Abstract
Background
Obesity is known as a major risk factor for postoperative vein thrombosis. Thromboelastography (TEG) is used to monitor viscoelastic features of blood clots. The aim of this study was to determine hypercoagulable states in patients undergoing bariatric surgery and to assess dynamics of coagulation parameters in the perioperative setting using TEG.
Material/Methods
We included 60 consecutive patients undergoing bariatric surgery. TEG alterations were assessed at 4 time points: at baseline, after the surgery, and on postoperative day 1 (POD1) and 2 (POD2). Hypercoagulable state was defined when patients showed clot strength (G) of ≥11 dynes/cm2 or maximum amplitude (MA) ≥68 mm.
Results
Fourteen patients (23.3%) out of 60 showed hypercoagulability prior to surgery on TEG. Fibrinogen levels were significantly higher in the G ≥11 group compared to the G <11 group, at 4.2 and 3.8 g/l, respectively (p=0.02). Seventeen patients (28.3%) had MA ≥68 mm at baseline. Fibrinogen levels increased significantly from 3.90 at baseline to 4.16 g/l in POD2 (p<0.001). There was an increase in mean reaction time from baseline (6.74 s) to POD2 (7.43 s, p=0.022). We found a correlation between baseline fibrinogen levels and MA (R=0.431, p=0.001) or G (R=0.387, p=0.003). ROC curve analysis showed that fibrinogen levels can predict clot strength (G) ≥11 dynes/cm2 with AUC=0.680 (p=0.044).
Conclusions
A considerable proportion of patients referred to bariatric surgery show a trend towards hypercoagulability on TEG. This study shows the potential of hypercoagulation monitoring by TEG in the perioperative setting of bariatric surgery.
MeSH Keywords: Bariatric Surgery, Obesity, Thrombelastography, Thrombophilia
Background
Increasing prevalence of morbid obesity has augmented the demand for bariatric surgery, which is so far the most effective and durable therapy for the treatment of morbidly obese patients [1]. Deep vein thrombosis (DVT) and pulmonary embolism (PE) are the major risk factors of mortality after bariatric surgery [2–4]; venous thrombosis of mesenteric vessels may also contribute to significant morbidity [5]. Although the risk of postoperative DVT following bariatric surgery does not exceed 2% in most studies, there are currently no strategies to identify individuals who have the highest risk for DVT [3]. Diagnosis of DVT in morbidly obese patients is a challenging clinical task because a considerable proportion of cases remains asymptomatic [4]. To date, there are different prophylactic strategies available for prevention of DVT; however, optimal personalized prophylactic regimes in individual patients are yet not defined [5,6]. Anticoagulation with low molecular weight heparin, anti-factor Xa inhibitors, and unfractionated heparin are widely used in bariatric surgery for prevention of DVT and PE, but appropriate dosing of anticoagulation is still controversial [7]. Furthermore, the risk of DVT among morbidly obese patients undergoing bariatric surgery appears to persist despite the use of aggressive prophylactic anticoagulation therapy [3]. Identification of individuals with increased risk for DVT is also crucial for the balance between DVT prevention and the risk of bleeding, which is another major complication of bariatric surgery [8].
The pathogenesis of hypercoagulability in obese individuals is a target of many research groups [9]. Recently, many different and complex changes in plasma coagulation factors have been described in patients with obesity [10]. Obesity is linked to elevated expression of the prothrombotic molecules plasminogen activator inhibitor-1 and tissue factor (TF) and increased platelet activation [9]. A significant increase in median levels of circulating microparticles was observed in obese patients compared to controls, including annexin V-MP, endothelial-derived, leukocyte-derived, and tissue factor bearing MP compared to controls [11]. The same study also showed that obese patients had a significantly higher median peak thrombin and increased median endogenous thrombin potential compared to controls [11]. The same group of researchers has shown that after sleeve gastrectomy, the decrease of BMI in morbidly obese subjects was matched with a decrease of circulating microparticles of endothelial, platelet, and leukocyte origin [12].
Thromboelastography (TEG) is used to monitor viscoelastic properties of blood clots, from formation to lysis [13]. Over the last decade, TEG has gained significant importance in the management of high-risk bleeding populations, liver transplantation, and certain other clinical conditions [14]. Although TEG is mostly used to monitor hypocoagulability states, recent evidence shows the importance of TEG in evaluation of hypercoagulability in the perioperative setting [15,16]. Thromboelastography has recently gained wide scientific attention in obesity and hypercoagulation trends observed in these individuals. A clear relationship between hypercoagulability detected by whole-blood thromboelastometry and aggregometry and increased fat mass has been shown [10]. Several studies addressed the role of hypercoagulability monitoring in the perioperative period of bariatric surgery [17,18]. A recent systematic review about hypercoagulable state and TEG indicated that further studies are needed to determine the ultimate role of this method for prediction of hypercoagulable states and postoperative thrombosis [19,20]. The aim of this study was to determine hypercoagulability states in obese patients undergoing bariatric surgery and to assess the alterations of coagulation parameters in the perioperative setting using TEG. We selected the cutoff values of TEG parameters for defining hypercoagulability based on the studies discussed above [15,17,21]. We also evaluated the potential of clinical and standard laboratory tests to predict hypercoagulable states in patients referred to bariatric surgery.
Material and Methods
Study population
Sixty consecutive patients undergoing laparoscopic bariatric surgery (gastric bypass or gastric plication) were recruited at the Department of Surgery, Lithuanian University of Health Sciences Hospital from October 2014 to June 2015. All the patients underwent general anesthesia in normothermal conditions and were given standardized thromboembolic prophylaxis with 500 ml 6% Dextran 70 (Fresenius, Poland) after induction of anesthesia and Fraxiparine 0.3 ml sc (GlaxoSmithKline, Poland) starting in the morning of POD1 until discharge from the hospital. Detailed clinical characteristics of the study population are provided in Table 1. We excluded patients on anticoagulation therapy, as well as those with chronic kidney/liver failure, coagulation disorders (liver cirrhosis, known hematological, or hereditary coagulation disorder), previous history of DVT, or using oral contraception. The patients were followed for clinically evident thrombotic complications for 1 year after the study by arranging clinical appointments 1 month and 1 year after the surgery. All patients signed an informed consent form to participate in the study. The study was approved by the regional Kaunas Ethics Committee (BE-2-10).
Table 1.
Clinical characteristics of study participants.
| All patients (n=60) | G <11 (n=46) | G >11 (n=14) | p value | MA <68 (n=43) | MA >68 (n=17) | p value | |
|---|---|---|---|---|---|---|---|
| Sex/male, n (%) | 18 (30.0) | 14 (30.4) | 4 (25.0) | 0.259 | 15 (34.8) | 3 (17.6) | 0.189 |
| Age, years ±SD | 39.1±11.9 | 37.8±11.8 | 43.6±11.7 | 0.117 | 37.1±11.5 | 44.2±11.8 | 0.046 |
| BMI, kg/m2 ±SD | 47.5 (8.5) | 47.8±9.1 | 46.3±6.4 | 0.499 | 47.6±9.4 | 47.0±6.2 | 0.775 |
| Surgery type/gastric bypass, n (%) | 38 (63.3) | 30 (65.2) | 8 (57.1) | 0.211 | 27 (62.7) | 11 (64.7) | 0.768 |
| Smokers, n (%) | 21 (35.0) | 15 (32.6) | 6 (42.8) | 0.192 | 14 (32.5) | 7 (41.2) | 0.528 |
| AH, n (%) | 39 (65.0) | 29 (63.0) | 10 (71.4) | 0.231 | 27 (62.7) | 12 (70.5) | 0.568 |
| CRP, mg/l ±SD | 6.1±4.5 | 6.3±4.8 | 5.8±3.6 | 0.682 | 6.3±4.8 | 5.8±3.7 | 0.668 |
| ESR, mm/h ±SD | 16.2±10.1 | 16.1±10.1 | 16.6±9.4 | 0.865 | 15.3±9.6 | 18.6±10.3 | 0.261 |
| D-dimers, mg/l ±SD | 0.49±0.35 | 0.47±0.32 | 0.63±0.48 | 0.268 | 0.46±0.32 | 0.60±0.44 | 0.245 |
| Fibrinogen (g/l) | 3.90±0.75 | 3.80±0.80 | 4.23±0.51 | 0.021 | 3.8±0.8 | 4.2±0.5 | 0.025 |
| Platelet count | 246±76 | 244±78 | 249±70 | 0.821 | 243±81 | 262±73 | 0.389 |
| aPTT | 33.9±5.4 | 33.9±4.9 | 34.9±6.8 | 0.608 | 33.7±4.9 | 34.6±6.4 | 0.604 |
| INR | 0.99±0.1 | 0.99±0.1 | 0.99±0.1 | 1 | 0.99±0.1 | 0.99±0.1 | 1 |
Data are presented as n (percentage) and mean ± standard deviation. P values represent statistical comparison between patients with G ≥11 vs. G <11 dynes/cm2 and MA ≥68 vs. MA <68 mm. P values where calculated using unpaired t-test. G – clot strength (dynes/cm2); MA – maximum amplitude (millimeters); BMI – body mass index; AH – arterial hypertension; CRP – C-reactive protein; ESR – erythrocyte sedimentation rate; aPTT – activated partial thromboplastin time; INR – international normalized ratio.
Thromboelastography measurements
Thromboelastography was performed using the TEG® 5000 Thromboelastograph® Hemostasis Analyzer System (Braintree, USA). Thromboelastographic alterations were assessed at 4 time points: at baseline prior to induction of anesthesia, immediately after the end of the surgery, and on the morning of postoperative days 1 (POD1) and 2 (POD2).
The following parameters of thromboelastography were assessed: r-time (reaction time, min.; normal range [NR]: 5–10); k-time (clotting time, min; NR: 1–3); α-angle (degrees; NR: 53–72); MA parameter (maximum amplitude, millimeters; NR: 50–70); and G (clot strength, dynes per square centimeter; NR: 4.6–10.9). All TEG measurements were performed as described previously [18]. Briefly, mixing 1 ml of citrated blood with kaolin by an inversion technique was performed by the physician conducting TEG. Then, 20 μl of 0.2 M calcium chloride was added to the plain TEG cup, followed by 340 μl of the blood/kaolin mix.
Statistical analysis
Data normality was assessed using Kolmogorov-Smirnov test. Data (age, BMI, coagulation parameters, and TEG values) are presented as mean values and standard deviation (SD). Means were compared using paired or unpaired t tests, as appropriate. Qualitative variables were compared using the chi-square or Fisher’s exact test, as appropriate. Spearman correlation analysis was performed to determine the correlation between clinical or standard laboratory test values (CRP, ESR, D-dimers, and fibrinogen) and TEG parameters. Receiver operating characteristic (ROC) curve analysis was performed to determine optimal fibrinogen levels for prediction of hypercoagulable states G ≥11 dynes/cm2 and MA ≥68 mm. P<0.05 value was considered to be statistically significant. Statistical analysis was performed using the SPSS 20.0 software package (Chicago, IL).
Results
Characteristics of the study group
The study included 60 consecutive patients (18 males and 42 females) referred to bariatric surgery, with a mean age of 39.1±11.9 years (age range 18–62) and a mean BMI of 47.5±8.5 years (BMI range 34.3–84.9). The mean duration of surgical procedure was 91.4±20.0 min. Gastric bypass surgery was performed in 38 (63.3%) patients and gastric plication in 22 (36.7%) patients. Twenty-one patients (35%) were active smokers at the time of the surgery, and 39 patients (65%) had previous diagnosis of arterial hypertension. We followed all our patients for clinically evident thrombotic complications for 1 year after the study and none of them had thrombotic events during the follow-up period (data not shown). A summary of patients’ characteristics is presented in Table 1.
Preoperative hypercoagulability rates determined by TEG
Forty-six patients (76.7%) had G parameter <11 and 14 patients (23.3%) exhibited hypercoagulability prior to surgery, as defined by G value ≥11 (Table 1). Comparison of clinical parameters and standard laboratory tests between the 2 groups (G <11 vs. G ≥11) did not reveal significant differences in BMI and CRP levels (Table 1). The age of patients in the G <11 group was lower (37.8) than in the G ≥11 group (43.6), but the difference was not significant (p=0.117). The only parameter that was significantly higher in the G ≥11 group compared to the G <11 group was fibrinogen levels, which were 4.23 and 3.8 g/l, respectively (p=0.021; Table 1). Among all study participants, 17 patients (28.3%) had MA ≥68 mm and 43 patients (71.7%) had MA <68 mm. Patients with MA ≥68 were significantly older (44.2 years) than in the group of patients with MA <68 mm (37.1 years; p=0.046; Table 1). Fibrinogen levels were also higher in the hypercoagulable group (MA ≥68 mm) than in the remaining subjects with MA <68 mm (Table 1, p=0.025)
Dynamics of TEG parameters, fibrinogen, and d-dimers in the perioperative period of bariatric surgery
Mean values of TEG parameters in the perioperative period of study participants are summarized in Table 2. There was an increase in r-time from preoperative levels (6.74 s) to POD1 (6.96 s), but it reached significance only at POD2 (7.43 s, p=0.022; Table 2). K-time and a-angle remained at levels similar to the initial TEG results recorded on POD1 and POD2 (Table 2). There was a trend towards hypercoagulability and higher values of MA and G compared with initial TEG values and results obtained on POD2; however, the differences were not significant (Table 2). Dynamics of fibrinogen and D-dimer during the perioperative period are also presented in Table 2. There was a significant increase in D-dimer levels in the postoperative period after bariatric surgery. Average fibrinogen levels also increased significantly from basal values prior to surgery, from 3.90 to 4.16 g/l on POD2 (p<0.001, Table 2).
Table 2.
Dynamics of TEG parameters, fibrinogen, and D-dimers in the perioperative period of bariatric surgery.
| TEG parameter | Initial | Post surgery | POD1 | POD2 | p value (initial vs. post surgery) | p value (initial vs. POD1) | p value (initial vs. POD2) |
|---|---|---|---|---|---|---|---|
| r-time (min) | 6.74±1.61 | 6.02±1.41 | 6.96±1.66 | 7.43±1.55 | 0.003 | 0.371 | 0.022 |
| k-time (min) | 2.21±0.87 | 1.99±0.77 | 2.03±0.72 | 2.09±1.13 | 0.094 | 0.174 | 0.505 |
| α-angle (deg) | 60.99±8.45 | 63.02±8.26 | 62.41±7.63 | 62.71±9.84 | 0.127 | 0.29 | 0.293 |
| MA (mm) | 65.6±4.66 | 63.57±5.52 | 65.10±4.54 | 66.11±4.87 | 0.132 | 0.391 | 0.608 |
| G (dyne/cm2) | 9.83±2.15 | 9.48±2.35 | 9.61±1.96 | 10.07±2.21 | 0.255 | 0.416 | 0.575 |
| Fibrinogen (g/l) | 3.90±0.75 | – | 3.79±0.59 | 4.16±0.62 | – | 0.109 | 0.012 |
| D-dimer (mg/l) | 0.49±0.35 | – | 1.41±1.04 | 0.95±0.47 | – | <0.001 | <0.001 |
P values were calculated using paired t-test; significant p -values are marked in bold.
Dynamics of coagulation status in the perioperative period of bariatric surgery
We also aimed to assess the changes in hypercoagulability status from baseline to POD2. At baseline, 46 patients (76.7%) had G parameter <11 (Table 3), but on POD2 13.0% of this group had G ≥11. Out 14 patients (23.3%) showing hypercoagulability prior to surgery, as defined by G value ≥11, 12 patients remained hypercoagulable on POD2 (Table 3). Prior to surgery, 17 patients (28.3%) had MA ≥68 mm and 14 of them remained hypercoagulable on POD2 (Table 3). Forty-three patients (71.7%) had MA <68 mm at baseline, but 7 of them became hypercoagulable by POD2.
Table 3.
Dynamics of hypercoagulability status among bariatric patients from baseline to POD2.
| Baseline | Dynamics of coagulability status from baseline to POD2 | ||||
|---|---|---|---|---|---|
| n | % | n | % | ||
| G ≥11 | 14 | 23.3 | G ≥11 | 12 | 85.7 |
| G <11 | 46 | 76.7 | G <11 | 2 | 14.3 |
| G ≥11 | 6 | 13.0 | |||
| G <11 | 40 | 87.0 | |||
| MA ≥68 | 17 | 28.3 | MA ≥68 | 14 | 82.4 |
| MA <68 | 43 | 71.7 | MA <68 | 3 | 17.6 |
| MA ≥68 | 7 | 16.3 | |||
| MA <68 | 36 | 83.7 | |||
G – clot strength (dynes/cm2); MA – maximum amplitude (millimeters); POD2 – postoperative day 2.
Analysis of correlation between TEG parameters and clinical or laboratory parameters
To assess the relationship between clinical variables and standard laboratory parameters with baseline TEG values, we performed correlation analysis (Table 4). We did not observe a correlation between BMI and any of TEG parameters. Similarly, markers of inflammation, CRP, and erythrocyte sedimentation rate (ESR) also did not correlate with coagulation parameters recorded by TEG (Table 4). Preoperative fibrinogen levels were associated with k-time (R=−0.313, p=0.018), α-angle (R=0.333, p=0.011), MA (R=0.431, p=0.001), and G (R=0.387, p=0.003; Table 4). Interestingly, we also observed a weak but significant correlation between age and r-time (R=−0.280, p=0.03), k-time (R=−0.259, p=0.046), and α-angle (R=0.260, p=0.045, Table 4).
Table 4.
Correlation analysis between TEG parameters and clinical or laboratory parameters prior to bariatric surgery.
| BMI | CRP | ESR | Fibrinogen | Age | PLT | D-dimer | aPTT | INR | ||
|---|---|---|---|---|---|---|---|---|---|---|
| r-time | r | −.002 | .078 | .183 | −.103 | −.280 | .152 | −.121 | .299 | −.034 |
| P value | .990 | .562 | .161 | .446 | .030 | .246 | .384 | .020 | .795 | |
| k-time | r | −.115 | −.111 | .130 | −.313 | −.259 | .038 | −.113 | −.243 | −.097 |
| P value | .381 | .413 | .322 | .018 | .046 | .773 | .414 | .062 | .460 | |
| α angle | r | .047 | .048 | −.102 | .333 | .260 | −.028 | .117 | .124 | .114 |
| P value | .722 | .724 | .437 | .011 | .045 | .835 | .399 | .345 | .385 | |
| MA | r | .053 | .140 | .066 | .431 | .238 | .134 | .146 | .092 | .026 |
| P value | .686 | .299 | .615 | .001 | .067 | .308 | .293 | .486 | .847 | |
| G | r | .019 | .086 | .057 | .387 | .207 | .125 | .138 | .115 | .019 |
| P value | .884 | .526 | .665 | .003 | .112 | .341 | .321 | .382 | .887 |
r – Pearson correlation coefficient; r-time – reaction time (min); k-time – clotting time (min); α-angle (degrees); G – clot strength (dynes/cm2); MA – maximum amplitude (millimeters); BMI – body mass index; CRP – C-reactive protein; ESR – erythrocyte sedimentation rate; PLT – platelet count; aPTT – activated partial thromboplastin time; INR – international normalized ratio. Significant correlations and p-values are marker in bold.
Plasma fibrinogen levels show moderate predictive value for identifying individuals with G ≥11 dynes/cm2 or MA ≥68 mm
To evaluate the potential of plasma fibrinogen levels to predict hypercoagulability measured by TEG, we performed ROC curve analysis. Figure 1 shows that plasma fibrinogen level could predict clot strength (G) ≥11 dynes/cm2 with area under the curve (AUC)=0.680 (95% confidence interval (CI): 0.53–0.80, p=0.044). The best cutoff point for fibrinogen to predict G ≥11 was 3.97 g/l (sensitivity of 78.6% and specificity of 67.4%). ROC curve analysis of plasma fibrinogen levels to predict MA ≥68 mm produced an AUC of 0.679 (95% CI: 0.53–0.83, p=0.041, Figure 2). The best cutoff point for fibrinogen to predict MA ≥68 was 3.85 g/l, with sensitivity of 80.0% and specificity of 64.3% (Figure 2).
Figure 1.
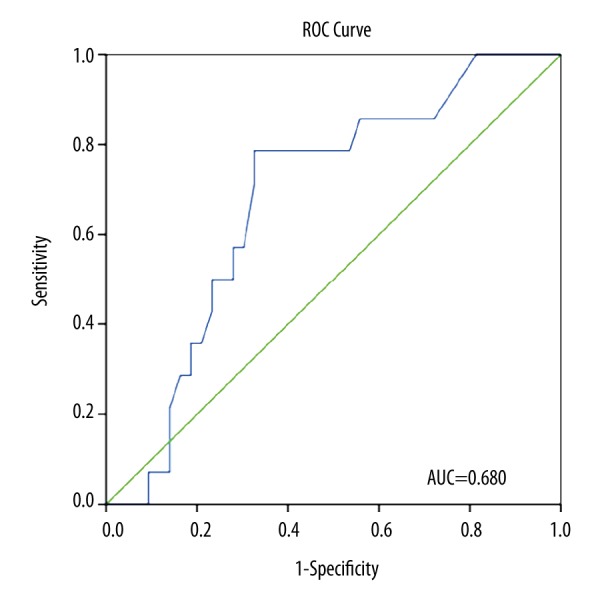
Relative operating characteristics (ROC) curve of plasma fibrinogen levels for prediction of G ≥11 dynes/cm2 (area under the curve [AUC]=0.680, 95% confidence interval [CI] 0.53–0.80, p=0.044). The optimal cutoff point for fibrinogen to predict G ≥11 was 3.97 g/l (sensitivity of 78.6%, specificity of 67.4%).
Figure 2.
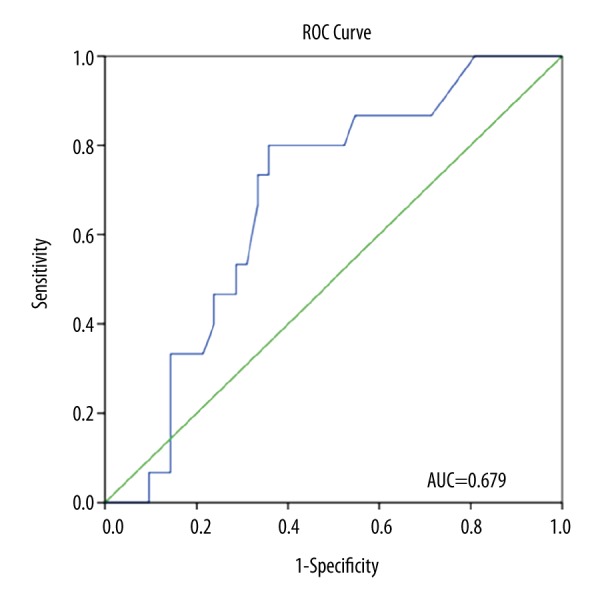
Receiver operating characteristics (ROC) curve of plasma fibrinogen levels for predicting MA ≥68 mm (area under the curve [AUC]=0.679, 95% confidence interval [CI] 0.53–0.83, p=0.041). The optimal cutoff point for fibrinogen to predict MA ≥68 was 3.85 g/l (sensitivity of 80.0%, specificity of 64.3%).
Discussion
The global increase in obesity rates has boosted the demand for bariatric surgery [22]. Much research has been targeted at establishment of optimal prophylactic anticoagulation regimens in bariatric surgery; however, a consensus over this strategy has not been achieved [7]. Although the incidence of thromboembolic complications in patients undergoing bariatric surgery is not very high, the mortality rates associated with PE in this subgroup of patients is striking [3]. Routine coagulation tests, including aPTT, INR, and platelet count, are not able to predict hypercoagulative status in the perioperative period [7]. Therefore, alternative testing and monitoring of coagulation status for identification of individuals at increased risk of VTE is needed.
In the present study we aimed to evaluate coagulation status in patients undergoing bariatric surgery and to assess dynamics of coagulation parameters in the perioperative setting using TEG. The major finding of our study is that many patients referred to bariatric surgery show a trend towards hypercoagulability on TEG. We used TEG cutoff values of G ≥11 dynes/cm2 and MA ≥68 mm to define hypercoagulability based on previous studies that have shown these thresholds to be linked with thrombotic events [15,17,21]. Our results also show that fibrinogen level is the only indicator of hypercoagulative state among the routinely used laboratory parameters; however, used alone, it is not able accurately predict hypercoagulative status. We believe that the results of our study add new insights into the monitoring of coagulation status in bariatric surgery.
An increasing number of studies show the importance of TEG for monitoring of coagulation status in the perioperative period [15,16]. The major finding of the present study is that many patients referred to bariatric surgery show hypercoagulability as determined by TEG in the perioperative setting. Based on results of previous studies, we used G ≥11 dynes/cm2 to define a hypercoagulable state [15,17]. Kashuk et al. found that strongest predictor of VTE in surgical patients was elevated G value; for every 1 dyne/cm2 increase in G, the odds of a VTE increased by 25% [15]. A significant proportion (23%) of our patients were in hypercoagulable state prior to surgery, which supports previous data obtained by ROTEM analysis [17]. It is important to point out that 18 patients (30%) also had G ≥11 at POD2. We routinely stop low molecular weight heparins (LMWH) on discharge and this group of patients might benefit from extended antithrombotic prophylaxis, but this remains to be assessed in further studies. Interestingly, we followed all our patients for clinically evident thrombotic complications for 1 year after the study and none of them had thrombotic events during the follow-up period.
The other TEG parameter that may be used to identify hypercoagulable states and to predict DVT in the postoperative period is MA [19]. Recent studies have also shown that MA parameter ≥68 mm is linked with increased risk of thrombotic complications and postoperative myocardial infarction [21]. It is worth pointing out that in our study 17 out 60 patients had MA ≥68 mm prior to surgery, but this proportion did not increase dramatically at POD2 (28.3% and 35.0%, respectively); however, Forfori et al. [18] showed a substantial (from 20% to 50%) increase in the percentage of patients with MA ≥68 mm by POD3. A potential reason why we did not observe a significant increase in the proportion of patients with MA ≥68 on POD2 could be the shorter overall surgery time (91.4±20.0 min) as compared to the Forfori et al. study [18], in which average duration of surgery was 120±37 min. Another reason might be related to the different regimens used for thrombosis prophylaxis. In our study, patients received dextran solution during induction of anesthesia. Dextran is known for its anti-platelet activity realized through reduction of platelet adhesion to von Willebrand factor and impairment of platelet activation by thrombin [23]. We also observed a significant increase in r-time from preoperative levels to POD2. These results indicate that a prophylactic regimen of 0.3 ml Fraxiparine qd started on POD1 seems to reduce activation of the coagulation system in the postoperative period, similar to the prophylaxis regimen based solely on Fraxiparine [18].
Pivalizza et al. were the first to show that morbidly obese patients have accelerated fibrin formation and higher MA values than lean patients referred to elective surgery, as determined by TEG [24]. In our study, the patients with clot strength G ≥11 dynes/cm2 had higher average plasma fibrinogen concentration than patients in the G <11 group, and these findings support previously published results [17]. We observed significant correlations between fibrinogen concentration and k-time, α-angle, MA, and G values as determined by TEG. To evaluate the potential of plasma fibrinogen levels to predict hypercoagulability measured by TEG, we performed ROC curve analysis. Plasma fibrinogen level predicted clot strength (G) ≥11 dynes/cm2 with an AUC=0.680 and the optimal cutoff point for fibrinogen to predict G ≥11 was 3.97 g/l. Similarly, ROC curve analysis of plasma fibrinogen levels to predict MA ≥68 mm resulted in AUC of 0.679 with an optimal cutoff point for fibrinogen at 3.85 g/l. Similar cutoff values of fibrinogen for prediction of hypercoagulability were observed in a Spanish study [17]. Our results show that fibrinogen level is the only indicator of hypercoagulative state among routinely used laboratory parameters; however, moderate AUC values obtained in the study should be reassessed in larger studies. It is worth pointing out that some studies have shown that hyperfibrinogenemia is a potential factor in heparin resistance, and these patients might need enhanced VTE prophylaxis regimens [20].
Obese patients have a higher prevalence of inherited and acquired thrombophilias compared to the general population [25]. Diabetes, which is closely linked to obesity, is also related to increased incidence of recurrent DVT [26]. A recent study has reported that the inflammation biomarker CRP was associated with hypercoagulable states evaluated by ROTEM in a group of patients referred to bariatric surgery [17]. In the present study we did not observe a significant correlation between TEG parameters and CRP or ESR levels. Several previous studies have shown that BMI increases the risk of VTE [27,28]. In the present study, BMI did not correlate with TEG values, which was also observed previously by a research group [17]. A recent study showed that central obesity, but now overall weight, is linked with increased thrombin generation [29] and is a better predictor of complications [17,28].
This study has certain limitations that need to be acknowledged. Firstly, it was not aimed at determining the optimal anticoagulation regimes for bariatric surgery, and that remains a challenging task in further studies. We have selected the TEG cutoff values based on some previous studies that have shown these thresholds to be linked with the risk of thrombotic events and used them in earlier studies: G ≥11 dynes/cm2 [15,17] and MA parameter MA ≥68 mm [21]; however, we agree that the prediction of prothrombotic states based on TEG results only is difficult and further research in the field is required. We also did not use Doppler ultrasound to monitor subclinical vein thrombosis; therefore, we might have missed some cases of obscure vein thrombosis in the follow-up. To date, it is not clear whether patients showing hypercoagulation parameters on TEG should be managed differently, and the optimal strategies for DVT prevention in this group have yet to be established in well-designed randomized clinical trials. Interestingly, some studies have suggested the need for extended anticoagulation regimens after bariatric surgery because some DVT occurs later after surgery [27]. We speculate that patients showing hypercoagulability on POD2 by TEG might benefit from receiving a prolonged prophylactic anticoagulation, but this needs to be assessed in further studies. Potentially, a combination of TEG results and other coagulation markers could provide more accurate prediction models, and this needs to be established in the future. Bariatric surgery is clearly associated with significant weight loss and will retain a major role in treatment of morbid obesity [26]. Furthermore, the balance between the risk of bleeding and risk of thrombotic complications remains very important, posing further challenges in the field.
Conclusions
A considerable proportion of patients referred to bariatric surgery show a trend towards hypercoagulability as determined by TEG. This study shows the potential of hypercoagulation monitoring by TEG in the perioperative setting of bariatric surgery. Plasma fibrinogen levels show moderate ability to predict hypercoagulation as determined by TEG.
Footnotes
Source of support: Departmental sources
References
- 1.Hughes V. Weight-loss surgery: A gut-wrenching question. Nature. 2014;511:282–84. doi: 10.1038/511282a. [DOI] [PubMed] [Google Scholar]
- 2.Stein PD, Matta F. Pulmonary embolism and deep venous thrombosis following bariatric surgery. Obes Surg. 2013;23:663–68. doi: 10.1007/s11695-012-0854-2. [DOI] [PubMed] [Google Scholar]
- 3.Jamal MH, Corcelles R, Shimizu H, et al. Thromboembolic events in bariatric surgery: A large multi-institutional referral center experience. Surg Endosc. 2014;29:376–80. doi: 10.1007/s00464-014-3678-4. [DOI] [PubMed] [Google Scholar]
- 4.Alsina E, Ruiz-Tovar J, Alpera MR, et al. Incidence of deep vein thrombosis and thrombosis of the portal–mesenteric axis after laparoscopic sleeve gastrectomy. J Laparoendosc Adv Surg Tech. 2014;24:601–5. doi: 10.1089/lap.2014.0125. [DOI] [PubMed] [Google Scholar]
- 5.Muneer M, Abdelrahman H, El-Menyar A, et al. Portomesenteric vein thrombosis after laparoscopic sleeve gastrectomy: 3 case reports and a literature review. Am J Case Rep. 2016;17:241–47. doi: 10.12659/AJCR.896892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikesaka R, Delluc A, Le Gal G, Carrier M. Efficacy and safety of weight-adjusted heparin prophylaxis for the prevention of acute venous thromboembolism among obese patients undergoing bariatric surgery: A systematic review and meta-analysis. Thromb Res. 2014;133:682–87. doi: 10.1016/j.thromres.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Bakirhan K, Strakhan M. Pharmacologic prevention of venous thromboembolism in obese patients. J Thromb Thrombolysis. 2013;36:247–57. doi: 10.1007/s11239-012-0844-1. [DOI] [PubMed] [Google Scholar]
- 8.Samama CM, Godier A. Perioperative deep vein thrombosis prevention: What works, what does not work and does it improve outcome? Curr Opin Anesthesiol. 2011;24:166–70. doi: 10.1097/ACO.0b013e328343cd4b. [DOI] [PubMed] [Google Scholar]
- 9.Samad F, Ruf W. Inflammation, obesity, and thrombosis. Blood. 2013;122:3415–22. doi: 10.1182/blood-2013-05-427708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campello E, Spiezia L, Zabeo E, et al. Hypercoagulability detected by whole blood thromboelastometry (ROTEM®) and impedance aggregometry (MULTIPLATE®) in obese patients. Thromb Res. 2015;135:548–53. doi: 10.1016/j.thromres.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Campello E, Zabeo E, Radu CM, et al. Hypercoagulability in overweight and obese subjects who are asymptomatic for thrombotic events. Thromb Haemost. 2015;113:85–96. doi: 10.1160/TH14-02-0156. [DOI] [PubMed] [Google Scholar]
- 12.Campello E, Zabeo E, Radu CM, et al. Dynamics of circulating microparticles in obesity after weight loss. Intern Emerg Med. 2016;11:695–702. doi: 10.1007/s11739-016-1397-7. [DOI] [PubMed] [Google Scholar]
- 13.Luddington RJ. Thrombelastography/thromboelastometry. Clin Lab Haematol. 2005;27:81–90. doi: 10.1111/j.1365-2257.2005.00681.x. [DOI] [PubMed] [Google Scholar]
- 14.Whiting D, DiNardo JA. TEG and ROTEM: technology and clinical applications. Am J Hematol. 2014;89:228–32. doi: 10.1002/ajh.23599. [DOI] [PubMed] [Google Scholar]
- 15.Kashuk JL, Moore EE, Sabel A, et al. Rapid thrombelastography (r-TEG) identifies hypercoagulability and predicts thromboembolic events in surgical patients. Surgery. 2009;146:764–72. doi: 10.1016/j.surg.2009.06.054. [DOI] [PubMed] [Google Scholar]
- 16.Mahla E, Lang T, Vicenzi MN, et al. Thromboelastography for monitoring prolonged hypercoagulability after major abdominal surgery. Anesth Analg. 2001;92:572–77. doi: 10.1097/00000539-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Taura P, Rivas E, Martinez-Palli G, et al. Clinical markers of the hypercoagulable state by rotational thrombelastometry in obese patients submitted to bariatric surgery. Surg Endosc. 2014;28:543–51. doi: 10.1007/s00464-013-3203-1. [DOI] [PubMed] [Google Scholar]
- 18.Forfori F, Ferro B, Mancini B, et al. Role of thrombolestagrophy in monitoring perioperative coagulation status and effect of thromboprophylaxis in bariatric surgery. Obes Surg. 2012;22:113–18. doi: 10.1007/s11695-011-0443-9. [DOI] [PubMed] [Google Scholar]
- 19.Dai Y, Lee A, Critchley LAH, White PF. Does thromboelastography predict postoperative thromboembolic events? A systematic review of the literature. Anesth Analg. 2009;108:734–42. doi: 10.1213/ane.0b013e31818f8907. [DOI] [PubMed] [Google Scholar]
- 20.Saner FH. Rotational thrombelastometry: A step forward to safer patient care? Crit Care. 2014;18:706. doi: 10.1186/s13054-014-0706-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCrath DJ, Cerboni E, Frumento RJ, et al. Thromboelastography maximum amplitude predicts postoperative thrombotic complications including myocardial infarction. Anesth Analg. 2005;100:1576–83. doi: 10.1213/01.ANE.0000155290.86795.12. [DOI] [PubMed] [Google Scholar]
- 22.Dietz WH, Baur LA, Hall K, et al. Management of obesity: Improvement of health-care training and systems for prevention and care. Lancet. 2015;385:2521–33. doi: 10.1016/S0140-6736(14)61748-7. [DOI] [PubMed] [Google Scholar]
- 23.Jones CI, Payne DA, Hayes PD, et al. The antithrombotic effect of dextran-40 in man is due to enhanced fibrinolysis in vivo. J Vasc Surg. 2008;48:715–22. doi: 10.1016/j.jvs.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Pivalizza EG, Pivalizza PJ, Weavind LM. Perioperative thromboelastography and sonoclot analysis in morbidly obese patients. Can J Anaesth. 1997;44:942–45. doi: 10.1007/BF03011965. [DOI] [PubMed] [Google Scholar]
- 25.Overby DW, Kohn GP, Cahan MA, et al. Prevalence of thrombophilias in patients presenting for bariatric surgery. Obes Surg. 2009;19:1278–85. doi: 10.1007/s11695-009-9906-7. [DOI] [PubMed] [Google Scholar]
- 26.Faber DR, de Groot PG, Visseren FLJ. Role of adipose tissue in haemostasis, coagulation and fibrinolysis. Obes Rev. 2009;10:554–63. doi: 10.1111/j.1467-789X.2009.00593.x. [DOI] [PubMed] [Google Scholar]
- 27.Raftopoulos I, Martindale C, Cronin A, Steinberg J. The effect of extended post-discharge chemical thromboprophylaxis on venous thromboembolism rates after bariatric surgery: a prospective comparison trial. Surg Endosc. 2008;22:2384–91. doi: 10.1007/s00464-008-0031-9. [DOI] [PubMed] [Google Scholar]
- 28.Livingston EH. Pitfalls in using BMI as a selection criterion for bariatric surgery. Curr Opin Endocrinol Diabetes Obes. 2012;19:347–51. doi: 10.1097/MED.0b013e328357f0b8. [DOI] [PubMed] [Google Scholar]
- 29.Beijers HJBH, Ferreira I, Spronk HMH, et al. Body composition as determinant of thrombin generation in plasma: The Hoorn study. Arterioscler Thromb Vasc Biol. 2010;30:2639–47. doi: 10.1161/ATVBAHA.110.211946. [DOI] [PubMed] [Google Scholar]


