Abstract
Early post-natal life is a key time for the development of the immune system and colonization of the host by microbiota. Recent studies have shown that specific limbs of the immune system can be regulated by microbiota in a time restricted period during this period. Studies in mouse models have shown that perturbations of the microbiota during early life can cause immune effects that can persist into adulthood and create increased host susceptibility to certain diseases. Here, we discuss the role of early life regulation of the immune system by microbiota and how it can be related to allergy development.
Humans are colonized by trillions of microorganisms that includes fungi, archaea, viruses, protozoans and bacteria. The highest number of these microbes can be found in the intestinal tract and in the colon in particular. The past decades have shown a growing interest in studying the phenotype and function of the microbiota in the gut and its roles in immunity and health outcomes. Analyses of microbiota composition in adult humans have revealed numerous associations between specific bacterial phyla and disease and elucidated a number of pathways by which this may occur. The earliest example is perhaps the relationship described between the gut microbiome and the phyla levels changes associated with metabolic pathways and obesity1,2. Since that time, there have been numerous other examples where alterations in the microbiota composition or the function of the microbiota as imputed by metagenomics or direct analysis of the microbiota are associated with human diseases. For example, type 2 diabetes is characterized by decreased quantities of butyrate producing bacteria3 in comparison to cardiovascular disease which is associated with increased gut microbiota that drive the production of inflammatory lipid mediators4. In the gut itself, microbial dysbiosis is also strongly associated with inflammatory bowel disease development5. This latter example has been quite instructive as it been difficult to determine whether the microbial alterations are primary and causative or secondary events that are derived from intestinal inflammation itself6.
Of recent interest is the possibility that many of the disease associated microbial changes that have been observed and their immune consequences may originally develop during the earliest days of life. During the early post-natal period of life, the microbiota is in the process of colonizing the host and its composition is highly unstable. This time of early life bacterial colonization also correlates with the development of the immune system and its education to tolerate its environment to fight pathogens and avoid allergy and autoimmunity. Increasing evidence shows that certain immune cell populations can be regulated by microbiota in a time restricted fashion. Further, perturbations of the microbiota during a specific early life time-period can have persisting effects on the immune system later in life. This has predicted the existence of a window of opportunity during the early life of the host which is amenable to environmental manipulation. This review describes the immune processes that are regulated by microbiota specifically during early life and their relation to disease development and allergy in particular.
Establishment of microbiota during early life
Mucosal tissues are colonized by microbes that have the capacity to interact with the host. In the adult, the gut microbiota, can regulate the host’s immune response7, metabolism8 and digestion9, which in the latter case provides the mucosa with specific enzymes and other proteins that wouldn’t be otherwise produced10. Four principal phyla generally comprise a healthy gut microbiome, Bacteroidetes, Firmicutes, Proteobacteria and Actinobacteria.
Although the fetal compartment has for long be considered as sterile, the colonization of the host by microbes seem to be initiated before birth. Maternal derived bacteria can be isolated from the umbilical cord blood of healthy neonates born by Caesarian (C)-section11. Bacteria are detected in the meconium of preterm human babies12. Labeled Enterococcus faecium orally inoculated to pregnant mice can be retrieved in the meconium of the fetus one day before birth13. In addition, a recent study has shown that, using a mutant strain of Escherichia coli, transient intestinal colonization of a pregnant mouse can exert effects on the immune system of the offspring14.
Newborns are exposed to a large diversity of maternal bacteria during birth. Logically, the composition of the newborn microbiota is deeply influenced by the mode of delivery and exposure to bacteria. The microbiota composition of infants born by vaginal birth is similar to maternal vaginal and gut microbiota15 while babies born by C-section harbor a microbiota resembling the human skin microbiota16. These differences in microbiota composition between vaginal and C-section born infants are persistent and microbes associated with C-section birth can still be detected 2 years after birth17. The infant microbiota structure is highly unstable and has low diversity18 compared to the adult19. The first major shift in intestinal microbiota composition was initially thought to be associated with the introduction of solid food20–22. A more recent study has shown that the differences in microbial composition and function associated with solid food introduction does not become apparent until the infant discontinues breast feeding suggesting that the latter rather than the transition to a solid food diet is the major factor that determines a shift of the microbial ecology toward an adult like configuration in 12 month-old infants15. The human intestinal microbiota further evolves with age and stabilizes after 3 years of life23. However, even at 5 years after birth the gut microbiota may still not be definitely established24. Thus, the precise time after which the microbiota definitely switches to an individual specific adult type remains to be firmly determined. Dietary and environmental factors are important in molding the composition of the microbiota after birth. Antibiotic usage25 and breast feeding/formula feeding26,27 are associated with modifications of microbiota composition. The genotype of infants can also influence the early gut microbiota composition as shown in infants carrying the human leukocyte antigen DQ2 haplotype28. The infant microbiota therefore evolves with age and reflects the history of exposure to external factors as well as host genetics before stabilizing to an individual adult microbiota arrangement (Figure 1).
Figure 1.
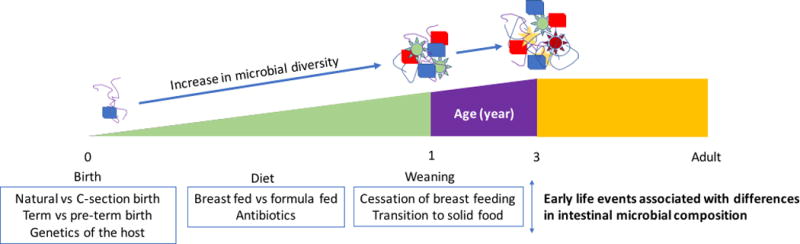
Evolution of microbiota from birth to adult life.
Microbial diversity increases from birth to 3 years of life and stabilizes. Genetics, the type of birth, diet and environmental factors are associated with differences in intestinal microbial composition.
This period of microbiota evolutiopn to an adult configuration during early life coincides with development of the immune system. Emerging evidence from rodent studies suggest that part of the immune system is educated by microbiota during early life in a time restricted fashion. This has been recognized to occur during the neonatal period of life especially before weaning29. However, the specific details of these temporal effects rodent and especially in the human remain to be defined. It has also been shown that the immune influences induced by the microbiota during this specific window of time may be a determining factor in resistance or susceptibility to diseases such as allergy during infancy and potentially in the adult.
Early life colonization and allergic diseases
The increased incidence of allergy worldwide in the context of progressive urbanization and industrialization has led to investigations about the influence of environmental and dietary factors associated with “westernized” countries that may be involved in the development of allergies. It is now well established that early life sensitization to allergens influence the susceptibility to allergic disease development in later life30,31. The “hygiene hypothesis” is based on the initial observation that family size and position in the household in childhood was associated with the development of hay fever, asthma and atopic dermatitis suggesting that exposure to microbial agents during early life can protect against the development of allergic diseases32,33. The implication of this hypothesis is that an absence of proper microbial exposure during early life can lead to increased susceptibility to these disorders. In a subsequent publication, Bach colleagues suggested that this hypothesis may have important implications for a broad range of autoimmune conditions by noting the inverse correlation between decreased infectious disease exposure in western societies and the increasing incidence of numerous autoimmune conditions34.
Clinical studies have provided evidence for a link between the composition of the bacteria in early life and the development of allergy. The composition of the lung microbiome has been identified as being altered in allergic diseases and asthma in particular. A higher bacterial burden and diversity is observed in the lower airways of asthmatic patients compared to healthy subjects35. The Proteobacteria phylum is particularly enriched in asthmatic patients35,36. This excess of Proteobacteria is interestingly reminiscent of observations in inflammatory bowel disease raising the possibility that such changes are secondary events6. A definitive association between dysbiosis in the airway and asthma is further limited by the inclusion in these studies of patients treated with inhaled corticosteroids that may influence the composition of the airway microbiome. In parallel, perturbations in airway colonization have been associated with early life asthma37. In this study, colonization of the oropharynx with a diverse microbial species at one month of age was associated with an increased risk of wheezing and development of asthma by the age of 5 years old. Colonization of the nasopharynx in early life has also been shown to influence the risk of recurrent wheezing and asthma development38. Indeed, early life asymptomatic colonization with Streptococcus microbial species is identified as a strong asthma predictor.
Similar to the airway, early life perturbations of the intestinal microbiota has also been shown to be associated with allergic diseases. An increased risk of allergic sensitization and allergic rhinitis, but not asthma, in the first 6 years of life is correlated with reduced bacterial diversity of the infant's intestinal flora39. Infants with atopic eczema exhibit a lower diversity of the gut microbiota40. Moreover, differences in the neonatal gut microflora may precede the development of atopy41. Together, these studies suggest that how the host is colonized by microbes during early life in different mucosal tissues may influence the later development of allergy.
Early life regulation of the immune system by microbiota and susceptibility to allergy
Experimental studies of germ free (GF) compared to specific pathogen free (SPF) mice demonstrate that the microbiota is deeply implicated in the maturation of the immune system. The regulation of the immune system by microbiota may be initiated prenatally via the transplacental transfer of molecular signatures from the microbiota to the developing fetus which in the case examined is associated with the early post-natal development of group 3 innate lymphoid cells14. It is interesting that this period coincides with the transplacental movement of IgG and that these observed effects were extinguished in JH−/− mice which lack B cells suggesting that they may be causally linked. Post-natally, maternal breast milk contains anti-commensal antibodies that helps dampen T cell responses toward microbiota in suckling pups42.
In the adult, GF mice are known to present smaller lymphoid structures including Peyer’s patches, mesenteric lymph nodes and isolated lymphoid follicles43–45. These major structural defects are associated with a decrease in the activation and/or frequency of lymphoid cells in the intestines and other organs including αβ T cells46, B cells47 and innate lymphoid cells48,49 that can be rescued by conventionalization with SPF microbiota at any age. However, the observation that transcriptional differences can be observed in the intestines of GF mice conventionalized during adult life with SPF microbiota compared to those that are conventionalized at birth50 support the notion that some immune cells abnormalities observed in GF mice cannot be normalized by reintroducing commensal bacteria in the adult but are instead regulated by microbiota only during a specific time period associated with early life. Further, it is now recognized that when this early life “window of opportunity” is not properly exposed to microbiota in mouse models, the effects of this inadequate exposure to the early life immune system can persist into adult life and thus cause permanent consequences for the health of the host29. Several lines of evidence reveal an association between specific early-life dysregulation of the immune system by microbiota and the development of allergic diseases.
iNKT cells and eosinophilic esophagitis
Eosinophilic esophagitis (EoE) is a chronic allergen-mediated esophageal disease induced by T-helper 2 cytokines that is characterized by esophageal dysfunction and eosinophil inflammation. The antigens that trigger the disease appear to be predominantly found in food although their nature remains undefined. EoE is a recently recognized condition that exhibits an increasing prevalence in developed and developing countries worldwide51,52. Environmental exposures seem to predispose to EoE. As an example, early life antibiotic treatment and cesarean delivery are associated with the development of EoE suggesting that microbiota may have a role in determining the risk for the development of this disease53. EoE was recently shown to be closely associated with a minor specialized subset of T cells: the invariant natural killer T cell (iNKT). iNKT cells use an αβ T-cell receptor heterodimer with limited diversity for the recognition of exogenous (bacterial and non-bacterial) and endogenous (self) lipid antigens specifically presented by the major histocompatibility-complex class I –like molecule CD1d54. Artificially induced EoE by peanut allergen exposure in mouse is dependent on iNKT cells, as CD1d deficient mice that lack iNKT cells are protected from disease induction55. In parallel, a clinical study of children with EoE showed that transcripts for the chemokine CXCL16, the iNKT T cell receptor marker Vα24 and Cd1d are upregulated in esophageal biopsies of children with EoE especially during early life56. It was also observed that successful treatment of EoE patients with dietary elimination of allergens leads to reduction of the transcriptional signatures associated with the infiltrating iNKT cells and normalization of the transcript levels for CXCL16 and CD1d. Moreover, this study showed that EoE and the aforementioned increase in CD1d-restricted iNKT cell responses is potentially associated with the use of antibiotics during the first year of life based upon retrospective surveys implicating a role for microbiota during early life in the regulation of iNKT cell responses to EoE associated allergens in later life. Interestingly, the microbiota is a known regulator of iNKT cell presence and function in mouse. iNKT cells play an important role in the control of commensal and pathogenic microbiota57,58. At the same time, the microbiota regulates iNKT cells. In adult GF mice, for example, iNKT cells accumulate in the colon and lung, leading to an increased susceptibility to experimental model of colitis and airway hyperresponsivness (AHR) that is associated with an increased expression of CXCL1659. The increased quantity of iNKT cells in these tissues and susceptibility to the associated diseases can be normalized by colonization with standard microbiota or treatment with a CXCL16 neutralizing antibody during neonatal, but not adult post-weaned life. This regulation of CXCL16 is associated with microbiota mediated epigenetic regulation by the microbiota59. CXCL16 can therefore regulate iNKT cell accumulation in lung and colon in a microbiota dependent but also age and tissue specific manner. In addition, monocolonization with the human bacterium Bacteroides fragilis or treatment with a Bacteroides fragilis derived glycosphingolipid during neonatal life can normalize the increased number of iNKT cells in the adult colon but not in the lung in a CXCL16 independent manner, supporting the idea that tissue specific microbes can regulate iNKT cell function60. Further, these same studies showed that mice which were not conventionalized during the neonatal period or were not monocolonized with B. fragilis during this time were shown to highly susceptible to later life environmental triggers of experimental colitis, using oxazolone61, or asthma, using ovalbumin sensitization62, both of which are known to be CD1d-dependent responses63,64. Together, these studies show that microbiota can regulate iNKT cells during an early life restricted window of time and influence later life susceptibility to inflammatory diseases such as allergy in mice. The mouse and human studies described above suggest a prominent role of early life microbiota in the regulation of iNKT cells and development of later life susceptibility to allergic diseases (Figure 2).
Figure 2.
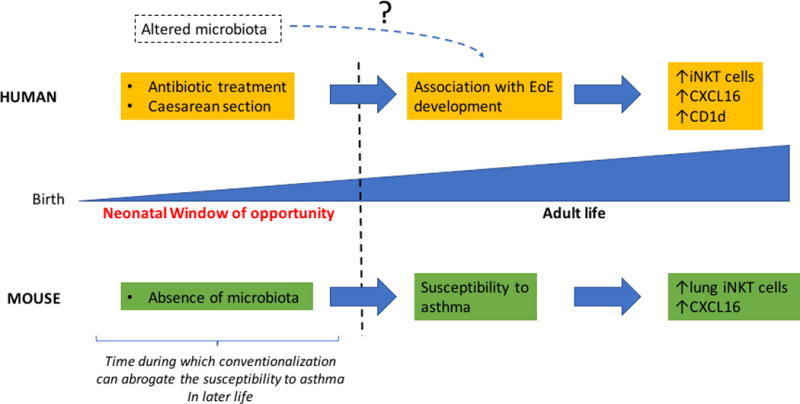
Role of early life microbiota in the regulation of iNKT cells and development of allergic disease in human and mouse.
Caesarean section and antibiotic treatment are associated with modifications of the microbiota in early life and to EoE development in later human life. Whether these microbial modifications are directly related to these diseases in humans remains to be established. EoE development is an allergic disease that depends on iNKT cell activation. iNKT cell numbers as well as CXCL16 and CD1d expression are increased in EoE patients less than 5 years of age. These increases are normalized by an elimination diet. An absence of microbiota in GF mice during early life leads to an accumulation of lung iNKT cells, increased CXCL16 expression and increased susceptibility to asthma in later life. Conventionalization with microbiota during a specific early life time frame, but not thereafter, can normalize the number of iNKT cells in the lung and susceptibility to colitis and asthma.
Regulatory T cells and Asthma
The type of environment an individual is exposed to determine the range of antigens that are encountered by the immune system. Environments that are rich in microbial products have been observed to significantly decrease susceptibility to specific allergic diseases. As an example, exposure to farms during early childhood provides a protective effect against allergy development including diseases such as asthma65. Microbial product exposure during childhood, as measured by endotoxin levels in mattress dust, is associated with protection against hay fever, atopic asthma and atopic sensitization66. Interestingly, this protective effect has been observed in farming and non-farming households in a rural environment66. Farm dust and endotoxin protect against allergy at least in part through a mechanism involving a modification of communication between epithelial cells and dendritic cells in a pathway that is dependent on the ubiquitin modifying enzyme A20, which normally negatively regulates these responses67,68. In parallel, maternal farm exposure is associated with increased T regulatory cells (Treg) function in the cord blood of the pregnant mothers and protection from asthma in their offspring69. Further, the environment is known to influence the composition of the microbiota. Accordingly, children with a farm lifestyle have different microbial diversity than those with less contact with farm animals and farm products70. Therefore, diversity of the intestinal microbiota may be involved in the protection from allergy observed in children exposed to a farm lifestyle and this may be correlated to specific modifications of the immune system during early life. Work from Renz and colleagues have in fact identified a number of potential candidate microorganisms that may be involved in providing protection from asthma71. Interestingly, regulation of Treg cells by microbiota during early life seems to play an especially important role in the development of allergic diseases in response to environmental exposure based upon studies in mice. Mice exposed to house dust mite (HDM) antigens during the first two weeks of life but not thereafter are protected from allergic airway inflammation due to the emergence of an induced (helios negative) Treg cell subset72. Emergence of Helios negative Treg cells after administration of HDM is dependent upon microbial exposure and correlates with an increase in the bacterial load and Bacteroidetes phyla in the lung. Moreover, the expansion of Treg cells can be abrogated by blocking programmed death ligand protein 1 (PD-L1) in early life resulting in increased asthma susceptibility. In another study, Treg cells have the capacity to accumulate in the mice skin in response to colonization with a skin commensal bacterial strain specifically during a window of time that consists of two weeks after birth73. In this study, the accumulation of Treg in the skin during neonatal life is required to establish tolerance to commensal derived antigens in the adult.
Antibiotic use in neonatal mice drives changes in microbiota that are associated with an alteration in colon associated Tregs and an increased susceptibility to airway hyperresponsiveness74. Consistent with this, pre and post-natal exposure to antibiotics in humans is associated with an increased risk for developing asthma75. Together these studies show that Treg cell function can be regulated specifically during early life by microbiota and exposure to specific environmental factors that may regulate their numbers and function (Figure 3). Further, such early life regulation of Treg cells may have effects that persist into adult life and affect the predisposition to allergy.
Figure 3.
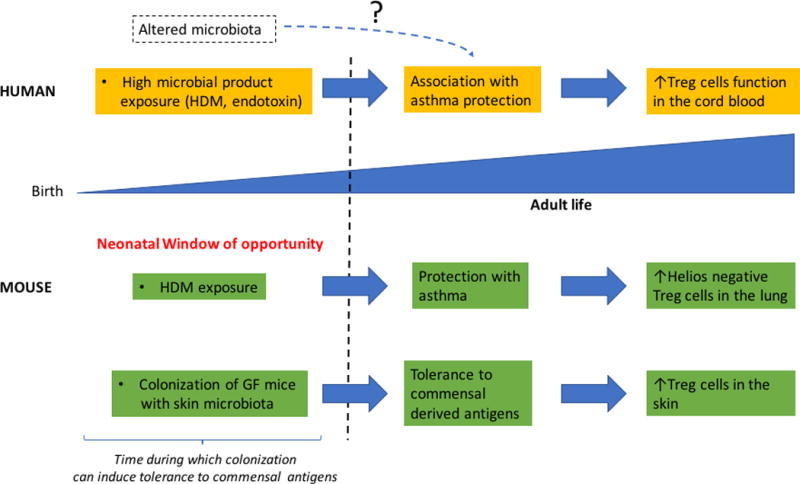
Role of early life microbiota in the regulation of Treg cells and development of allergic diseases in human and mouse.
High microbial exposure during early life is associated with asthma protection as well as increased Treg cells function in the cord blood of the fetus. In mice, HDM exposure during early life but not thereafter protects from asthma due to the emergence of an induced (helios negative) Treg cells. Treg cells have the capacity to accumulate in the skin of mice in response to colonization with a skin commensal bacterial strain specifically during early life and are required to establish tolerance to commensal derived antigens in the adult. HDM, house dust mite.
IgE and anaphylactic reaction
B cells are the source of different classes of immunoglobulins with different functions. Among them, IgE antibodies can bind Fc receptors on the surface of mast cells upon recognition of specific antigen and lead to their activation. The activation of mast cells by IgE is central in the development of allergic diseases because it can trigger the sudden release of granules into the blood stream that contain large amounts of inflammatory mediators such as histamine which lead to anaphylactic reactions. GF mice are well known to have decreased levels of other antibody isotypes such as IgA and IgG1 that can be normalized by conventionalization by microbiota in adult mice47. In contrast, GF mice and mice with low diversity microbiota develop elevated IgE levels in the serum that is associated with an increased level of IgE isotype switching within mucosal sites in a pathway that is dependent on CD4 T-cells and interleukin-476. These elevated levels of IgE in the serum lead to an increase in IgE binding to the mast cell surface and exaggerated oral induced systemic anaphylaxis. Only conventionalization with standard microbiota within 4 weeks after birth can normalize the IgE levels in the adult mice. Interestingly, a correlation has been observed between specific fecal microbial species in the infant and IgE mediated food allergy77. These studies showed that microbiota can regulate IgE expression in an age restricted fashion during early life in mice and that dysbiosis during infancy may be a predisposing factor to IgE mediated allergy development.
Concluding remarks
Among other hypotheses about the etiology of allergy, the “hygiene hypothesis” states that the exposure to microbial agents during early life can protect against the development of allergic diseases and vice-versa. This hypothesis has been increasingly gaining acceptance as it is becoming increasingly clear that exposure to microbiota during a specific early life window of time is essential for the normal development of certain elements of the immune system. The perturbations that develop when appropriate microbial signals are not received during this “window of opportunity” may have long-lasting effects on the immune system whose duration is still yet to be defined resulting in susceptibility to diseases associated with these tissue compartments such as colitis, asthma, atopic dermatitis, anaphylaxis and potentially others. The exact time frame of the “window of opportunity” where interventions for allergic protection may be successful still needs to be determined as well as the relevant equivalence for translation into human studies. As there is strong epidemiologic correlation between early life exposures associated with removal (e.g. antibiotics) or exposure (e.g. rural exposures) to microbes during early life in humans and allergy, understanding these pathways is of outmost importance in order to develop beneficial interventions in humans for protecting against allergic diseases. In the future, one of the principal challenges will be to determine the mechanisms that allow the immune system to be regulated by microbiota in a time restricted fashion.
Acknowledgments
R.S.B. is consultant to Symbiotix Biotherapies, Inc, that is developing microbial agents which regulate the immune system. R.S.B. is supported by NIH DK44319 and the Harvard Digestive Diseases Center (DK0034854). T.G is supported by the Crohn’s and Colitis Foundation of America.
This was supported by NIH RO1 DK44319 and Harvard Digestive Diseases Center (R.S.B) and Crohn’s and Colitis Foundation of America grant number 418509 (T.G)
MAD Glossary
- Archaea
A domain of primitive single-celled microorganisms that lack a nucleus. as well as organelles. Archaea and bacteria make up the two domains of Prokaryotae. Archaea are thought to have arose from extreme settings, such as hot, salty, and/or acidic environments
- Diversity
A term utilized in bacterial community metrics analysis that includes both richness (the number of different species represented in the community) and evenness (how equal the abundances of the species are).
- Enzyme
A20 A cytoplasmic zinc finger protein that inhibits nuclear factor kappa-B (NF-κB) activity and tumor necrosis factor (TNF)-mediated programmed cell death. NF-κB signaling cascades are heavily controlled by ubiquitination, and several proteins including A20 may interfere with these processes. Genome wide association studies (GWAS) have identified A20 as a susceptibility gene in inflammatory disease
- Group 3 Innate Lymphoid Cells (ILC3s)
Innate immune cells with lymphoid features that do not have antigen receptors. They can quickly respond to multiple tissue-derived factors, such as cytokines, eicosanoids, and alarmins, by producing multiple proinflammatory and immunoregulatory cytokines. ILCs classification into groups based on their transcription factors and cytokines parallels helper T-cell subsets. ILC3s resemble TH17 cells. ILC3s are defined by the production of IL-17A and IL-22 and by expression of retinoic acid–related orphan receptor γt (RORγt). They have been implicated in immunity against extracellular bacteria and autoimmune diseases
- Helios negative T regulatory cell
Natural T regulatory cells (nTreg) are Foxp3+ and usually are thymic derived. However, some Foxp3+ Tregs can be peripherally induced. A subset of Treg cells express Helios, a transcription factor whose function in Tregs is poorly understood, but may represent an additional way to differentiate subsets of Tregs
- Invariant natural killer T cells
A subset of lymphocytes that express surface molecules characteristic of both NK (CD16) and T cells (CD3). All NKT cells recognize lipids bound to CD1, an MHC-like molecule. They are capable of rapidly secreting cytokines following stimulation. The TCR α chains in invariant NKT cells have limited diversity and are characterized by a unique Vα24-Jα18 rearrangement
- Protozoans
Motile single-celled organisms, such as amoeba, containing nuclei and organelles. They are often divided based on kinetic properties. Examples include Plasmodium spp., Entamoeba histolytica, Trypanosoma spp., Leishmania spp., Giardia lamblia, Cryptosporidium, and Toxoplasma gondii.
References
- 1.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: Human gut microbes associated with obesity. Nature. 2006;444:1022–3. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 2.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–4. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, DuGar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hold GL, Smith M, Grange C, Watt ER, El-Omar EM, Mukhopadhya I. Role of the gut microbiota in inflammatory bowel disease pathogenesis: what have we learnt in the past 10 years? World J Gastroenterol. 2014;20:1192–210. doi: 10.3748/wjg.v20.i5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng MY, Inohara N, Nuñez G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017;10:18–26. doi: 10.1038/mi.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–23. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mardinoglu A, Shoaie S, Bergentall M, Ghaffari P, Zhang C, Larsson E, et al. The gut microbiota modulates host amino acid and glutathione metabolism in mice. Mol Syst Biol. 2015;11:834. doi: 10.15252/msb.20156487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes. 2012;3:289–306. doi: 10.4161/gmic.19897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donia MS, Fischbach MA. HUMAN MICROBIOTA. Small molecules from the human microbiota. Science. 2015;349:1254766. doi: 10.1126/science.1254766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiménez E, Fernández L, Marín ML, Martín R, Odriozola JM, Nueno-Palop C, et al. Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr Microbiol. 2005;51:270–4. doi: 10.1007/s00284-005-0020-3. [DOI] [PubMed] [Google Scholar]
- 12.Moles L, Gómez M, Heilig H, Bustos G, Fuentes S, de Vos W, et al. Bacterial diversity in meconium of preterm neonates and evolution of their fecal microbiota during the first month of life. PLoS One. 2013;8:e66986. doi: 10.1371/journal.pone.0066986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiménez E, Marín ML, Martín R, Odriozola JM, Olivares M, Xaus J, et al. Is meconium from healthy newborns actually sterile? Res Microbiol. 2008;159:187–93. doi: 10.1016/j.resmic.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Gomez de Aguero M, Ganal-Vonarburg SC, Fuhrer T, Rupp S, Uchimura Y, Li H, et al. The maternal microbiota drives early postnatal innate immune development. Science (80-) 2016;351:1296–302. doi: 10.1126/science.aad2571. [DOI] [PubMed] [Google Scholar]
- 15.Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe. 2015;17:690–703. doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–5. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. 2014;63:559–66. doi: 10.1136/gutjnl-2012-303249. [DOI] [PubMed] [Google Scholar]
- 18.Arrieta M-C, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B. The Intestinal Microbiome in Early Life: Health and Disease. Front Immunol. 2014;5:427. doi: 10.3389/fimmu.2014.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Claesson MJ, Cusack S, O’Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A. 2011;108(Suppl):4586–91. doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fallani M, Amarri S, Uusijarvi A, Adam R, Khanna S, Aguilera M, et al. Determinants of the human infant intestinal microbiota after the introduction of first complementary foods in infant samples from five European centres. Microbiology. 2011;157:1385–92. doi: 10.1099/mic.0.042143-0. [DOI] [PubMed] [Google Scholar]
- 21.Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A. 2011;108(Suppl):4578–85. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergström A, Skov TH, Bahl MI, Roager HM, Christensen LB, Ejlerskov KT, et al. Establishment of intestinal microbiota during early life: a longitudinal, explorative study of a large cohort of Danish infants. Appl Environ Microbiol. 2014;80:2889–900. doi: 10.1128/AEM.00342-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–7. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng J, Ringel-Kulka T, Heikamp-de Jong I, Ringel Y, Carroll I, de Vos WM, et al. Discordant temporal development of bacterial phyla and the emergence of core in the fecal microbiota of young children. ISME J. 2016;10:1002–14. doi: 10.1038/ismej.2015.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reinhardt C, Reigstad CS, Bäckhed F. Intestinal microbiota during infancy and its implications for obesity. J Pediatr Gastroenterol Nutr. 2009;48:249–56. doi: 10.1097/mpg.0b013e318183187c. [DOI] [PubMed] [Google Scholar]
- 26.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, et al. Factors Influencing the Composition of the Intestinal Microbiota in Early Infancy. Pediatrics. 2006;118 doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 27.Klonowski KD, Williams KJ, Marzo AL, Blair Da, Lingenheld EG, Lefrançois L. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20:551–62. doi: 10.1016/s1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- 28.Olivares M, Neef A, Castillejo G, Palma G De, Varea V, Capilla A, et al. The HLA-DQ2 genotype selects for early intestinal microbiota composition in infants at high risk of developing coeliac disease. Gut. 2015;64:406–17. doi: 10.1136/gutjnl-2014-306931. [DOI] [PubMed] [Google Scholar]
- 29.Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science (80-) 2016:352. doi: 10.1126/science.aad9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perzanowski MS, Chew GL, Divjan A, Jung KH, Ridder R, Tang D, et al. Early-life cockroach allergen and polycyclic aromatic hydrocarbon exposures predict cockroach sensitization among inner-city children. J Allergy Clin Immunol. 2013;131:886–93. doi: 10.1016/j.jaci.2012.12.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lynch SV, Wood RA, Boushey H, Bacharier LB, Bloomberg GR, Kattan M, et al. Effects of early-life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. J Allergy Clin Immunol. 2014;134:593–601.e12. doi: 10.1016/j.jaci.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–60. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu AH. Revisiting the hygiene hypothesis for allergy and asthma. J Allergy Clin Immunol. 2015;136:860–5. doi: 10.1016/j.jaci.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Bach J-F. The Effect of Infections on Susceptibility to Autoimmune and Allergic Diseases. N Engl J Med. 2002;347:911–20. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 35.Huang YJ, Nelson CE, Brodie EL, DeSantis TZ, Baek MS, Liu J, et al. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127:372–381.e3. doi: 10.1016/j.jaci.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, et al. Disordered Microbial Communities in Asthmatic Airways. PLoS One. 2010;5:e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bønnelykke K, et al. Childhood Asthma after Bacterial Colonization of the Airway in Neonates. N Engl J Med. 2007;357:1487–95. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 38.Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, et al. The Infant Nasopharyngeal Microbiome Impacts Severity of Lower Respiratory Infection and Risk of Asthma Development. Cell Host Microbe. 2015;17:704–15. doi: 10.1016/j.chom.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bisgaard H, Li N, Bonnelykke K, Chawes BLK, Skov T, Paludan-Müller G, et al. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol. 2011;128:646–652.e5. doi: 10.1016/j.jaci.2011.04.060. [DOI] [PubMed] [Google Scholar]
- 40.Abrahamsson TR, Jakobsson HE, Andersson AF, Björkstén B, Engstrand L, Jenmalm MC. Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol. 2012;129:434–440.e2. doi: 10.1016/j.jaci.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 41.Kalliomäki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol. 2001;107:129–34. doi: 10.1067/mai.2001.111237. [DOI] [PubMed] [Google Scholar]
- 42.Koch MA, Reiner GL, Lugo KA, Kreuk LSM, Stanbery AG, Ansaldo E, et al. Maternal IgG and IgA Antibodies Dampen Mucosal T Helper Cell Responses in Early Life. Cell. 2016;165:827–41. doi: 10.1016/j.cell.2016.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–85. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 44.Mosconi I, Geuking MB, Zaiss MM, Massacand JC, Aschwanden C, Kwong Chung CKC, et al. Intestinal bacteria induce TSLP to promote mutualistic T-cell responses. Mucosal Immunol. 2013;6:1157–67. doi: 10.1038/mi.2013.12. [DOI] [PubMed] [Google Scholar]
- 45.Baptista AP, Olivier BJ, Goverse G, Greuter M, Knippenberg M, Kusser K, et al. Colonic patch and colonic SILT development are independent and differentially regulated events. Mucosal Immunol. 2013;6:511–21. doi: 10.1038/mi.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Umesaki Y, Setoyama H, Matsumoto S, Okada Y. Expansion of alpha beta T-cell receptor-bearing intestinal intraepithelial lymphocytes after microbial colonization in germ-free mice and its independence from thymus. Immunology. 1993;79:32–7. [PMC free article] [PubMed] [Google Scholar]
- 47.Crabbé PA, Nash DR, Bazin H, Eyssen H, Heremans JF. Immunohistochemical observations on lymphoid tissues from conventional and germ-free mice. Lab Invest. 1970;22:448–57. [PubMed] [Google Scholar]
- 48.Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, et al. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Satoh-Takayama N, Vosshenrich CAJ, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–70. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 50.El Aidy S, Hooiveld G, Tremaroli V, Bäckhed F, Kleerebezem M. The gut microbiota and mucosal homeostasis: colonized at birth or at adulthood, does it matter? Gut Microbes. 4:118–24. doi: 10.4161/gmic.23362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi YN, Sun SJ, Xiong LS, Cao QH, Cui Y, Chen MH. Prevalence, clinical manifestations and endoscopic features of eosinophilic esophagitis: a pathological review in China. J Dig Dis. 2012;13:304–9. doi: 10.1111/j.1751-2980.2012.00593.x. [DOI] [PubMed] [Google Scholar]
- 52.Spergel JM, Book WM, Mays E, Song L, Shah SS, Talley NJ, et al. Variation in prevalence, diagnostic criteria, and initial management options for eosinophilic gastrointestinal diseases in the United States. J Pediatr Gastroenterol Nutr. 2011;52:300–6. doi: 10.1097/MPG.0b013e3181eb5a9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Radano MC, Yuan Q, Katz A, Fleming JT, Kubala S, Shreffler W, et al. Cesarean section and antibiotic use found to be associated with eosinophilic esophagitis. J Allergy Clin Immunol Pract. 2014;2:475–477.e1. doi: 10.1016/j.jaip.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 54.van Dieren JM, van der Woude CJ, Kuipers EJ, Escher JC, Samsom JN, Blumberg RS, et al. Roles of CD1d-restricted NKT cells in the intestine. Inflamm Bowel Dis. 2007;13:1146–52. doi: 10.1002/ibd.20164. [DOI] [PubMed] [Google Scholar]
- 55.Rajavelu P, Rayapudi M, Moffitt M, Mishra A, Mishra A. Significance of para-esophageal lymph nodes in food or aeroallergen-induced iNKT cell-mediated experimental eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol. 2012;302:G645–54. doi: 10.1152/ajpgi.00223.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lexmond WS, Neves JF, Nurko S, Olszak T, Exley MA, Blumberg RS, et al. Involvement of the iNKT cell pathway is associated with early-onset eosinophilic esophagitis and response to allergen avoidance therapy. Am J Gastroenterol. 2014;109:646–57. doi: 10.1038/ajg.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nieuwenhuis EES, Matsumoto T, Exley M, Schleipman RA, Glickman J, Bailey DT, et al. CD1d-dependent macrophage-mediated clearance of Pseudomonas aeruginosa from lung. Nat Med. 2002;8:588–93. doi: 10.1038/nm0602-588. [DOI] [PubMed] [Google Scholar]
- 58.Nieuwenhuis EES, Matsumoto T, Lindenbergh D, Willemsen R, Kaser A, Simons-Oosterhuis Y, et al. Cd1d-dependent regulation of bacterial colonization in the intestine of mice. J Clin Invest. 2009;119:1241–50. doi: 10.1172/JCI36509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke a, et al. Microbial Exposure During Early Life Has Persistent Effects on Natural Killer T Cell Function. Science (80-) 2012;336:489–93. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.An D, Oh SF, Olszak T, Neves JF, Avci FY, Erturk-Hasdemir D, et al. Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell. 2014;156:123–33. doi: 10.1016/j.cell.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boirivant M, Fuss IJ, Chu A, Strober W. Oxazolone colitis: A murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J Exp Med. 1998;188:1929–39. doi: 10.1084/jem.188.10.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nials AT, Uddin S. Mouse models of allergic asthma: acute and chronic allergen challenge. Dis Model Mech. 2008;1:213–20. doi: 10.1242/dmm.000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heller F, Fuss IJ, Nieuwenhuis EE, Blumberg RS, Strober W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity. 2002;17:629–38. doi: 10.1016/s1074-7613(02)00453-3. [DOI] [PubMed] [Google Scholar]
- 64.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9:582–8. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 65.Riedler J, Braun-Fahrländer C, Eder W, Schreuer M, Waser M, Maisch S, et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet (London, England) 2001;358:1129–33. doi: 10.1016/S0140-6736(01)06252-3. [DOI] [PubMed] [Google Scholar]
- 66.Braun-Fahrländer C, Riedler J, Herz U, Eder W, Waser M, Grize L, et al. Environmental Exposure to Endotoxin and Its Relation to Asthma in School-Age Children. N Engl J Med. 2002;347:869–77. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 67.Schuijs MJ, Willart MA, Vergote K, Gras D, Deswarte K, Ege MJ, et al. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science (80-) 2015;349:1106–10. doi: 10.1126/science.aac6623. [DOI] [PubMed] [Google Scholar]
- 68.Ma A, Malynn BA. A20: linking a complex regulator of ubiquitylation to immunity and human disease. Nat Rev Immunol. 2012;12:774–85. doi: 10.1038/nri3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schaub B, Liu J, Höppler S, Schleich I, Huehn J, Olek S, et al. Maternal farm exposure modulates neonatal immune mechanisms through regulatory T cells. J Allergy Clin Immunol. 2009;123:774–82.e5. doi: 10.1016/j.jaci.2009.01.056. [DOI] [PubMed] [Google Scholar]
- 70.Dicksved J, Flöistrup H, Bergström A, Rosenquist M, Pershagen G, Scheynius A, et al. Molecular fingerprinting of the fecal microbiota of children raised according to different lifestyles. Appl Environ Microbiol. 2007;73:2284–9. doi: 10.1128/AEM.02223-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pfefferle PI, Renz H. Microbial Exposure and Onset of Allergic Diseases - Potential Prevention Strategies? Allergol Int. 2014;63:3–10. doi: 10.2332/allergolint.13-RAI-0671. [DOI] [PubMed] [Google Scholar]
- 72.Gollwitzer ES, Saglani S, Trompette A, Yadava K, Sherburn R, McCoy KD, et al. Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nat Med. 2014;20:642–7. doi: 10.1038/nm.3568. [DOI] [PubMed] [Google Scholar]
- 73.Scharschmidt TC, Vasquez KS, Truong H-A, Gearty SV, Pauli ML, Nosbaum A, et al. A Wave of Regulatory T Cells into Neonatal Skin Mediates Tolerance to Commensal Microbes. Immunity. 2015;43:1011–21. doi: 10.1016/j.immuni.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Russell SL, Gold MJ, Hartmann M, Willing BP, Thorson L, Wlodarska M, et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 2012;13:440–7. doi: 10.1038/embor.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Metsälä J, Lundqvist A, Virta LJ, Kaila M, Gissler M, Virtanen SM. Prenatal and post-natal exposure to antibiotics and risk of asthma in childhood. Clin Exp Allergy. 2015;45:137–45. doi: 10.1111/cea.12356. [DOI] [PubMed] [Google Scholar]
- 76.Cahenzli J, Köller Y, Wyss M, Geuking MB, McCoy KD. Intestinal microbial diversity during early-life colonization shapes long-term IgE levels. Cell Host Microbe. 2013;14:559–70. doi: 10.1016/j.chom.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ling Z, Li Z, Liu X, Cheng Y, Luo Y, Tong X, et al. Altered fecal microbiota composition associated with food allergy in infants. Appl Environ Microbiol. 2014;80:2546–54. doi: 10.1128/AEM.00003-14. [DOI] [PMC free article] [PubMed] [Google Scholar]


