Abstract
Previous studies revealed that Igf2 and Mpr/Igf2r are imprinted in eutherian mammals and marsupials but not in monotremes or birds. Igf2 lies in a large imprinted cluster in eutherians, and its imprinting is regulated by long-range mechanisms. As a step to understand how the imprinted cluster evolved, we have determined a 490-kb chicken sequence containing the orthologs of mammalian Ascl2/Mash2, Ins2 and Igf2. We found that most of the genes in this region are conserved between chickens and mammals, maintaining the same transcriptional polarities and exon–intron structures. However, H19, an imprinted noncoding transcript, was absent from the chicken sequence. Chicken ASCL2/CASH4 and INS, the orthologs of the imprinted mammalian genes, showed biallelic expression, further supporting the notion that imprinting evolved after the divergence of mammals and birds. The H19 imprinting center and many of the local regulatory elements identified in mammals were not found in chickens. Also, a large segment of tandem repeats and retroelements identified between the two imprinted subdomains in mice was not found in chickens. Our findings show that the imprinted genes were clustered before the emergence of imprinting and that the elements associated with imprinting probably evolved after the divergence of mammals and birds.
Genomic imprinting, a germ-line-specific epigenetic modification of the genome, causes parental-origin-specific expression of a small subset of genes (up to a few hundred) in eutherian mammals such as humans and mice (Tilghman 1999; Ferguson-Smith and Surani 2001; Reik and Walter 2001). The imprinted genes play crucial roles in embryonic development and growth regulation (Solter 1988; Surani et al. 1990; Cattanach and Beechey 1997). They also affect behavior and several disease phenotypes (Falls et al. 1999). However, the reasons for the evolution of imprinting is not well understood. Also, although CpG methylation is clearly involved in the imprinting processes (Li 2002; Kaneda et al. 2004), the precise mechanisms governing imprinting are yet to be elucidated.
Recent studies revealed that Igf2, a paternally expressed gene in eutherians, shows the same imprinted expression pattern in marsupials (such as opossums) (O'Neill et al. 2000). However, this gene is biallelically expressed in monotremes (such as platypuses and echidnas) and birds (such as chickens) (O'Neill et al. 2000; Nolan et al. 2001; Yokomine et al. 2001). Similarly, it was shown that Mpr/Igf2r, a gene located on a different chromosome, is maternally expressed in both eutherians and marsupials, but biallelically expressed in monotremes and birds (Killian et al. 2000; Nolan et al. 2001; Yokomine et al. 2001). These results are consistent with the conflict hypothesis of imprinting evolution (Moore and Haig 1991), which predicts that imprinting would be favored by viviparous animals.
A striking feature of the imprinted genes is their tendency to form clusters, which is most probably associated with the mechanisms of imprinting. Igf2 is contained in a large imprinted gene cluster (imprinted domain) on Chromosome 7 in mice and Chromosome 11 in humans (Reik and Maher 1997). The human domain is responsible for Beckwith-Wiedemann syndrome (BWS), an imprinting-associated fetal overgrowth syndrome. The domain is ∼1 Mb in size and contains 13 imprinted genes. Evidence indicates that the domain is composed of two subdomains, which are, respectively, regulated by an imprinting center (IC) (Leighton et al. 1995b; Caspary et al. 1998; Horike et al. 2000; Fitzpatrick et al. 2002). The sequence elements in the ICs and many local regulatory elements involved in the allele-specific expression of the genes are conserved between humans and mice (Ainscough et al. 2000; Bell and Felsenfeld 2000; Hark et al. 2000; Ishihara et al. 2000; Drewell et al. 2002).
In order to understand how the long-range imprinting mechanisms evolved during mammalian evolution, it is important to know the structural and functional properties of the orthologous region of nonimprinted vertebrate species. Chickens provide an excellent model for such a comparative study because they have been an important experimental system in many fields of biology including developmental biology. In addition to the easy access to the embryo, the increase in genomic resources is enabling chicken research to contribute to the functional analysis of the vertebrate genome (Brown et al. 2003).
Here we report the DNA sequence of a 0.5-Mb chicken region containing IGF2. This enables us for the first time to make large-scale structural and functional comparisons of an imprinted mammalian region with the orthologous region from an oviparous vertebrate species. We show that the chicken orthologs of Ascl2/Mash2 and Ins2 are not imprinted, supporting the idea that birds do not have genomic imprinting. We also show that most of the elements involved in imprinting in humans and mice are not present in chickens. The present work provides the basis to study and understand how an imprinted region evolved and how imprinting is regulated.
Results
Isolation and sequencing of chicken BAC clones
To isolate bacterial artificial chromosome (BAC) clones containing the chicken region orthologous to the imprinted Ascl2/Mash2–Igf2–H19 region, a White Leghorn BAC library (Hori et al. 2000) was screened with a PCR-amplified IGF2 probe (Yokomine et al. 2001). A total of six BAC clones were obtained (26D12, 90B1, 192C9, 283C3, 411E9, and 457F4). Based on the data from sequence-tagged site (STS) content analysis and restriction fingerprinting, the clones 26D12 and 192C9 were selected for large-scale sequencing (Fig. 1). To isolate clones extending to more 3′, an end probe was produced from clone 192C9 and used to rescreen the BAC library. Two clones were obtained (27B1 and 161D9). The clone 161D9 was subjected to sequencing because this clone contained the TNNT3/TNT (the ortholog for mouse Tnnt3) marker and thus should be longer than the other.
Figure 1.
Overview of the ASCL2/CASH4-IGF2 region on chicken Chromosome 5 and the orthologous region on mouse Chromosome 7. The locations of the chicken BAC clones that were sequenced in this study are shown as horizontal bars. The DDBJ/GenBank/EMBL accession numbers of the sequences are indicated in parentheses.
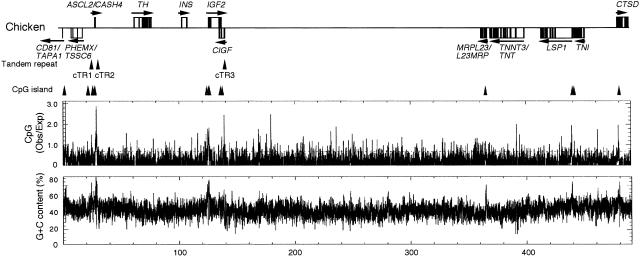
The chicken sequence of 490,074 bp contained eight previously reported genes/transcripts, ASCL2/CASH4, TH, INS, IGF2, CIGF, TNNT3/TNT, TNI, and CTSD (Fig. 2). Among these, TH, INS, IGF2, TNNT3/TNT, TNI, and CTSD were known to be the orthologs of mouse Th, Ins2, Igf2, Tnnt3, Tni, and Ctsd, respectively. CIGF was previously identified as an antisense transcript at the IGF2 locus (Taylor et al. 1991). ASCL2/CASH4, a member of the achaete–scute gene family (Henrique et al. 1997), was now revealed to be the ortholog of mouse Ascl2/Mash2, a gene essential for placental development (Guillemot et al. 1994). In addition to these eight genes/transcripts, four genes orthologous to mouse Cd81/Tapa1, Phemx/Tssc6, Mrpl23/L23mrp, and Lsp1, respectively, were identified by data analysis (see below) (Fig. 2).
Figure 2.
Overall structure and gene arrangement of the chicken region. Arrangement of genes (boxes) is illustrated at the top of each panel. Horizontal arrows or arrowheads above or below the genes indicate directions of transcription. Upward arrowheads indicate positions of CpG islands and tandem repeats. Observed/Expected ratio of CpG dinucleotide per 100 bp and G+C content per 100 bp are graphically shown under the gene arrangement.
We recently reported a 0.6-Mb mouse sequence corresponding to this sequence (Shirohzu et al. 2004). Comparisons of the two sequences revealed that the gene order and transcriptional polarities are conserved except that two genes/transcripts are missing in chickens (see below). The gene distance, however, varied greatly between the two species. For example, a chicken region spanning from CD81/TAPA1 to IGF2 (∼140 kb) was three times smaller than the orthologous region in mice (Figs. 1 and 2). The more compact organization of the chicken region was due to shorter intergenic distances. The absence of the 210-kb region composed of tandem repeats and retroelements, which was found between Th and Ins2 in mice (Shirohzu et al. 2004), was another reason for the more compact structure. In contrast, the intergenic region between chicken IGF2 and MRPL23/L23MRP (∼223 kb) was twice as large as the corresponding region in mice.
Identification and characterization of chicken CD81/TAPA1, PHEMX/TSSC6, MRPL23/L23MRP, and LSP1
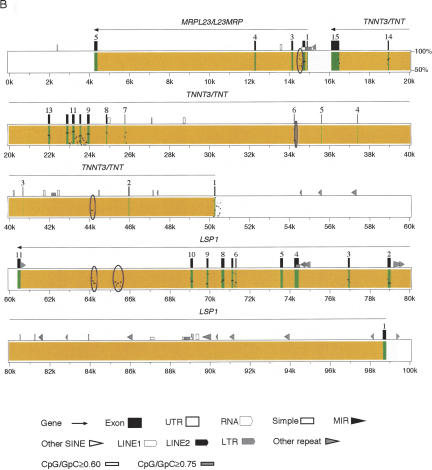
Chicken CD81/TAPA1, PHEMX/TSSC6, MRPL23/L23MRP, and LSP1 were identified by making use of BLAST, GENSCAN, and a large-scale sequence comparison program, PipMaker (Schwartz et al. 2000). Figure. 3 shows the results of PipMaker analyses, which were particularly useful in detecting the new genes. The percent identity plot studies highlighted the well-conserved exon regions in the chicken sequence.
Figure 3.
Sequence comparison between chickens and mice from CD81/TAPA1 to IGF2 (A) and from MRPL23/L23MRP to LSP1 (B). Percent identity plot analysis was done using the PipMaker software using the chicken sequence as a reference. The order and arrangement of the chicken genes are shown at the top. The dots and short horizontal bars correspond to the segments of sequence conservation. The regions corresponding to the exons and introns of the chicken genes are shown in green and yellow, respectively. Conserved sequences in the noncoding regions, including those in introns, are circled.
One of the genes newly identified was CD81/TAPA1, a gene encoding a member of the transmembrane-4 superfamily. A 105-bp region located at ∼2.2 kb from the 5′-end of our chicken sequence showed a 78.2% similarity with the exon 1 sequence of mouse Cd81/Tapa1. This chicken exon contained an initiation codon and an open reading frame, which codes for the first 22 amino acids (MGVEGCTKCIKYLLFVFNFVFN) of the presumed chicken CD81/TAPA1 protein (GenBank accession no. AB101638). The amino acid sequence was identical with that of the N-terminal part of human CD81/TAPA1, but there was one amino acid difference (20V to I) when compared with the mouse protein. We then examined the tissue distribution of CD81/TAPA1 transcripts by RT-PCR and found that it is expressed ubiquitously just as in mice (Andria et al. 1991; data not shown).
Six evolutionarily conserved DNA segments were identified in the 8–25-kb region of the chicken sequence (Fig. 3). The segments were 54.8%–64.9% identical in sequence with exons 2, 3, 4, 5, 6, and 7 of mouse Phemx/Tssc6, a transcript with potential tumor suppressor activity (Paulsen et al. 2000). We then examined the presence of the transcripts in chicken tissues by RT-PCR using two primer pairs. With the primers for exons 2 and 7, four PCR products were detected in all embryonic stages and adult tissues that we examined (Fig. 4A). The largest and most abundant product (isoform 1) contained all the predicted exons (GenBank accession no. AB101639) (Fig. 4B). This transcript species had a single open reading frame, and its deduced amino acid sequence exhibited 45.6% and 42.2% identity with the human and the mouse PHEMX/TSSC6 protein, respectively (Fig. 4C). The second largest product (isoform 2) lacked the exon 6 sequence, the third one (isoform 3) lacked exons 4 and 6, and the smallest one (isoform 4) lacked exons 4, 5, and 6 (Fig. 4A). None of these alternative-splicing events would cause a frameshift (Fig. 4B). With the primers for exons 3 and 6, two other alternative splicing products were identified (isoforms 5 and 6). Both of them lacked exon 5, but one (isoform 5) contained an additional 68-bp sequence from intron 3 (exon 3′) (Fig. 4A). This insertion was predicted to cause a frameshift resulting in aberrant amino acids and stop codons within the exon 4 region. Although multiple splicing variants have also been observed for mouse Phemx/Tssc6 (Paulsen et al. 2000), the one corresponding to isoform 5 has not been reported.
Figure 4.
Structure and expression of chicken PHEMX/TSSC6. (A) RT-PCR analysis of chicken PHEMX/TSSC6 in whole chick embryos and adult tissues. GAPD was used as a housekeeping control. Splicing variants, revealed by sequencing of the PCR products, are shown (isoforms 1–6). (B) Nucleotide sequence and predicted amino acid sequence of chicken PHEMX/TSSC6 cDNA (isoform 1; GenBank accession no. AB101639). Arrowheads indicate the positions of exon–intron boundaries. (C) Alignment of the predicted amino acid sequences of human (GenBank accession no. AF125569), mouse (GenBank accession no. AJ279791), and chicken PHEMX/TSSC6. Amino acids conserved in more than two species are shaded.
The chicken ortholog of mammalian Mrpl23/L23mrp, a gene encoding a putative mitochondrial ribosomal protein (Tsang et al. 1995; Zubair et al. 1997), was located downstream of TNNT3/TNT. This gene consisted of five exons as its mammalian ortholog. The predicted protein product of the chicken MRPL23/L23MRP cDNA (GenBank accession no. AB101640) was 154 amino acids in size and displayed 58.0% and 54.0% identity with the human and the mouse ortholog, respectively (Fig. 5A,B). RT-PCR analyses showed that the chicken gene is ubiquitously expressed just as its mammalian orthologs (Tsang et al. 1995; Zubair et al. 1997; data not shown).
Figure 5.
Structure of chicken MRPL23/L23MRP. (A) Nucleotide sequence and predicted amino acid sequence of chicken MRPL23/L23MRP cDNA (GenBank accession no. AB101640). Arrowheads indicate the positions of exon–intron boundaries. (B) Alignment of the predicted amino acid sequences of human (GenBank accession no. BC027710), mouse (GenBank accession no. U84902), and chicken MRPL23/L23MRP.
Chicken LSP1 was identified upstream of TNNT3/TNT at a position orthologous to mammalian Lsp1. Mouse Lsp1 codes for a lymphocyte-specific calcium-binding protein with unknown function (Jongstra et al. 1988) and was previously mapped upstream of Tnnt3 (Misener et al. 1998). The chicken gene consisted of 11 exons as the mammalian ortholog. The 5′-end of the gene was tentatively assigned based on an expressed sequence tag (EST) sequence (riken1 8h20r1), which was assumed to be a full-length cDNA, from the BursaEst Database (http://swallow.gsf.de/dt40est.html). Exons 10 and 11, which code for the 3′-untranslated sequences, were predicted based on another EST sequence (GenBank accession no. AI979962). RT-PCR primers were designed according to these EST sequences, and the middle part of the cDNA was amplified and sequenced. The predicted protein product of the chicken LSP1 cDNA (GenBank accession no. AB101641) was 318 amino acids in size and displayed 34.8% and 36.5% identity with the human and the mouse ortholog, respectively (Fig. 6A,B). RT-PCR analyses revealed that chicken LSP1 is highly expressed in lymphoid tissues such as the spleen (data not shown). Expression was also detectable, however, at a comparable level in the ovary and at a lower level in the lung.
Figure 6.
Structure of chicken LSP1. (A) Nucleotide sequence and predicted amino acid sequence of chicken LSP1 cDNA (GenBank accession no. AB101641). Arrowheads indicate the positions of exon–intron boundaries. (B) Alignment of the predicted amino acid sequences of human (GenBank accession no. NM002339), mouse (GenBank accession no. NM019391), and chicken LSP1.
Chicken ASCL2/CASH4 and INS are not imprinted
Although it has been shown that chicken IGF2 is not imprinted (O'Neill et al. 2000; Nolan et al. 2001; Yokomine et al. 2001), the imprinting status of the other genes in this region is unknown. ASCL2/CASH4 is of special interest because its mouse ortholog Ascl2/Mash2 is imprinted to be silent on the maternal chromosome (Guillemot et al. 1995). However, its human ortholog ASCL2/HASH2 does not appear to be imprinted (Miyamoto et al. 2002). INS is another interesting gene because its mouse and human orthologs are imprinted in a tissue-specific way: they are expressed from the paternal allele in the yolk sac but expressed from both alleles in the pancreas (Giddings et al. 1994; Moore et al. 2001).
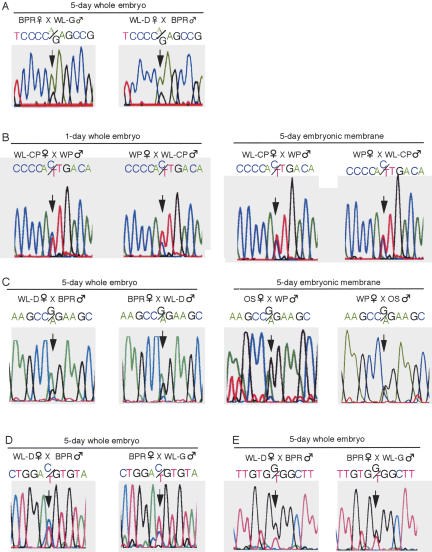
To study the allelic expression status of ASCL2/CASH4 and INS, as well as PHEMX/TSSC6 and TH, of which orthologs are not imprinted in mammals, we looked for single nucleotide polymorphisms (SNPs) in their transcribed regions that can be used to trace parental origin of the alleles. Comparisons of the PCR-amplified genomic sequences from six different chicken lines uncovered at least three SNPs for each gene (Table 1). The chicken lines with different SNP genotypes were reciprocally crossed, and the RT-PCR products from resulting embryos were sequenced. We first confirmed the biallelic expression of IGF2 in four informative embryos recovered at day 5 of development (5-d embryos) using the SNP identified previously (Fig. 7A; Yokomine et al. 2001). We then found that ASCL2/CASH4 is biallelically expressed in nine 1-d embryos (stage 5–6) and in the embryonic membranes (chorion, allantoic membrane, and yolk sac) of 14 5-d embryos (Fig. 7B). We also demonstrated biallelic expression of INS, PHEMX/TSSC6, and TH in all informative 5-d embryos (6, 11, and 8 embryos, respectively) (Fig. 7C–E). Biallelic expression of INS was also detected in the embryonic membranes, including the yolk sac, of nine 5-d embryos (Fig. 7C). Thus, all genes examined here, including ASCL2/CASH4 and INS, were not imprinted, although we cannot exclude the possibility that they are imprinted in limited tissues of the embryos or at other developmental stages.
Table 1.
DNA polymorphisms
| Gene | Location | Polymorphism (positiona) | Line or breedb |
|---|---|---|---|
| PHEMX/TSSC6 | Exon 4 | A→G (11,747) | BPR, WL-D, WL-G, WL-HA |
| Exon 3′ | T→C (21,082) | BPR, WL-HA | |
| C→T (21,098) | OS | ||
| ASCL2/CASH4 | Exon 1 | C→T (29,316) | BPR, WL-Cornell-P, WL-G |
| A→G (29,365) | BPR, WL-Cornell-P, WL-G | ||
| C→T (29,406) | BPR, WL-Cornell-P, WL-G | ||
| G→A (29,429) | BPR, OS | ||
| A→G (29,450) | BPR, OS, WL-Cornell-P, WL-G | ||
| C→T (29,534) | BPR, WL-G | ||
| T→C (29,563) | BPR, WL-Cornell-P, WL-G | ||
| T→C (29,570) | BPR, OS | ||
| TH | Exon 13 | C→T (86,656) | BPR |
| G→T (86,744) | BPR, OS | ||
| G→A (86,848) | WL-Cornell-P | ||
| INS | Exon 2 | C→T (106,014) | OS |
| G→A (106,015) | BPR, WL-Cornell-P, WL-HA | ||
| G→T (106,047) | OS |
The nucleotide position number is according to AP003796
(BRP), Barred Plymouth Rock; (OS), Oh-Shamo; (WL-Cornell-P), Cornell-P line of White Leghorn; (WL-D), D line of White Leghorn; (WL-G) G line of White Leghorn; (WL-HA), HA line of White Leghorn
Figure 7.
Imprinting status of chicken IGF2 (A), ASCL2/CASH4 (B), INS (C), and the control genes PHEMX/TSSC6 (D) and TH (E). Biallelic expression was revealed in informative embryos and embryonic membranes including the yolk sac by RT-PCR and sequencing. The SNPs used to distinguish the parental alleles were 1242A/G for IGF2 (Yokomine et al. 2001), 29365A/G for ASCL2/CASH4, 106015G/A for INS, 11747A/G for PHEMX/TSSC6, and 86744G/T for TH. In B and D, the data for the other strand are shown. Three additional SNPs confirmed the biallelic expression of ASCL2/CASH4 (data not shown).
Absence of H19 and Nctc1 in chickens
In eutherian mammals, a maternally expressed imprinted transcript, H19, is located between Igf2 and Mrpl23/L23mrp, at about one-third of the way from Mrpl23/L23mrp (Figs. 1 and 2). H19 does not code for a protein but is expressed at high levels in a wide array of mesodermal and endodermal tissues. Approximately 17 kb 3′ to mouse H19, another noncoding transcript called Nctc1 is present (Ishihara et al. 1998). Nctc1 is expressed in the skeletal muscle and shows biallelic expression. No sequence orthologous to H19 or Nctc1 was found in the chicken sequence. In fact, no transcript or exon-like sequence was found in the 223-kb chicken region between IGF2 and MRPL23/L23MRP by BLAST search for ESTs or GENSCAN search for exons. While Nctc1 is not conserved even in mammals (e.g., humans do not have this transcript), the existence of chicken H19 was previously suggested by a zoo blot analysis (Brannan et al. 1990). The possibility that chickens have H19 somewhere else in the genome remains to be investigated.
G+C content and CpG islands
It has been proposed that the imprinted genes of this domain tend to possess two or more CpG islands upstream of and/or within the gene (Onyango et al. 2000). The base composition and CpG frequency of our chicken sequence were analyzed by the computer software cpgplot (Larsen et al. 1992). The G+C content was not much different from that of the corresponding mouse sequence (46.5% vs. 47.1%), both of which were above the average G+C content of the vertebrate genomes (∼40%). Thirteen CpG islands were identified in the chicken sequence (Fig. 2; Table 2), according to the criteria by Gardiner-Garden and Frommer (1987). Among these, three were associated with ASCL2/CASH4 and five with IGF2. Thus, the multiple CpG island rule may also apply to the chicken orthologs, and it is clear that this is not sufficient for imprinting.
Table 2.
CpG islands
| Gene | Location | Length (bp) |
|---|---|---|
| CD81/TAPA1 | Intron 1 | 206 |
| ASCL2/CASH4 | Upstream | 304 |
| Upstream | 773 | |
| Upstream ∼ exon 1 | 551 | |
| IGF2 | Upstream | 223 |
| Upstream | 906 | |
| Upstream | 321 | |
| Intron 1 | 252 | |
| Exon 3 | 363 | |
| MRPL23/L23MRP | Upstream ∼ exon 1 | 234 |
| LSP1 | Intron 1 | 491 |
| Intron 1 | 312 | |
| CTSD | Upstream ∼ exon 1 | 442 |
Tandem repeats
Association with tandem repeats has been suggested to be a feature of mammalian imprinted genes (Neumann et al. 1995). Indeed, tandem repeats have been shown to be associated with functional imprinting at the mouse Impact and Rasgrf1 loci (Okamura et al. 2000; Yoon et al. 2002).
A program called Tandem Repeat Finder (Benson 1999) was used to identify tandem repeats. We identified three tandem repeats (cTR1–cTR3) with a unit size of 6 bp or more (Fig. 2; Table 3). The repeat cTR3, a 14-time repetition of a 18-bp sequence, was located in the 3′-flanking region of IGF2. The repeats cTR1 and cTR2 were located in the 5′-flanking region and the 3′ untranslated region, respectively, of ASCL2/CASH4: The repeat cTR1 was a 26-time repetition of a 16-bp sequence, and cTR2 was a 29-time repetition of a 6-bp sequence (Table 3). Thus, although repeats identical or very similar to cTR1–cTR3 were not present in mice, the orthologs of imprinted genes tended to possess tandem repeats.
Table 3.
Tandem repeats
| Position | Unit size (bp) | Consensus sequence | Copy number | ||
|---|---|---|---|---|---|
| Chicken | cTR1 | 26,747–27,120a | 16 | TGGCCATGGGGTTGAG | 26 |
| cTR2 | 29,282–29,776a | 6 | TGGGGT | 29 | |
| cTR3 | 139,920–140,195a | 18 | AGC(G/A)TG(G/A)TGGCCTCCATC | 14 |
The nucleotide position numbers are according to AP003796
We recently reported that a 210-kb region between mouse Th and Ins2 contains numerous tandem repeats, which could serve either as a boundary between the two imprinted subdomains or as a target for epigenetic chromatin modifications leading to imprinting (Shirohzu et al. 2004). No such tandem repeat was detected in our chicken sequence.
The IC and differentially methylated regions (DMRs)
The key regulatory elements involved in imprinting are likely to be conserved among imprinted species but may be absent from nonimprinted species. Our results that the chicken genes of the region are not imprinted provided a unique opportunity to investigate this possibility.
Previous studies showed that a 2.0-kb differentially methylated region (DMR) located 1.2 kb upstream of H19 serves as an IC: it is essential for both silencing of paternal H19 and silencing of maternal Igf2 (Thorvaldsen et al. 1998). This IC contains multiple binding sites for CTCF, a methylation-sensitive factor that mediates chromatin insulator activity (Bell and Felsenfeld 2000; Hark et al. 2000). Another putative CTCF-dependent insulator, which may serve as an imprinted/nonimprinted boundary, was identified between H19 and Mrpl23/L23mrp (Ishihara and Sasaki 2002). We therefore scanned the chicken sequence for potential CTCF sites, from IGF2 to MRPL23/L23MRP. With the consensus sequence that previously identified putative insulators (Chao et al. 2002; Ishihara and Sasaki 2002), we found no potential CTCF sites in this chicken region.
Mouse Igf2 has three DMRs, two of which are paternally methylated: DMR1 upstream of the fetal promoter contains a GCF2-binding site and acts as a methylation-sensitive silencer (Constancia et al. 2000; Eden et al. 2001); DMR2 in the last exon contains a methylation-sensitive activator (Murrell et al. 2001). The function of the maternally methylated DMR0 at the placenta-specific promoter is currently unknown. Despite our careful examination, chicken IGF2 did not have a sequence similar to DMR0 or DMR1. However, since the 54-bp core of DMR2 is located within the protein-coding region of the last exon (Murrell et al. 2001), we were not surprised to find that it is conserved in chickens (64.8% nucleotide identity). Among the eight differentially methylated CpGs within the core, four were conserved in chickens.
Methylation status of the region corresponding to DMR2
Since the 54-bp core of Igf2 DMR2 was the only DMR conserved in chickens, we were interested to know the methylation status of the chicken sequence. By bisulfite methylation analysis, we found that the overall methylation level at seven CpG sites (among which four were conserved) of the region is 58% (65/112), 62.5% (70/112), and 87.1% (122/140) in 5-d whole embryos (where allelic expression of IGF2 was studied) (Fig. 7A), 7-d whole embryos and peripheral blood, respectively (Fig. 8). Although we were not able to distinguish between the parental alleles because of the lack of SNPs, most of the sites were rather uniformly methylated or unmethylated (Fig. 8), suggesting that there is no allelic bias in methylation.
Figure 8.
Bisulfite methylation analysis of the chicken IGF2 region homologous to the 54-bp core of the mouse DMR2. CpG dinucleotides are underlined. The mouse CpG sites are numbered according to Murrell et al. (2001). Filled circles indicate methylated sites and open circles unmethylated sites. There is no indication for allelic difference in DNA methylation.
Other local regulatory elements
At about 40 kb 3′ to mouse Igf2, there is a conserved segment called A6A4, which contains two DNaseI-hypersensitive sites (Koide et al. 1994). A targeted deletion of this segment has led to biallelic Igf2 expression in the brain and a relaxation of Igf2 imprinting in the skeletal muscle, suggesting a tissue-specific silencer activity (Ainscough et al. 2000; Jones et al. 2001). Also, two DNA segments, termed H19 upstream conserved regions (HUCs), have been shown to act as enhancers in a range of mesodermal tissues (Drewell et al. 2002). However, we were not able to identify sequences similar to these in the chicken sequence between IGF2 and MRPL23/L23MRP.
We previously identified 10 conserved noncoding segments (CS1–10), which are located between H19 and Mrpl23/L23mrp (Ishihara et al. 2000). It was shown by transgenic assays that seven of them possess tissue-specific enhancer activities (Ishihara et al. 2000). In fact, two were identical with the previously reported endoderm-specific enhancers shared by Igf2 and H19 (Leighton et al. 1995b). Although we attempted to identify sequences similar to the enhancers in the chicken IGF2–MRPL23/L23MRP region, no significant homology was detected. The tissue-specific enhancers in chickens, if present, may be much diverged from those in the mammalian species.
Conserved noncoding sequences
Having established that most regulatory elements identified in mammals are not conserved in chickens, we asked whether there are any noncoding homologies (excluding the promoters) between mice and chickens. A PipMaker analysis revealed several conserved noncoding sequences (Fig. 3). Table 4 summarizes those that showed highest sequence identities (>50%) over 50 bp.
Table 4.
Conserved noncoding sequences
| Gene | Location | Size (bp) | Size in mouse (bp) | Identity (%) |
|---|---|---|---|---|
| ASCL2/CASH4 | Downstream 10 kb | 115 | 119 | 68.3 |
| Downstream 15 kb | 51 | 52 | 75.0 | |
| Downstream 18 kb | 179 | 167 | 66.7 | |
| MRPL23/L23MRP | Intron 2 | 175 | 182 | 58.0 |
| TNNT3/TNT | Intron 2 | 64 | 64 | 65.6 |
| Intron 5 | 90 | 87 | 60.0 | |
| LSP1 | Intron 10 | 327 | 303 | 51.6 |
| Intron 10 | 154 | 161 | 64.6 |
There were three conserved noncoding sequences in the intergenic region between ASCL2/CASH4 and TH (Fig. 3; Table 4). The sequences were 51 bp to 179 bp in size, and their identity to the corresponding mouse sequence ranged from 66.7% to 75.0%. The findings suggest that ASCL2/CASH4, TH, or both are regulated by evolutionarily conserved distant control elements. Conserved noncoding sequences were also identified in introns (Fig. 3; Table 4). MRPL23/L23MRP had a conserved 175-bp sequence in intron 2, which showed 58.0% identity with the corresponding 182-bp mouse sequence. TNNT3/TNT had two conserved intronic sequences: a 64-bp sequence in intron 2 was 65.6% identical with the corresponding mouse sequence; a 90-bp sequence in intron 5 was 60.0% identical with its mouse homolog. LSP1 had two conserved sequences in intron 10: a 327-bp and a 154-bp sequence, which, respectively, showed 51.6% and 64.6% identity with their mouse homolog. All these intronic similarities are most probably involved in the regulation of the relevant genes.
Discussion
We have determined the sequence of a 0.5-Mb chicken region orthologous to the imprinted Ascl2/Mash2–Igf2–H19 cluster on mouse distal Chromosome 7 and on human Chromosome 11 (Reik and Maher 1997). The region was previously mapped to chicken Chromosome 5 by fluorescent in situ hybridization (Yokomine et al. 2001). The determined sequence contained eight previously reported genes/transcripts (ASCL2/CASH4, TH, INS, IGF2, CIGF, TNNT3/TNT, TNI, and CTSD) and four newly identified genes (CD81/TAPA1, PHEMX/TSSC6, MRPL23/L23MRP, and LSP1). Comparisons between the chicken and mouse sequences revealed that most of the genes are conserved between the two species, with notable exceptions of H19 and Nctc1. The conserved genes maintain the same gene order, exon–intron structures, and transcriptional polarities.
Among the conserved genes, ASCL2/CASH4 was of special interest since its mammalian ortholog Ascl2/Mash2 is only expressed in diploid trophoblast cells and required for development of the placenta (Guillemot et al. 1994). Chicken ASCL2/CASH4 is most probably involved in neural development (Henrique et al. 1997), and thus the gene provides a good example of functional diversification during vertebrate evolution. The role in neural development is not unexpected because the members of this basic helix–loop–helix transcription factor family are often involved in neural development in many species.
Having identified the genes/transcripts of the chicken region, we examined whether the orthologs of the mammalian imprinted genes show imprinted expression patterns. We found that chicken ASCL2/CASH4 and INS are not imprinted in developing embryos or in the embryonic membranes, including the yolk sac. Together with the previous data that chicken IGF2 and MPR1 are not imprinted (O'Neill et al. 2000; Nolan et al. 2001; Yokomine et al. 2001), our findings support the idea that genomic imprinting does not exist in chickens. This is consistent with the conflict theory (Moore and Haig 1991), or the kinship theory (Wilkins and Haig 2003), which states that imprinting evolved as a result of conflicting interests between the paternal and maternal genes over allocation of maternal resources to the offspring. The theory predicts that imprinting would be more favored by viviparous species than oviparous species.
Thus, the sequence reported here for the first time enabled us to compare in detail a mammalian imprinted region with its orthologous region from a nonimprinted vertebrate species. The presence of the same gene cluster in the imprinted and nonimprinted species suggests that the imprinting of the genes in this cluster evolved on a domain basis, but not on a gene-by-gene basis. Several sequence elements involved in imprinting and long-distance regulation of the cluster have been identified in mice and humans. We found that such elements of the region, including the H19 IC, DMRs, and HUCs, are not conserved in chickens. The only exception was the 54-bp core of Igf2 DMR2, which is a part of the protein-coding region, but it did not show allele-specific differential methylation in chickens. Thus, it is likely that the IC and other local regulatory elements involved in the imprinted expression of the genes evolved after the diversification of mammals and birds.
In this connection, it is interesting that not only the H19 IC but also H19 itself was not found in the chicken sequence. Mammalian H19 encodes no protein, and a germ-line deletion of mouse H19 and its IC showed that their sole function may be to imprint Igf2 and Ins2 (Leighton et al. 1995a). It is therefore tempting to speculate that H19 and the linked IC were acquired by horizontal gene transfer in an ancestral species of mammals and caused imprinting. A possible link between parasitic DNA and imprinting was discussed previously (Barlow 1993; Yoder et al. 1997). Alternatively, the common ancestor of mammals and birds might have had this gene. Then this nonessential gene was lost in birds while it acquired a new function (imprinting) in mammals.
It is also noteworthy that chickens lack the large cluster of tandem repeats and retroelements, which we recently identified in the mouse genome between Th and Ins2 (Shirohzu et al. 2004). Approximately 46% of the 210-kb mouse region consists of retroelements such as LINE-1 and IAP with 30% of the remaining being tandem repeats and, despite the heterochromatin-like sequence composition, this region shows asynchronous replication between the parental chromosomes. Whether this repeat-rich region has any biological function remains to be tested, but the presence of a similar retroelement-rich region in humans (between ASCL2/HASH2 and TH) and the lack of its equivalence in chickens suggest a functional correlation.
Recently, Walter and Paulsen (2003) found that many imprinted genes, including those in the BWS cluster, have imprinted as well as nonimprinted paralogs, which are often linked to the other imprinted clusters. This finding has led them to propose that duplications as well as translocations and transpositions dispersed the imprinted genes and clusters throughout the genome. Since we showed that the same gene cluster is conserved in mammals and chickens, such dynamic events that affect the gene arrangement must have occurred prior to the emergence of imprinting. It is likely that many regulatory elements, noncoding RNA genes, and retroelements were then brought into the cluster during early mammalian evolution and eventually caused imprinting in a common ancestor of eutherians and marsupials.
Methods
Isolation and sequencing of BAC clones
Chicken BAC clones 26D12 and 192C9 were obtained from a White Leghorn library (Hori et al. 2000) with a PCR-amplified probe from the last coding exon of chicken IGF2 (primers: 5′-GAGAGCTTCCAGAAGCCATCTC-3′ and 5′-GCCCAACTGTCCCTTCGTAAGT-3′) (Yokomine et al. 2001) by colony hybridization. Then a 192C9-end STS (primers: 5′-CATGAGAAGTGACTTTCTGAAGCC-3′ and 5′-CCTGTCCCTGTGTTGCAGATGAG-3′) was used as a probe to isolate 27B1 and 161D9. Chicken STS primers used to assess the STS content of the BAC clones were TH, 5′-AGAGGACTGGCTTCCAGCTCCG-3′ and 5′-CTGGGAGAACTGGGCAAACGTCT-3′; INS, 5′-GGCTCTCTGGATCCGATCAC-3′ and 5′-GGCTGCTCGACATCCCGTCG-3′; TNNT3/TNT, 5′-GAGGAAAAGGCACGGAGAGAGG-3′ and 5′-CTTTGCCAGATAGCTGCTGTATGA-3′. A combined shotgun (Fleischmann et al. 1995) and nested-deletion (Hattori et al. 1997) strategy was adopted to sequence the BAC inserts as described (Hattori et al. 2000). Sequence data were assembled by Phred-Phrap and Sequencher software (Gene Codes).
Sequence data analysis
Database homology search and gene predictions were performed with BLAST (http://www.ncbi.nlm.nih.gov/BLAST/) and GENSCAN (http://genes.mit.edu/GENSCAN.html), respectively. CpG islands were predicted by using cpgplot (http://bioweb.pasteur.fr/seqanal/interfaces/cpgplot.html). Large-scale DNA sequence alignment was performed with Advanced PipMaker (http://nog.cse.psu.edu/pipmaker/). To eliminate spurious matches resulting solely from low and high complexity repeats, we masked the reference sequence using RepeatMasker (http://repeatmasker.genome.washington.edu/cgi-bin/RepeatMasker) before performing the PipMaker analysis. Tandem Repeats Finder (http://c3.biomath.mssm.edu/trf.html) was used to identify tandem repeats in the sequence.
RT-PCR
For tissue distribution analysis, alternative splicing analysis, and allelic expression analysis, reverse transcription of total RNA from whole chick embryos and adult tissues was performed using M-MLV reverse transcriptase (GIBCO BRL) according to the manufacturer's protocol. PCR was carried out under the following conditions: 30 cycles of 94°C for 30 sec, appropriate annealing temperature for 30 sec, and 72°C for 30 sec, with a final extension at 72°C for 5 min. The annealing temperature was 65°C for CD81/TAPA1, 70°C for PHEMX/TSSC6, 68°C for TH, 65°C for MRPL23/L23MRP and 60°C for LSP1. The primers used were CD81/TAPA1, 5′-TTGGCCTCAGACCGGGAGCTG-3′ and 5′-GATGAAGTTGAAGACGAAGAGCAG-3′; PHEMX/TSSC6 exons 2/7, 5′-TTTCTACGCCGGCGTCTTCCTCA-3′ and 5′-CTTCCTCGCAAGGTGTATTTGCCT-3′; PHEMX/TSSC6 exons 3/6, 5′-TTTGCTCTGGCCTTCTGTGGGATG-3′ and 5′-CGTGGAGACAAAGCCCATGTGCTT-3′; TH, 5′-TGTGTCTGAGAGCTTCAGTGATGC-3′ and 5′-GAAGCTGGCTTTCAGTAAAGCAGG-3′; MRPL23/L23MRP, 5′-ACCCCTTGTACCAGCTGGGTGG-3′ and 5′-AACTGCACGGTGTCCTCAGGCT-3′; LSP1, 5′-ATTGCTCCAGTCTGTCATCTATC-3′ and 5′-GCAGAGGAGGGCTTCAATGGCA-3′. ASCL2/CASH4 and INS cDNAs were amplified by nested PCR. First PCR was carried out under the following condition: 10 cycles of 94°C for 30 sec, appropriate annealing temperature for 30 sec, and 72°C for 30 sec, with a final extension at 72°C for 5 min. The annealing temperature was 68°C for ASCL2/CASH4 and 65°C for INS. The primers used were ASCL2/CASH4, 5′-GAGGAGCAGGAACTGCTGGATTTC-3′ and 5′-GCTGTGGAAGACCATAGGAATCGA-3′; INS, 5′-AACCAGCTATGCAGCTGCCAACC-3′ and 5′-GAGTAAGTGTATGTCTGTGCCCGC-3′. Using an aliquot of the products as a template, a second PCR was done for 30 cycles with the same parameters. The primers used were ASCL2/CASH4, 5′-ACCAGCTGGCTTGGGAGCTACTGA-3′ and 5′-CCAATGCCTTTGACAACCTGTTGG-3′; INS, 5′-GGAGAGCGTGGCTTCTTCTACTC-3′ and 5′-GAGTAAGTGTATGTCTGTGCCCGC-3′.
Identification of SNPs and allelic expression analysis
To identify SNPs within the transcribed regions, PCR primers were designed to amplify the exons of each gene. The primers were PHEMX/TSSC6 exons 3 and 3′, 5′-ACCTCTCAAGAGTCGAGCAGCTC-3′ and 5′-TGCTCTAGCTTTAACCAGGCTTGC-3′; PHEMX/TSSC6 exon 4, 5′-TGGCAAAAGGCTGGTTCTGGAGG-3′ and 5′-TGTCACAGGCACTTCTGTTTCTGTA-3′; PHEMX/TSSC6 exon 5, 5′-TGTTGTCACACAACCTAAACACGT-3′ and 5′-ATAGCATGGTAGGCACCTGCTTAG-3′; PHEMX/TSSC6 exon 6, 5′-TTCCATGGTGGAGGCCATCACCA-3′ and 5′-CATCCTCTGCAGACTGGAACTCAT-3′; ASCL2/CASH4 exon 1, 5′-ACCAGCTGGCTTGGGAGCTACTGA-3′ and 5′-GCTGTGGAAGACCATAGGAATCGA-3′; TH exon 13, 5′-ACTACGCAGCACATATCAAGAGGC-3′ and 5′-GAAGCTGGCTTTCAGTAAAGCAGG-3′; INS exon 1, 5′-TCACGTCAAAGGAGCTGAGGGAC-3′ and 5′-GGACATTCCTTGTGTCACCATCAAA-3′; INS exon 2, 5′-CAGCTCTTCACTTACACACCTGGT-3′ and 5′-GTGGTGTCCCCTCCACAAGAAAC-3′. PCR was done using genomic DNA as a template under the following condition: 30 cycles of 94°C for 30 sec, 65°C for 30 sec, and 72°C for 30 sec, with a final extension at 72°C for 5 min. PCR products were purified using Microcon TM-100 (Millipore) and sequenced using BigDye Terminator Cycle Sequencing Ready Reaction Kit (PE Applied Biosystems) and the ABI 377 DNA Sequencer.
Following appropriate crosses, total RNA was obtained from F1 embryos or the tissues from F1 individuals heterozygous for the SNP. Allelic expression status of PHEMX/TSSC6 was examined by sequencing the RT-PCR products amplified with the primer set “exons 3/6.” Since this primer set amplifies multiple alternative splicing products, the RT-PCR product from the most abundant isoform, isoform 5, was purified and sequenced. Allelic expression status of ASCL2/CASH4, TH, and INS was examined by sequencing the RT-PCR products amplified with the primers described in the “RT-PCR” section.
Bisulfite methylation analysis
For analysis, 1 μg of DNA isolated from peripheral blood was subjected to bisulfite methylation analysis (Frommer et al. 1992). The bisulfite treatment was carried out with EZ DNA Methylation Kit (Zymo Research). Semi-nested PCR was preformed to amplify the chicken region homologous to the DMR2 of mouse Igf2. The primers were bisIGF2-P1, 5′-GTGTTGATATTGTGTTGTTTTTTTTTTT-3′; bisIGF2-P3 (outer), 5′-CCACCCCTCCTTACTTATATCATTT-3′; bisIGF2-P2 (inner), 5′-TAACTTCCTCAACTACTTACAACCC-3′. PCR was carried out under the following conditions: first round of amplification: 5 cycles of 95°C for 1 min, 50°C for 2 min, and 72°C for 3 min; and 25 cycles of 94°C for 1 min, 50°C for 2 min, and 72°C for 2 min, with a final extension at 72°C for 7 min. Second round of amplification: 25 cycles of 94°C for 30 sec, 65°C, and then reduced by 0.5°C for each later cycle for 30 sec; and 72°C for 30 sec and 10 cycles of 94°C for 30 sec, 55°C for 30 sec, and 72°C for 30 sec; with a final extension at 72°C for 5 min. PCR products were gel-purified using QIAquick (QIAGEN), cloned using TOPO TA Cloning System (Invitrogen), and sequenced using BigDye Terminator Cycle Sequencing Ready Reaction Kit (PE Applied Biosystems) and ABI 377 DNA Sequencer.
Acknowledgments
This work was supported by Grant-in-Aid for Scientific Research on Priority Areas (C) “Genome Biology” from the Ministry of Education, Culture, Sports, Science and Technology of Japan and NIG Collaboration Research Program. We thank the members of Division of Human Genetics, Department of Integrated Genetics, National Institute of Genetics and Human Genome Research Group, Genomic Sciences Center, RIKEN, for technical support and discussions.
Article and publication are at http://www.genome.org/cgi/doi/10.1101/gr.2609605. Article published online before print in December 2004.
Footnotes
References
- Ainscough, J.F-X., John, R.M., Barton, S.C., and Surani, M.A. 2000. A skeletal muscle-specific mouse Igf2 repressor lies 40 kb downstream of the gene. Development 127: 3923-3930. [DOI] [PubMed] [Google Scholar]
- Andria, M.L., Hsieh, C.L., Oren, R., Francke, U., and Levy, S. 1991. Genomic organization and chromosomal localization of the TAPA-1 gene. J. Immunol. 147: 1030-1036. [PubMed] [Google Scholar]
- Barlow, D.P. 1993. Methylation and imprinting: From host defense to gene regulation. Science 260: 309-310. [DOI] [PubMed] [Google Scholar]
- Bell, A.C. and Felsenfeld, G. 2000. Methylation of a CTCF-dependent boundary controls imprinted expression of the Igf2 gene. Nature 405: 482-485. [DOI] [PubMed] [Google Scholar]
- Benson, G. 1999. Tandem repeats finder: A program to analyze DNA sequences. Nucleic Acids Res. 27: 573-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannan, C.I., Dees, E.C., Ingram, R.S., and Tilghman, S.M. 1990. The product of the H19 gene may function as an RNA. Mol. Cell. Biol. 10: 28-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, W.R.A., Hubbard, S.J., Tickle, C., and Wilson, S.A. 2003. The chicken as a model for large-scale analysis of vertebrate gene function. Nat. Rev. 4: 87-97. [DOI] [PubMed] [Google Scholar]
- Caspary, T., Cleary, M.A., Baker, C.C., Guan, X.J., and Tilghman, S.M. 1998. Multiple mechanisms regulate imprinting of the mouse distal chromosome 7 gene cluster. Mol. Cell. Biol. 18: 3466-3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattanach, B.N. and Beechey, C.V. 1997. Genomic imprinting in the mouse: Possible final analysis. In Genomic imprinting (eds. W. Reik and A. Surani), pp. 118-145. IRL Press at Oxford University Press, Oxford, UK.
- Chao, W., Huyuh, K.D., Spencer, R.J., Davidow, L.S., and Lee, J.T. 2002. CTCF, a candidate trans-acting factor for X-inactivation choice. Science 295: 345-347. [DOI] [PubMed] [Google Scholar]
- Constancia, M., Dean, W., Lopes, S., Moore, T., Kelsey, G., and Reik, W. 2000. Deletion of a silencer element in Igf2 results in loss of imprinting independent of H19. Nat. Genet. 26: 203-206. [DOI] [PubMed] [Google Scholar]
- Drewell, R.A., Arney, K.L., Arima, T., Barton, S.C., Brenton, J.D., and Surani, M.A. 2002. Novel conserved elements upstream of the H19 gene are transcribed and act as mesodermal enhancers. Development 129: 1205-1213. [DOI] [PubMed] [Google Scholar]
- Eden, S., Constancia, M., Hashimshony, T., Dean, W., Goldstein, B., Johnson, A.C., Keshet, I., Reik, W., and Cedar, H. 2001. An upstream repressor element plays a role in Igf2 imprinting. EMBO J. 20: 3518-3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falls, J.G., Pulford, D.J., Wylie, A.A., and Jirtle, R.L. 1999. Genomic imprinting: Implications for human disease. Am. J. Pathol. 154: 635-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson-Smith, A.C. and Surani, M.A. 2001. Imprinting and the epigenetic asymmetry between parental genomes. Science 293: 1086-1089. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick, G.V., Soloway, P.D., and Higgins, M.J. 2002. Regional loss of imprinting and growth deficiency in mice with a targeted deletion of KvDMR1. Nat. Genet. 32: 426-431. [DOI] [PubMed] [Google Scholar]
- Fleischmann, R.D., Adams, M.D., White, O., Clayton, R.A., Kirkness, E.F., Kerlavage, A.R., Bult, C.J., Tomb, J.F., Dougherty, B.A., Merrick, J.M., et al. 1995. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science 269: 496-512. [DOI] [PubMed] [Google Scholar]
- Frommer, M., McDonald, L.E., Millar, D.S., Collis, C.M., Watt, F., Grigg, G.W., Molloy, P.L., and Paul, C.L. 1992. A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc. Natl. Acad. Sci. 89: 1827-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner-Garden, M. and Frommer, M. 1987. CpG islands in vertebrate genomes. J. Mol. Biol. 196: 261-282. [DOI] [PubMed] [Google Scholar]
- Giddings, S.J., King, C.D., Harman, K.W., Flood, J.F., and Carnaghi, L.R. 1994. Allele specific inactivation of insulin 1 and 2, in the yolk sac, indicates imprinting. Nat. Genet. 6: 310-313. [DOI] [PubMed] [Google Scholar]
- Guillemot, F., Nagy, A., Auerbach, A., Rossant, J., and Joyner, A.L. 1994. Essential role of Mash-2 in extraembryonic development. Nature 371: 333-336. [DOI] [PubMed] [Google Scholar]
- Guillemot, F., Caspary, T., Tilghman, S.M., Copeland, N.G., Gilbert, D.J., Jenkins, N.A., Anderson, D.J., Joyner, A.L., Rossant, J., and Nagy, A. 1995. Genomic imprinting of Mash2, a mouse gene required for trophoblast development. Nat. Genet. 9: 235-242. [DOI] [PubMed] [Google Scholar]
- Hark, A.T., Schoenherr, C.J., Katz, D.J., Ingram, R.S., Levorse, J.M., and Tilghman, S.M. 2000. CTCF mediates methylation-sensitive enhancer-blocking activity at the H19/Igf2 locus. Nature 405: 486-489. [DOI] [PubMed] [Google Scholar]
- Hattori, M., Tsukahara, F., Furuhata, Y., Tanahashi, H., Hirose, M., Saito, M., Tsukuni, S., and Sakaki, Y. 1997. A novel method for making nested deletions and its application for sequencing of a 300 kb region of human APP locus. Nucleic Acids Res. 25: 1802-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori, M., Fujiyama, A., Taylor, T.D., Watanabe, H., Yada, T., Park, H.S., Toyoda, A., Ishii, K., Totoki, Y., Choi, D.K., et al. 2000. The DNA sequence of human chromosome 21. Nature 405: 311-319. [DOI] [PubMed] [Google Scholar]
- Henrique, D., Tyler, D., Kintner, C., Heath, J.K., Lewis, J.H., Ish-Horowicz, D., and Storey, K.G. 1997. cash4, a novel achaete–scute homolog induced by Hensen's node during generation of the posterior nervous system. Genes & Dev. 11: 603-615. [DOI] [PubMed] [Google Scholar]
- Hori, T., Asakawa, S., Itoh, Y., Shimizu, N., and Mizuno, S. 2000. Wpkci, encoding an altered form of PKCI, is conserved widely on the avian W chromosome and expressed in early female embryos: Implication of its role in female sex determination. Mol. Biol. Cell 11: 3645-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horike, S., Mitsuya, K., Meguro, M., Kotobuki, N., Kashiwagi, A., Notsu, T., Schulz, T.C. Shirayoshi, Y., and Oshimura, M. 2000. Targeted disruption of the human LIT1 locus defines a putative imprinting control element playing an essential role in Beckwith-Wiedemann syndrome. Hum. Mol. Genet. 9: 2075-2083. [DOI] [PubMed] [Google Scholar]
- Ishihara, K. and Sasaki, H. 2002. An evolutionarily conserved putative insulator element near the 3′ boundary of the imprinted Igf2/H19 domain. Hum. Mol. Genet. 11: 1627-1636. [DOI] [PubMed] [Google Scholar]
- Ishihara, K., Kato, R., Furuumi, H., Zubair, M., and Sasaki, H. 1998. Sequence of a 42-kb mouse region containing the imprinted H19 locus: Identification of a novel muscle-specific transcription unit showing biallelic expression. Mamm. Genome 9: 775-777. [DOI] [PubMed] [Google Scholar]
- Ishihara, K., Hatano, N., Furuumi, H., Kato, R., Iwaki, T., Miura, K., Jinno, Y., and Sasaki, H. 2000. Comparative genomic sequencing identifies novel tissue-specific enhancers and sequence elements for methylation-sensitive factors implicated in Igf2/H19 imprinting. Genome Res. 10: 664-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, B.K., Levorse, J., and Tilghman, S.M. 2001. Deletion of a nuclease-sensitive region between the Igf2 and H19 genes leads to Igf2 misregulation and increased adiposity. Hum. Mol. Genet. 10: 807-814. [DOI] [PubMed] [Google Scholar]
- Jongstra, J., Tidmarsh, G.F., Jongstra-Bilen, J., and Davis, M.M. 1988. A new lymphocyte-specific gene which encodes a putative Ca2+-binding protein is not expressed in transformed T-lymphocyte lines. J. Immunol. 141: 3999-4004. [PubMed] [Google Scholar]
- Kaneda, M., Okano, M., Hata, K., Sado, T., Tsujimoto, N., Li, E., and Sasaki, H. 2004. Essential role for de novo DNA methyltransferase Dnmt3a in paternal and maternal imprinting. Nature 429: 900-903. [DOI] [PubMed] [Google Scholar]
- Killian, J.K., Byrd, J.C., Jirtle, J.V., Munday, B.L., Stoskopf, M.K., MacDonald, R.G., and Jirtle, R.L. 2000. M6P/IGF2R imprinting evolution in mammals. Mol. Cell 5: 707-716. [DOI] [PubMed] [Google Scholar]
- Koide, T., Ainscough, J., Wijerde, M., and Surani, M.A. 1994. Comparative analysis of Igf2/H19 imprinted domain: Identification of a highly conserved intergenic DNase I hypersensitive region. Genomics 24: 1-8. [DOI] [PubMed] [Google Scholar]
- Larsen, F., Gundersen, G., Lopez, R., and Prydz, H. 1992. CpG islands as gene markers in the human genome. Genomics 13: 1095-1107. [DOI] [PubMed] [Google Scholar]
- Leighton, P.A., Ingram, R.S., Eggenschwiler, J., Efstratiadis, A., and Tilghman, S.M. 1995a. Disruption of imprinting caused by deletion of the H19 gene region in mice. Nature 375: 34-39. [DOI] [PubMed] [Google Scholar]
- Leighton, P.A., Saam, J.R., Ingram, R.S., Stewart, C.L., and Tilghman, S.M. 1995b. An enhancer deletion affects both H19 and Igf2 expression. Genes & Dev. 9: 2079-2089. [DOI] [PubMed] [Google Scholar]
- Li, E. 2002. Chromatin modification and epigenetic reprogramming in mammalian development. Nat. Rev. Genet. 3: 662-673. [DOI] [PubMed] [Google Scholar]
- Misener, V.L., Wielowieyski, A., Brennan, L.A., Beebakhee, G., and Jongstra, J. 1998. The mouse Lsp1 and Tnnt3 genes are 4.3 kb apart on distal mouse chromosome 7. Mamm. Genome 9: 846-848. [DOI] [PubMed] [Google Scholar]
- Miyamoto, T., Hasuike, S., Jinno, Y., Soejima, H., Yun, K., Miura, K., Ishikawa, M., and Niikawa, N. 2002. The human ASCL2 gene escaping genomic imprinting and its expression pattern. J. Assist. Reprod. Genet. 19: 240-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, T. and Haig, D. 1991. Genomic imprinting in mammalian development: A parental tug-of-war. Trends Genet. 7: 45-49. [DOI] [PubMed] [Google Scholar]
- Moore, G.E., Abu-Amero, S.N., Bell, G., Wakeling, E.L., Kingsnorth, A., Stanier, P., Jauniaux, E., and Bennett, S.T. 2001. Evidence that insulin is imprinted in the human yolk sac. Diabetes 50: 199-203. [DOI] [PubMed] [Google Scholar]
- Murrell, A., Heeson, S., Bowden, L., Constancia, M., Dean, W., Kelsey, G., and Reik, W. 2001. An intragenic methylated region in the imprinted Igf2 gene augments transcription. EMBO Rep. 2: 1101-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann, B., Kubicka, P., and Barlow, D.P. 1995. Characteristics of imprinted genes. Nat. Genet. 9: 12-13. [DOI] [PubMed] [Google Scholar]
- Nolan, C.M., Killian, J.K., Petitte, J.N., and Jirtle, R.L. 2001. Imprint status of M6P/IGF2R and IGF2 in chickens. Dev. Genes Evol. 211: 179-183. [DOI] [PubMed] [Google Scholar]
- Okamura, K., Hagiwara-Takeuchi, Y., Li, T., Vu, T.H., Hirai, M., Hattori, M., Sakaki, Y., Hoffman, A.R., and Ito, T. 2000. Comparative genome analysis of the mouse imprinted gene Impact and its nonimprinted human homolog IMPACT: Toward the structural basis for species-specific imprinting. Genome Res. 10: 1878-1889. [DOI] [PubMed] [Google Scholar]
- O'Neill, M.J., Ingram, R.S., Vrana, P.B., and Tilghman S.M. 2000. Allelic expression of IGF2 in marsupials and birds. Dev. Genes Evol. 210: 18-20. [DOI] [PubMed] [Google Scholar]
- Onyango, P., Miller, W., Lehoczky, J., Leung, C.T., Birren, B., Wheelan, S., Dewar, K., and Feinberg, A.P. 2000. Sequence and comparative analysis of the mouse 1-megabase region orthologous to the human 11p15 imprinted domain. Genome Res. 10: 1697-1710. [DOI] [PubMed] [Google Scholar]
- Paulsen, M., El-Maarri, O., Engemann, S., Strodicke, M., Franck, O., Davies, K., Reinhardt, R., Reik, W., and Walter, J. 2000. Sequence conservation and variability of imprinting in the Beckwith-Wiedemann syndrome gene cluster in human and mouse. Hum. Mol. Genet. 9: 1829-1841. [DOI] [PubMed] [Google Scholar]
- Reik, W. and Maher, E.R. 1997. Imprinting in clusters: Lessons from Bechwith-Wiedemann syndrome. Trends Genet. 13: 330-334. [DOI] [PubMed] [Google Scholar]
- Reik, W. and Walter, J. 2001. Genomic imprinting: Parental influence on the genome. Nat. Rev. Genet. 2: 21-32. [DOI] [PubMed] [Google Scholar]
- Schwartz, S., Zhang, Z., Frazer, K.A., Smit, A., Riemer, C., Bouck, J., Gibbs, R., Hardison, R., and Miller, W. 2000. PipMaker—A web server for aligning two genomic DNA sequences. Genome Res. 10: 577-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirohzu, H., Yokomine, T., Sato, C., Kato, R., Toyoda, A., Purbowasito, W., Suda, C., Mukai, T., Hattori, M., Okumura, K., et al. 2004. A 210-kb segment of tandem repeats and retroelements located between imprinted subdomains of mouse distal chromosome 7. DNA Res. 11: 325-334. [DOI] [PubMed] [Google Scholar]
- Solter, D. 1988. Differential imprinting and expression of maternal and paternal genomes. Annu. Rev. Genet. 22: 127-146. [DOI] [PubMed] [Google Scholar]
- Surani, M.A., Kothary, R., Allen, N.D., Singh, P.B., Fundele, R., Furguson-Smith, A.C., and Barton, S.C. 1990. Genomic imprinting and development in the mouse. Development Suppl. 89-98. [PubMed]
- Taylor, E.R., Seleiro, E.A.P., and Brickell, P.M. 1991. Identification of antisense transcripts of the chicken insulin-like growth factor-II gene. J. Mol. Endocrinol. 7: 145-154. [DOI] [PubMed] [Google Scholar]
- Thorvaldsen, J.L., Duran, K.L., and Bartolomei, M.S. 1998. Deletion of the H19 differentially methylated domain results in loss of imprinted expression of H19 and Igf2. Genes & Dev. 12: 3693-3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman, S.M. 1999. The sins of the fathers and mothers: Genomic imprinting in mammalian development. Cell 96: 185-193. [DOI] [PubMed] [Google Scholar]
- Tsang, P., Gilles, F., Yuan, L., Kuo, Y.-H., Lupu, F., Samara, G., Moosiksuwan, J., Goye, A., Zelenetz, A.D., Selleri, L., et al. 1995. A novel L23-related gene 40-kb downstream of the imprinted H19 gene is biallelically expressed in mid-fetal and adult human tissues. Hum. Mol. Genet. 4: 1499-1507. [DOI] [PubMed] [Google Scholar]
- Walter, J. and Paulsen, M. 2003. The potential role of gene duplications in the evolution of imprinting mechanisms. Hum. Mol. Genet. 12: R215-R220. [DOI] [PubMed] [Google Scholar]
- Wilkins, J.F. and Haig, D. 2003. What good is genomic imprinting: The function of parent-specific gene expression. Nat. Rev. Genet. 4: 359-368. [DOI] [PubMed] [Google Scholar]
- Yoder, J.A., Walsh, C.P., and Bestor, T.H. 1997. Cytosine methylation and the ecology of intragenomic parasites. Trends Genet. 13: 335-340. [DOI] [PubMed] [Google Scholar]
- Yokomine, T., Kuroiwa, A., Tanaka, K., Tsudzuki, Y., Matsuda, Y., and Sasaki H. 2001. Sequence polymorphisms, allelic expression status and chromosome locations of chicken IGF2 and MPR1 genes. Cytogenet. Cell Genet. 93: 109-113. [DOI] [PubMed] [Google Scholar]
- Yoon, B.J., Herman, H., Sikora, A., Smith, L.T., Plass, C., and Soloway, P.D. 2002. Regulation of DNA methylation of Rasgrf1. Nat. Genet. 30: 92-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubair, M., Hilton, K., Saam, J.R., Surani, M.A., Tilghman, S.M., and Sasaki, H. 1997. Structure and expression of the mouse L23mrp gene downstream of the imprinted H19 gene: Biallelic expression and lack of interaction with the H19 enhancers. Genomics 45: 290-296. [DOI] [PubMed] [Google Scholar]
Web site references
- http://bioweb.pasteur.fr/seqanal/interfaces/cpgplot.html; CpG plot.
- http://c3.biomath.mssm.edu/trf.html; Tandem Repeats Finder.
- http://genes.mit.edu/GENSCAN.html; GENSCAN.
- http://nog.cse.psu.edu/pipmaker/; PipMaker.
- http://repeatmasker.genome.washington.edu/cgi-bin/RepeatMasker; RepeatMasker.
- http://swallow.gsf.de/dt40est.html; BursaEst Database.
- http://www.ncbi.nlm.nih.gov/BLAST/; BLAST.