Abstract
Background
The purpose of this paper is to describe the establishment of the Advanced Clinical Monitoring of ART Project in Ethiopia for monitoring and evaluation of the longitudinal effectiveness of the ART program and to show the opportunities it presents. This cohort was established in response to the 2005 call by WHO for establishing additional mechanisms for stronger monitoring of ART and the need for creating the platform to generate evidence to guide the care given for the ever increasing number of patients on ART in Ethiopia.
Method
A participatory and multi-stage process which started from a consensus building workshop and steered by a mother protocol as well as guiding documents which dictated the degree of engagement and expectations was followed. The primary and secondary aims of the study were agreed upon. A multi-site longitudinal observational clinical cohort was established by a consortium of stakeholders including seven Ethiopian medical schools and their affiliated referral hospitals, John Hopkins University, Ethiopian Public Health Institute, Ministry of Science and Technology, US Centers for Disease Prevention and Control - CDC-Ethiopia, and the Federal Ministry of Health. Adult and adolescent cohorts covering the age range of 14+ years) and pediatric cohorts covering those below age 14 years were the two main cohorts. During the initial recruitment of these cohorts information was extracted from existing documents for a total of 2,100 adult participants. In parallel, a prospective cohort of 1,400 adult and adolescent patients were enrolled for ART initiation and follow-up. Using similar recruitment procedures, a total of 120 children were enrolled in each of retrospective and prospective cohorts. Replacement of participants were made in subsequent years based on lost follow up and death rates to maintain adequacy of the sample to be followed-up.
Achievements
Between January 2005 and August 2013 a total of 4,339 patients were followed for a median of 41.6 months and data on demographic characteristics, baseline and ongoing clinical features, hospitalization history, medication and laboratory information were collected. 39,762 aliquots and 25,515 specimens of plasma and dryblood-spots respectively were obtained and stored longitudinally from October 2009 to August 2013. The project created a research platform for researchers, policy and decision makers. Moreover, it encouraged local and international investigators to identify and answer clinically and programmatically relevant research questions using the available data and specimens. Calls for concept notes paired with multiple trainings to stimulate investigators to conduct analyses further boosted the potential for doing research.
Conclusions
A comprehensive and resourceful mechanism for scientific inquiry was established to support the national HIV/ART program. With meaningful involvement and defined roles, establishment of a study, which involved multiple institutions and investigators, was possible. Since ACM is the largest multi-site clinical cohort of patients on antiretroviral treatment in Ethiopia—which can be used for research and for improving clinical management—considering options to sustain the project is crucial.
Keywords: Ethiopia, HIV clinical cohort, Antiretroviral therapy, Establishing Longitudinal Cohort Study, ART Monitoring and Evaluation
Introduction
The Ethiopian government began a limited program of making antiretroviral therapy (ART) available for a fee in 2003(1). In early 2005, ART was made available nationwide without payment, with support from the Ethiopian government, the U.S. President's Emergency Program for AIDS Relief (PEPFAR), and the Global Fund to Fight AIDS, Tuberculosis and Malaria(2).
The prevalence of HIV in 2014 was 1.2% (3) and the incidence rate was reported as 15,000 by 2016(4). With 79.0% coverage of eligible patients, Ethiopia had put 492,649 patients on ART by the end of 2013(5). The PEPFAR program provided technical and financial assistance to support the rapid nation-wide scale up of free ART(6). Ethiopia used the WHO consolidated guideline of ART for treatment and preventing HIV infection (7)
The introduction of free ART was paired with introduction of monitoring and evaluation (M&E) tools(8) to routinely track key program performance and outcome indicators. This routine M&E system provided limited and primarily aggregated information about the effectiveness of antiretroviral treatment. The data were not sufficiently detailed to explore important demographic, clinical, laboratory or pharmaceutical determinants of treatment effectiveness.
Moreover, a WHO consultation on development of a Standardized ARV Treatment Outcome Monitoring System held in October, 2005 called for the development of a standardized outcome monitoring system that would provide additional information on treatment program success at facility, subnational and national levels. Consequently, a study allowing the detailed monitoring of a cohort of patients participating in the national free ART program at the referral hospitals of seven Ethiopian university medical schools was initiated. The Advanced Clinical Monitoring of ART in Ethiopia study (ACM) was designed to provide the platform for advanced monitoring of the ART program in Ethiopia..
Longitudinal cohort studies of HIV/AIDS patients in a clinic setting have been critical in fostering our understanding both of the natural history of HIV disease and of the effectiveness of treatment in a real-world setting.
The purpose of ACM was to provide comprehensive data about the study cohort, and to make these data available to health care providers, hospital and clinic managers, researchers, national and regional health officials and other policy makers, enabling on-going improvements in prevention and treatment of HIV in Ethiopia based on critical analysis of data/specimens through research.
The objectives of the ACM included the following: supporting the Ethiopian antiretroviral treatment program with the intention of improving ART delivery by establishing a multi-site patient registry, cohort database and specimen repository, supporting the successful implementation of ART programs at each participating hospital by providing information that enable better patient level care, and clinic management; and, supporting the national ART program by providing detailed information about care processes, treatment adherence, virologic indicators and patient outcomes from the selected sites. The project also intended to facilitate operational research to refine national and international implementation strategies for ART program.
Methods
The cohort establishment process, began from a consensus building workshop with participation of the collaborating institutes and others who were involved in HIV care and treatment. The primary and secondary aims of the study were defined in this meeting. The primary aims included: (a) evaluation of treatment effectiveness, (b) assessment of monitoring protocols, (c) assessment of adverse effects of treatment, (d) assessment of adherence, and, (e) insight into potential causes of early mortality. The secondary aims included: (a) assessment of guideline compliance, (b) monitoring of drug resistance development, (c) assessment of the impact of PMTCT on treatment effectiveness, (d) assessment of utilization rates for various kinds of care, and (4) an assessment of access to ART care.
The study design of ACM was an ambi-directional follow up study which included retrospective data abstraction and prospective data collection. Its establishment required site-specific follow up cohorts, and a governance structure with supporting documentation and legal backing. The following are the key approaches followed in establishing this clinical cohort.
Structure and Governance
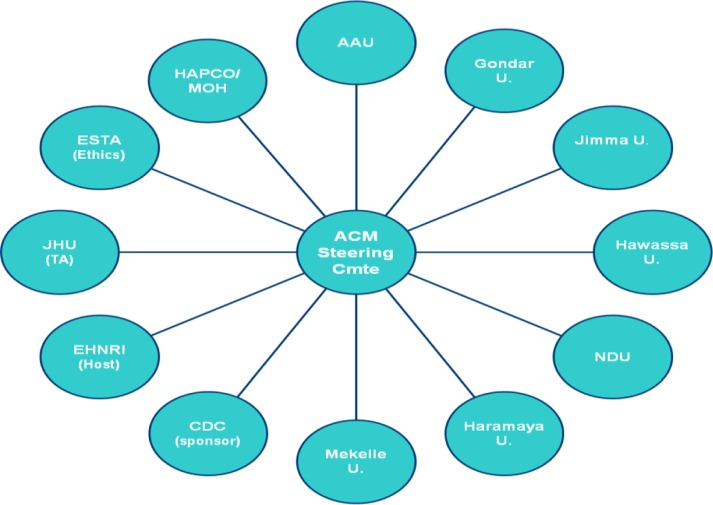
The seven Ethiopian medical faculties with their affiliated hospitals, and five participating institutions, agreed to collaborate in the development, management and governance of the ACM project (Figure 1). The collaborating institutions were:
The Federal Ministry of Health of Ethiopia through the HIV/AIDS/STIs Prevention and Control Program (HAPCO)
Addis Ababa University/Tikur Anbessa Hospital (AAU)
National Defense University/Armed Forces Teaching General Hospital (NDU)
Gondar University/Gondar University Hospital
Jimma University/Jimma University Specialized Hospital
Hawassa University/Hawassa University Referral Hospital
Haramaya University/Hiwot Fana Hospital
Mekelle University/Mekelle Hospital
The Ethiopian Public Health Institute (EPHI, formerly called “Ethiopian Health and Nutrition Research Institute” or EHNRI)
Ministry of Science and Technology (formerly called “Ethiopian Science and Technology Commission” or ESTA)
The Johns Hopkins University - Bloomberg School of Public Health (JHU)
The United States Centers for Disease Control & Prevention (CDC-Ethiopia).
Figure 1.
Advanced clinical monitoring (ACM) project organization, January 1, 2005 to August 31, 2013, Ethiopia ACM.
The organizations agreed to work under a Memorandum of Understanding (MOU) with a Steering Committee making key decisions and providing guidance, and a Project Implementation Office (PIO) (located at EPHI) overseeing day-to-day implementation of activities.
Each stakeholder had designated responsibilities, as stipulated in the MOU, to achieve the common goal of the project. The seven Ethiopian universities and their respective hospitals were considered as study sites because these facilities were engaged in provision of HIV care services including ART, and followed a high volume of patients. Moreover, they were located in geographically distant places covering a wide catchment population of the country, and were equipped with infrastructure and research centers.
Site level offices were established in the ART clinics of the hospitals to manage data extraction, data entry, enrollment and data as well as specimen collection. There were 3 employees (Site Study Coordinator, Data Manager and Junior Data Manager) serving the ACM project in each of the seven sites.
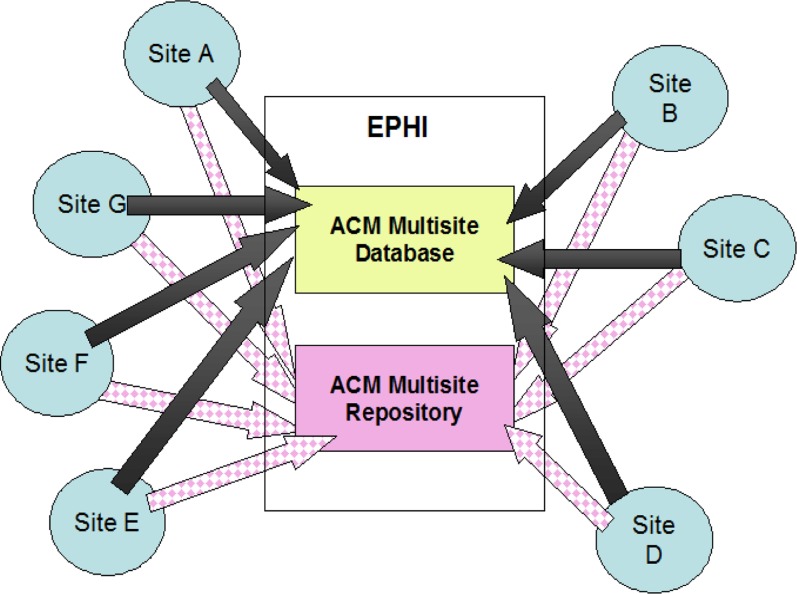
The other stakeholders included: (a) Ethiopian Federal Ministry of Health represented by the HIV/AIDS/STIs Prevention and Control Program/HAPCO (HAPCO was the owner of the national HIV program and responsible for guiding the National ART Program), (b) The Federal Ministry of Science and Technology provided regulatory guidance, (c) The Johns Hopkins University Bloomberg School of Public Health through a CDC-funded project in Ethiopia called the Technical Support for the Ethiopian HIV/AIDS ART Initiative (TSEHAI) served as the PIO, to providing technical guidance and support to the national program, regional health bureaus, and hospitals and clinics participating in the free ART program, (d) CDC-Ethiopia was the funding agency, (e) The Ethiopian Public Health Institute (previously known as Ethiopian Health and Nutrition Research Institute - EHNRI), was the hub of the collaboration where the central data and specimens were kept and the coordination office was stationed (see Fig 1 and 2).
Fig 2.
Specimen and Data Flow of the advanced clinical monitoring (ACM) project, January 1, 2005 to August 31, 2013, Ethiopia. Specimens from the 2 sites in Addis Ababa were being sent same day as collected and those from out of Addis were being sent within 2 weeks after partial processing.
Note: communication was bidirectional with the intent of ensuring quality of data and specimens.
Organization of the project - Each collaborating institution signed an MOU, and a letter of support was provided by the funding agency (CDC). According to the MOU, a Steering Committee, formed by members selected from each participating institution, was the governing body of the consortium. The Steering Committee was composed of representatives who were elected by the member institutions. The Steering Committee was governed by a bylaws. There were sub-committees which served specific purposes. The Steering Committee had standing meetings on a quarterly basis. Extra-ordinary meetings were held as deemed necessary. The establishment of the collaboration through the signing of the MOU was accomplished over a period of two and a half years. An umbrella study protocol was also developed during this period and was approved by the national research ethics review committee under the Ministry of Science and Technology and the Institutional Review Boards of EPHI and the Johns Hopkins University Bloomberg School of Public Health, as well as the science office of CDC. Several amendments to the umbrella protocol were made during the follow up time and approved by the different ethics committees to address emerging issues with the most recent version of the umbrella protocol being version 4.0.
The day-to-day activities of ACM were managed by the PIO under the directions of the Steering Committee. An electronic data management system was created by the PIO. The database included seven-site level databases and a central database at EPHI where clinical and laboratory data from the seven sites were merged, cleaned and stored for analytic use.
Specimen collection and storage mechanism was also established which included central storage in −80 degree Celsius deep freezers at EPHI. Data collection and data quality assurance activities were handled by study-site level study staff who worked at the seven hospitals. These staff were employed by the project and supervised by the PIO. They were expected to work collaboratively with staff members of the clinics, laboratories and pharmacies. All study sites had office space dedicated for ACM. The specimen collection was done by the hospital laboratory staff and in return they were receiving payment for the additional activities of collecting, processing and shipping of specimens related directly to the ACM study.
Since ACM project was created as a research platform for scientific inquiries, mechanisms to encourage and enhance the practice of research were put in place.
One mechanism included calls for concept notes. The calls were disseminated widely and the Research and Scientific Inquiry Sub-committee, working under direction of the main Steering Committee, reviewed concept notes and protocols, and guided researchers to align concept notes/protocols with the primary and secondary aims of ACM and to focus research questions on relevant and high priority areas for the national ART program. The key functional units in facilitating the actual implementation of this were the PIO and the host institution.
Daily Implementation - having a governance structure, some guiding documents and a mother protocol was not considered sufficient for smooth running. An office responsible for the actual execution was needed. Since the recipient of the funding was JHU, it was tasked to establish the PIO which was mandated to oversee the daily activities. The PIO had dual reporting lines - to the Steering Committee and to the JHU-TSEHAI project office.
Host Institution for data and specimen - Since the seven participating local institutions were all interested in being the host, a mechanism for fair selection had to be established. The Steering Committee agreed on the following criteria to select the host institution: mandate (does the institution have a related mandate relevant to ACM?), neutrality (is the institution free of conflict of interest?), location (accessible to most participating institutions), potential to take over (able to maintain the cohort after external funding phased out), utilization potential (which institution can use the data and specimens optimally?) and management capacity (which institution can manage the project more resourcefully?). EPHI was selected as the host institution because it was the research arm of the Federal Ministry of Health, with unique technical and physical capabilities to handle the biological data, while at the same time was neutral to all contributing institutions.
Epidemiological Cohort Development
The two primary cohorts of ACM were 1) an adult and adolescent cohort, and 2) a pediatric cohort, created by considering age during the consent process. The primary cohorts were each sub-divided into database and repository cohorts. While data were collected on both cohorts, plasma and DBS specimens were collected every six months only from participants in the repository cohort. The ACM enrolled a group of initial patients and then recruited subsequent replacement participants each year. The number of replacement participants was determined based on the number of participants lost to follow up and the number of cohort members who died in the previous calendar year. Each cohort had a retrospective period (data collected from active participants collected prior to enrollment) and a prospective period (data collected from active participants during enrollment and onwards) (Table 1).
Table 1.
Total number of participants enrolled in advanced clinical monitoring (ACM) project by study-site, January 1, 2005 to August 31, 2013, Ethiopia.
| University | Hospital | Cohorts | |
| Adults (Age≥ 14) | Children (Age <14) | ||
| Addis Ababa University | Tikur Anbessa Hospital | 573 | 63 |
| National Defense University | Armed Forces General Hospital | 558 | 41 |
| Gondar University | Gondar Referral Hospital | 620 | 65 |
| Jimma University | Jimma Referral Hospital | 544 | 49 |
| Mekelle University | Mekelle Referral Hospital | 590 | 54 |
| Haramaya University | Hiwot Fana Referral Hospital | 535 | 49 |
| Hawassa University | Hawassa Univ. Referral Hospital | 542 | 56 |
| Total | 3,962 | 377 | |
Adult and Adolescent Cohort (Age >14 years) - The initial group of participants (400 per site) enrolled in this cohort were divided into existing (ART-experienced) and new (ART-naïve) groups. A total of 300 ART experienced participants were sampled from a registry of patients receiving ART care at each site. In addition, 100 ART-naïve patients were recruited prospectively to constitute the initial cohort. Subsequently, each study-site planned and enrolled on a replacement basis, to ensure representativeness, a total of at least 60 new participants in a given calendar year per the protocol.
Pediatric Cohort (Age < 14 years) - The initial group of participants (40 per site) enrolled in this cohort were divided into existing (ART-experienced) and new (ART-naïve) groups. A total of 200 ART experienced participants were sampled from a registry of patients receiving ART care in the study sites. In addition, 20 ART-naïve patients were recruited prospectively to constitute the initial cohort. Subsequently, each site planned and enrolled on a replacement basis, to ensure representativeness, a total of at least 10 new participants in a given calendar year.
Adult and Adolescent Repository Cohort (Age >14 years) - The initial group of participants (100 per site) were enrolled prospectively into the adult and adolescent repository cohort. Each site sought to recruit all eligible participants from the Database Adult and Adolescent Cohort at their ART initiation visit immediately following their consent to participate in the database cohort. Once the site reached a total of 100 participants, subsequent enrollment was on a replacement basis as sample size fell below 100.
Pediatric Repository Cohort (Age <14 years) - The initial group of participants (20 per site) were prospectively enrolled into the pediatric repository cohort. Each site sought to recruit all eligible participants from the Database Pediatrics Cohort at their ART initiation visit immediately following their consent to participate in the database cohort. Once the site reached a total of 20 participants, subsequent enrollment was on a replacement basis as sample size fell below 20.
Participant Enrolment and Follow up - Site level enrollment and data/specimen collection activities were done according to standard operating procedures. All patients who initiated ART on or after January 1, 2005 were eligible for enrollment regardless of their status at the time of enrollment. Given the retrospective nature of some of the data, the sampling frame included patients who had died by the time the study began. All enrolled participants were given unique identifiers and random selection was done so that all eligible patients had equal chance of being approached for enrollment
Patients who were alive and randomly selected as potential participants were approached when they came for their routine visit and, in a confidential setting, asked if they wanted to provide informed consent to participate in the ACM. If a participant agreed to participate in ACM, they signed a consent form and were enrolled in the database cohort. Parental consent was obtained for those pediatric participants <10 years of age and those older than 10 years of age, in addition to the written parental consent, a verbal assent was also obtained from the children. Participants were compensated for their time. If patients declined to participate, the next eligible patient was approached. Participants enrolled in only the database cohort were expected to provide data during their routine follow up visits; while those enrolled in both the database and repository cohorts were expected to come to the facility for routine visits and provide specimens every six months.
Sample selection and size determination - Retrospective cohort participants were selected randomly from the list of all potentially eligible registered patients from the ART database. All participants were given an equal chance of being selected. A computer program using uniform distribution was applied to generate random numbers to select study participants. A total of 2,100 ART experienced participants were selected from the seven sites. Prospective cohort participants were selected randomly at ART initiation until the required sample size was reached.
The sample size for cohort development was determined considering the available resources and multiple analyses and research topics raised by investigators. Various intra cluster correlation coefficient (ICC) values were considered for site comparison. Hence a total of 2,800 adults, with 700 participants in repository cohort, and 280 pediatric participants, with 140 in the repository cohort, was considered a sufficient sample size to answer project objectives.
Data and Specimen Management Information System Development
Data on clinical and demographic characteristics, ongoing medical care, and clinical and laboratory outcomes were collected on all participants in the cohort. Plasma specimens were also obtained and stored every six months for viral load and resistance testing, and for other studies to be specified on subsamples of the overall cohort.
The project used the National ART forms (ART intake forms, ART follow up forms, laboratory and Pharmacy Drug Dispense form) and ACM specific forms (Patient Information form (PATI), Outpatient care (°CARE), Outpatient Clinical Laboratory Abstraction form (°CLA), Tuberculosis abstraction form (TB), Hospitalization form (HOSP), Visit form (VIST), Termination (TERM), Mortality form (MORT), Maternity Data Abstraction form (MATR) and Specimen Collection and Processing form (SCPF). ACM specific instruments were pre-tested and modifications made regularly through version control.
Except for specimen collection purposes with specific appointments and corresponding coverage of transportation expenses, patient visits were limited to the required clinical visits for routine care and no study-specific appointments were given to patients.
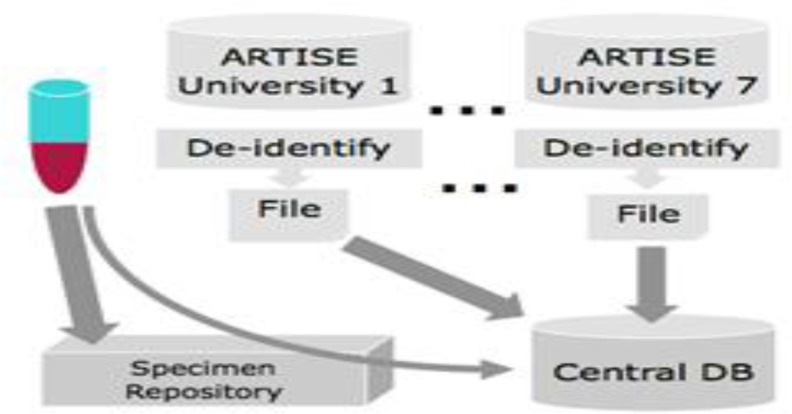
Antiretroviral Treatment Information System for Ethiopia (ARTISE), an ACCESS-based database, was deployed at each participating site to collect patient data. ARTISE functionality included a data entry template, patient tracking, database backup, generating ad hoc reports and de-identification programming. The De-identification process eliminated patient identifiers information like names, address, medical card numbers, and all possible combination keys, etc. before data extraction and transportation. The ARTISE system linked the unique ACM ID number with a one to one function to all participants so that data and specimen users were blinded while accessing the resources centrally. On a quarterly basis, a de-identified ARTISE backup was transported to Ethiopian Public Health Institute to be consolidated and stored in a SQL-based Central Database (CDB). CDB was created through a structured process of requirements, specification, design, implementation, testing, and deployment. The functions of the CDB included ARTISE site data consolidation, provision of coding guide for each exported data field, laboratory information management for specimens, analytical dataset export, and built-in and ad-hoc reporting (Fig 3).
Figure 3.
Data and specimen flow to central data and specimen warehouse at Ethiopian Public Health Institute (EPHI), January 1, 2005 to August 31, 2013, Ethiopia. All de-identified data was gathered in the central database, a SQL server based system developed specifically for this purpose.
Laboratory Management - As shown in Figure 2, specimens were collected from all ART naïve repository participants at the time of enrollment and every 6 months thereafter for 24 months; subsequent specimen collections were every 12 months. With the exception of Addis Ababa sites (Tikur Anbessa and Armed Forces Hospitals), plasma and DBS specimens were collected, processed and transported from each site to EPHI on a bi-monthly basis.
All whole blood specimens collected from participants in sites outside of Addis (5 out 7 sites) were used to provide Dried Blood Spots (DBS) and the remaining were processed into plasma aliquots and stored temporarily on site at −20-degree freezer. The processed plasma aliquots and DBS were shipped to the central repository at EPHI within 15 days of collection. All plasma specimens were transported using dry ice to maintain the cold chain. Whole blood specimens collected from participants in the Addis sites (2 out 7 sites) were transported within six hours of collection to EPHI for further processing into DBS and plasma aliquots. All specimens received at the central repository in EPHI were counterchecked with corresponding documentation to ensure accuracy and timeliness and were permanently stored in −80 °C deep freezers. In addition to storing the merged site datasets, the SQL-based central database also stored the repository database and mapped all the specimens for easy retrieval.
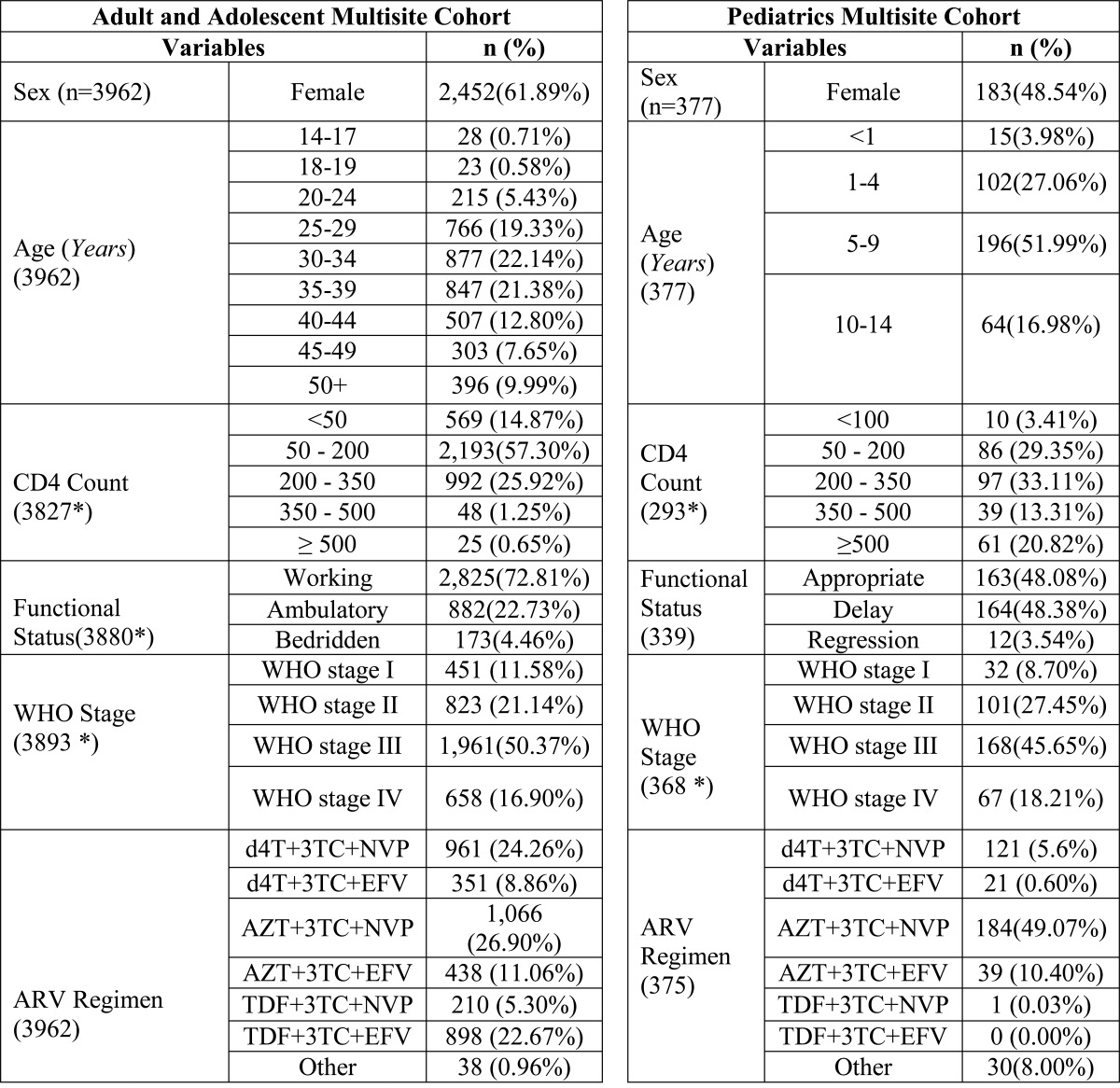
Data Preparation and Analysis- In general, customized datasets were prepared for researchers based on variables needed to answer their research questions. Data cleaning and preparation of datasets were the mainstay of the data management unit. An overall descriptive analysis of the patients in the database is provided in Table 2.
Table 2.
Patient Characteristics of advanced clinical monitoring (ACM) participants at ART initiation, January 1, 2005 to August 31, 2013, Ethiopia.
There are some missing values
Ethical review
Given the fact that multiple Ethiopian universities, JHU and CDC had their own institutional review boards (IRBs), approval at multiple levels appeared to be required. To expedite the process, the EPHI institutional review board was designated to represent all the local university IRBs. Following the review and approval from the EPHI IRB, the protocol was simultaneously submitted to the National Research and Ethics Committee at the Ministry of Science and Technology in Ethiopia, the IRB at Johns Hopkins University Bloomberg School of Public Health, and the science office of the CDC.
This process, with parallel review by three different institutions, with different time intervals for review, where each of the IRBs could require changes that then needed to be re-reviewed, delayed the initiation of the study. Final clearance was secured nine months after submission of the first version of the ACM protocol. Another challenge that further prolonged the review process was the gap in the Ethiopian Research Ethics Guidelines regarding use of data obtained from patients who were already dead or who were lost to follow up and could not be tracked to seek informed consent. This lack of guidance led to multiple consultative meetings between the research team and the National Research Ethics Committee to reach consensus. While the study was allowed to use data from patients who had died, the IRB was not willing to allow inclusion of patients who were not in follow up and whose final status was unknown.
ACM secured required approvals for continuation on an annual basis.
Accomplishments
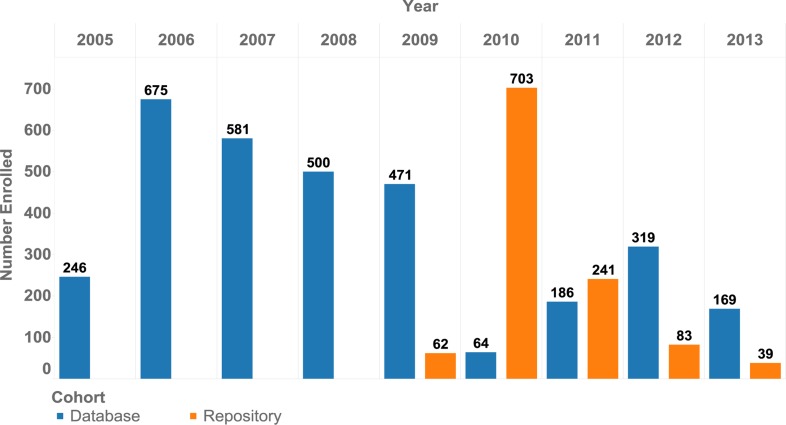
After three years of preparatory work, the ACM project initiated enrollment in 2009. As shown in Table 1 and Figure 4, a total of 3,962 adults and 377 children were enrolled in the study. The sites had comparable contributions with a mean of 566 adults (542–620) and 54 children (41–65) per site.
Figure 4.
Advanced clinical monitoring (ACM) multisite database and repository cohort enrollment by year, January 1, 2005 to August 31, 2013, Ethiopia.
ACM provided collaborators the opportunity to form a research consortium which involved seven local universities, one international university, CDC, the Ministry of Science and Technology, the Ethiopian Public Health Institute and the Ministry of Health. To the best of our knowledge ACM continues to be the largest research collaboration in the country. In addition to the fact that it serves as a platform for national and international collaboration, the lessons learned in terms of processes for establishing a large multi-site cohort study are worth discussing.
As summarized in Table 2 and 3a, among the adults, 62% (n=2,452) were female and 38% (n=1,510) were male. Most of the participants were in the 25–39 year age category, and only 10% were older than 50 years. At the time of enrollment, the majority of the study participants (57%) had a baseline CD4 count of 50–200 and more than 66% of the participants were in WHO stage 3 and 4.
Table 3a.
Total number of participants enrolled in advanced clinical monitoring (ACM) project by cohort, January 1, 2005 to August 31, 2013, Ethiopia.
| Primary ACM cohorts |
Cohort type | Gender | Total | |
| Male | Female | |||
| Multisite Adult and Adolescent |
Database Cohort | 1,125 | 1,855 | 2,980 |
| Repository Cohort | 385 | 597 | 982 | |
| Multisite Pediatrics | Database Cohort | 125 | 106 | 231 |
| Repository Cohort | 69 | 77 | 146 | |
| Total | 1,704 | 2,635 | 4,339 | |
At initiation of treatment, the most commonly prescribed NRTIs for retrospective cohort patients were AZT (38%) and d4T (33%). In 2009, following the introduction of Tenofovir, the trend changed to TDF as the most prescribed NRTI (53.6%). Efavirenz (EFV) and NVP were the only NNRTIs prescribed, with NVP accounting for 56.5%. Cotrimoxazole prophylactic treatment (CPT) was prescribed to more than 90% of the patients, but INH was given to only 4% (n=160). TB treatment was initiated for 15.4% (n=604) of patients. As seen on table 3b, with a total of 39,762 aliquots and 25,515 DBS, a large pool of specimens was created. The mean follow-up period of patients on ART was 44 months with median follow-up of 41.6 months.
Table 3b.
Total number of samples collected by visit for repository cohort participant in advanced clinical monitoring (ACM) project, September 2009 to August 31, 2013, Ethiopia
| Laboratory sample/Specimen |
Visits after ART Initiation for Repository Cohorts (months) | ||||||||
| M0 | M6 | M12 | M18 | M24 | M30 | M36 | M42 | Total | |
| Total number of aliquots stored |
8,762 | 6,985 | 6,290 | 5,756 | 5,128 | 4,056 | 2,429 | 356 | 39,762 |
| Total number of DBS spots collected |
5,640 | 4,465 | 3,995 | 3,650 | 3,295 | 2,595 | 1,615 | 260 | 25,515 |
The project conducted workshops and advanced data analysis trainings for participating university investigators to support their studies and answer relevant scientific inquiries using available data and the specimen repository more resourcefully.
The ACM platform enabled researchers to determine predictors of survival among adults on ART (15), common causes of hospitalization and outcomes among pediatric patients on ART (16), magnitude of drug toxicity (17), timeliness of HIV care (18) and mortality of patients on ART with TB co-infection (19). Answers to more research questions are in the pipeline and the authors anticipate many more research activities based on the data and specimens collected by ACM.
Challenges
Funding - Though this project was of national importance, it was solely funded by PEPFAR through CDC-Ethiopia. When the CDC funding came to an end, active follow up of participants and data and specimen collection were suspended. However, with the support of EPHI, use of the available data and specimens to address key research and programmatic questions is being encouraged and facilitated.
Data use - Although the data and specimens were being analyzed to address research aims, little was done early on to support public health decisionmaking, and therefore data and specimens were not likely to been used to their full potential.
Specimen use - All research activities requiring use of specimens needed to come with associated funding. Except for the limited viral loads done through the CDC funding, no advanced tests have been done. The routine laboratory investigations including CD4, blood chemistry and other basic laboratory data are available at the facility level and results are documented and captured on the ACM database.
Discussion and Lessons Learned
Establishment of the ACM project responded to the need for an African cohort that would enable certain research questions to be addressed. The five-country study by Dalal et al.(13), reflected on the need for a longitudinal as opposed to cross sectional study, and the ACM Project demonstrates its feasibility. The retention rate at month 6 was more than 80 percent, which is higher than found by Dalal et.al (13). Publications showing the process of establishment of specific cohort studies are very scarce.
There are over 30 large HIV cohort studies ongoing, according to the Forum for Collaborative HIV Research. Some of the largest are Euro-SIDA with 60 clinical centers in Europe, the Swiss HIV Cohort with 7 centers, and the HIV Research Network with 17 centers in the U.S.(10) However, the vast majority of these multi-site cohort studies are being carried out in high resource settings - U.S., Europe and/or Australia. ACM has been able to stimulate generation of local evidence.
Seven essential research questions were answered in the first round of research activity, and an additional 18 are in the pipeline.
With more than 4,000 patients, ACM is larger than many of the cohort studies in Africa. The AFRICOS study has 3,600 patients. The multi-centre prospective cohort network of pediatric clinics in West Africa, which involves seven countries, has 1,415 participants. But it was comparable to the cohort study involving 3 countries by Koole et al. which has 4,147 patients included though it was a retrospective cohort study (14)
ACM is a multi-site clinical cohort with the potential to provide valuable information about the effectiveness of the ART program, the survival of patients on ART, incidence of ART-related toxicities, and other more research questions. The fact that ACM has readily available longitudinally-collected specimens with corresponding clinical and socio-demographic data makes it an ideal platform for researchers. Establishing and running a multi-site cohort study requires creation of a functional system that will guide the collaboration. A multi-site, multi-partner clinical cohort establishment also requires a long-term commitment and advance planning for maintenance of the cohort.
Establishing the cohort -Starting with a clear study purpose, and ensuring meaningful involvement of all stakeholders by creating the venue for consultative engagement, were crucial. Developing the rules of collaboration as well as defining the roles and responsibilities of each institution at the inception played a crucial role in building ownership. The discussion of how to integrate the implementation office and its functions, including the data and specimen warehousing within EPHI, gave ACM the chance to continue even after donor funding was discontinued.
Maintaining the cohort - Since there are close to 4,000 participants enrolled in ACM, and since there is a need for site level staff, maintaining follow up of participants at the ACM study sites would have required continued funding. However, given that all the structural issues were addressed, maintaining ACM was not significantly challenging. Though enrollment have been suspended, we have learned that ACM has an invaluable pool of data and specimens which can be used to inform the ART program nationally, and HIV/AIDS research globally.
Ethical clearance process - Though the process was not as fast as we desired, delegation of one IRB to be the local IRB on other institutions' behalf expedited the process. Parallel submission of the protocol has also enabled somewhat faster clearance.
Stimulating scientific inquiries - Posting calls for concept notes, followed by organizing consultative workshops gave investigators the chance to review scientific questions. Examination of data during the consultative workshops also helped in stimulating research by the busy clinicians.
Limitations - The project was not able to start participant enrollment at the beginning of ART initiation in 2005, hence we were forced to include a retrospective cohort design with the intention of understanding the early years of the ART program. The transition away from donor funding was challenging and alternative funding mechanisms were not put in place by the time funding from CDC ended, resulting in suspension of the project.
Future analyses - The findings of the aforementioned seven sub-studies have shown us that many important questions can be answered using the ACM cohort. Although additional research questions can be answered using the ACM data, it is strongly recommended that future studies be geared towards informing decisions of the national program, in addition to regional and global issues. More emphasis should be placed on timely availability and use of the data, on increased networking, and on creating strong collaboration with in-country and external collaborators.
Acknowledgment
The Research has been supported by the President's Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) under the terms of Cooperative Agreement with Johns Hopkins Bloomberg School of Public Health number PS000858.
Disclaimer
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Attribution/ Acknowledgement
The Research has been supported by the President's Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) under the terms of Cooperative Agreement with Johns Hopkins Bloomberg School of Public Health number PS000858. We would like to express our gratitude to the providers who routinely documented the status of their patients on the standard forms and last but not least, we would like to thank all the study subjects who consented and were followed in the project.
References
- 1.Ministry of Health of Ethiopia, author. Guideline for use of Anti Retroviral Drugs in Ethiopia FMOH, DACA and FHAPCO. 2003
- 2.Federal HIV/AIDS Prevention and Control Office, author. Country progress report on the HIV response. 2014 Report.
- 3.UNAIDS, author. AIDS Info 2014. 2015. Nov 3, retrieved from: https://www.google.com.et/webhp?sourceid=chrome-instant&ion=1&espv=2&ie=UTF8#q=UNAIDS.+AIDS+Info.+2014.on.
- 4.Minstry of Health of Ethiopia, author. Annual Performance Report EFY 2006 (2013/14) Addis Ababa: Federal Ministry of Health; 2013/14 Contract No.: 02/14. [Google Scholar]
- 5.Ethiopian Public Health Institute, author. HIV related estimates and projections for Ethiopia. 2012 Report.
- 6.PEPFAR, author. Ethiopia 2014 Country Operational Plan Executive Summary. 2014
- 7.World_Health_Organisation, author. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva, Switzerland: 2013. [PubMed] [Google Scholar]
- 8.Federal HIV/AIDS Prevention and Control Office, author. National Monitoring and Evaluation Framework for the Multi-sectoral Response to HIV/AIDS in Ethiopia. Addis Ababa: FHAPCO; 2003. [Google Scholar]
- 9.Monitoring the output and outcome of antiretroviral therapy (ART) programmes. Geneva: WHO, UNAIDS, and partners, Mövenpick Hotel; 2005. Oct 10–12, [Google Scholar]
- 10.World Health Organization, author. Progress on Global Access to HIV Antiretroviral Therapy: A Report on “3 by 5” and Beyond. 2006 Mar
- 11.AFRICOS Study. 2013. retrieved from: www.hirresearch.org/africos.
- 12.Dicko Arrive E, Amghar F, Aka H, Dior AE, Bouah H, et al. Pediatric IeDEA West Africa Working Group, author. HIV status disclosure and retention in care in HIV infected Adolescents on antiretroviral thearapy (ART) in wes Africa. PloS One. 2012;7(3):e33690. doi: 10.1371/journal.pone.0033690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalal S, Holmes MD, Laurence C, BAjunirwe F, Guwatudde D, Njelekela M, et al. Feasibility of a large cohort study in sub-saharan Africa assessed through four-country study. Glob Health Action. 2015:8. doi: 10.3402/gha.v8.27422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koole O, Tsui S, Wabwire-Mangen F, Kwesigabo G, Menten J, Mulenda M, et al. Retention and Risk factors among adults in antiretorival treatment programmes in Tanzania, Uganda and Zambia. Tropical Medicne and Intenational Health:TM&IH. 19(12):1397–1410. doi: 10.1111/tim.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fekade Daniel, Woldegebriel Teklu, Teklu Alula M, Damen Melake, Abdella Saro, Baraki Neaga, et al. Predictors of survival among Adult Ethiopian Patients in the National ART program at Sevent Univeristy Teaching Hospitals: A prospective Cohort Study. EJHS. 27(1.3):63–71. doi: 10.4314/ejhs.v27i1.7s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haileamlak Abraham, Hagos Tesfalem, Abebe Workeabeba, Abraham Loko, Asefa Henok, Teklu Alula M. Predictors of Hospitalization among Children on ART in Ethiopia: a Cohort study. EJHS. 27(1.2):39–52. doi: 10.4314/ejhs.v27i1.6s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gudina Esayas Kebede, Teklu Alula M, Berhan Asres, Gebreegziabhier Atsbeha, Siyoum Teshome, Nega Abiy, et al. Magnitude of Antiretroviral Drug Toxicity in Adult HIV patients in Ethiopian: A cohort study at seven teaching hospitals. EJHS. 27(1.2):39–52. doi: 10.4314/ejhs.v27i1.5s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teklu Alula M, Delele Kesetebirhan, Abraha Mulu, Belayhun Bekele, Gudina Esayas Kebede, Nega Abiy. Exploratory Analysis of Time from HIV Diagnosis to ART Start, FActors and effect on survival: A longitudinal follow sup study at seven teaching hospitals in Ethiopia. EJHS. 27(1.2):17–28. doi: 10.4314/ejhs.v27i1.3s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teklu Alula M, Nega Abiy, Mamuye Admasu Tenna, et al. Factors Associated with Mortality of TB/HIV Co-infected Patients in Ethiopia. EJHS. 27(1.2):29–38. doi: 10.4314/ejhs.v27i1.4s. [DOI] [PMC free article] [PubMed] [Google Scholar]