Abstract
Metal alloys are frequently used as implant materials in veterinary medicine. Recent studies suggest that many alloys induce both local and systemic inflammatory responses. In this study, 37 rhesus macaques with long-term skull-anchored percutaneous titanium alloy implants (duration, 0 to 14 y) were evaluated for changes in their hematology, coagulation, and serum chemistry profiles. Negative controls (n = 28) did not have implants. Macaques with implants had higher plasma D-dimer and lower antithrombin III concentrations than nonimplanted animals. In addition, animals with implants had higher globulin and lower albumin and calcium concentrations compared with nonimplanted macaques. Many of these changes were positively correlated with duration of implantation and the number of implants. Chronic bacterial infection of the skin was present around many of the implant sites and within deeper tissues. Representative histopathology around the implant site of 2 macaques revealed chronic suppurative to pyogranulomatous inflammation extending from the skin to the dura mater. X-ray fluorescence microscopy of tissue biopsies from the implant site of the same 2 animals revealed significantly higher levels of free metal ions in the tissue, including titanium and iron. The higher levels of free metal ions persisted in the tissues for as long as 6 mo after explantation. These results suggest that long-term skull-anchored percutaneous titanium alloy implants can be associated with localized inflammation, chronic infection, and leaching of metal ions into local tissues.
Abbreviations: ATIII, antithrombin III
Cephalic implants comprising titanium alloys are commonly used in many animal models for neurologic research. Although these implants are essential to researcher, the chronic presence of a large metallic foreign body presents its own obstacles, including leaching of alloys, promotion of chronic inflammation, and a nidus for infection and biofilm formation. Percutaneous implants of various materials are prone to infection in many human specialties, including oral, orthopedic, and neurosurgery,10,17 even under optimal conditions.
The most common metal in cephalic implants in research settings is titanium. This transition metal is noted for its corrosion resistance and biocompatibility within the human body.11,35 When titanium is used as a prosthetic material, such as in periodontal implants, prosthetic joints, or fracture repair, its mechanical and chemical properties and its interaction with the host are of prime importance for long-term clinical success of the implant. Titanium alloys are more commonly used than pure titanium, to improve the tensile strength and yield strength of the implant.19 Previous generations of titanium alloys were composed of vanadium and aluminum (for example, Ti–6Al–4V), although research has shown that these implants may have toxic effects resulting from the leaching of these components.20,31,46,51,61 Newer titanium alloy compositions show improved biocompatibility and stability and include niobium, zirconium, and molybdenum (for example, Ti–13Nb–13Zr and Ti–12Mo–6Zr),20,55,70,73 and new ways of synthesizing high-strength titanium are being developed. Modifications to the surface coating of the titanium and related alloys are also being developed, to both improve biocompatibility and decrease biofilm formation.18,26,42,58,65 These improved titanium-based alloys have dramatically improved the stability and longevity of implants and reduced the amount of associated local tissue inflammation16,37
Recent literature has revealed the metal–tissue interaction of titanium within the host and suggests that galvanism, the corrosion of the alloy, may induce and sustain a local host immune response, jeopardizing the stability of the surgical implant as well as the health of the local tissue.7 In addition, serum titanium levels often are significantly elevated in patients with implants and are correlated with the type of the implant and the duration of implantation.14,36,40 Neurobiology research often uses percutaneous skull-anchored implants of various conformations, frequently with rhesus macaques (Macaca mulatta) as the experimental subjects.1,2 To date, no studies have evaluated the systemic effects of these long-term implants in rhesus macaques.
In this study, we evaluated the effects of long-term skull-anchored percutaneous titanium alloy implants in rhesus macaques. Histologic evaluation revealed chronic pyogranulomatous inflammation, and X-ray fluorescence microscopy demonstrated metal ions that were distributed within the granulation tissue around the implant site and that persisted for as long as 6 mo after explantation. In addition, chronic bacterial infection frequently was present at the site of implantation. Furthermore, basic blood analysis and assessment of coagulation parameters revealed a trend toward a prothrombotic and proinflammatory state.
Materials and Methods
Animals.
Chinese-origin rhesus macaques (Macaca mulatta; n = 56; 37 with implants, 28 without implants; 45 male, 20 female; age, 6 to 19 y) were evaluated for this study. Animals had one or more skull-anchored percutaneous titanium alloy implants types as a consequence of their use in cognitive neuroscience research. The composition of the titanium alloys varied depending on the laboratory and implant distributor; other metals in the alloy included, but were not limited to, iron, aluminum, niobium, molybdenum, and vanadium. Macaques were divided into groups according to implant status: cephalic restraint pedestal only, n = 18 (16 male, 2 female); cephalic restraint pedestal and a cephalic recording chamber, n = 19 (16 male, 3 female); and no implant, n = 28 (13 male, 15 female). Briefly, the cephalic restraint pedestals are percutaneous titanium alloy posts that are anchored to the skull with titanium alloy screws; the cephalic recording chambers are percutaneous recording chambers that lead to the brain and are usually made of acrylic or CILUX plastic and anchored to skull by either ceramic or titanium alloy cortical screws.1,2 The duration of implantation ranged from 0 to 14 y (0 y signifies more than 6 mo but less than 1 y of implantation). Chronic bacterial infections were frequently present around the base of the cephalic restraint pedestals or within the cephalic recording chambers. The implants were cleaned as frequently as necessary with sterile saline, 0.05% chlorhexidine solution, or 1% to 2% povidone–iodine solution, according to the recommendation of the Division of Comparative Medicine veterinary staff, to reduce the animals’ infectious and inflammatory burden and to decrease stress. More specifically, all cephalic implants were inspected at least once weekly for any signs of infection or contamination. If mild but chronic signs were evident, a minimum of twice weekly (3 to 4 d apart) inspection and cleaning was required. For infected implant margins or for margins with obvious signs of inflammation, dehiscence, or necrosis, the veterinary staff was consulted, given that these cases needed to be assessed regarding the need for daily care to treat the infection and prevent tissue devitalization. Bacterial cultures and antibiotic susceptibility testing were performed, and macaques received oral or injectable antibiotics as needed.
All study animals were indoor-housed (either singly or paired) in an AAALAC-accredited facility. Commercial primate chow (Lab Diet 5038, PMI Nutrition International, St Louis, MO) was fed twice daily. Environmental enrichment was provided daily in the form of toys, videos, and seasonal fruits, vegetables, and other treats. While quarantined, macaques were screened for endoparasites, Salmonella spp., Shigella spp., other enteric pathogens, tuberculosis, and a battery of viral agents including simian retrovirus, Macacine herpesvirus type 1 (B virus), simian T-lymphotrophic virus 1, measles virus, and SIV. With the exception of scattered positive measles antibody titers, all of the animals were free of these agents. Macaques were tested and confirmed negative for enteric pathogens annually and for tuberculosis, fecal endoparasites, and B virus semiannually. All of the macaques were on IACUC-approved experimental protocols and were not implanted solely for the purposes of the current study.
Blood collection, processing, and testing.
All macaques were sedated with ketamine (10 mg/kg IM; Zoetis, Florham Park, NJ) or tiletamine–zolazepam (5 mg/kg IM; Zoetis). Blood collected from the femoral vein was dispensed into 2 (2.9-mL) sodium citrate (3.2%) tubes, a 1.2-mL EDTA tube, and a serum-separator vacuum phlebotomy tube (Sarstedt, Nümbrecht, Germany). The first sodium citrate tube was discarded to minimize the chance that endothelial and platelet activation from the initial venipuncture would result in spurious biomarker readings (that is, ‘2 tube technique’).41 Platelet-poor plasma was prepared within 4 h of phlebotomy. Platelet-poor plasma was collected from the citrate tubes by 2 sequential centrifugations at 1900 × g for 10 min, separated into 200-μL aliquots, and stored at –80 °C until analyzed. CBC analysis was performed inhouse (Hemavet 950 FS, Drew Scientific, Oxford, CT), and a serum biochemistry profile was performed by a commercial laboratory (IDEXX Laboratories, Westbrook, ME). Assessment of coagulation biomarkers, including protein C, antithrombin III (ATIII), D-dimer, and soluble P-selectin (that is, sCD62P), were performed as previously described.23 Briefly, bead-based fluorescence technology was used in previously validated sandwich ELISA to obtain quantitative outputs for coagulation biomarkers.
Histopathology.
Postmortem tissue samples were collected from the implant area; the pathologist was not blinded to sample collection or evaluation. At the time of this study, representative samples were collected from only 3 macaques, because the number of animals available at the end of the study limited collection. Biopsies were not collected from additional animals, because this study was designed to be as minimally invasive as possible, with no testing or sample collection performed outside of health-screening events. Rhesus 1 had no implant during specimen collection but 6 mo prior had a grade 2 titanium cephalic restraint pedestal (President Titanium, Hanson, MA) with Ti cortex self-tapping screws (diameter, 2.0 to 2.7 mm) made from both commercially pure grade 4 titanium and Ti–6Al–7Nb alloys (Synthes, West Chester, PA). Rhesus 2 had 2 implants for a total duration of 6 y—a grade 2 titanium cephalic restraint pedestal (President Titanium, Hanson, MA) with 7-mm cortical screws made from Ti–6Al–4V alloy (Veterinary Orthopedics Implants, St. Augustine, FL) and a CILUX plastic recording chamber, which contained no metal (Crist Instruments, Hagerstown, MD). Rhesus 3 had no implant and served as the negative control. The samples were fixed in 10% formalin, embedded in paraffin, and processed routinely. All tissue blocks were sectioned at 4 µm and stained with hematoxylin and eosin; sections were evaluated by a board-certified veterinary pathologist. Selected slides were also stained with special stains, including Brown and Brenn, Fontana–Masson, Perls Prussian blue, and rubeanic acid.5
X-ray fluorescence microscopy.
X-ray fluorescence microscopy was performed as previously described on the same tissue specimens that were evaluated for histopathology.38 Briefly, paraffin-embedded tissue samples were cut into sections (thickness, 9 μm), placed onto polyethylene nahphalate slides, and deparaffinized. Laser-capture microscopy was performed (Arcturus Veritas platform, ThermoFisher Scientific, Waltham, MA) to excise areas of tissue with black pigment, which subsequently were mounted on Si3N4 membrane grids (2.0 × 2.0 mm). The samples were excited with incident synchrotron X-rays of 10 keV for elemental Kα characteristic emission lines. Elemental profiles were obtained by using synchrotron scanning X-ray fluorescence microscopy at the Advanced Photon Source of the Argonne National Laboratory (Lemont, IL).
Statistical analysis.
Because of the large numbers of nonimplanted and implanted animals and the difficulty in obtaining baseline data for each animal, statistical analysis was performed to compare nonimplanted with implanted macaques in terms of serum biochemistry, CBC, and coagulation biomarker quantitative data. The data were analyzed (GraphPad Software, La Jolla, CA) by using 2-tailed Student t tests assuming either equal or unequal variance, depending on the result of an F test, with a P value of less than 0.05 on the F test considered to signify unequal variance between the 2 groups. A P value of less than 0.05 was considered significant in the Student t test. Prior evaluation of the coagulation biomarkers panel confirmed minimal to no effect of age or sex on the plasma values of protein C, ATIII, D-dimer, and sCD62P.23 Pearson correlation analysis verified the lack of correlation between animal age and number of implants and between animal age and duration of implantation, signifying that all statistically significant differences in hematologic parameters reported here are most likely related to implant status.
Results
Local tissue effects of implantation.
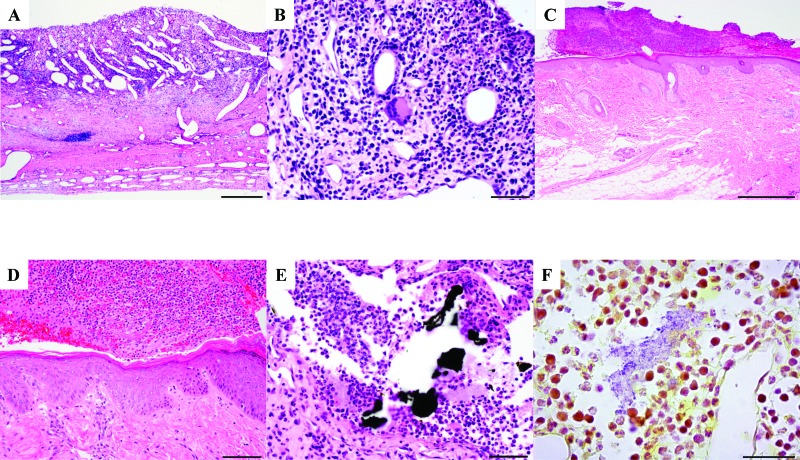
Histopathology was performed on tissue surrounding the implant site in rhesus macaques to examine the cellular changes associated with chronic titanium implantation. At time of tissue collection, chronic purulent exudate was on the skin around the implant site of rhesus 2 and was occasionally found within deeper tissues. In rhesus 1, the dura mater was disrupted by black granular material surrounded by woven bone fragments and few multinucleated giant cells (foreign body type; Figure 1 A and B). The dura mater of rhesus 1 had black accellular material surrounded by degenerate neutrophils and proteinaceous material (Figure 1 D). In addition, gram-positive bacteria surrounded by inflammatory cells were present in the dura mater (Figure 1 E). In rhesus 2, the epidermis was expanded by acanthosis and orthokeratosis. The epidermis was covered by fibrin, hemorrhage, neutrophils, and macrophages. The superficial dermis was infiltrated by macrophages and lymphocytes (Figure 1 C and D). The samples from these 2 macaques were both compared with cranial tissue biopsies from the normal control (macaque 3), which had no abnormal changes or inflammatory cell infiltrates. Histopathology around the implant site of both implanted animals revealed chronic pyogranulomatous inflammation extending from the skin to the dura mater.
Figure 1.
Histopathology of tissue surrounding the implant site. (A) The epidermis was covered by serocellular exudate (crust). Magnification, 4× (scale, 500 μm). (B) The epidermis was expanded by acanthosis and orthokeratosis and covered by serocellular exudate composed of fibrin, hemorrhage, and inflammatory cells. Magnification, 40× (scale, 50 μm). (C) The dura mater was expanded by diffuse chronic–active pyogranulomatous inflammation and ectatic blood vessels. Magnification, 10× (scale, 200 μm). (D) A higher-magnification image from panel C shows inflammation composed of a mixture of granulocytes, mononuclear cells, and few multinucleated giant cells. Magnification, 40× (scale, 50 μm). (E) The dura mater is disrupted by multiple, variably shaped patches of black acellular material surrounded by degenerate neutrophils and proteinaceous material. Magnification, 40× (scale, 50 μm). Hematoxylin and eosin stain (panels A through E). (F) Aggregates of gram-positive bacteria are surrounded by inflammatory cells within the dura mater. Modified Brown and Brenn stain; magnification, 40× (scale, 50 μm).
Fontana–Masson staining of biopsy sections confirmed that the black pigment was foreign material and not melanin. Perls Prussian blue and von Kossa stains were performed to confirm the presence of iron and mineral, respectively, in the biopsy sections. These results indicate the presence of a black foreign material in the tissue surrounding the implant site and an acute and chronic pyogranulomatous inflammation associated with this foreign material. These 2 implanted animals also had both intra- and extracellular bacteria in deeper tissues and superficial purulent material. In fact, many of the animals had chronic bacterial infections at the site of implantation, which were confirmed with routine culture of either the skin surrounding the cephalic restraint pedestal or within the cephalic recording chamber.6,69
X-ray fluorescence microscopy of tissues surrounding the implant site.
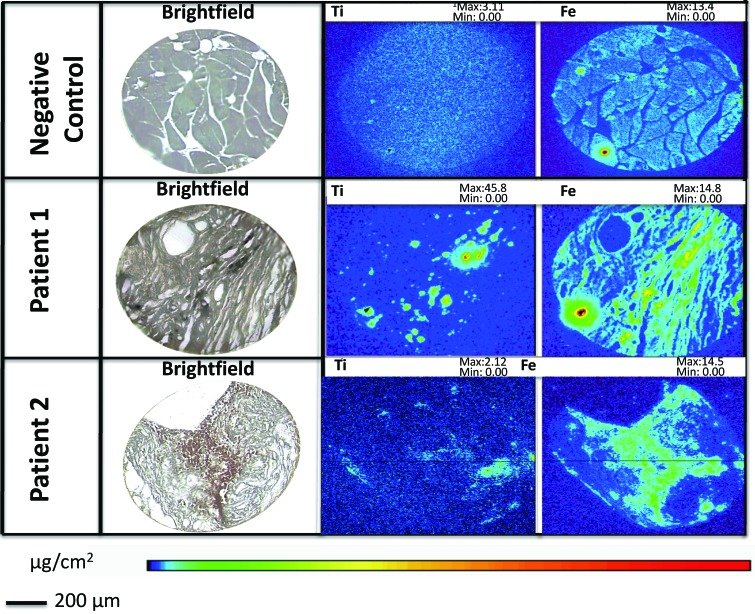
X-ray fluorescence microscopy was used to examine whether the black foreign material in the tissue around the implant site was of metal origin, and if so, to determine the metal composition (Figure 2, Table 1) Both rhesus 1 and 2 had a large amount of titanium and iron deposition in the tissue samples. Although the increase in iron of both rhesus could be due to the composition of the titanium alloy, the large amount of iron in rhesus 2 also correlated with the presence of RBC. In the negative control, no titanium was identified in the tissue samples, and only traces of iron were present. The presence of titanium and a small amount of iron was confirmed in the areas of the black material (as seen in the brightfield image) of the 2 macaques with titanium implants that were evaluated. Imaging confirmed the leakage of Ti ions from the implants into local tissues; the results also indicated that other metals, such as Cu, also leaked into surrounding tissues.
Figure 2.
X-ray fluorescence microscopy of tissue surrounding the implant site. The negative control (rhesus 3, top) show no Ti metal deposition. Rhesus macaques 1 (middle) and 2 (bottom) show a moderate amount of Ti deposition around the tissues as well as small to moderate amounts of Fe deposition.
Table 1.
Total metal content (fg) according to X-ray fluorescent microscopy
| Ti | Fe | |
| Rhesus 1 | 15 × 104 | 17 × 104 |
| Rhesus 2 | 3.7 × 104 | 21 × 104 |
| Rhesus 3 | 2.4 × 104 | 3.4 × 104 |
Rhesus macaque 3 was the negative control.
Association of implantation with a proinflammatory state.
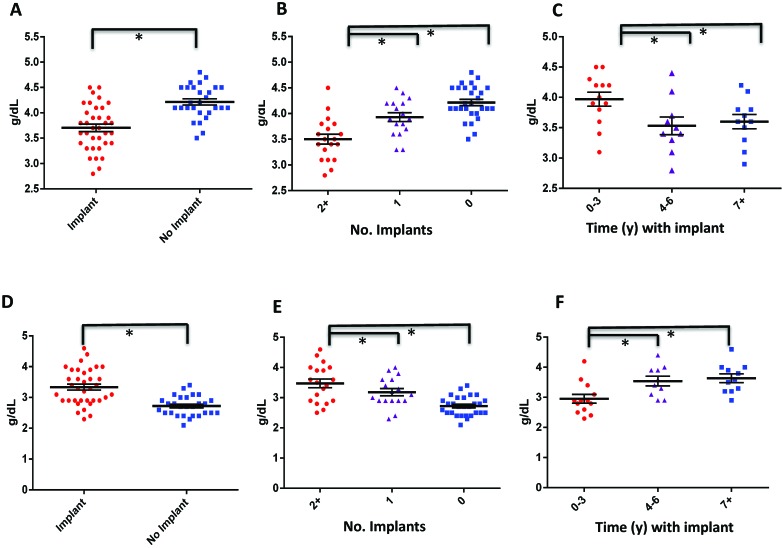
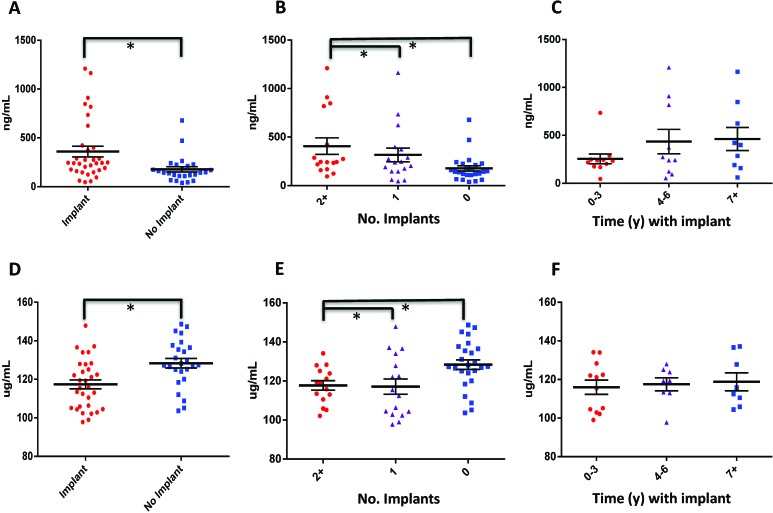
Macaques with implants had lower albumin and calcium concentrations than those without implants (3.7 ± 0.4 g/dL and 9.1 ± 0.4 mg/dL compared with 4.2 ± 0.3 g/dL and 9.5 ± 0.6 mg/dL, respectively; P < 0.01); these differences were also significantly negatively correlated with the number of implants involved and the duration of implantation (Figure 3 A through C; Tables 2 and 3). In addition, globulin levels were significantly elevated in implanted compared with control animals (3.3 ± 0.6 g/dL compared with 2.7 ± 0.3 g/dL; P < 0.01); these differences were also positively correlated with the number of implants and duration of implantation (Figure 3 D through F; Tables 2 and 3).
Figure 3.
Titanium implantation is likely associated with a proinflammatory state. (A through C) Albumin and (D through F) globulin concentrations (mean ± SE) were compared (A and D) between implanted and nonimplanted macaques, (B and E) between animals that had 2 or more implants, 1 implant, or no implant, and (C and F) between animals that were implanted for 0 to 3 y, 4 to 6 y, or 7 y or more. *, Statistically significant relationship (P < 0.05).
Table 2.
Summary of biomarker statistics
| No implant | Implant | Pa | 1 implant | Pb | 2 or more implants | Pc | |||
| D-dimer (ng/mL) | 178.1 ± 137.5 | 360.6 ± 312.5 | 0.01 | 317.0 ± 287.6 | 0.04 | 406.9 ± 340.1 | 0.00 | ||
| Antithrombin III (μg/mL) | 128.4 ± 12.4 | 117.4 ± 12.9 | 0.00 | 117.1 ± 15.8 | 0.01 | 117.7 ± 9.4 | 0.01 | ||
| Protein C (μg/mL) | 3.4 ± 0.7 | 3.6 ± 1.0 | 0.38 | 3.4 ± 1.0 | 0.85 | 3.8 ± 1.0 | 0.15 | ||
| sCD62P (ng/platelet × 106) | 0.14 ± 0.0 | 0.15 ± 0.1 | 0.52 | 0.14 ± 0.0 | 0.42 | 0.1 ± 0.1 | 0.73 | ||
| WBC (×103/μL) | 7.09 ± 3.40 | 7.0 ± 2.3 | 0.92 | 6.4 ± 2.6 | 0.49 | 7.6 ± 2.0 | 0.60 | ||
| ALP (IU/L) | 205.7 ± 154.3 | 153.8 ± 92.9 | 0.10 | 171.7 ± 111.0 | 0.43 | 143.2 ± 72.9 | 0.11 | ||
| ALT (IU/L) | 31.9 ± 20.9 | 25.5 ± 19.0 | 0.20 | 26.1 ± 18.7 | 0.35 | 24.5 ± 20.1 | 0.23 | ||
| AST (IU/L) | 30.4 ± 7.8 | 29.2 ± 7.2 | 0.52 | 29.4 ± 6.6 | 0.67 | 29.1 ± 8.0 | 0.57 | ||
| Albumin (g/dL) | 4.2 ± 0.3 | 3.7 ± 0.4 | 0.00 | 3.9 ± 0.4 | 0.01 | 3.5 ± 0.4 | 0.00 | ||
| Globulin (g/dL) | 2.7 ± 0.3 | 3.3 ± 0.6 | 0.00 | 3.2 ± 0.5 | 0.00 | 3.5 ± 0.4 | 0.00 | ||
| Total protein (g/dL) | 6.9 ± 0.4 | 6.9 ± 1.2 | 0.74 | 7.1 ± 0.5 | 0.20 | 7.0 ± 0.4 | 0.74 | ||
| BUN (mg/dL) | 14.6 ± 5.3 | 15.2 ± 4.4 | 0.62 | 15.5 ± 4.2 | 0.55 | 14.3 ± 4.0 | 0.86 | ||
| Creatinine (mg/dL) | 0.9 ± 0.3 | 1.1 ± 0.3 | 0.00 | 1.1 ± 0.2 | 0.01 | 1.1 ± 0.3 | 0.01 | ||
| Glucose (mg/dL) | 64.6 ± 12.9 | 63.2 ± 8.6 | 0.60 | 63.5 ± 5.5 | 0.73 | 63.1 ± 11.0 | 0.67 | ||
| Calcium (mg/dL) | 9.5 ± 0.6 | 9.2 ± 0.4 | 0.00 | 9.2 ± 0.4 | 0.07 | 9.1 ± 0.3 | 0.00 | ||
| Phosphorus (mg/dL) | 3.5 ± 1.2 | 3.4 ± 0.9 | 0.82 | 3.4 ± 1.1 | 0.76 | 3.4 ± 0.8 | 0.95 | ||
| Platelets (×103/μL) | 382.5 ± 86.7 | 394.1 ± 97.9 | 0.62 | 379.3 ± 86.5 | 0.91 | 409.0 ± 109.7 | 0.36 | ||
| Hct (%) | 38.5 ± 4.3 | 39.2 ± 5.1 | 0.58 | 40.7 ± 4.3 | 0.10 | 37.7 ± 5.6 | 0.59 | ||
| MPV (fL) | 13.3 ± 2.4 | 13.1 ± 2.5 | 0.74 | 13.2 ± 2.5 | 0.89 | 13.0 ± 2.5 | 0.69 | ||
| MCV (fL) | 71.6 ± 4.4 | 69.3 ± 7.7 | 0.17 | 71.44 ± 5.6 | 0.94 | 67.3 ± 8.9 | 0.04 | ||
| MCHC (g/dL) | 31.7 ± 0.8 | 31.3 ± 1.6 | 0.20 | 31.0 ± 1.7 | 0.05 | 31.6 ± 1.5 | 0.71 | ||
| Cholesterol (mg/dL) | 158.6 ± 33.0 | 122.1 ± 25.7 | 0.00 | 120.8 ± 25.3 | 0.00 | 123.3 ± 26.8 | 0.00 | ||
| Total bilirubin (mg/dL) | 0.2 ± 0.1 | 0.14 ± 0.1 | 0.03 | 0.16 ± 0.1 | 0.25 | 0.13 ± 0.1 | 0.01 | ||
| Lipase (U/L) | 34.5 ± 30.1 | 33.7 ± 29.3 | 0.91 | 28.2 ± 20.6 | 0.44 | 38.8 ± 35.5 | 0.65 | ||
| Creatine kinase (U/L) | 541.2 ± 346.8 | 513.6 ± 306.7 | 0.74 | 494.9 ± 377.9 | 0.68 | 523.5 ± 244.4 | 0.85 | ||
| Amylase (U/L) | 275.7 ± 101.6 | 273.8 ± 78.6 | 0.93 | 272.3 ± 91.8 | 0.91 | 273.2 ± 68.2 | 0.99 | ||
| γ-Glutamyl transferase (IU/L) | 58.4 ± 24.1 | 51.8 ± 16.8 | 0.20 | 54.3 ± 17.1 | 0.54 | 49.4 ± 16.6 | 0.16 |
Unpaired Student t test between no implant and implant (regardless of number)
Unpaired Student t test between no implant and 1 implant
Unpaired Student t test between no implant and 2 or more implants
Table 3.
Summary of statistics regarding duration of implantation
| 0 to 3 y | Pa | 4 to 6 y | 7 y or more | Pb | Pc | |
| D-dimer (ng/mL) | 255.73 ± 171.48 | 0.19 | 435.38 ± 399.02 | 462.31 ± 358.73 | 0.11 | 0.88 |
| Antithrombin III (μg/mL) | 115.98 ± 12.84 | 0.77 | 117.54 ± 9.57 | 118.85 ± 13.23 | 0.63 | 0.82 |
| Albumin (g/dL) | 3.97 ± 0.42 | 0.03 | 3.53 ± 0.46 | 3.60 ± 0.39 | 0.04 | 0.71 |
| Globulin (g/dL) | 2.95 ± 0.53 | 0.01 | 3.54 ± 0.49 | 3.64 ± 0.48 | 0.00 | 0.66 |
| Creatinine (mg/dL) | 1.12 ± 0.31 | 0.54 | 1.19 ± 0.23 | 1.13 ± 0.24 | 0.92 | 0.57 |
| Calcium (mg/dL) | 9.29 ± 0.32 | 0.05 | 9.01 ± 0.31 | 9.19 ± 0.41 | 0.50 | 0.27 |
| MCV (fL) | 70.04 ± 6.05 | 0.94 | 69.86 ± 5.56 | 67.26 ± 11.43 | 0.46 | 0.52 |
| MCHC (g/dL) | 30.73 ± 2.41 | 0.22 | 31.71 ± 0.48 | 31.80 ± 0.96 | 0.18 | 0.79 |
| Cholesterol (mg/dL) | 127.46 ± 29.68 | 0.43 | 118.20 ± 23.90 | 119.00 ± 26.91 | 0.48 | 0.94 |
| Total bilirubin (mg/dL) | 0.15 ± 0.07 | 0.88 | 0.15 ± 0.05 | 0.13 ± 0.05 | 0.28 | 0.31 |
Unpaired Student t test between 0 to 3 y and 4 to 6 y
Unpaired Student t test between 0 to 3 y and 7 y or more
Unpaired Student t test between 4 to 6 y and 7 y or more
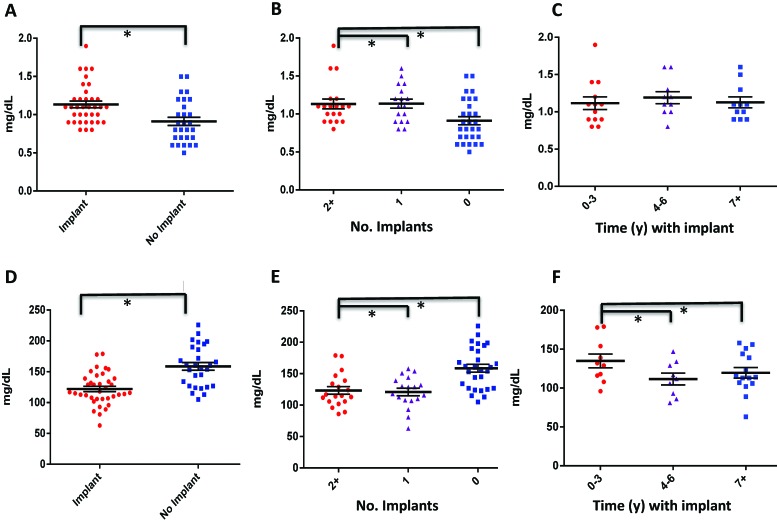
Other clinical chemistry alterations observed in the implanted compared with control macaques included significantly higher creatinine concentrations (1.1 ± 0.3 mg/dL compared with 0.9 ± 0.3 mg/dL; P < 0.01); these differences were positively correlated with the number of implants but not the duration of implantation (Figure 4 A through C; Tables 2 and 3). In addition, cholesterol was lower in animals with implants (122 ± 26 mg/dL compared with 159 ± 33 mg/dL; P < 0.01); these differences were negatively correlated with the number of implants but not the duration of implantation (Figure 4 D through F; Tables 2 and 3). Urinalyses were not performed on these animals; therefore renal function was not thoroughly evaluated in this study.
Figure 4.
Titanium implantation is likely associated with a proinflammatory state. (A through C) Creatinine and (D through F) cholesterol were compared (A and D) between implanted and nonimplanted macaques, (B and E) between animals that had 2 or more implants, 1 implant, or no implant, and (C and F) between animals that were implanted for 0 to 3 y, 4 to 6 y, or 7 y or more. *, Statistically significant relationship (P < 0.05).
Pearson correlation analysis of these clinical chemistry parameters showed no significant correlation of age and biomarker concentration (Table 4). There was slight positive correlation between macaque age and the number of years with an implant but no significant correlation between albumin or globulin level and age (Table 4).
Table 4.
Pearson correlation between animal age and biomarker
| Pearson correlation coefficient | Strength of association | |
| Antithrombin III | 0.04 | Negligible |
| D-dimer | −0.30 | Weak negative |
| Albumin | −0.24 | Negligible |
| Globulin | 0.61 | Weak positive |
| Creatinine | −0.16 | Negligible |
| Calcium | −0.43 | Weak negative |
| MCV | −0.29 | Negligible |
| MCHC | 0.27 | Negligible |
| Total bilirubin | 0.12 | Negligible |
| No. of implants | 0.12 | Negligible |
| Duration (y) of | 0.57 | Weak positive |
| implantation |
Strength of association: Pearson R of –0.7 to –0.3, weak negative association; –0.3 to +0.3, negligible or no association; +0.3 to +0.7, weak positive association
Association between implantation and a procoagulant state.
Analysis revealed that macaques with implants had higher plasma D-dimer and lower ATIII concentrations (360.6 ± 312.5 ng/mL and 117.4 ± 12.9 μg/mL, respectively) than did nonimplanted animals (178.1 ± 137.5 ng/mL and 128.4 ± 12.4 μg/mL, respectively). In addition, the number of implants, but not the duration of implantation, had a significant effect on levels of D-dimer and ATIII. (Figure 5, Tables 2 and 3). Concentrations of protein C and sCD62P did not differ between controls and animals with implants. Pearson correlation analysis of these markers showed no significant effect of age on biomarker concentration (Table 4). The increased D-dimers (a by-product of fibrin degradation) and decreased ATIII level (a major endogenous anticoagulant) suggest a hypercoagulable state associated with chronic titanium implantation and infection.
Figure 5.
Titanium implantation is associated with a likely procoagulant state. (A through C) D-dimer and (D through F) antithrombin III concentrations were compared (A and D) between implanted and nonimplanted macaques, (B and E) between animals that had 2 or more implants, 1 implant, or no implant, and (C and F) between animals that were implanted for 0 to 3 y, 4 to 6 y, or 7 y or more. *, Statistically significant relationship (P < 0.05).
Discussion
In this study, we have shown that percutaneous, static, chronic titanium alloy implants are associated with localized bacterial infection and inflammation, leaching of metal ions into the surrounding tissues, and various associated systemic hematologic changes. We have previously documented isolation of mixed bacterial infections from cephalic implants in this population.6,69 In addition, recent case reports have documented life-threatening infections associated with cephalic implants in rhesus macaques from other colonies.34
The development of physiologically inert synthetic materials for orthopedic implantation is of major importance. A variety of metals are used in surgical implantation, and over time, appear to be correlated with adverse local and systemic effects. Of note, cobalt–chromium alloys, previously used in total hip arthroplasties, can experience long-term corrosion and wear of the implant, resulting in local tissue necrosis, osteolysis, and implant failure.13,47,64 In addition, these alloys have the capability to release metal ions both in the local tissue environment as well as into the systemic circulation, resulting in metallosis.32,33,36 New metal alloys and synthetic polymers have been developed to prevent these adverse effects.66
Titanium alloys were previously perceived to be relatively inert within the host, until recent biomaterials research elucidated the potential for titanium ion leakage and its associated adverse effects. Like cobalt–chromium alloys, titanium can result in elevated serum metal ion levels after implantation.36,39,45,57 A growing body of literature in oral surgery research shows that release of titanium ions from implant surfaces results in increases in inflammation and alveolar bone resorption.63 In addition, titanium ions have been associated with carcinogenic and mutagenic activity within the oral cavity.4,12,54,59,62,68 One recent case report demonstrated the development of a pseudotumor caused by titanium particles from a total hip prosthesis, suggesting that titanium, like cobalt–chromium, may result in metallosis and its associated adverse systemic effects.56 Although most studies attribute ion-leakage to implant wear, a recent study on cochlear implants used X-ray fluorescence microscopy to demonstrate ‘passive’ surface deterioration of medical titanium in the absence of wear.3
Numerous mechanisms could mediate degradation of metal implants within the body, including wear and corrosion. The macaques we studied had static cranial implants and persistent localized bacterial infection; therefore, corrosion is the most likely cause of metal-ion leaching. Local inflammation, pH, and bacterial load all play important roles in the corrosion of titanium implants.7,9 Histopathology was performed in only 3 animals in this study; however, those evaluations of tissue from around the implants showed that the areas of metal deposition appear to be a focus of acute and chronic inflammation (Figure 1 A and B). Free metal ions have been shown to cause local pain and swelling, with or without infection, in the region of implant insertion.68
The implanted macaques in the current study displayed chronic low-grade bacterial infection and inflammation surrounding the implant sites. Studies have shown that titanium can serve as a nidus for bacterial biofilm formation.15,22,30,52 A physiologic pH of 4 to 7 in the local environment has an additive effect with LPS, promoting the corrosion of titanium and its alloys, suggesting that the presence of bacteria around the implant site could produce an environment conducive to implant surface corrosion.71 Implant corrosion and subsequent metal ion leakage activate host complement, and phagocytized metal particles induce the release of inflammatory cytokines from macrophages, which potentially resulted in sustained inflammation and loosening of implants in our animals.7,59 In this cohort of macaques with cephalic implants, we are unable to determine whether the leaching of the titanium ions incited inflammation and predisposed the animals to chronic infection, or whether the presence of chronic infection caused a local environmental change that promoted ion leakage. Based on the current evidence, we cannot conclude that the implant material served as a chronic source of infection for the implanted animals, but it likely plays an important role.21,24,25,50
Although the local effects of titanium alloy implantation with concurrent chronic bacterial infection have been studied in both humans and animals, the systemic effects in general are not well studied. In the current study, macaques with titanium alloy implants had biochemical alterations that can be consistent with chronic inflammation and that were not seen in nonimplanted animals; these alterations included increased levels of globulin, which can be a positive acute-phase protein, and decreased concentrations of albumin, which is a negative acute-phase protein. Globulins represent a wide spectrum of proteins, and increases in several types of globulins are consistent with inflammation. Determining the specific types of globulins present was beyond the scope of this study. Because albumin is the main carrier protein for calcium, calcium levels are often decreased when albumin levels are low, as in the current case.15 We think the decreased albumin concentrations in these macaques were due to inflammation because other common causes of decreased albumin, such as endoparasites and hepatic or renal pathology, were unlikely given the routine screening for endoparasites and lack of strong convincing biochemical evidence indicative of hepatic or renal pathology. In addition, note that creatinine was elevated, but BUN was not,16 and neither liver function nor urine parameters were assessed; therefore other causes of decreased albumin cannot be entirely ruled out. Given that the 2 implanted macaques that underwent histopathologic evaluation had inflammatory changes and that many of the animals with implants had superficial bacterial infections, the combination of low albumin and high globulin levels in implanted macaques is likely due to local inflammation. Furthermore, systemic amyloidosis, an indicator of chronic systemic inflammation, is common in captive macaques and has been frequently noted in our animal population, with 2 cases reported in animals with cephalic implants.53 Taken together, these changes suggest that static, cephalic, titanium-alloy implants are likely associated with systemic inflammation.
Although many publications in both humans and animals show that acute implantation with metal alloys is associated with changes in systemic coagulation, most of those studies were performed in peracute and acute settings, with follow-up being limited to a few weeks or months after surgery.27,60,70 The etiology of coagulation status changes in those cases might reflect tissue trauma during surgery, anesthesia, metal implantation, or a combination thereof. The current study evaluated coagulation biomarkers at 1 to 14 y after implantation, and, to our knowledge, is the first to measure long-term changes in coagulation parameters after metal implantation. The implanted animals in this study displayed both a significant elevation in D-dimer and a significant decrease in ATIII levels. D-dimer is a fibrin degradation product that forms when crosslinked fibrin is cleaved by plasmin and is therefore an indicator of fibrinolytic activity; elevations in D-dimer are suggestive of ongoing clot lysis or a hyperfibrinolytic state.67 ATIII, an anticoagulant that is synthesized in the liver, binds and inhibits several coagulation factors, including factors II and X.49 These changes in D-dimer and ATIII levels suggest that chronic titanium alloy implant-induced bacterial infection may be associated with a systemic procoagulant state. Although D-dimer values were significantly increased in our macaques (Figure 2), the data range widely, including a few outliers with very elevated D-dimer levels, which might influence the overall analysis. ATIII is considered a negative acute-phase protein; therefore the chronic–active inflammation and infection may be the reason for the lower ATIII levels. The loss of ATIII through the kidneys or decreased production of ATIII by the liver cannot entirely be ruled out because, as previously stated, urinalyses and liver function tests were not done.44 In addition, when interpreting these findings, one must take into account that presence of chronic bacterial colonization and inflammation associated with the implants in this colony. These findings may have a major effect on the monitoring and follow-up care of animals with chronic metal implants, and future studies evaluating coagulation markers in these subjects are warranted to evaluate whether they have an increased risk for thrombus formation.
The implants we evaluated are similar to other titanium alloy implants, which are exposed to the external environment, such as external fixators and cochlear implants. Although many of our animals had implants with concurrent bacterial infections, it was not possible to discern whether the systemic changes in inflammation and coagulation were due solely to implant status, the presence of bacterial infection, or another unrelated etiology. Bacterial contamination and biofilm formation is common in implants that are exposed to the external environment.6,28,29,43,48 Implants for deep brain stimulation—which are made from a variety of metal alloys, protected from the outside environment, and placed in the same anatomic location as the implants our animals—are similarly subject to biofilm formation.8 No comprehensive studies to date evaluate either the long-term effects of implantation on coagulation status or the effects of biofilm presence on implants upon the coagulation system. Research in this area may improve our understanding of the pathophysiology of metal ion leakage, local tissue inflammation or infection, and system changes in inflammation and coagulation status, and could thereby eventually provide clinical utility.
The current study determined the systemic effects of chronic, percutaneous, static cranial implantation of macaques with titanium alloys. The findings indicate that chronic bacterial infection and likely a proinflammatory and procoagulant state are common in rhesus macaques with cephalic implants. Biopsy and histopathologic evaluation of tissue surrounding the implant site showed evidence of simultaneous active, chronic inflammation and bacterial infection. X-ray fluorescence microscopy confirmed the leaching of titanium ions into the local tissue, which appeared to persist for as long as 6 mo after removal of the implant. Some limitations in this study include the lack of sham surgery controls and the inability to measure serum titanium levels in these animals, because serum for metal analysis was not collected at the time of this study. Local bacterial infection in the area surrounding the implant site was also common in the macaques and might be a confounding factor in this study.
Acknowledgments
Funding support was provided by grant nos. T32-0D019078-27 (JGF), P30-ES002109 (JGF), and P50-GM021700 (RGT) and the Harvard NeuroDiscovery Center. This research used resources of the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357. We acknowledge Alyssa Pappa for assistance in manuscript preparation.
References
- 1.Adams DL, Economides JR, Jocson CM, Horton JC. 2007. A biocompatible titanium headpost for stabilizing behaving monkeys. J Neurophysiol 98:993–1001. [DOI] [PubMed] [Google Scholar]
- 2.Adams DL, Economides JR, Jocson CM, Parker JM, Horton JC. 2011. A watertight acrylic-free titanium recording chamber for electrophysiology in behaving monkeys. J Neurophysiol 106:1581–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Addison O, Davenport AJ, Newport RJ, Kalra S, Monir M, Mosselmans JF, Proops D, Martin RA. 2012. Do ‘passive’ medical titanium surfaces deteriorate in service in the absence of wear? J R Soc Interface 9:3161–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arillo A, Melodia F, Frache R. 1987. Reduction of hexavalent chromium by mitochondria: methodological implications and possible mechanisms. Ecotoxicol Environ Saf 14:164–177. [DOI] [PubMed] [Google Scholar]
- 5.Bancroft JD, Gamble M. 2008. Theory and practice of histologic techniques, 6th ed. Edinburgh (United Kingdom): Churchill Livingstone. [Google Scholar]
- 6.Bergin IL, Chien CC, Marini RP, Fox JG. 2000. Isolation and characterization of Corynebacterium ulcerans from cephalic implants in macaques. Comp Med 50:530–535. [PubMed] [Google Scholar]
- 7.Bhola R, Bhola SM, Mishra B, Olson DL. 2011. Corrosion in titanium dental implants and prostheses—a review. Trends Biomater Artif Organs 25:34–46. [Google Scholar]
- 8.Bjerknes S, Skogseid IM, Saehle T, Dietrichs E, Toft M. 2014. Surgical site infections after deep brain stimulation surgery: frequency, characteristics, and management in a 10-year period. PLoS One 9:e105288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bozzini B, Carlino P, D'Urso L, Pepe V, Mele C, Venturo F. 2008. Effects of peracetic acid on the corrosion resistance of commercially pure titanium (grade 4). J Mater Med 19:3443–3453. [DOI] [PubMed] [Google Scholar]
- 10.Campoccia D, Montanaro L, Arciola CR. 2006. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 27:2331–2339. [DOI] [PubMed] [Google Scholar]
- 11.Cheng X, Roscoe SG. 2005. Corrosion behavior of titanium in the presence of calcium phosphate and serum proteins. Biomaterials 26:7350–7356. [DOI] [PubMed] [Google Scholar]
- 12.Cohen MD, Kargacin B, Klein CB, Costa M. 2008. Mechanisms of chromium carcinogenicity and toxicity. Crit Rev Toxicol 23:255–281. [DOI] [PubMed] [Google Scholar]
- 13.Cooper HJ, Urban RM, Wixson RL, Meneghini RM, Jacobs JJ. 2013. Adverse local tissue reaction arising from corrosion at the femoral neck–body junction in a dual-taper stem with a cobalt–chromium modular neck. J Bone Joint Surg Am 95:865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cundy TP, Cundy WJ, Antoniou G, Sutherland LM, Freeman BJ, Cundy PJ. 2014. Serum titanium, niobium, and aluminium levels 2 y following instrumented spinal fusion in children: does implant surface area predict serum metal ion levels? Eur Spine J 23:2393–2400. [DOI] [PubMed] [Google Scholar]
- 15.Dal Agnol CZ, Stefenon L, van De Sande FH, Della Bona Á, Cenci MS, Webber B, Rodrigues LB, dos Santos LR. 2015. Microcosm biofilm formation on titanium surfaces. J Mater Res 18:677–682. [Google Scholar]
- 16.Dalal A, Pawar V, McAllister K, Weaver C, Hallab NJ. 2012. Orthopedic implant cobalt-alloy particles produce greater toxicity and inflammatory cytokines than titanium alloy and zirconium alloy-based particles in vitro, in human osteoblasts, fibroblasts, and macrophages. J Biomed Mater Res A 100A:2147–2158. [DOI] [PubMed] [Google Scholar]
- 17.Darouiche RO. 2004. Treatment of infections associated with surgical implants. N Engl J Med 350:1422–1429. [DOI] [PubMed] [Google Scholar]
- 18.Diefenbeck M, Schrader C, Gras F, Muckley T, Schmidt J, Zankovych S, Bossert J, Jandt KD, Volpel A, Sigusch BW, Schubert H, Bischoff S, Pfister W, Edel B, Faucon M, Finger U. 2016. Gentamicin coating of plasma chemical oxidized titanium alloy prevents implant-related osteomyelitis in rats. Biomaterials 101:156–164. [DOI] [PubMed] [Google Scholar]
- 19.Donachie M., Jr 2000Introduction to selection of titanium alloys, p 5–12. In: Titanium: a technical guide, 2nd ed. Materials Park (OH): ASM International. [Google Scholar]
- 20.Elias CN, Lima JHC, Valiev R, Meyers MA. 2008. Biomedical applications of titanium and its alloys. JOM 60:46–49. [Google Scholar]
- 21.Ettinger M, Calliess T, Kielstein JT, Sibai J, Bruckner T, Lichtinghagen R, Windhagen H, Lukasz A. 2015. Circulating biomarkers for discrimination between aseptic joint failure, low-grade infection, and high-grade septic failure. Clin Infect Dis 61:332–341. [DOI] [PubMed] [Google Scholar]
- 22.Ferreira Ribeiro C, Cogo-Muller K, Franco GC, Silva-Concilio LR, Sampaio Campos M, de Mello Rode S, Claro Neves AC. 2016. Initial oral biofilm formation on titanium implants with different surface treatments: an in vivo study. Arch Oral Biol 69:33–39. [DOI] [PubMed] [Google Scholar]
- 23.Frydman GH, Davis N, Beck PL, Fox JG. 2015. Helicobacter pylori eradication in patients with immune thrombocytopenic purpura: a review and the role of biogeography. Helicobacter 20:239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gristina AG, Costerton JW. 1985. Bacterial adherence to biomaterials and tissue. The significance of its role in clinical sepsis. J Bone Joint Surg Am 67:264–273. [PubMed] [Google Scholar]
- 25.Gristina AG, Oga M, Webb LX, Hobgood CD. 1985. Adherent bacterial colonization in the pathogenesis of osteomyelitis. Science 228:990–993. [DOI] [PubMed] [Google Scholar]
- 26.Harrasser N, Jussen S, Banke IJ, Kmeth R, von Eisenhart-Rothe R, Stritzker B, Gollwitzer H, Burgkart R. 2015. Antibacterial efficacy of titanium-containing alloy with silver-nanoparticles-enriched diamond-like carbon coatings. AMB Express 5:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hermanides J, Huijgen R, Henny CP, Mohammad NH, Hoekstra JB, Levi MM, DeVries JH. 2009. Hip surgery sequentially induces stress hyperglycaemia and activates coagulation. Neth J Med 67:226–229. [PubMed] [Google Scholar]
- 28.Jennison T, McNally M, Pandit H. 2014. Prevention of infection in external fixator pin sites. Acta Biomater 10:595–603. [DOI] [PubMed] [Google Scholar]
- 29.Kos MI, Stenz L, Francois P, Guyot JP, Schrenzel J. 2009. Immunodetection of Staphylococcus aureus biofilm on a cochlear implant. Infection 37:450–454. [DOI] [PubMed] [Google Scholar]
- 30.Koseki H, Yonekura A, Shida T, Yoda I, Horiuchi H, Morinaga Y, Yanagihara K, Sakoda H, Osaki M, Tomita M. 2014. Early staphylococcal biofilm formation on solid orthopaedic implant materials: in vitro study. PLoS One 9:e107588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krewski D, Yokel RA, Nieboer E, Borchelt D, Cohen J, Harry J, Kacew S, Lindsay J, Mahfouz AM, Rondeau V. 2007. Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J Toxicol Environ Health B Crit Rev 10 Suppl 1:1–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon YM, Fehring TK, Lombardi AV, Barnes CL, Cabanela ME, Jacobs JJ. 2014. Risk stratification algorithm for management of patients with dual modular taper total hip arthroplasty: consensus statement of the American Association of Hip and Knee Surgeons, the American Academy of Orthopaedic Surgeons, and the Hip Society. J Arthroplasty 29:2060–2064. [DOI] [PubMed] [Google Scholar]
- 33.Kwon YM, Lombardi AV, Jacobs JJ, Fehring TK, Lewis CG, Cabanela ME. 2014. Risk stratification algorithm for management of patients with metal-on-metal hip arthroplasty: consensus statement of the American Association of Hip and Knee Surgeons, the American Academy of Orthopaedic Surgeons, and the Hip Society. J Bone Joint Surg Am 96:e4. [DOI] [PubMed] [Google Scholar]
- 34.Leblanc M, Berry K, McCort H, Reuter JD. 2013. Brain abscess in a rhesus macaque (Macaca mulatta) with a cephalic implant. Comp Med 63:367–372. [PMC free article] [PubMed] [Google Scholar]
- 35.Lemons JE, Venugopalan R, Lucas LC. 1999. Corrosion and biodegradation p 155–170. In: von Recum AF. Handbook of biomaterials evaluation. New York (NY): Taylor and Francis. [Google Scholar]
- 36.Levine BR, Hsu AR, Skipor AK, Hallab NJ, Paprosky WG, Galante JO, Jacobs JJ. 2013. Ten-year outcome of serum metal ion levels after primary total hip arthroplasty: a concise follow-up of a previous report. J Bone Joint Surg Am 95:512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Yang C, Zhao H, Qu S, Li X, Li Y. 2014. New developments of Ti-based alloys for biomedical applications. Materials (Basel) 7:1709–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu G, Huang W, Moir RD, Vanderburg CR, Lai B, Peng Z, Tanzi RE, Rogers JT, Huang X. 2006. Metal exposure and Alzheimer's pathogenesis. J Struct Biol 155:45–51. [DOI] [PubMed] [Google Scholar]
- 39.Lukina E, Laka A, Kollerov M, Sampiev M, Mason P, Wagstaff P, Noordeen H, Yoon WW, Blunn G. 2016. Metal concentrations in the blood and tissues after implantation of titanium growth guidance sliding instrumentation. Spine J.16:380–388. [DOI] [PubMed] [Google Scholar]
- 40.McGarry S, Morgan SJ, Grosskreuz RM, Williams AE, Smith WR. 2008. Serum titanium levels in individuals undergoing intramedullary femoral nailing with a titanium implant. J Trauma 64:430–433. [DOI] [PubMed] [Google Scholar]
- 41.Meyers K, Wardrop JJ.1991. Platelets and coagulation, p 87–136. In: Cotter SM. Comparative transfusion medicine. San Diego (CA): Academic Press. [DOI] [PubMed] [Google Scholar]
- 42.Mishra SK, Teotia AK, Kumar A, Kannan S. 2016. Mechanically tuned nanocomposite coating on titanium metal with integrated properties of biofilm inhibition, cell proliferation, and sustained drug delivery. Nanomedicine.13:23–35. [DOI] [PubMed] [Google Scholar]
- 43.Moroni A, Vannini F, Mosca M, Giannini S. 2002. State of the art review: techniques to avoid pin loosening and infection in external fixation. J Orthop Trauma 16:189–195. [DOI] [PubMed] [Google Scholar]
- 44.Niessen RW, Lamping RJ, Jansen PM, Prins MH, Peters M, Taylor FB, Jr, de Vijlder JJ, ten Cate JW, Hack CE, Sturk A. 1997. Antithrombin acts as a negative acute-phase protein as established with studies on HepG2 cells and in baboons. Thromb Haemost 78:1088–1092. [PubMed] [Google Scholar]
- 45.Nuevo- Ordóñez Y, Montes-Bayon M, Blanco-Gonzalez E, Paz-Aparicio J, Raimundez JD, Tejerina JM, Pena MA, Sanz-Medel A. 2011. Titanium release in serum of patients with different bone fixation implants and its interaction with serum biomolecules at physiological levels. Anal Bioanal Chem 401:2747–2754. [DOI] [PubMed] [Google Scholar]
- 46.Park YJ, Song YH, An JH, Song HJ, Anusavice KJ. 2013. Cytocompatibility of pure metals and experimental binary titanium alloys for implant materials. J Dent 41:1251–1258. [DOI] [PubMed] [Google Scholar]
- 47.Park YS, Moon YW, Lim SJ, Yang JM, Ahn G, Choi YL. 2005. Early osteolysis following second-generation metal-on-metal hip replacement. J Bone Joint Surg Am 87:1515–1521. [DOI] [PubMed] [Google Scholar]
- 48.Pawlowski KS, Wawro D, Roland PS. 2005. Bacterial biofilm formation on a human cochlear implant. Otol Neurotol 26:972–975. [DOI] [PubMed] [Google Scholar]
- 49.Perry DJ. 1994. Antithrombin and its inherited deficiencies. Blood Rev 8:37–55. [DOI] [PubMed] [Google Scholar]
- 50.Phaff M, Aird J, Rollinson PD. 2015. Delayed implants sepsis in HIV-positive patients following open fractures treated with orthopaedic implants. Injury 46:590–594. [DOI] [PubMed] [Google Scholar]
- 51.Rae T. 1981. The toxicity of metals used in orthopaedic prostheses. An experimental study using cultured human synovial fibroblasts. J Bone Joint Surg Br 63-B:435–440. [DOI] [PubMed] [Google Scholar]
- 52.Ribeiro M, Monteiro FJ, Ferraz MP. 2012. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacteria–material interactions. Biomatter 2:176–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rice KA, Chen ES, Metcalf Pate KA, Hutchinson EK, Adams RJ. 2013. Diagnosis of amyloidosis and differentiation from chronic, idiopathic enterocolitis in rhesus (Macaca mulatta) and pig-tailed (M. nemestrina) macaques. Comp Med 63:262–271. [PMC free article] [PubMed] [Google Scholar]
- 54.Ryberg D, Alexander J. 1990. Mechanisms of chromium toxicity in mitochondria. Chem Biol Interact 75:141–151. [DOI] [PubMed] [Google Scholar]
- 55.Saini M, Singh Y, Arora P, Arora V, Jain K. 2015. Implant biomaterials: a comprehensive review. World J Clin Cases 3:52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sakamoto M, Watanabe H, Higashi H, Kubosawa H. 2015. Pseudotumor caused by titanium particles from a total hip prosthesis. Orthopedics 39:e162–e165. [DOI] [PubMed] [Google Scholar]
- 57.Sarmiento- González A, Encinar JR, Marchante-Gayon JM, Sanz-Medel A. 2008. Titanium levels in the organs and blood of rats with a titanium implant, in the absence of wear, as determined by double-focusing ICP–MS. Anal Bioanal Chem 393:335–343. [DOI] [PubMed] [Google Scholar]
- 58.Schmidmaier G, Lucke M, Wildemann B, Haas NP, Raschke M. 2006. Prophylaxis and treatment of implant-related infections by antibiotic-coated implants: a review. Injury 37 Suppl 2:S105–S112. [DOI] [PubMed] [Google Scholar]
- 59.Snow ET, Xu LS. 1991. Chromium(III) bound to DNA templates promotes increased polymerase processivity and decreased fidelity during replication in vitro. Biochemistry 30:11238–11245. [DOI] [PubMed] [Google Scholar]
- 60.Sokolowska B, Piecuch W, Walter-Croneck A, Dmoszynska A, Furmanik F. 2002. [[Evaluation of selected parameters of blood coagulation and the fibrinolysis system in patients undergoing total hip replacement]] Przegl Lek 59:502–508.[Article in Polish]. [PubMed] [Google Scholar]
- 61.Song YH, Kim MK, Park EJ, Song HJ, Anusavice KJ, Park YJ. 2014. Cytotoxicity of alloying elements and experimental titanium alloys by WST1 and agar overlay tests. Dent Mater 30:977 –983. [DOI] [PubMed] [Google Scholar]
- 62.Stearns DM, Courtney KD, Giangrande PH, Phieffer LS, Wetterhahn KE. 1994. Chromium(VI) reduction by ascorbate: role of reactive intermediates in DNA damage in vitro. Environ Health Perspect 102 Suppl 3:21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wachi T, Shuto T, Shinohara Y, Matono Y, Makihira S. 2015. Release of titanium ions from an implant surface and their effect on cytokine production related to alveolar bone resorption. Toxicology 327:1–9. [DOI] [PubMed] [Google Scholar]
- 64.Walsh CP, Hubbard JC, Nessler JP, Markel DC. 2015. MRI findings associated with recalled modular femoral neck rejuvenate and ABG implants. J Arthroplasty 30:2021–2026. [DOI] [PubMed] [Google Scholar]
- 65.Wang J, Li J, Guo G, Wang Q, Tang J, Zhao Y, Qin H, Wahafu T, Shen H, Liu X, Zhang X. 2016. Silver-nanoparticles-modified biomaterial surface resistant to Staphylococcus: new insight into the antimicrobial action of silver. Sci Rep 6:32699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Webster TJ, Patel AA, Rahaman MN, Sonny Bal B. 2012. Antiinfective and osteointegration properties of silicon nitride, poly(ether ether ketone), and titanium implants. Acta Biomater 8:4447–4454. [DOI] [PubMed] [Google Scholar]
- 67.Weisel JW, Dempfle CE. 2013. Fibrinogen structure and function, p 254–271. In: Marder VJ, Aird WC, Bennett JS, Schulman S, White GC., II Hemostasis and thrombosis, 6th ed. Philadelphia (PA):Lipinscott Williams and Wilkins. [Google Scholar]
- 68.Wetterhahn KE, Demple B, Kulesz MM, Copeland ES. 1992. Workshop report from the Division of Research Grants, National Institutes of Health. Metal carcinogenesis: a chemical pathology study section workshop. Cancer Res 52:4058–4063. [PubMed] [Google Scholar]
- 69.Woods SE, Lieberman MT, Lebreton F, Trowel E, de la Fuente-Nunez C, Dzink-Fox J, Gilmore MS, Fox JG. 2017. Characterization of multidrug-resistant Enterococcus faecalis isolated from cephalic recording chambers in research macaques (Macaca spp.).PLoS One 12:e0169293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang D, Guo Z, Shao H, Liu X, Ji Y. 2012. Mechanical properties of porous Ti–Mo and Ti–Nb alloys for biomedical application by gelcasting. Procedia Eng 36:160–167. [Google Scholar]
- 71.Yang Y, Yao Z, Dai W, Shi P, Luo L, Zhang C. 2014. Changes of thrombelastography in patients undergoing elective primary total knee and total hip replacement with low-molecular heparin prophylaxis. J Orthop Surg Res 9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu F, Addison O, Baker SJ, Davenport AJ. 2015. Lipopolysaccharide inhibits or accelerates biomedical titanium corrosion depending on environmental acidity. Int J Oral Sci 7:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu ZT, Zhang MH, Tian YX, Cheng J, Ma XQ, Liu HY, Wang C. 2014. Designation and development of biomedical Ti alloys with finer biomechanical compatibility in long-term surgical implants. Front Mater Sci 8:219–229. [Google Scholar]