Abstract
BACKGROUND
In order to address the pre- and perioperative need for visualization and prediction of patient-specific anatomy for surgical planning, endoscopic neurosurgeons have increasingly relied on computerized navigation devices to guide their surgical approaches.
OBJECTIVE
This manuscript aims to review: 1) the use of neuronavigation in endoscopic neurosurgery for pre-operative planning, 2) the intraoperative advantages of neuronavigation in endoscopic neurosurgery, and 3) the effects of navigation guidance on operative time, registration accuracy, brain shift, and avoidance of complications. Limitations of the current neuroendoscopic navigation literature will be discussed.
METHODS
We conducted a search using PubMed-MEDLINE; the keywords “stereotactic navigation AND endoscopic surgery” and “simulation AND endoscopic neurosurgery”. 36 studies were identified that addressed the use of neuronavigation in endoscopic neurosurgery. These studies were then further analyzed for topics relevant to computerized neuroendoscopy and reviewed for the purposes of this article.
CONCLUSION
Three-dimensional, frameless neuronavigation systems are useful in endoscopic neurosurgery to assist in the pre-operative planning of potential trajectories and to help localize the pathology of interest. Neuronavigation appears to be accurate to < 1–2 mm without issues related to brain shift. Further work is necessary in the investigation of the effect of neuronavigation on operative time, cost, and patient-centered outcomes.
Keywords: Endoscopic, stereotactic navigation, neuronavigation, frameless, image-guided
Introduction
A recurrent problem faced by endoscopic neurosurgeons is the ability to visualize and predict the location of important anatomic structures in a relatively small working environment. In endoscopic trans-sphenoidal surgery of the pituitary gland, the ability to predict the location of the carotid artery behind the sphenoid bone is paramount to prevent accidental vascular injury. In re-operative cases, normal anatomy can be distorted and the risk of iatrogenic complications can significantly increase.1 To address these potential pitfalls, endoscopic neurosurgeons have increasingly relied on computerized navigation devices to guide their surgical approaches. There are several navigation systems currently available on the market such as the Brainlab Curve (AG, Feldkirchen, Germany) and Medtronic StealthStation S7 (Medtronic, Minneapolis, MN).2 This manuscript aims to review the use of three-dimensional (3D) computerized neuronavigation systems in endoscopic neurosurgery for pre-operative planning and several of its key intraoperative advantages through discussion of an illustrative case of odontoidectomy with intraoperative neuronavigation. In addition, the effects of navigation guidance in regards to operative time, registration accuracy, brain shift, and complications will be discussed. Finally, the limitations of the current neuronavigation in neuroendoscopy literature will be discussed.
Illustrative Case: Navigation Guidance in Endoscopic Odontoidectomy
Navigation guidance has been utilized in endoscopic odontoidectomy.3–5 We report the case of a 49-year-old woman who initially presented complaining of severe frontal tussive headaches accompanied by imbalance, dizziness, nausea, and perioral and lingual numbness for the previous seven months. At the time, the patient’s exam was normal with the exception of bilateral fine beating nystagmus noted upon sustained horizontal gaze. MRI of the brain and cervical spine revealed a type I Chiari malformation with downward tonsilar displacement of 12 mm, no associated syrinx, and right-sided foraminal stenosis secondary to bulging discs at the cervical level (C) 3/4, and C 4/5 (Figure 1). Given the unremitting symptomatology with medical therapy, the patient underwent a decompressive suboccipital craniectomy and laminectomy of C1 with an uneventful post-operative course.
Figure 1. Pre-operative MRI of the brain and cervical spine.

(A) Pre-operative sagittal and axial T2-weighted magnetic resonance imaging scans demonstrating a Type I Chiari Malformation with associated basilar invagination. (B) There is ventral and dorsal medullary compression by the odontoid process and cerebellar tonsils, respectively.
Six weeks post-operatively, the patient presented with a recurrence of the tussive frontal headache exacerbated with flexion of the head, neck pain with radiation to the shoulders bilaterally, dizziness, imbalance, dysmetria, ataxia, paresthesias of the upper and lower extremities, and difficulty with ambulation and dexterity. Physical examination revealed a wide-based gait with the inability to toe or heel walk or maintain a tandem gait, diffuse weakness and hyperactive reflexes throughout, and diminished sensation following a stocking glove distribution. As shown in Figure 1, MRI of the brain and cervical spine revealed a ventral cervicomedullary junction deformity, an acute angle between the dens and the clivus, and stable cerebellar ectopia. Due to the interval development of symptoms suggestive of cervical myelopathy and findings on MRI, the patient underwent a staged endoscopic transnasal odontoidectomy followed by occipitocervical fusion. The interval development of symptomatic atlanto-axial instability was likely exacerbated by the initial decompression. However, it is unclear if the patient had pre-existing instability before surgery. Unfortunately, pre-operative flexion/extension imaging of the cervical spine was not available.
As demonstrated in Figures 2–4, stereotactic neuronavigation (Brainlab AG, Feldkirchen, Germany and Surgical Theatre, LLC, Mayfield Village, OH) was essential in the determination of the operative approach (transnasal versus transoral), and navigation was used throughout the case with excellent accuracy. Based on the results of imaging, a trans-nasal entry point was selected instead of a trans-oral approach. The reason was due to a tortuous left carotid artery that was directly anterior to the C2 vertebral body. A trans-oral approach may have increased the risk of accidental carotid injury given the angle of approach; therefore a slight more superior trajectory angle was selected (trans-nasal). An extradural odontoid, C1 process, and basilar invagination resection was performed using a transclival C1 approach. Following drilling of the inferior clivus, the underlying tectorial membrane and dura were identified. The anterior tubercle of C1 was drilled until the identification of C2, and the angulation of C2 was shaped to an anterior angulation. The odontoid process was drilled using an eggshell technique. A 30-degree endoscope in combination with intraoperative navigation was used to identify decompression of the spinal cord, as pictured in Figure 5. Occipitocervical fusion occurred 4 days post-operatively, as demonstrated in Figure 6, and the patient was discharged after an uneventful post-operative course 8 days following the first operation. The patient’s post-operative course remained unremarkable and her myelopathic symptoms were steadily improving.
Figure 2. 3D-reconstructed pre-operative images displayed by the Surgical Theatre™ planning station (Surgical Theatre, LLC, Mayfield Village, OH).

(A) (blue asterix) A tortuous left internal carotid artery is seen directly anterior to the C2 vertebral body and odontoid process. (B) A stereotactic registration wand was used to plan the surgical approach (maroon asterix). Based on this imaging, a trans-nasal approach was selected given the location of the left internal carotid artery.
Figure 4. 3D-reconstructed coronal view demonstrating a “probe’s eye” view through the nasal septum.

The anterior face of C1-2 is seen in the center of the circle (blue asterix).
Figure 5. Comparison of pre-operative trajectory planning and resultant post-operative decompression.

(A) Sagittal T1-weighted MRI demonstrating the surgical approach (endoscopic trans-nasal, red arrow). (B) Post-operative T1-weighted MRI demonstrating resection of anterior arch of C1 and odontoid process (blue asterix). There is less ventral compression of the medulla and craniocervical junction. (C) Post-operative midline sagittal CT scan demonstrating resection of the anterior and posterior arch of C1 and the odontoid process (blue asterix). (D) Coronal CT view demonstrating satisfactory resection of the odontoid tip (blue asterix).
Figure 6. Post-operative lateral flexion-extension cervical x-rays.

Post-operative lateral flexion-extension cervical x-rays after occiput-C4 instrumentation and fusion demonstrating dynamic stability and preservation of cervical lordosis.
In this case study, stereotactic neuronavigation was crucial for pre-operative trajectory planning (transnasal versus transoral) and intraoperative navigation. This patient experienced no adverse events and improved post-operatively. This informative case adds to the growing literature in navigation-assisted endoscopic odontoidectomy, demonstrating the safe and effective use of neuronavigation for pre-operative planning and intraoperative navigation in endoscopic odontoidectomy.
Methods
A review of the available literature utilizing PubMed/MEDLINE and the following search terms: “stereotactic navigation AND endoscopic surgery” and “simulation AND endoscopic neurosurgery” was performed. The former resulted in 63 total results, 49 of which were related to the research question by title, and the latter resulted in 82 total results, 60 of which were related to the research question by title. The abstract and the methods section of each related manuscript were reviewed. Manuscripts that were not related to navigation in endoscopic neurosurgery or were performed solely in cadavers were excluded. All of the remaining 36 studies were reviewed in their entirety.
Results
The Current State of Navigation Guidance in Endoscopic Neurosurgery
Navigation and Pre-Operative Planning
There is substantial evidence to support the utility of neuronavigation in preoperative planning of burr hole placement6–9 and trajectory selection2,6–12 in a variety of neurosurgical cases, including third ventriculostomy,2,6,12,13 brain biopsy,9 colloid cyst aspiration,8,13 and arachnoid cyst fenestration2 (Table 1). Based on these studies, it appears that stereotactic navigation may be most useful for entrance site selection and pre-selection of trajectories in cases using multiple entry sites,7 and in endoscopic colloid cyst surgery due to the variability of the optimal entry point.8 In surgeries demonstrating an advantage for stereotactic navigation in pre-operative planning, neuronavigation was of less utility intra-operatively.11 For example, when optic navigation was performed in combination with continuous navigation of the endoscope, this resulted in a difficulty in establishing a line-of-sight.2 Overall, the review of more than 244 cases employing stereotactic navigation demonstrated pre-operative advantages for using neuronavigation in target localization,14 surgical access,6–940 and trajectory planning compared to conventional localization methods.2,6–12
Table 1.
Pre-operative planning: Advantages of computer-assisted navigation in endoscopic neurosurgery
| Author & Year | Number ofPatients | Procedure | Advantages |
|---|---|---|---|
| Rhoten et al., 19976 | 11 | Ventricular fenestration, shunting, third ventriculostomy | Entry site and trajectory planning Target localization |
| Grunert et al., 199820 | 11 | . | Trajectory planning |
| Matula et al., 199814 | 5 | . | Target localization |
| Rohde et al., 199812 | 12 | Third ventriculostomy, cystoventriculostomy, ventriculoatrial shunt, cystoperitoneal shunt, intracranial cysts | Trajectory planning |
| Burtscher et al., 200311 | 5 | Third ventriculostomy and fenestration | Trajectory planning Fixed suction mouthpiece for pre-operative planning without patient present |
| Souweidane et al., 200813 | 4 | Colloid cyst aspiration, third ventriculostomy | Access optimization |
| Leonardo et al., 20097 | 44* | Ventricular access via catheter tracking | Entry site and trajectory planning in cases with multiple entry points |
| Tanei et al., 20129 | 6 | Parenchymal biopsy | Entry point and trajectory planning Target localization |
| Mert et al., 20132 | 52 | Transphenoidal procedures, third ventriculostomy, cyst puncture | Trajectory planning |
| Rangel-Castilla et al., 20148 | 39 | Colloid cyst resection | Trajectory and entry site planning |
Navigation was variably employed
Intraoperative Advantages of Navigation Guidance
In addition to the pre-operative advantages of navigation guidance in surgical planning, neuronavigation has been shown to have many advantages during surgery, including anatomical identification of key structures2,5,15 and real-time intraoperative guidance6,12,16 (Table 2). Neuronavigation allows for the real-time, accurate intraoperative sampling of multiple lesions using a single burr hole16 and intraparenchymal tumor resection due to the difficulty of externally visualizing anatomic landmarks.12,16,17 Neuronavigation is limited in its ability to display the sella’s relationship to surrounding anatomy and the sphenoid sinus’ contours when compared to 3D reconstructions.18 The use of neuronavigation may also impact the extent of tumor resection,19 with a meta-analysis demonstrating an association between navigation and either near-complete or complete tumor resection (p<0.0003),20 and an additional report of higher resection volumes in endoscopic biopsy when compared to open or stereotactic biopsies (p<0.05).21 Further investigation is still needed into the effect of intraoperative navigation on the avoidance of injury, with very limited reporting on safety outcomes at present.22,23
Table 2.
Intraoperative navigation: Advantages of computer-assisted navigation in endoscopic neurosurgery
| Author & Year | Number of Patients | Procedure | Major Findings |
|---|---|---|---|
| Rhoten et al., 19976 | 11 | Ventricular fenestration, shunting, third ventriculostomy | Navigation of the ventricles |
| Matula et al., 199814 | 5 | . | Possible time-saving effect |
| Rohde et al., 199812 | 12 | Third ventriculostomy, cystoventriculostomy, ventriculoatrial shunt, cystoperitoneal shunt, intracranial cysts | Intraoperative navigation |
| Kuroki et al., 200223 | 92 | Pituitary surgery | Possible effects on time-saving and increased safety |
| Burtscher et al., 200311 | 5 | Third ventriculostomy and fenestration | Possible time-saving effect |
| Dubin et al., 200515 | Dacryocystorhinostomy and optic and orbital nerve decompression | Anatomical identification | |
| Akai et al., 200817 | 3 | Astrocytoma and lymphoma resection/biopsy, radiation necrosis | Navigating the brain parenchyma |
| Leng et al., 20095 | 1 | Endonasal cervicomedullary junction decompression | Vascular localization Possible time-saving effect |
| Conley et al., 201119 | 4 | Maxillary sinusitis-dentigerous cyst, orbital osteoma, clival lesion, stenosis of the frontal recess | Complete or good resection |
| Tsuda et al., 201121 | 9 | Tumor biopsy | Increased resection volume Possible time-saving effect over open procedures |
| Barber et al., 201320 | . | Tumor resection | Complete or near-complete resection |
| Constantini et al., 201329 | 45 | Biopsy | Diagnostic rate similar in cases with and without navigation in a review |
| Mert et al., 20132 | 52 | Transphenoidal procedures, third ventriculostomy, cyst puncture | Anatomic orientation |
| Nagahisa et al., 201316 | 21 | Intraparenchymal biopsies | Navigation of the brain parenchyma Sampling of multiple lesions using one burr hole |
| Ali et al., 201522 | 3 | Endonasal dacryolocalization for nasolacrimal drainage obstructions | Avoiding injury |
| Inoue et al., 201518 | 99 | Transphenoidal pituitary surgery | Navigation inferior to 3D reconstructions in anatomic presentation |
Effect of Neuronavigation on Operative Time
There is clinical equipoise regarding the effect of neuronavigation on the operative duration of endoscopic procedures. The possibility of a timesaving effect has previously been reported,5,11,14,23 and Tsuda et al. included a statistical analysis showing a timesaving effect, with the procedure duration of open biopsy of 4.4 hours, endoscopic biopsy 2.2 hours, and stereotactic biopsy 1 hour (p<0.01).21 Further investigations into the effect of stereotactic navigation on procedure duration with statistical analysis powered to detect differences in operative time are necessary, as shorter operative times could translate to substantial cost savings.
Accuracy of Neuronavigation and Brain Shift
In order to optimize safety during endoscopic neurosurgery, navigation guidance requires highly accurate registration. Of the reviewed studies,4,12,14,21,24–26 the most accurate target registration had a minimum error of 0.8 mm and mean error of 1.2 mm.4 Registration accuracy was found to be study-dependent, with toleration of inaccuracies up to 3.5 mm reported.12 There was no difference between endoscopic biopsies that employed navigation versus open or stereotactic biopsies with navigation, but there was a trend toward higher accuracy in neuroendoscopy, warranting further investigation with an appropriately powered study.21 Brain shift did not present a problem for intraoperative accuracy in the studies included in this review11,27,28 (Table 3).
Table 3.
Accuracy achieved in studies employing computer-assisted navigation in endoscopic neurosurgery
| Author & Year | Number of Patients | Procedure | Major Findings |
|---|---|---|---|
| Gunkel et al., 199725 | 11 | . | 1–2 mm accuracy |
| Matula et al., 199814 | 5 | . | 2 mm error but not separated from microscopic cases |
| Rohde et al., 199812 | 12 | Third ventriculostomy, cystoventriculostomy, ventriculoatrial shunt, cystoperitoneal shunt, intracranial cysts | 3.5 mm accuracy |
| Gumprecht et al., 200028 | 5 | Ventriculostomy, arachnoid cyst removal, partial astrocytoma resection, colloid cyst removal | No problems with brain shift |
| Krombach et al., 200227 | 23 | Hydrocephalus, intracranial cysts | No problems with brain shift |
| Burtscher et al., 200311 | 5 | Third ventriculostomy and fenestration | No problems with brain shift |
| Bumm et al., 200526 | 10 | Paranasal anterior sphenoid wall resection, frontal sinus ostium opening and enlargement, frontal and ethmoid sinus tissue biopsy | Similar accuracy to paranasal sinus surgery |
| Monin et al., 200524 | 1 | Optic nerve sheath venous hemangioma resection | 1.7 mm accuracy |
| Tsuda et al., 201121 | 9 | Tumor biopsy | Trend toward higher accuracy of aim for the target |
| Choudhri et al., 20144 | 5 | Odontoidectomy | 1.2 mm mean error rate |
Complications of Neuronavigation in Neuroendoscopy
Endoscopic neurosurgery employing neuronavigation is not without complications, and navigation guidance has not been shown to reduce adverse event rates in neuroendoscopy.20,29 Investigators have faced many challenges in endoscopic neurosurgery, especially in the ability to address intraoperative hemorrhage,17 and the intraoperative advantages of neuronavigation do not address the problem of hemostasis. Inaccuracies in neuronavigation could lead to safety problems, as the toleration of registration errors of up to 3 mm12 could potentially result in accidental vascular injury. The operation of neuronavigation is also prone to human error, with the inadvertent resection of normal brain tissue during tumor resection due to accidental powering off of equipment a possibility.
Applications in Pediatric Neurosurgery
Navigation guidance in endoscopic neurosurgery has not been limited to utilization in the adult population; however, the application of neuronavigation in pediatric neurosurgery is limited by the use of invasive head holders. A technique in which a suction mouthpiece replaces the invasive head holder used in adult operations has been successfully employed in a 20-month-old patient.11 Frameless neuronavigation has been reported in operations for hydrocephalus in patients as young as 6-weeks-old.30
Discussion
Limitations of the Current Literature
The literature on navigation in neuroendoscopy and endoscopic sinus surgery is restricted by the lack of statistical analyses, small patient cohorts, and limited description of the effects of navigation in endoscopic procedures.15,31–33 For example, investigators have explored the effect of navigation guidance on skin incision location, soft tissue dissection, surgical orientation, and operative duration during craniotomy, but the same level of detailed description does not exist in neuroendoscopy.34 At times, the reporting of neuroendoscopic procedures using navigation guidance were not separate from microscopic and open procedures, limiting the ability to draw neuroendoscopic-specific conclusions.35 In order to draw strong conclusions about the clinical utility of navigation guidance in neuroendoscopy, further investigation with pre-planned hypotheses and analyses specific to neuroendoscopic cases are needed. In addition, studies ought to explore the effects of navigation-assisted neuroendoscopy on cost and patient-centered outcomes, including post-operative recovery and patient satisfaction.
Conclusion
This manuscript reviewed the use of neuronavigation in endoscopic neurosurgery for pre-operative planning of trajectories2,6–12 and entrance site6–9 and localization of the pathology of interest.6,9 Furthermore, the intraoperative advantages of neuronavigation in anatomic localization or orientation were discussed.2,5,12,15–17 Neuronavigation in endoscopic surgery was highly accurate,4 and brain shift did not present a problem for the studies included in this review.11,27,28 Further work is necessary in the investigation of the effect of neuronavigation on operative time, cost, and patient-centered outcomes.
Figure 3. Pre-operative surgical planning using 3D reconstructions rendered by Surgical Theatre™ (Surgical Theatre, LLC, Mayfield Village, OH).
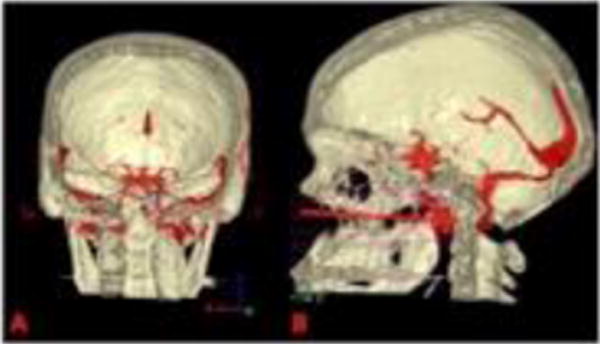
(A) In this coronal view, the vertebrobasilar junction is seen in relation to the clivus (blue asterix). (B) In this sagittal view, multiple trans-nasal trajectories are projected. The final intraoperative trajectory is displayed (red arrow).
Highlights.
Pre-operative trajectory planning is optimized with neuronavigation
Neuronavigation aids in localizing intraoperative pathology
Neuronavigation may be accurate to < 1–2 mm with no brain shift-related problems
Acknowledgments
RKS was supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number TL1TR001434.
Financial Support: None
Abbreviations
- 3D
Three-dimensional
- C
cervical level
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: JBB is a consultant for and owns shares in Surgical Theater, LLC.
References
- 1.Boling CC, Karnezis TT, Baker AB, et al. Multi-institutional study of risk factors for perioperative morbidity following transnasal endoscopic pituitary adenoma surgery. International forum of allergy & rhinology. 2016;6(1):101–107. doi: 10.1002/alr.21622. [DOI] [PubMed] [Google Scholar]
- 2.Mert A, Gan LS, Knosp E, Sutherland GR, Wolfsberger S. Advanced cranial navigation. Neurosurgery. 2013;72(Suppl 1):43–53. doi: 10.1227/NEU.0b013e3182750c03. [DOI] [PubMed] [Google Scholar]
- 3.Dasenbrock HH, Clarke MJ, Bydon A, et al. Endoscopic image-guided transcervical odontoidectomy: outcomes of 15 patients with basilar invagination. Neurosurgery. 2012;70(2):351–359. doi: 10.1227/NEU.0b013e318230e59a. discussion 359–360. [DOI] [PubMed] [Google Scholar]
- 4.Choudhri O, Mindea SA, Feroze A, Soudry E, Chang SD, Nayak JV. Experience with intraoperative navigation and imaging during endoscopic transnasal spinal approaches to the foramen magnum and odontoid. Neurosurgical focus. 2014;36(3):E4. doi: 10.3171/2014.1.FOCUS13533. [DOI] [PubMed] [Google Scholar]
- 5.Leng LZ, Anand VK, Hartl R, Schwartz TH. Endonasal endoscopic resection of an os odontoideum to decompress the cervicomedullary junction: a minimal access surgical technique. Spine. 2009;34(4):E139–143. doi: 10.1097/BRS.0b013e31818e344d. [DOI] [PubMed] [Google Scholar]
- 6.Rhoten RL, Luciano MG, Barnett GH. Computer-assisted endoscopy for neurosurgical procedures: technical note. Neurosurgery. 1997;40(3):632–637. doi: 10.1097/00006123-199703000-00042. discussion 638. [DOI] [PubMed] [Google Scholar]
- 7.Leonardo J, Hanel RA, Grand W. Endoscopic tracking of a ventricular catheter for entry into the lateral ventricle: technical note. Minimally invasive neurosurgery : MIN. 2009;52(5–6):287–289. doi: 10.1055/s-0029-1243241. [DOI] [PubMed] [Google Scholar]
- 8.Rangel-Castilla L, Chen F, Choi L, Clark JC, Nakaji P. Endoscopic approach to colloid cyst: what is the optimal entry point and trajectory? Journal of neurosurgery. 2014;121(4):790–796. doi: 10.3171/2014.5.JNS132031. [DOI] [PubMed] [Google Scholar]
- 9.Tanei T, Nakahara N, Takebayashi S, et al. Endoscopic biopsy for lesions located in the parenchyma of the brain: preoperative planning based on stereotactic methods. Technical note. Neurologia medico-chirurgica. 2012;52(8):617–621. doi: 10.2176/nmc.52.617. [DOI] [PubMed] [Google Scholar]
- 10.Grunert P, Muller-Forell W, Darabi K, et al. Basic principles and clinical applications of neuronavigation and intraoperative computed tomography. Computer aided surgery : official journal of the International Society for Computer Aided Surgery. 1998;3(4):166–173. doi: 10.1002/(SICI)1097-0150(1998)3:4<166::AID-IGS6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 11.Burtscher J, Sweeney R, Bale R, Eisner W, Twerdy K. Neuroendoscopy based on computer assisted adjustment of the endoscope holder in the laboratory. Minimally invasive neurosurgery : MIN. 2003;46(4):208–214. doi: 10.1055/s-2003-42348. [DOI] [PubMed] [Google Scholar]
- 12.Rohde V, Reinges MH, Krombach GA, Gilsbach JM. The combined use of image-guided frameless stereotaxy and neuroendoscopy for the surgical management of occlusive hydrocephalus and intracranial cysts. British journal of neurosurgery. 1998;12(6):531–538. doi: 10.1080/02688699844385. [DOI] [PubMed] [Google Scholar]
- 13.Souweidane MM, Hoffman CE, Schwartz TH. Transcavum interforniceal endoscopic surgery of the third ventricle. Journal of neurosurgery. Pediatrics. 2008;2(4):231–236. doi: 10.3171/PED.2008.2.10.231. [DOI] [PubMed] [Google Scholar]
- 14.Matula C, Rossler K, Reddy M, Schindler E, Koos WT. Intraoperative computed tomography guided neuronavigation: concepts, efficiency, and work flow. Computer aided surgery : official journal of the International Society for Computer Aided Surgery. 1998;3(4):174–182. doi: 10.1002/(SICI)1097-0150(1998)3:4<174::AID-IGS7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 15.Dubin MG, Kuhn FA. Stereotactic computer assisted navigation: state of the art for sinus surgery, not standard of care. Otolaryngologic clinics of North America. 2005;38(3):535–549. doi: 10.1016/j.otc.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 16.Nagahisa S, Watabe T, Sasaki H, et al. Surgical navigation-assisted endoscopic biopsy is feasible for safe and reliable diagnosis of unresectable solid brain tumors. Neurosurgical review. 2013;36(4):595–600. doi: 10.1007/s10143-013-0467-9. discussion 600–591. [DOI] [PubMed] [Google Scholar]
- 17.Akai T, Shiraga S, Sasagawa Y, Okamoto K, Tachibana O, Lizuka H. Intraparenchymal tumor biopsy using neuroendoscopy with navigation. Minimally invasive neurosurgery : MIN. 2008;51(2):83–86. doi: 10.1055/s-2007-1004562. [DOI] [PubMed] [Google Scholar]
- 18.Inoue A, Ohnishi T, Kohno S, et al. Utility of three-dimensional computed tomography for anatomical assistance in endoscopic endonasal transsphenoidal surgery. Neurosurgical review. 2015;38(3):559–565. doi: 10.1007/s10143-015-0625-3. [DOI] [PubMed] [Google Scholar]
- 19.Conley DB, Tan B, Bendok BR, et al. Comparison of Intraoperative Portable CT Scanners in Skull Base and Endoscopic Sinus Surgery: Single Center Case Series. Skull base : official journal of North American Skull Base Society … [et al] 2011;21(4):261–270. doi: 10.1055/s-0031-1280681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barber SM, Rangel-Castilla L, Baskin D. Neuroendoscopic resection of intraventricular tumors: a systematic outcomes analysis. Minimally invasive surgery. 2013;2013:898753. doi: 10.1155/2013/898753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuda K, Ishikawa E, Zaboronok A, et al. Navigation-guided endoscopic biopsy for intraparenchymal brain tumor. Neurologia medico-chirurgica. 2011;51(10):694–700. doi: 10.2176/nmc.51.694. [DOI] [PubMed] [Google Scholar]
- 22.Ali MJ, Naik MN. Image-Guided Dacryolocalization (IGDL) in Traumatic Secondary Acquired Lacrimal drainage Obstructions (SALDO) Ophthalmic plastic and reconstructive surgery. 2015;31(5):406–409. doi: 10.1097/IOP.0000000000000502. [DOI] [PubMed] [Google Scholar]
- 23.Kuroki A, Kayama T. Endoscopic approach to the pituitary lesions: contemporary method and review of the literature. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2002;56(Suppl 1):158s–164s. doi: 10.1016/s0753-3322(02)00228-7. [DOI] [PubMed] [Google Scholar]
- 24.Monin DL, Blumner K, Cohen NA, Brooks JS, Chen C, Kennedy DW. Endoscopic resection of a venous hemangioma of the optic nerve sheath. Ear, nose, & throat journal. 2005;84(9):586, 588, 590. passim. [PubMed] [Google Scholar]
- 25.Gunkel AR, Freysinger W, Martin A, et al. Three-dimensional image-guided endonasal surgery with a microdebrider. The Laryngoscope. 1997;107(6):834–838. doi: 10.1097/00005537-199706000-00020. [DOI] [PubMed] [Google Scholar]
- 26.Bumm K, Wurm J, Bohr C, Zenk J, Iro H. New endoscopic instruments for paranasal sinus surgery. Otolaryngology–head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2005;133(3):444–449. doi: 10.1016/j.otohns.2005.05.046. [DOI] [PubMed] [Google Scholar]
- 27.Krombach GA, Rohde V, Haage P, Struffert T, Kilbinger M, Thron A. Virtual endoscopy combined with intraoperative neuronavigation for planning of endoscopic surgery in patients with occlusive hydrocephalus and intracranial cysts. Neuroradiology. 2002;44(4):279–285. doi: 10.1007/s00234-001-0731-5. [DOI] [PubMed] [Google Scholar]
- 28.Gumprecht H, Trost HA, Lumenta CB. Neuroendoscopy combined with frameless neuronavigation. British journal of neurosurgery. 2000;14(2):129–131. doi: 10.1080/02688690050004552. [DOI] [PubMed] [Google Scholar]
- 29.Constantini S, Mohanty A, Zymberg S, et al. Safety and diagnostic accuracy of neuroendoscopic biopsies: an international multicenter study. Journal of neurosurgery. Pediatrics. 2013;11(6):704–709. doi: 10.3171/2013.3.PEDS12416. [DOI] [PubMed] [Google Scholar]
- 30.Mangano FT, Limbrick DD, Jr, Leonard JR, Park TS, Smyth MD. Simultaneous image-guided and endoscopic navigation without rigid cranial fixation: application in infants: technical case report. Neurosurgery. 2006;58(4 Suppl 2) doi: 10.1227/01.NEU.0000205297.39862.33. ONS-E377; discussion ONS-E377. [DOI] [PubMed] [Google Scholar]
- 31.Melada A, Heinrich Z, Chudy D, Scap M, Rotim K. The difference between ultrasound-guided and stereotactic-guided neurosurgical procedures. Minimally invasive neurosurgery : MIN. 2000;43(3):149–152. doi: 10.1055/s-2000-8335. [DOI] [PubMed] [Google Scholar]
- 32.Toffel PH. Secure endoscopic sinus surgery with partial middle turbinate modification: a 16-year long-term outcome report and literature review. Current opinion in otolaryngology & head and neck surgery. 2003;11(1):13–18. doi: 10.1097/00020840-200302000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Fatemi N, Dusick JR, Gorgulho AA, et al. Endonasal microscopic removal of clival chordomas. Surgical neurology. 2008;69(4):331–338. doi: 10.1016/j.surneu.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 34.Enchev YP, Popov RV, Romansky KV, Marinov MB, Bussarsky VA. Neuronavigated surgery of intracranial cavernomas–enthusiasm for high technologies or a gold standard? Folia medica. 2008;50(2):11–17. [PubMed] [Google Scholar]
- 35.Caversaccio M, Langlotz F, Nolte LP, Hausler R. Impact of a self-developed planning and self-constructed navigation system on skull base surgery: 10 years experience. Acta oto-laryngologica. 2007;127(4):403–407. doi: 10.1080/00016480601002104. [DOI] [PubMed] [Google Scholar]


