 ± 0.26 mEq/L [n = 11]; P < 0.05) and approached significance at 2400 (isoflurane, 4.75 ± 0.15 mEq/L [n = 6]; CO2, 5.45 ± 0.23 mEq/L [n = 12]; P = 0.0638). Circadian rhythmicity was evident in both groups. Blood potassium peaked at 1600 in the isoflurane group but at 0400 in the CO2 group, with nadirs at 1200 and 2400, respectively (Figure 7).
Figure 7.
Circadian rhythm of arterial blood potassium (mEq/L) concentrations in rats that underwent isoflurane anesthesia (blue) compared with brief CO2 anesthesia (amber). Black bars represent the dark phase; *, significant (P < 0.05) difference between groups. Values are double plotted to show rhythmicity. The total numbers of samples at time points 0400, 0800, 1200, 1600, 2000, and 2400 were 6 in all cases for the isoflurane group and 11, 12, 12, 12, 11, 12 for the CO2 group, respectively. For reference, the gray line represents the vendor-reported mean value for Sprague–Dawley rats.30
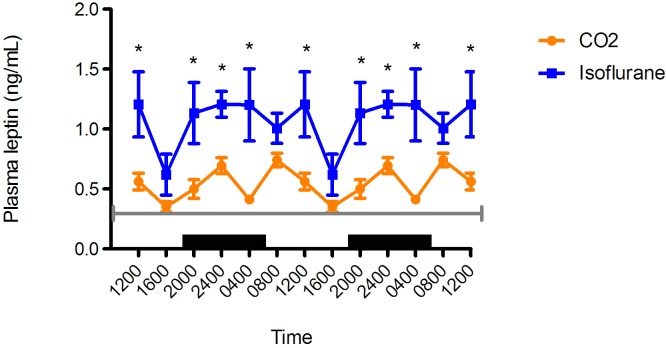
Plasma leptin.
Blood leptin concentrations followed a prominent circadian rhythm in the CO2 anesthesia group but not in the isoflurane anesthesia group (Figure 8). The isoflurane group demonstrated 2 peaks, with the one at 2400 higher than that at 1200. Two peaks in blood leptin concentration also occurred in the CO2 anesthesia group, with that at 0800 greater than the second peak at 2400. Leptin concentrations were significantly higher at all time points in the isoflurane group than the CO2 anesthesia group (P < 0.05 for all time points except 1600 [P = 0.0588]).
Figure 8.
Circadian rhythm of arterial plasma leptin (ng/mL) concentrations in rats that underwent isoflurane anesthesia (blue) compared with brief CO2 anesthesia (amber). Black bars represent the dark phase; *, significant (P < 0.05) difference between groups. Values are double plotted to show rhythmicity. The total numbers of samples at time points 0400, 0800, 1200, 1600, 2000, and 2400 were 6, 6, 5, 5, 6, and 6 for the isoflurane group and 6, 9, 7, 11, 6, and 8 for the CO2 group, respectively. For reference, the gray line represents the mean values reported in Sprague–Dawley rats.45
Discussion
The interpretation of behavioral signs related to pain and stress during anesthesia remains a topic of debate. Both of our anesthetized groups exhibited nose twitching, rising, and a period of excitement,2,27,55 which was much longer in the isoflurane group and might have been due to the differences in induction time. Some isoflurane-anesthetized rats exhibited paddling after the loss of the righting reflex, often considered an adverse effect associated with neurologic excitement.26 Some CO2-anesthetized rats exhibited nose touching, which some authors interpret as an expression of pain.4,14 Pain is a subjective measurement,4 and conflicting reports in humans indicate no pain to a range of pain sensations depending on the CO2 concentration to which they are exposed.24 Recent studies advocate nasal application of CO2 to relieve migraine headaches and symptoms of allergic rhinitis.12,51 Like all other drugs, CO2 can have vastly different effects depending on species, total dosage, concentration, and duration of application.4,12,51 The maintenance of body weight and food and water intake throughout the experiment in both groups give indirect but credible measurements of animal wellbeing.
In addition, isoflurane has a pungent odor.2 Inhalation for as few as 10 s at various concentrations produced coughing, burning, and irritation or other discomfort in humans.2,24,50 Other adverse effects commonly reported include nausea, vomiting, and headaches, which also are associated with occupational exposure to isoflurane.29 Therefore, when comparing CO2 and isoflurane in regards to pungency, irritability, and possible pain sensations, both gasses induce negative effects.2,3,23,39 Due to interspecies differences in sensitivity to CO2, directly studying isoflurane and CO2 inhalation for their irritant qualities in rats seems prudent.
Although both methods of anesthesia had relatively short induction and recovery times, isoflurane induction was 14-fold slower and recovery 7-fold slower than those for brief CO2 anesthesia. The duration of action for isoflurane was too short for our blood-draw procedure, because the rats did not stay in a surgical plane long enough after removal from the anesthesia chamber; therefore, we added nosecone maintenance to the isoflurane anesthesia protocol. During brief CO2 anesthesia, the anesthetic depth gets deeper27 after removal from the anesthetic chamber and before recovery. Because of this difference, CO2 need not be supplied continually in the chamber or via nosecone for maintenance, and doing so may be the cause of the adverse effects2 previously reported for CO2 anesthesia, presumably due to overdosage and toxicity, resulting in increased morbidity and mortality.
For circadian studies, these seemingly small differences in induction and recovery times are important because each group of rats (n = 6 minimum) must be sampled within 1 h of each other. For example, blood for the 0400 time point had to be collected between 0330 and 0430. As in our previous publications,18,19,53 4 test groups each with 6 rats means that 24 rats have to be sampled in that hour, or 2.5 min for each procedure. This goal was easily achieved by using brief CO2 anesthesia and a single investigator; however, isoflurane anesthesia required 2 investigators, who each sampled 12 rats during a 2-h period. Importantly, isoflurane anesthesia enabled only half the number of animals in twice the amount of time by using more staff, compared with CO2 anesthesia.
Our results show that when using isoflurane anesthesia, blood melatonin levels were more than 70% lower than those in rats exposed to brief CO2, as demonstrated in previous studies under similar conditions.18-21 A normal melatonin signal is essential for circadian regulation of physiology and metabolism.18,19,22,37,41,43,46,53 Isoflurane clearly disrupts the melatonin signal and thus biochemical homeostasis. Isoflurane may have suppressed the activity of aralalkylamine N-acetyltransferase, the rate-limiting enzyme for the synthesis of melatonin from serotonin.41 Alternatively, isoflurane may have inhibited the pineal uptake of tryptophan, which is required for pineal melatonin production. Melatonin has been reported to exhibit potent analgesic properties, and its effectiveness is comparable to that of morphine.46 The current study, to our knowledge, is the first to examine the effects of isoflurane on the nocturnal circadian melatonin signal in male Sprague–Dawley rats. Other investigators found similar rhythms of melatonin concentration after decapitation in Wistar rats9 as those in our Sprague–Dawley rats under CO2 anesthesia. The preservation of the melatonin signal during CO2 anesthesia may serve to reinforce some degree of analgesia, which might be lost during isoflurane anesthesia.
At multiple time points, corticosterone levels were higher in the isoflurane-anesthetized group than the CO2 anesthesia group, indicative of a physiologic stress response. This result has been supported by other studies.5 In addition, brief CO2 anesthesia did not increase other important stress indicators, including prolactin, corticosterone,52 and adrenocorticotrophic hormone,45 when investigators compared CO2 with decapitation without anesthesia. In general, corticosterone concentrations can vary considerably depending on animal handling procedures, various stressors, anesthetic used, and time of day;5,16 therefore the corticosterone results should be interpreted in conjunction with other indices of animal wellbeing.
We found that, at all time points, isoflurane-anesthetized rats exhibited marked hyperglycemia compared with the CO2 group; this finding corroborates the results of our previous studies in Sprague–Dawley rats19,53 and reported breeder control data (mean, 146.3 mg/dL).28 In addition, the insulin peak in the isoflurane group occurred 8 h prior to the CO2 group. Isoflurane is well known to inhibit insulin secretion in rats, humans, and dogs, concomitantly causing an increase in glucose concentrations or glucose intolerance by changing normal protein, fatty acid, and glucose metabolism.5,30,49 The current study also found that rats anesthetized with isoflurane had an abolished TFA rhythm, hyperglycemia, and a shift in the insulin peak.
Plasma TFA levels were lower in isoflurane-anesthetized rats than CO2-anesthetized rats, and—importantly—the normal circadian rhythm had completely disappeared,41 although both groups maintained comparable body weights. Neither of our experimental groups was fasted prior to anesthesia, but a previous study found that fasted rats anesthetized with isoflurane had decreased nonesterified fatty acids compared with control animals.5 Our results regarding TFA concentrations and rhythmicity in CO2-anesthetized rats are consistent with other reports18,27 and those for isoflurane in dogs.30 Isoflurane may act on the suprachiasmatic nuclei, the circadian pacemaker, in a manner that mimics the effects of constant bright light at night,19 as exhibited by the hyperglycemia, hyperinsulinemia, and the abolished TFA rhythm. These rapid and robust changes in metabolism imply a direct, central action.30 Further investigation is needed in rats to determine the mechanisms of isoflurane-induced changes in the circadian regulation of metabolism.
Isoflurane causes dose-dependent respiratory depression in rats33,36 and mice,13,26 which in turn causes hypercapnia, respiratory acidosis, and lactic acidosis.36 The isoflurane-anesthetized rats in our current study exhibited lactic acidosis, validating other studies.5,31 Interestingly, other authors have found that isoflurane (1%) eliminates CO2 chemosensation in rat brainstem neurons, thereby blocking the body's natural ventilatory response to hypercapnia42 and hypoxia,15 resulting in alterations of respiratory physiology42 and cardiovascular parameters7,8 that may contraindicate halogenated anesthetics for these studies.
One surprising finding from our study is the significantly lower potassium concentrations for most time points and the 12-h shift in its peak in the isoflurane group compared with the CO2 group. These dramatic differences in potassium in our study suggests alteration of the K+ channel as a viable mechanism of action.25
We noted higher leptin levels in animals undergoing isoflurane anesthesia compared with brief CO2 anesthesia. Leptin is produced by adipose tissue and acts on metabolic, respiratory, and cardiovascular physiologic functions in addition to its well-known direct influences on obesity, appetite, and metabolic syndrome.6,47 Increased leptin concentrations typically cause a decrease in appetite in mice for as long as 3 d after anesthesia.13 However, we measured food and water intake every 2 to 3 d; this interval is too long for the evaluation of postanesthesia changes in food intake. Serum leptins in rats anesthetized with isoflurane in our study follow the same pattern that emerged in animals fed a high-fat diet, both having significantly reduced melatonin and effects associated with metabolic syndrome, such as hyperinsulinemia and hyperglycemia.47 Both isoflurane and high-fat diets cause circadian disruption and increase plasma levels of these hormones, in line with obesity and metabolic syndrome.6,47 Intense investigation is underway to elucidate the mechanism behind leptins’ actions on blood pressure and ventilatory function. Various authors suggest that leptin depends on the melanocortin system by activation of proopiomelanocortin neurons and melanocortin 4 receptors 6 which, on depolarization in multiple hypothalamic nuclei and downstream pathways, modulate metabolism, cardiovascular function, and respiratory function, particularly ventilatory responses to hypercapnia. Melatonin, which was decreased in isoflurane-anesthetized rats, synchronizes circadian rhythms of these physiologic functions.46 In addition, high leptin levels abolish the Marey reflex, a baroreceptor response in the carotid sinus that helps regulate high arterial blood pressure by decreasing the heart rate.48 When drugs or disease blunt these baroreceptors, the lack of adequate maintenance of systemic arterial blood pressure contributes to morbidity and mortality.48 These previous authors concluded isoflurane anesthesia should not be used if lowered blood pressure, a ramped rennin–angiotensin system, and decreases of blood pressure regulation would affect the study.8,42
In future studies, we plan to introduce an established behavioral ethogram to obtain more precise behavioral assessments between the 2 anesthesia groups. In addition, recording food, dietary and water intake, as well as body weights, at precise intervals in relation to anesthesia events and with greater frequency will allow us to correlate metabolic findings to each anesthesia event. Finally, repeating CO2 anesthesia chamber measurements with a meter that has a higher range would enable us to quantify the exact dosage of CO2 required for anesthesia.
The findings of our current study must be differentiated from those of studies that use CO2 for prolonged anesthesia or euthanasia, given that they report adverse effects that are likely due to overdosage and resulting toxicity. Here we demonstrated that, unlike brief CO2 anesthesia, isoflurane anesthesia in rats significantly disrupts normal circadian rhythms and blood levels of melatonin, TFA, potassium, glucose, corticosterone, lactate, and leptin. We conclude that brief CO2 anesthesia is superior to isoflurane anesthesia in rats when used for short procedures, because it preserves normal physiologic circadian function that contributes to the maintenance of animal health and wellbeing and supports unambiguous scientific outcomes.
Acknowledgments
This work was supported by NIH grant no. 1 R25 RR032028 (MAW, TGO, and RPB), Tulane University School of Medicine and Louisiana Cancer Research Consortium Startup Grant no. 631455 (DEB), and an AALAS Grant for Laboratory Animal Science (GLAS) Award (RTD). We thank Michael Webb for his outstanding care of the animals and Erin Dauchy for her technical training and statistical expertise.
References
- 1.Ahlers I, Ahlersova E, Smajda B, Sedlakova A. 1980. Circadian rhythm of serum and tissue lipids in fed and fasted rats. Physiol Bohemoslov 29:525–533. [PubMed] [Google Scholar]
- 2.Altholtz LY, Fowler KA, Badura LL, Kovacs MS. 2006. Comparison of the stress response in rats to repeated isoflurane or CO2:O2 anesthesia used for restraint during serial blood collection via the jugular vein. J Am Assoc Lab Anim Sci 45:17–22. [PubMed] [Google Scholar]
- 3.Amparan AA, Djoufack-Momo SM, Grunden B, Boivin GP. 2014. Exposure of research personnel to carbon dioxide during euthanasia procedures. J Am Assoc Lab Anim Sci 53:376–380. [PMC free article] [PubMed] [Google Scholar]
- 4.Anton F, Euchner I, Handwerker HO. 1992. Psychophysical examination of pain induced by defined CO2 pulses applied to the nasal mucosa. Pain 49:53–60. [DOI] [PubMed] [Google Scholar]
- 5.Arnold M, Langhans W. 2010. Effects of anesthesia and blood sampling techniques on plasma metabolites and corticosterone in the rat. Physiol Behav 99:592–598. [DOI] [PubMed] [Google Scholar]
- 6.Bassi M, Furuya WI, Zoccal DB, Menani JV, Colombari E, Hall JE, da Silva AA, do Carmo JM, Colombari DS. 2015. Control of respiratory and cardiovascular functions by leptin. Life Sci 125:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baxter MG, Murphy KL, Crosby G, Culley DJ. 2008. Different behavioral effects of neurotoxic dorsal hippocampal lesions placed under either isoflurane or propofol anesthesia. Hippocampus 18:245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bencze M, Behuliak M, Zicha J. 2013. The impact of 4 different classes of anesthetics on the mechanisms of blood pressure regulation in normotensive and spontaneously hypertensive rats. Physiol Res 62:471–478. [DOI] [PubMed] [Google Scholar]
- 9.Benot S, Molinero P, Soutto M, Goberna R, Guerrero JM. 1998. Circadian variations in the rat serum total antioxidant status: correlation with melatonin levels. J Pineal Res 25:1–4. [DOI] [PubMed] [Google Scholar]
- 10.Brosnan RJ, Eger EI, 2nd, Laster MJ, Sonner JM. 2007. Anesthetic properties of carbon dioxide in the rat. Anesth Analg 105:103–106. [DOI] [PubMed] [Google Scholar]
- 11.Cardinali DP, Scacchi PA. 2010. Chronophysiology of melatonin: therapeutical implications. Open Neuroendocrinol J 3:72–84. [Google Scholar]
- 12.Casale TB, Korenblat PE, Meltzer EO, Yen K, Bhatnagar A. 2011. Nasal carbon dioxide for the symptomatic treatment of perennial allergic rhinitis. Ann Allergy Asthma Immunol 107:364–370. [DOI] [PubMed] [Google Scholar]
- 13.Cesarovic N, Nicholls F, Rettich A, Kronen P, Hassig M, Jirkof P, Arras M. 2010. Isoflurane and sevoflurane provide equally effective anaesthesia in laboratory mice. Lab Anim 44:329–336. [DOI] [PubMed] [Google Scholar]
- 14.Conlee KM, Stephens ML, Rowan AN, King LA. 2005. Carbon dioxide for euthanasia: concerns regarding pain and distress, with special reference to mice and rats. Lab Anim 39:137–161. [DOI] [PubMed] [Google Scholar]
- 15.Cotten JF. 2013. TASK1 (KCNK3) and TASK3 (KCNK9) tandem-pore potassium-channel antagonists stimulate breathing in isoflurane-anesthetized rats. Anesth Analg 116:810–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D'Agostino J, Vaeth GF, Henning SJ. 1982. Diurnal rhythm of total and free concentrations of serum corticosterone in the rat. Acta Endocrinol (Copenh) 100:85–90. [DOI] [PubMed] [Google Scholar]
- 17.Danneman PJ, Stein S, Walshaw SO. 1997. Humane and practical implications of using carbon dioxide mixed with oxygen for anesthesia or euthanasia of rats. Lab Anim Sci 47:376–385. [PubMed] [Google Scholar]
- 18.Dauchy RT, Dauchy EM, Hanifin JP, Gauthreaux SL, Mao L, Belancio VP, Ooms TG, Dupepe LM, Jablonski MR, Warfield B, Wren MA, Brainard GC, Hill SM, Blask DE. 2013. Effects of spectral transmittance through standard laboratory cages on circadian metabolism and physiology in nude rats. J Am Assoc Lab Anim Sci 52:146–156. [PMC free article] [PubMed] [Google Scholar]
- 19.Dauchy RT, Dauchy EM, Tirrell RP, Hill CR, Davidson LK, Greene MW, Tirrell PC, Wu J, Sauer LA, Blask DE. 2010. Dark-phase light contamination disrupts circadian rhythms in plasma measures of endocrine physiology and metabolism in rats. Comp Med 60:348–356. [PMC free article] [PubMed] [Google Scholar]
- 20.Dauchy RT, Hoffman AE, Wren-Dail MA, Hanifin JP, Warfield B, Brainard GC, Xiang S, Yuan L, Hill SM, Belancio VP, Dauchy EM, Smith K, Blask DE. 2015. Daytime blue light enhances the nighttime circadian melatonin inhibition of human prostate cancer growth progression. Comp Med 65:473–485. [PMC free article] [PubMed] [Google Scholar]
- 21.Dauchy RT, Wren MA, Dauchy EM, Hoffman AE, Hanifin JP, Warfield B, Jablonski MR, Brainard GC, Hill SM, Mao L, Dobek GL, Dupepe LM, Blask DE. 2015. The influence of red light exposure at night on circadian metabolism and physiology in Sprague–Dawley rats. J Am Assoc Lab Anim Sci 54:40–50. [PMC free article] [PubMed] [Google Scholar]
- 22.Dispersyn G, Pain L, Touitou Y. 2010. Propofol anesthesia significantly alters plasma blood levels of melatonin in rats. Anesthesiology 112:333–337. [DOI] [PubMed] [Google Scholar]
- 23.Djoufack-Momo SM, Amparan AA, Grunden B, Boivin GP. 2014. Evaluation of carbon dioxide dissipation within a euthanasia chamber. J Am Assoc Lab Anim Sci 53:404–407. [PMC free article] [PubMed] [Google Scholar]
- 24.Doi M, Ikeda K. 1993. Airway irritation produced by volatile anaesthetics during brief inhalation: comparison of halothane, enflurane, isoflurane, and sevoflurane. Can J Anaesth 40:122–126. [DOI] [PubMed] [Google Scholar]
- 25.Enyedi P, Czirjak G. 2010. Molecular background of leak K+ currents: 2-pore domain potassium channels. Physiol Rev 90:559–605. [DOI] [PubMed] [Google Scholar]
- 26.Fish RE, Brown MJ, Danneman PJ, Karas AZ. 2008. Anesthesia and analgesia in laboratory animals. New York (NY):Elsevier. [Google Scholar]
- 27.Forslid A, Ingvar M, Rosen I, Ingvar DH. 1986. Carbon dioxide narcosis: influence of short-term high concentration carbon dioxide inhalation on EEG and cortical evoked responses in the rat. Acta Physiol Scand 127:281–287. [DOI] [PubMed] [Google Scholar]
- 28.Giknis MLA, Clifford CB. [Internet]. 2006. Clinical laboratory parameters for Crl:CD (SD) rats. Charles River Laboratories. [Cited 09 August 2016]. Available at: http://www.criver.com/files/pdfs/rms/cd/rm_rm_r_clinical_parameters_cd_rat_06.aspx
- 29.Gupta A, Stierer T, Zuckerman R, Sakima N, Parker SD, Fleisher LA. 2004. Comparison of recovery profile after ambulatory anesthesia with propofol, isoflurane, sevoflurane, and desflurane: a systematic review. Anesth Analg 98:632–641. [DOI] [PubMed] [Google Scholar]
- 30.Horber FF, Krayer S, Miles J, Cryer P, Rehder K, Haymond MW. 1990. Isoflurane and whole-body leucine, glucose, and fatty acid metabolism in dogs. Anesthesiology 73:82–92. [DOI] [PubMed] [Google Scholar]
- 31.Horn T, Klein J. 2010. Lactate levels in the brain are elevated upon exposure to volatile anesthetics: a microdialysis study. Neurochem Int 57:940–947. [DOI] [PubMed] [Google Scholar]
- 32.Hu S, Bai XD, Liu XQ, Wang HB, Zhong YX, Fang T, Zhou FQ. 2013. Pyruvate Ringer's solution corrects lactic acidosis and prolongs survival during hemorrhagic shock in rats. J Emerg Med 45:885–893. [DOI] [PubMed] [Google Scholar]
- 33.Imai A, Steffey EP, Farver TB, Ilkiw JE. 1999. Assessment of isoflurane-induced anesthesia in ferrets and rats. Am J Vet Res 60:1577–1583. [PubMed] [Google Scholar]
- 34.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 35.Kärkelä J, Vakkuri O, Kaukinen S, Huang WQ, Pasanen M. 2002. The influence of anaesthesia and surgery on the circadian rhythm of melatonin. Acta Anaesthesiol Scand 46:30–36. [DOI] [PubMed] [Google Scholar]
- 36.Kohler I, Meier R, Busato A, Neiger-Aeschbacher G, Schatzmann U. 1999. Is carbon dioxide (CO2) a useful short-acting anaesthetic for small laboratory animals? Lab Anim 33:155–161. [DOI] [PubMed] [Google Scholar]
- 37.Leary S, Underwood W, Anthony R, Cartner S, Corey D, Grandin T, Greenacre CB, Gwaltney-Bran S, McCrackin MA, Meyer R, Miller D, Shearer J, Yanong R.[Internet]. 2013. AVMA guidelines for the euthanasia of animals: 2013 edition. [Cited 01 June 2016]. Available at: https://www.avma.org/KB/Policies/Documents/euthanasia.pdf
- 38.Lutz LJ, Milde JH, Milde LN. 1990. The cerebral functional, metabolic, and hemodynamic effects of desflurane in dogs. Anesthesiology 73:125–131. [DOI] [PubMed] [Google Scholar]
- 39.Marrino P, Gavish D, Shafrir E, Eisenberg S. 1987. Diurnal variations of plasma lipids, tissue and plasma lipoprotein lipase, and VLDL secretion rates in the rat. A model for studies of VLDL metabolism. Biochim Biophys Acta 920:277–284. [DOI] [PubMed] [Google Scholar]
- 40.Massey CA, Iceman KE, Johansen SL, Wu Y, Harris MB, Richerson GB. 2015. Isoflurane abolishes spontaneous firing of serotonin neurons and masks their pH–CO2 chemosensitivity. J Neurophysiol 113:2879–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naguib M, Baker MT, Spadoni G, Gregerson M. 2003. The hypnotic and analgesic effects of 2-bromomelatonin. Anesth Analg 97:763–768. [DOI] [PubMed] [Google Scholar]
- 42.Naguib M, Gottumukkala V, Goldstein PA. 2007. Melatonin and anesthesia: a clinical perspective. J Pineal Res 42:12–21. [DOI] [PubMed] [Google Scholar]
- 43.Nazian SJ. 2001. Leptin secretion from the epididymal fat pad is increased by the sexual maturation of the male rat. J Androl 22:491–496. [PubMed] [Google Scholar]
- 44.Pepelko WE, Dixon GA. 1975. Arterial blood gases in conscious rats exposed to hypoxia, hypercapnia, or both. J Appl Physiol 38:581–587. [DOI] [PubMed] [Google Scholar]
- 45.Reed B, Varon J, Chait BT, Kreek MJ. 2009. Carbon dioxide-induced anesthesia results in a rapid increase in plasma levels of vasopressin. Endocrinology 150:2934–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reiter RJ, Robinson J. 1995. Melatonin: your body's natural wonder drug. New York (NY): Bantam Books. [Google Scholar]
- 47.Rios-Lugo MJ, Cano P, Jimenez-Ortega V, Fernandez-Mateos MP, Scacchi PA, Cardinali DP, Esquifino AI. 2010. Melatonin effect on plasma adiponectin, leptin, insulin, glucose, triglycerides, and cholesterol in normal and high fat-fed rats. J Pineal Res 49:342–348. [DOI] [PubMed] [Google Scholar]
- 48.Shiry LJ, Hamlin RL. 2011. Measuring high pressure baroreceptor sensitivity in the rat. J Pharmacol Toxicol Methods 64:97–101. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka T, Nabatame H, Tanifuji Y. 2005. Insulin secretion and glucose utilization are impaired under general anesthesia with sevoflurane as well as isoflurane in a concentration-independent manner. J Anesth 19:277–281. [DOI] [PubMed] [Google Scholar]
- 50.TerRiet MF, DeSouza GJ, Jacobs JS, Young D, Lewis MC, Herrington C, Gold MI. 2000. Which is most pungent: isoflurane, sevoflurane, or desflurane? Br J Anaesth 85:305–307. [DOI] [PubMed] [Google Scholar]
- 51.Tzabazis AZ, Niv SH, Manering NA, Klyukinov M, Cuellar JM, Bhatnagar A, Yeomans DC. 2010. Trigeminal antihyperalgesic effect of intranasal carbon dioxide. Life Sci 87:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Urbanski HF, Kelley ST. 1991. Sedation by exposure to a gaseous carbon dioxide-oxygen mixture: application to studies involving small laboratory animal species. Lab Anim Sci 41:80–82. [PubMed] [Google Scholar]
- 53.Wren MA, Dauchy RT, Hanifin JP, Jablonski MR, Warfield B, Brainard GC, Blask DE, Hill SM, Ooms TG, Bohm RP., Jr 2014. Effect of different spectral transmittances through tinted animal cages on circadian metabolism and physiology in Sprague–Dawley rats. J Am Assoc Lab Anim Sci 53:44–51. [PMC free article] [PubMed] [Google Scholar]
- 54.Wu LY, Juan CC, Ho LT, Hsu YP, Hwang LS. 2004. Effect of green tea supplementation on insulin sensitivity in Sprague–Dawley rats. J Agric Food Chem 52:643–648. [DOI] [PubMed] [Google Scholar]
- 55.Zhang F, Zhu ZQ, Liu DX, Zhang C, Gong QH, Zhu YH. 2014. Emulsified isoflurane anesthesia decreases brain-derived neurotrophic factor expression and induces cognitive dysfunction in adult rats. Exp Ther Med 8:471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]