Abstract
Patient: Male, 55
Final Diagnosis: Hepatitis C
Symptoms: Icterus
Medication: —
Clinical Procedure: —
Specialty: Endocrinology and Metabolic
Objective:
Unusual clinical course
Background:
Chronic hepatitis C virus (HCV) infection is associated with increased insulin resistance and risk of type 2 diabetes. Successful antiviral treatment can improve insulin resistance and allow a reduction in blood glucose-lowering treatment. There have been case reports of a reduced insulin requirement in this situation, although 1 case in which insulin was stopped exhibited a subsequent deterioration in glycemic control.
Case Report:
A 55-year-old Italian man was diagnosed with HCV infection in 2000 at the age of 39 years and with type 2 diabetes 6 years later. He was started on metformin but progressed to multiple daily insulin injections after 3 years. He was treated with pegylated interferon, ribavirin, and telaprevir over 12 months from early 2013, and achieved a sustained virologic response and normalization of hepatic function within 6 months of starting therapy. He was subsequently able to reduce his insulin doses from 0.56 to 0.44 U/kg/day over the next 2 years and, based on a random serum C-peptide of 1.73 nmol/L (fasting reference range 0.37–1.47 nmol/L) in the presence of serum glucose 7.9 mmol/L (143 mg/dL) and negative glutamic acid decarboxylase antibodies, he accelerated withdrawal and stopped insulin 6 months later. He is currently taking linagliptin 5 mg daily with good glycemic control. His body mass index and HbA1c have remained <25 kg/m2 and <6.0% (<42 mmol/mol), respectively, throughout.
Conclusions:
This case shows that complete withdrawal of long-term insulin therapy may be possible after HCV treatment has induced a sustained virologic response.
MeSH Keywords: Antiviral Agents; Diabetes Mellitus, Type 2; Hepatitis C, Chronic; Insulin
Background
There is evidence of a relationship between type 2 diabetes and chronic hepatitis C virus (HCV) infection, which appears to be largely mediated by viremia-associated increased insulin resistance (IR) [1]. Individuals infected with HCV genotype 1 or 4 exhibit the greatest IR [2] and IR is a positive predictor of the response to antiviral therapy [3]. In addition to these observations, it is well established that treatment with conventional interferon-based regimens [4] as well as novel direct-acting antiviral (DAA) therapies [5] improves glycemic control in people with HCV infections and diabetes, especially those involving genotype 1. Reports of individual cases have shown that some people taking oral agents, including metformin, sulfonylureas, and dipeptidyl peptidase 4 (DPP4) inhibitors, can withdraw from these therapies completely [6,7], while insulin-treated individuals can reduce their doses [8] or stop treatment [9] after antiviral therapy. Unfortunately, glycemic control worsened within 6 months after completion of HCV treatment in 2 of these cases, with a consequent need for therapeutic intensification whether there was [7] or was not [9] a sustained virologic response (SVR).
We report the case of an insulin-treated man with HCV who has successfully withdrawn from long-term insulin treatment over a 2-year period subsequent to achieving an SVR during DAA-based therapy.
Case Report
A 55-year-old Italian man without a family history of diabetes was found to have HCV infection in the year 2000 when he was age 39 years. He was treated with interferon-ribavirin in 2006 but did not respond. He was diagnosed with type 2 diabetes at that time and was started on metformin monotherapy. In 2009, he was admitted with severe abdominal pain, acute hepatic decompensation, and marked hyperglycemia with ketosis. He was changed from metformin to multiple daily injections (MDI) of insulin and had a second unsuccessful interferon-ribavirin treatment course. His diabetes was subsequently well managed. He performed self-monitoring of blood glucose (SMBG) at least 4 times per day and experienced non-severe hypoglycemia only occasionally.
He was referred for further hepatologic assessment in 2012. At that time, infection with HCV genotype 1 was confirmed (viral load 5.84 log IU/mL), and imaging and biopsy showed evidence of cirrhosis. He was treated with pegylated interferon, ribavirin, and telaprevir over a period of 12 months from early in 2013, and achieved an SVR and normalization of hepatic function within 6 months of starting therapy. A surveillance liver ultrasound at 6 months showed a probable small 17-mm hepatocellular carcinoma (HCC), which was successfully treated with microwave ablation. He has remained aviremic with no recurrence of HCC or new lesions on further surveillance imaging.
When he started his third course of HCV treatment, he had a body mass index (BMI) of 24.2 kg/m2 and was taking glargine at night and pre-meal soluble human insulin 3 times a day at a total insulin dose of 0.56 U/kg/day (Figure 1). Although he did not experience an increase in the frequency or severity of hypoglycemic episodes during HCV treatment and did not change his diet or exercise program, his HbA1c decreased from 5.6% (38 mmol/mol) to 4.9% (30 mmol/mol) in the presence of a normal total hemoglobin concentration and no hemoglobinopathy. It had increased to 5.4% (36 mmol/mol) at 6 months after treatment but he had started to reduce his pre-meal insulin doses by 2 units on each occasion without a noticeable deterioration in his SMBG results. He viewed MDI insulin as essential but flexible treatment, and he was able to reduce the doses further over the next 2 years. Because of declining insulin requirements, he attended early in 2016 for measurement of a random serum C-peptide, which showed an increased concentration (1.73 nmol/L versus a fasting reference range of 0.37 to 1.47 nmol/L) in the presence of a serum glucose of 7.9 mmol/L (143 mg/dL) [10]. His glutamic acid decarboxylase (GAD) antibodies were negative. The rapidity with which he reduced his insulin treatment accelerated after this and he was able to withdraw from insulin completely without a deterioration in his HbA1c results by early August 2016 (Figure 1). His body weight fell from a peak of 75 kg during HCV treatment to 71 kg 3 months after complete withdrawal of insulin therapy.
Figure 1.
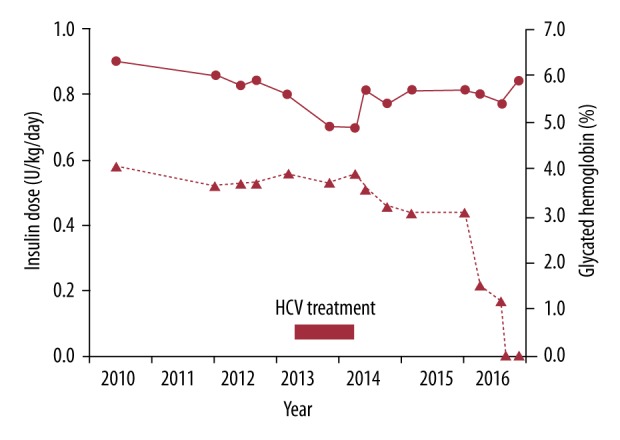
Insulin dose in U/kg/day (▴- - -▴) on the left axis and HbA1c (●—●) on the right axis before, during and after successful HCV treatment in a 55-year-old man.
He has been treated with linagliptin 5 mg daily during the 6 months since withdrawing from insulin. On this well-tolerated therapy, he maintains a pre-breakfast blood glucose concentration that is typically 6 mmol/L (110 mg/dL) or with post-prandial levels that range up to 11 mmol/L (200 mg/dL). He is currently undergoing periodic review with a view to stopping linagliptin should his near-normoglycemia persist. He remains a non-smoker, and in August 2016 was normotensive (supine blood pressure 101/77 mmHg) with a satisfactory serum lipid profile (serum LDL cholesterol 1.7 mmol/L or 66 mg/dL and serum triglycerides 0.7 mmol/L or 62 mg/dL). He has no microvascular or macrovascular complications as assessed through Fremantle Diabetes Study screening protocols [11].
Discussion
The present case is the first reported in the literature in which, following HCV therapy that produced an SVR, MDI insulin treatment was withdrawn completely without a subsequent deterioration in glycemic control. In other similar cases, a reduction but not cessation of insulin has been possible [8] or insulin was stopped acutely but glycemic control then worsened significantly within 6 months [9]. Although our patient coped well with MDI insulin treatment guided by regular SMBG over a 10-year period without any episodes of severe hypoglycemia, he has been pleased that he has been able to stop injections and to reduce the frequency of SMBG. He has consistently maintained a BMI in the normal range (<25 kg/m2) but the mild degree of weight loss since stopping insulin is also something he has valued.
Although the exact mechanisms through which HCV infection may perturb glucose tolerance are unclear, HCV is known to interact with proteins that modulate insulin signaling and to alter cytokine profiles that influence insulin secretion and action [12–14]. Abrogation of these effects through successful HCV treatment should improve IR and glycemia [4,5]. Indeed, hepatic transplantation also improves glucose tolerance in chronic HCV infection with reports of successful withdrawal of insulin [15] as in the present case. The protease inhibitor telaprevir is, as in our patient, effective against genotype 1 infections. It has been hypothesized that this drug has an independent blood glucose-lowering effect, perhaps through inhibition of the serine protease activity of DPP4, thus mimicking the action of drugs such as linagliptin [7]. The evidence for this comes from a single case report in which there was post-telaprevir treatment deterioration in glycemic control despite an SVR [7]. However, there was weight gain during treatment and no tests relevant to pancreatic beta cell function (such as islet autoantibodies and serum C-peptide) were performed, which complicates interpretation of the clinical course. By the same token, there have been reports of autoimmune diabetes presenting during interferon-based HCV treatment [16]. Although our case was diagnosed with diabetes at the time of his first unsuccessful interferon-ribavirin therapy, his progression to insulin took 3 years, he was GAD antibody-negative and he had an appropriate ‘stressed’ C-peptide response 10 years after diagnosis. There was, therefore, no suggestion that he had autoimmune diabetes even though he was lean and had no family history of type 2 diabetes.
Insulin requirements in the 2 years before successful HCV treatment in our case ranged between 0.52 and 0.58 U/kg/day, which is more than the 0.2–0.5 U/kg/day considered suitable for lean individuals (BMI <25 kg/m2) with type 2 diabetes [17]. This and his relative lack of hypoglycemia suggest that insulin was an appropriate form of treatment at that time. He was reluctant to initiate a more than modest reduction in insulin doses in the aftermath of antiviral treatment as he wished to continue with very tight glycemic control to prevent the long-term complications of diabetes. However, he was persuaded to follow a planned progressive dose reduction once the significance of the random serum C-peptide result was explained. Whether he will be able to come off all therapy for diabetes and maintain acceptable levels of glycemia even during periods of stress, such as due to infections, remains to be seen. At present, he is aware that his post-prandial blood glucose concentrations can be high relative to those he was recording on MDI insulin, so oral monotherapy appears appropriate.
Conclusions
The clinical implications of this and similar cases that have been published in the literature [6–9] are several. First, HCV infection should be considered as a potential factor, albeit infrequent [18], underlying newly presenting type 2 diabetes or an unexpected worsening of glycemic control in established diabetes. Second, successful treatment of HCV (the expected outcome in the era of DAA-based regimens [19]) may improve glycemic control and lead to a reduction in blood glucose-lowering therapy including insulin. The present case suggests that even longstanding insulin therapy may not be required if there is an SVR. Third, unless warranted by frequency and severity of hypoglycemia, we recommend that insulin dose reduction is done in a staged manner based on the results of tests such as ‘stressed’ serum C-peptide and islet autoantibodies, as well as increased SBMG and periodic HbA1c measurement. As was the case in our patient, people with diabetes are usually told that starting insulin is an irreversible therapeutic decision with potentially dire consequences if the injections are stopped. A slow withdrawal allows reassurance that this is the correct strategy with serial substitution of oral agents if required. As in our case, the improvements in psychological and physical wellbeing resulting from successful HCV treatment are likely to be augmented by simplification of diabetes self-management.
Acknowledgments
We thank the PathWest Laboratories, Perth, Western Australia for performing biochemical and serological testing.
Footnotes
Conflicts of interest
None.
References:
- 1.Negro F, Alaei M. Hepatitis C virus and type 2 diabetes. World J Gastroenterol. 2009;15:1537–47. doi: 10.3748/wjg.15.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kralj D, Virovic Jukic L, Stojsavljevic S, et al. Hepatitis C virus, insulin resistance, and steatosis. J Clin Transl Hepatol. 2016;4:66–75. doi: 10.14218/JCTH.2015.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasso A, Malfatti F, De Leo P, et al. Insulin resistance predicts rapid virological response in non-diabetic, non-cirrhotic genotype 1 HCV patients treated with peginterferon alpha-2b plus ribavirin. J Hepatol. 2009;51:984–990. doi: 10.1016/j.jhep.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Tai TY, Lu JY, Chen CL, et al. Interferon-alpha reduces insulin resistance and beta-cell secretion in responders among patients with chronic hepatitis B and C. J Endocrinol. 2003;178:457–65. doi: 10.1677/joe.0.1780457. [DOI] [PubMed] [Google Scholar]
- 5.Moucari R, Forestier N, Larrey D, et al. Danoprevir, an HCV NS3/4A protease inhibitor, improves insulin sensitivity in patients with genotype 1 chronic hepatitis C. Gut. 2010;59:1694–98. doi: 10.1136/gut.2010.219089. [DOI] [PubMed] [Google Scholar]
- 6.Doyle MA, Cooper C. Successful hepatitis C Antiviral therapy induces remission of type 2 diabetes: A case report. Am J Case Rep. 2015;16:745–50. doi: 10.12659/AJCR.895064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tallon de Lara P, Himschoot T, et al. Does telaprevir possess a direct anti-diabetic effect? Liver Int. 2014;34:967–69. doi: 10.1111/liv.12440. [DOI] [PubMed] [Google Scholar]
- 8.Pashun RA, Shen NT, Jesudian A. Markedly Improved glycemic control in poorly controlled type 2 diabetes following direct acting antiviral treatment of genotype 1 hepatitis C. Case Reports Hepatol. 2016;2016:7807921. doi: 10.1155/2016/7807921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tahrani A, Bowler L, Singh P, Coates P. Resolution of diabetes in type 2 diabetic patient treated with IFN-alpha and ribavirin for hepatitis C. Eur J Gastroenterol Hepatol. 2006;18:291–93. doi: 10.1097/00042737-200603000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Hope SV, Knight BA, Shields BM, et al. Random non-fasting C-peptide: Bringing robust assessment of endogenous insulin secretion to the clinic. Diabet Med. 2016;33:1554–58. doi: 10.1111/dme.13142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis TM, Bruce DG, Davis WA. Cohort profile: The Fremantle Diabetes Study. Int J Epidemiol. 2013;42:412–21. doi: 10.1093/ije/dys065. [DOI] [PubMed] [Google Scholar]
- 12.Milner KL, van der Poorten D, Trenell M, et al. Chronic hepatitis C is associated with peripheral rather than hepatic insulin resistance. Gastroenterology. 2010;138:932–41e1. doi: 10.1053/j.gastro.2009.11.050. –3. [DOI] [PubMed] [Google Scholar]
- 13.Vanni E, Abate ML, Gentilcore E, et al. Sites and mechanisms of insulin resistance in nonobese, nondiabetic patients with chronic hepatitis C. Hepatology. 2009;50:697–706. doi: 10.1002/hep.23031. [DOI] [PubMed] [Google Scholar]
- 14.Walsh MJ, Jonsson JR, Richardson MM, et al. Non-response to antiviral therapy is associated with obesity and increased hepatic expression of suppressor of cytokine signalling 3 (SOCS-3) in patients with chronic hepatitis C, viral genotype 1. Gut. 2006;55:529–35. doi: 10.1136/gut.2005.069674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vlaeminck-Guillem V, Guillem P, Dequiedt P, et al. Liver transplantation eliminates insulin needs of a diabetic patient. Diabetes Metab. 2000;26:493–96. [PubMed] [Google Scholar]
- 16.Blanchard E, Vickers CR, Samaras K. Not so sweet: Autoimmune diabetes mellitus on triple therapy for chronic hepatitis C infection. Diabet Med. 2015;32:e1–3. doi: 10.1111/dme.12585. [DOI] [PubMed] [Google Scholar]
- 17.Mudaliar S, Edelman SV. Insulin therapy in type 2 diabetes. Endocrinol Metab Clin North Am. 2001;30:935–82. doi: 10.1016/s0889-8529(05)70222-x. [DOI] [PubMed] [Google Scholar]
- 18.Davis TM, Peters KE, Bruce DG, Davis WA. Prevalence, incidence, and prognosis of hepatobiliary disease in community-based patients with type 2 diabetes: The Fremantle Diabetes Study. J Clin Endocrinol Metab. 2012;97:1581–88. doi: 10.1210/jc.2011-3232. [DOI] [PubMed] [Google Scholar]
- 19.Bidell MR, McLaughlin M, Faragon J, et al. Desirable characteristics of hepatitis C treatment regimens: A review of what we have and what we need. Infect Dis Ther. 2016;5:299–312. doi: 10.1007/s40121-016-0118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


