Abstract
Aim:
The purpose of this comparative study was to evaluate cognitive function in end-stage renal disease (ESRD) patients in comparison with age, sex, and level of education-matched control.
Materials and Methods:
This is a cross-sectional study involving 80 ESRD patients receiving maintenance hemodialysis treatment and recruited conservatively at the nephrology unit of our hospital. Eighty apparently healthy control, that were matched with the patients for age, sex and education, were also recruited. Following exclusion of depression and severe functional disability, a computer-assisted neuropsychological test, the FePsy, was used to assess memory, psychomotor speed, concentration and attention using simple auditory and visual reaction time tasks, recognition memory tests (RMTs), finger tapping task, and binary choice task (BCT) for both the ESRD patients and controls.
Results:
ESRD patients performed worse on simple auditory and visual reaction time tasks (P < 0.05), RMTs (P < 0.05), finger tapping task (P < 0.05), BCT, and computerized visual search task (P < 0.05).
Conclusion:
Performance in memory, psychomotor tasks, concentration, and attention tasks were found to be reduced among patients with ESRD compared with age, sex and level of education-matched control.
Keywords: Attention, cognition function, end stage renal disease, memory, psychomotor speed, Attention, la fonction cognitive, une maladie rénale au stade terminal, la mémoire, la vitesse psychomotrice
Résumé
But:
Le but de cette étude comparative a été pour évaluer la fonction cognitive en insuffisance rénale terminale (IRT) Patients en comparaison avec l’âge, le sexe et le niveau d’instruction de contrôle appariés.
Matériels et Méthodes:
Il s’agit d’une étude transversale impliquant 80 Insuffisance rénale terminale chez les patients recevant un traitement d’hémodialyse maintenance recrutés prudemment à l’unité de néphrologie de notre hôpital. 80 L’âge, le sexe et le niveau d’instruction de contrôle appariés ont été recrutés pour servir de témoins. Après l’exclusion de la dépression et de la déficience fonctionnelle grave, un test neuropsychologique assistée par ordinateur, l’FePsy, a été utilisé pour évaluer la mémoire, psychomoteur et l’attention la rapidité, la concentration à l’aide d’un simple délai de réaction auditive et visuelle des tâches, des tests de mémoire de reconnaissance (RMTs), avec les doigts de tâche, et choix binaire tâche (BCT) pour les patients et de contrôles de l’IRT.
Résultats:
les cas d’IRT effectué sur simple pire temps de réaction auditive et visuelle des tâches (P < 0,05), RMTs (P < 0,05), d’un doigt tapping tâche (P < 0,05), BCT, et informatisé recherche visuelle tâche (P < 0,05).
Conclusion:
les performances en mémoire, psychomoteur tâches, concentration et d’attention les tâches se sont avérés être réduite chez les patients atteints d’insuffisance rénale terminale par rapport avec l’âge, le sexe et le niveau de l’éducation contrôle appariés.
Introduction
Chronic kidney disease (CKD) is increasingly a public health problem.[1] It is characterized by progressive destruction of renal mass with irreversible sclerosis and loss of nephrons over a period of at least months to many years, depending on the underlying etiology.[2] Cognitive impairment is common in end-stage renal disease (ESRD) patients in comparison with the general population.[3,4] Previous studies have shown that CKD influences the cognitive function of adults.[5] Cognitive dysfunction may occur as a result of deficits in a number of domains of cognition including concentration, judgment, abstraction, memory, and executive function and this may impair the patients’ capability. There is a growing body of evidence that CKD is associated with comorbidities such as hypertension, diabetes mellitus (DM), hyperlipidemia, and stroke that are known to increase the risk of cognitive impairment.[6,7] Earlier studies have demonstrated that renal transplantation is associated with improvements in both neuropsychological tests such as the mini-mental state examination (MMSE), and neurophysiological markers of cognitive function measured using evoked potential latencies and electroencephalographic rhythms.[8,9,10] This cognitive impairment does not only have implication for the patient's ability to participate in adequate care of the ESRD[3] but also have major implications for informed consent in relation to dialysis initiation or maintenance, and ultimately, renal transplantation.[11,12]
In spite of the importance of knowing cognitive status of patients with ESRD, there is limited data, particularly from the developing countries where CKD is increasingly becoming a major public health problem. The purpose of this comparative study was to evaluate cognitive function in ESRD patients in comparison with age, sex and level of education-matched control.
Materials and Methods
Participants and study design
The study was cross-sectional in design. Eighty ESRD patients on maintenance hemodialysis, who consented to participate in the study, were recruited consecutively from the dialysis unit of Aminu Kano Teaching Hospital (AKTH) during the study period (from June 2010 to June 2014). Eighty healthy, age, sex and level of education-matched controls recruited from the general out-patient clinic and healthy volunteers among members of staff of the hospital.
The exclusion criteria were age <18 years, education <6 years, presence of comorbidities that could cause cognitive dysfunction (e.g., DM, hypertension, epilepsy, other metabolic diseases, cerebrovascular disease, Parkinson's disease, epilepsy, human immunodeficiency virus, brain tumor etc., subjects with psychiatric disorders were also excluded). Other exclusion criteria included drug abuse, current use of psychoactive drugs, history of pervious head injury with loss of consciousness, severe functional impairment (Karnofsky performance <50%, alcohol intake, presence of cardiac failure and use of anti-cholinergic medications). The same exclusion criteria were applied to the normal control subjects. A questionnaire was developed and applied to all subjects and emphasis in the questionnaire was on sociodemographic, anthropometric CKD-related data. The study participants were evaluated by way of a detailed general physical and neurological examination.
Data sources
Using a questionnaire-based proforma, we obtained information on sociodemographic characteristics and CKD-related information, history of hypertension, heart disease, previous stroke, and diabetes. Subsequently, all the patients had screening for functional performance and depression using Karnofsky scale and Hamilton and Anxiety Depression Scale (HADS) respectively. Those with Karnofsky performance more than 50% and no evidence of depression on HADS proceeded with the study. Results of blood count, coagulation tests, serum electrolytes, and glucose and renal function tests (urea, creatinine levels) were also obtained in cases.
Cognitive function measure
The cognitive assessment was conducted with the aid of a computer-assisted neuropsychological test battery called iron psychology (“FePsy” version 6.8, The Psychology company, Amsterdam, Netherlands).[13] FePsy, “The Iron Psyche” is a system for automated neuropsychological testing. It contains a set of highly sensitive computerized tests for cognitive neuropsychological functions and a relational database system for storage of the results.[13] FePsy had been validated for use in studies of cognitive functions.[14,15] The FePsy test has been validated for use among Nigerians and in patients with chronic renal impairment.[15,16] The tests employed in the present study included simple reaction time task, binary choice reaction time, computerized visual scanning task, recognition memory task and finger tapping test. The tests were administered in a quiet and well-lit room at a room temperature with the subject sitting at distance of 40–60 cm from the visual display screen of the computer. The memory function was assessed using the recognition memory test (RMT-word and figure). Mental or psychomotor speed was assessed using the simple reaction time test (auditory and visual). Binary choice task (BCT), which is a complex form of continuous performance test, was used in the study to assess psychomotor speed, attention, and concentration. The computer-assisted visual scanning task (CVST). Which could be used to detect presence of brain damage in the subject reflects accuracy and speed of responses and are evaluated within the context of visual (complex) information processing and perceptual-mental strategies.[15] Finger tapping test was also performed in the context of motor activation and fluency.
The higher the mean scores on auditory reaction test, visual reaction test, and computerized visual search task the poorer the cognitive performance. Conversely the higher the mean scores on recognition test, BCT, and tapping task (which are expressed as correct percent of total) the better the cognitive performance.
On conducting these neuropsychological tests, care was taken to ensure good brightness and contrast of screen, sufficient sound level of the speaker and to elucidate a history of photosensitive seizure from the patients.
Data analysis
Analysis of data was done using GraphPad Prism version 5.03 (GraphPad Software, Inc., CA 92037, USA). The normality of the numerical data was assessed using D’Agostino and Pearson Omnibus tests. Numerical data that were normally distributed were expressed as the mean ± standard deviation (SD). Comparisons of cognitive function parameters between ESRD patients and control subjects were performed using Student's independent sample t-test and Mann–Whitney test in the case of parametric and nonparametric data respectively. P <0.05 was considered statistically significant.
Ethical approval was obtained from the Ethical Committee of AKTH.
Results
During the study period, 80 patients and 80 age- and sex-matched control comprising 45 (56.2%) males and 35 (43.8%) females, in each arm, were evaluated. The mean age ± SD of the patients, were 49.85 ± 10.58 and 49.84 ± 10.55, respectively, the difference in their age was not statistically significant (P = 0.990). The median duration of CKD from diagnosis was 2 years (range 0.2–5 years) [Table 1].
Table 1.
Sociodemographic, clinical and laboratory characteristics of the study participants
| Variable | ESRD patients (%) | Control (%) | P |
|---|---|---|---|
| Mean age | 49.85±10.58 | 49.84±10.55 | 0.990 |
| Sex | |||
| Male | 45 | 45 | 1.000 |
| Female | 35 | 35 | |
| Level of education | |||
| Primary | 8 (10) | 8 (10) | 1.000 |
| Secondary | 50 (62.5) | 50 (62.5) | |
| Tertiary | 22 (27.5) | 22 (27.5) | |
| Occupation | |||
| Civil service | 28 (35) | 30 (37.5) | 0.848 |
| Homemaker | 19 (23.8) | 20 (25) | |
| Farming | 10 (12.5) | 7 (8.75) | |
| Trading | 10 (12.5) | 10 (12.5) | |
| Student | 5 (6.3) | 8 (6.3) | |
| Others | 8 (10) | 5 (10) | |
| Etiology | |||
| Chronic glomerulonephritis | 35 (43.8) | ||
| DM | 9 (11.3) | ||
| Hypertension | 29 (36.3) | ||
| Hypertension + DM | 6 (7.5) | ||
| Undefined | 1 (1.3) | ||
| Mean creatinine level | 486.72±266.4 | ||
| Mean urea level | 23.81±10.2 | ||
| Mean hemoglobin level | 7.3±1.8 | ||
| Median duration of CKD | 2 (0.2-5) years | ||
| Mean BMI | 17.84±7.9 |
ESRD=End stage renal disease, CKD=Chronic kidney disease, DM=Diabetes mellitus, BMI=Body mass index
Regarding etiology of CKD in the patients, 35 (43.8%) had chronic glomerulonephritis, 29 (36.2%) patients had hypertension only, 9 (11.2%) had type -2 DM only, 6 (7.6%) had hypertension and diabetes and 1 (1.2%) patient had unidentified etiology [Table 1].
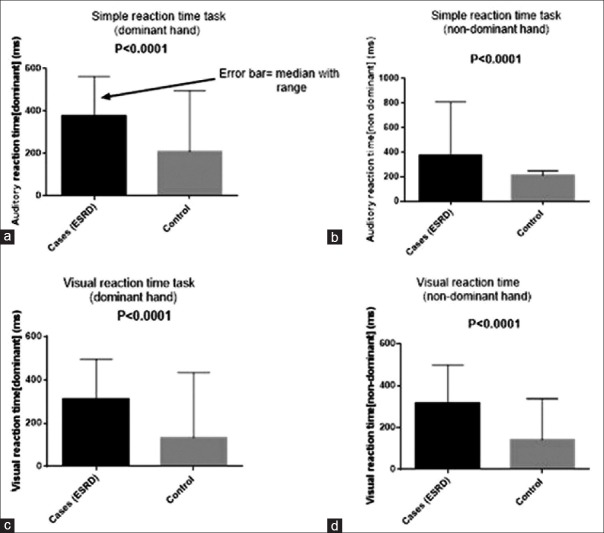
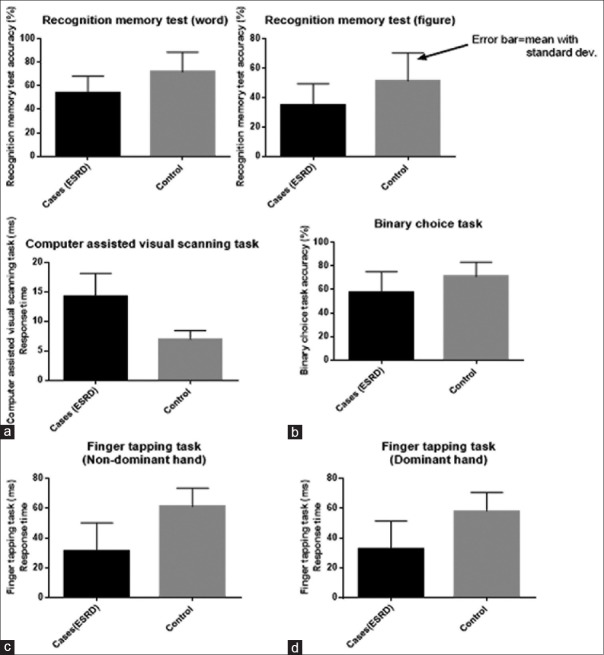
Considering test of psychomotor speed, median score of cognitive performance was significantly (P < 0.0001) prolonged in ESRD patients compared to control on dominant auditory reaction time (350.8 vs. 196.9 ms), nondominant auditory reaction times (375.9 vs. 204.6 ms), dominant visual reaction time (284.1 vs. 133.4 ms), nondominant auditory reaction times (310.0 vs. 141.9 ms) [Figure 1]. For test of memory; RMT was significantly (P < 0.0001) prolonged in ESRD patient than control as mean response time on visual RMT in ESRD in cases and control were 71.8 ± 16.8 ms and 53.9 ± 14.3 ms, respectively, and on word RMT in ESRD and cases were 51.4 ± 19.0 ms and 35.8 ± 14.3 ms, respectively. With the CVST which can distinguish brain damage from normal brain; mean response time was significantly (P < 0.0001) prolonged in ESRD patient than controls (14.23 ± 3.9 vs. 6.95 ± 1.5 respectively).
Figure 1.
Cognitive performance on: Auditory reaction test (a) dominant (b) nondominant, visual reaction time test (c) dominant (d) nondominant in end-stage renal disease and matched control
On assessment of attention, concentration and psychomotor speed with BCT, binary choice accuracy was lower in ESRD patients (57.94 ± 17.2) than in controls (71.07 ± 12.3), P < 0.05.
The mean finger tapping scores in ESRD patients and controls were 32.52 ± 18.8 and 58.17 ± 12.4 respectively in the dominant hand, and 31.2 ± 19.0 and 61.1 ± 12.3 respectively in the nondominant hand. P < 0.05 was considered statistically significant in each case [Figure 2].
Figure 2.
Cognitive performance on: (a) computer assisted visual task (b) binary choice task, finger tapping task (c) non-dominant (d) dominant in ESRD and matched control
Discussion
In the present study, we found a significant difference across all the cognitive domains assessed in ESRD patients in comparison with age, sex and level of education-matched control. This finding is in conformity with reports from other workers.[10,17,18,19]
Cognitive involvement and dementia are commonly seen in patients with CKD, particularly in subjects with advanced stage disease, but are poorly recognized by nephrologists.[20] Previous studies[11,17,18] reported a close association between CKD and cognitive impairment. In a study, the prevalence of mental impairment was as high as 30% among persons with kidney failure.[3] A systematic review estimated that as many as 60% of dialysis patients may meet diagnostic criteria for cognitive dysfunction.[21]
We found in this study that ESRD patients performed significantly worse than controls on memory tests. This finding is compatible with previous reports.[10,16] Our observation suggests that ESRD patients may be at higher risk of memory impairment than the general population. Similarly, there was demonstrable reduced performance in attention, concentration, visual processing speed, motor activation and fluency and psychomotor performance in ESRD patients. These findings are in agreement with reports from other studies.[22,23] However, relative reduction in attention and concentration seen in the current study is in contrast with the finding of Egbi et al.[10] This difference may not be unconnected with methodological differences. Cognitive symptoms appear to represent one of the core features of depressive disorders with an impact on many functional outcomes;[24,25] thus, deliberate effort was made in the present study to exclude depression and severe functional disability in the study patients.
Although this study was not designed to evaluate the relationship between cognitive performance and glomerular filtration rate as the focus of our study was ESRD, other studies have shown a graded association between CKD and cognitive impairment with different cognitive screening instruments, such as the six-item cognitive screening test derived from the widely used MMSE.[1,11,16] Other neuropsychological detecting for detecting impaired cognitive function in specific domains suggest that even small reductions in kidney function are associated with clinically significant consequences for cognitive functioning.[17,18]
Cognitive dysfunctions in CKD patients have implication for therapy and quality of life. For instance, cognitive impairment in ESRD patients that is severe enough to preclude reliable compliance is an established contraindication to kidney transplantation.[26] However, there is clear evidence that cognitive performance improves following renal transplantation; Griva et al. reported a remarkable improvement in cognitive functions compared to baseline values 6 months after transplantation.[22]
The mechanism underlying cognitive impairment in persons with ESRD is largely unknown. The mechanisms proposed as mediators of relations between kidney function and cognition are similar to those that have been advanced to explain relations between other risk factors for cardiovascular disease and cognition.[16] Body of evidence suggests that cerebrovascular disease may play a prominent role in the neuropathology of cognitive dysfunction in ESRD. The vascular beds of the brain and kidney have similar anatomic and hemodynamic features. Consequently, cognitive impairment in ESRD or CKD may be a reflection of vascular injury in end organs.[20] This relationship is further corroborated by neuroimaging studies which revealed a large vessel stroke, small vessel stroke, and white matter lesions.[27,28]
Given that cognitive impairment is associated with high risks of death, dialysis withdrawal, hospitalization, and disability among patients with ESRD, recognizing and effectively managing cognitive impairment may improve the care of persons with ESRD. Periodic screening is, therefore, needed to accurately identify patients with cognitive impairment to improve their care and quality of life. Screening for cognitive dysfunctions in persons with ESRD could begin with questioning of the caregivers as caregivers often notice cognitive deficits before they are apparent to clinicians, and their observations or the availability of a pre-ESRD cognitive assessment is useful for helping to establish the course of impairment. Preventive measure focusing on vascular risk factor modification, physical and cognitive activity, that have shown some promise in the general population, may be reasonably extended to the ESRD patients.
In spite of our findings, the current study has some limitations, the control, unlike ESRD patients, did not have extensive hematological and biochemical evaluations, thus, they might not have been perfect control. Nonetheless, the control was strictly matched in the areas known to influence cognitive functions i.e., age, sex and level of education.
Conclusion
This study showed a significant difference across all the cognitive domains assessed in ESRD patients in comparison with age, sex and level of education-matched controls. Our data further emphasize the need to assess CKD patients for cognitive impairment to improve their care and quality of life.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Madero M, Gul A, Sarnak MJ. Cognitive function in chronic kidney disease. Semin Dial. 2008;21:29–37. doi: 10.1111/j.1525-139X.2007.00384.x. [DOI] [PubMed] [Google Scholar]
- 2.Jacobson HR. Chronic renal failure: Pathophysiology. Lancet. 1991;338:419–23. doi: 10.1016/0140-6736(91)91042-s. [DOI] [PubMed] [Google Scholar]
- 3.Sehgal AR, Grey SF, DeOreo PB, Whitehouse PJ. Prevalence, recognition, and implications of mental impairment among hemodialysis patients. Am J Kidney Dis. 1997;30:41–9. doi: 10.1016/s0272-6386(97)90563-1. [DOI] [PubMed] [Google Scholar]
- 4.Fazekas G, Fazekas F, Schmidt R, Kapeller P, Offenbacher H, Krejs GJ. Brain MRI findings and cognitive impairment in patients undergoing chronic hemodialysis treatment. J Neurol Sci. 1995;134:83–8. doi: 10.1016/0022-510x(95)00226-7. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Fang C, Cai L, Dong B, Deng J. Chronic kidney disease and cognitive impairment among the very old in China. Aging Clin Exp Res Aug. 2015 Aug 12; doi: 10.1007/s40520-015-0433-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Koren-Morag N, Goldbourt U, Tanne D. Renal dysfunction and risk of ischemic stroke or TIA in patients with cardiovascular disease. Neurology. 2006;67:224–8. doi: 10.1212/01.wnl.0000229099.62706.a3. [DOI] [PubMed] [Google Scholar]
- 7.Tsagalis G, Akrivos T, Alevizaki M, Manios E, Stamatellopoulos K, Laggouranis A, et al. Renal dysfunction in acute stroke: An independent predictor of long-term all combined vascular events and overall mortality. Nephrol Dial Transplant. 2009;24:194–200. doi: 10.1093/ndt/gfn471. [DOI] [PubMed] [Google Scholar]
- 8.Kramer L, Madl C, Stockenhuber F, Yeganehfar W, Eisenhuber E, Derfler K, et al. Beneficial effect of renal transplantation on cognitive brain function. Kidney Int. 1996;49:833–8. doi: 10.1038/ki.1996.115. [DOI] [PubMed] [Google Scholar]
- 9.Mendley SR, Zelko FA. Improvement in specific aspects of neurocognitive performance in children after renal transplantation. Kidney Int. 1999;56:318–23. doi: 10.1046/j.1523-1755.1999.00539.x. [DOI] [PubMed] [Google Scholar]
- 10.Egbi OG, Ogunrin O, Oviasu E. Cognitive deficits of patients with chronic kidney disease and effect of hemodialysis. Greener J Med Sci. 2015;5:19–25. [Google Scholar]
- 11.Kurella M, Chertow GM, Luan J, Yaffe K. Cognitive impairment in chronic kidney disease. J Am Geriatr Soc. 2004;52:1863–9. doi: 10.1111/j.1532-5415.2004.52508.x. [DOI] [PubMed] [Google Scholar]
- 12.Rakowski DA, Caillard S, Agodoa LY, Abbott KC. Dementia as a predictor of mortality in dialysis patients. Clin J Am Soc Nephrol. 2006;1:1000–5. doi: 10.2215/CJN.00470705. [DOI] [PubMed] [Google Scholar]
- 13.Moerland MC, Aldenkamp AP, Alpherts WC. A neuropsychological test battery for the Apple IIE. Int J Man Mach Stud. 1986;25:453–86. [Google Scholar]
- 14.Miller EN, Satz P, Visscher B. Computerized and conventional neuropsychological assessment of HIV-1-infected homosexual men. Neurology. 1991;41:1608–16. doi: 10.1212/wnl.41.10.1608. [DOI] [PubMed] [Google Scholar]
- 15.Ogunrin O, Odiase FE. Predictive validity and usefulness of visual scanning task in HIV/AIDS – A case control analysis. [Last cited on 2015 Dec 03];Afr J Neurol Sci. 2008 26:45–52. Available from: http://www.ajol.info/index.php/ajns/article/view/7593 . [Google Scholar]
- 16.Elias MF, Elias PK, Seliger SL, Narsipur SS, Dore GA, Robbins MA. Chronic kidney disease, creatinine and cognitive functioning. Nephrol Dial Transplant. 2009;24:2446–52. doi: 10.1093/ndt/gfp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Etgen T. Kidney disease as a determinant of cognitive decline and dementia. Alzheimers Res Ther. 2015;7:29. doi: 10.1186/s13195-015-0115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buchman AS, Tanne D, Boyle PA, Shah RC, Leurgans SE, Bennett DA. Kidney function is associated with the rate of cognitive decline in the elderly. Neurology. 2009;73:920–7. doi: 10.1212/WNL.0b013e3181b72629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurella Tamura M, Wadley V, Yaffe K, McClure LA, Howard G, Go R, et al. Kidney function and cognitive impairment in US adults: The reasons for geographic and racial differences in stroke (REGARDS) study. Am J Kidney Dis. 2008;52:227–34. doi: 10.1053/j.ajkd.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurella Tamura M, Yaffe K. Dementia and cognitive impairment in ESRD: Diagnostic and therapeutic strategies. Kidney Int. 2011;79:14–22. doi: 10.1038/ki.2010.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pereira AA, Weiner DE, Scott T, Sarnak MJ. Cognitive function in dialysis patients. Am J Kidney Dis. 2005;45:448–62. doi: 10.1053/j.ajkd.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 22.Griva K, Thompson D, Jayasena D, Davenport A, Harrison M, Newman SP. Cognitive functioning pre- to post-kidney transplantation – A prospective study. Nephrol Dial Transplant. 2006;21:3275–82. doi: 10.1093/ndt/gfl385. [DOI] [PubMed] [Google Scholar]
- 23.Murray AM. Cognitive impairment in the aging dialysis and chronic kidney disease populations: An occult burden. Adv Chronic Kidney Dis. 2008;15:123–32. doi: 10.1053/j.ackd.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papazacharias A, Nardini M. The relationship between depression and cognitive deficits. Psychiatr Danub. 2012;24(Suppl 1):S179–82. [PubMed] [Google Scholar]
- 25.Atre-Vaidya N, Taylor MA, Seidenberg M, Reed R, Perrine A, Glick-Oberwise F. Cognitive deficits, psychopathology, and psychosocial functioning in bipolar mood disorder. Neuropsychiatry Neuropsychol Behav Neurol. 1998;11:120–6. [PubMed] [Google Scholar]
- 26.Kasiske BL, Cangro CB, Hariharan S, Hricik DE, Kerman RH, Roth D, et al. The evaluation of renal transplantation candidates: Clinical practice guidelines. Am J Transplant. 2001;1(Suppl 2):3–95. [PubMed] [Google Scholar]
- 27.Kobayashi S, Ikeda T, Moriya H, Ohtake T, Kumagai H. Asymptomatic cerebral lacunae in patients with chronic kidney disease. Am J Kidney Dis. 2004;44:35–41. doi: 10.1053/j.ajkd.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 28.Nakatani T, Naganuma T, Uchida J, Masuda C, Wada S, Sugimura T, et al. Silent cerebral infarction in hemodialysis patients. Am J Nephrol. 2003;23:86–90. doi: 10.1159/000068034. [DOI] [PubMed] [Google Scholar]