Abstract
Although alternative test methods based on the 3Rs (Replacement, Reduction, Refinement) are being developed to replace animal testing in reproductive and developmental toxicology, they are still in an early stage. Consequently, we aimed to develop alternative test methods in male animals using mouse spermatogonial stem cells (mSSCs). Here, we modified the OECD TG 489 and optimized the in vitro comet assay in our previous study. This study aimed to verify the validity of in vitro tests involving mSSCs by comparing their results with those of in vivo tests using C57BL/6 mice by gavage. We selected hydroxyurea (HU), which is known to chemically induce male reproductive toxicity. The 50% inhibitory concentration (IC50) value of HU was 0.9 mM, as determined by the MTT assay. In the in vitro comet assay, % tail DNA and Olive tail moment (OTM) after HU administration increased significantly, compared to the control. Annexin V, PI staining and TUNEL assays showed that HU caused apoptosis in mSSCs. In order to compare in vitro tests with in vivo tests, the same substances were administered to male C57BL/6 mice. Reproductive toxicity was observed at 25, 50, 100, and 200 mg/kg/day as measured by clinical measures of reduction in sperm motility and testicular weight. The comet assay, DCFH-DA assay, H&E staining, and TUNEL assay were also performed. The results of the test with C57BL/6 mice were similar to those with mSSCs for HU treatment. Finally, linear regression analysis showed a strong positive correlation between results of in vitro tests and those of in vivo. In conclusion, the present study is the first to demonstrate the effect of HU-induced DNA damage, ROS formation, and apoptosis in mSSCs. Further, the results of the current study suggest that mSSCs could be a useful model to predict male reproductive toxicity.
Keywords: Alternative test method, Mouse spermatogonial stem cells, Reproductive toxicity, DNA damage, Apoptosis
INTRODUCTION
Reproductive and developmental toxicity test methods evaluate toxic effects of substances on reproductive ability and developmental stages including germ cell growth, fertilization, pregnancy, birth and offspring growth. The Organization for Economic Co-Operation and Development (OECD) published Test Guidelines on in vivo reproductive and developmental toxicity test methods. The reproductive and developmental toxicity tests, however, require laboratory animals and costs more than other tests in existing OECD Test Guidelines since the entire reproductive and developmental stages have to be evaluated (1). Therefore, alternative test methods that replace the reproductive and developmental toxicity tests based on the 3R (Replacement, Reduction, Refinement) principles are required.
Existing reproductive and developmental toxicity tests usually evaluate teratogenesis, abortion, and offspring growth affected by exposure to toxic substances during pregnancy of female animals. However, male animals have been targeted in recent studies to predict reproductive and developmental toxicity (2,3). The European Union Research Laboratory for Alternative in Animal Testing (EURL ECVAM) published the Repair Proficient Comet assay (ReProComet assay) using frozen bovine sperm (ex vivo), computer-assisted sperm analysis (ex vivo), an assay using Leydig cells and Sertoli cells that exist around germ cells as alternative male fertility test methods (4). In vitro tests that directly evaluate sperm toxicity do not exist.
Spermatogonial stem cells (SSCs) are a precursor of germ cells. Mouse SSCs (mSSCs) are less populous (0.03%) than the other germ cells in the testis (5). Markers of germ cells (Piwil2, Dazl, Tex18 and Gfra1) and pluripotent markers (Oct4 and Sox2) are mainly expressed in mSSCs (6). In vitro mSSC culture methods have been developed since the 2000s. In a previous study, in vitro culture of mSSCs isolated from the neonatal testes (DBA/2 background mouse) was conducted (7). mSSCs were then isolated in the adult testes, and adult mouse unipotent mSSCs were converted into pluripotent stem cells (6,8). Recently, studies on mechanism and toxic effects of substances using mSSCs have been performed (9–12).
The comet assay, known as single-cell gel electrophoresis, is a test method to measure DNA damage in individual cells. The comet assay image looks like a ‘comet’ with a distinct head consisting of intact DNA and a tail, which contains damaged or broken pieces of DNA. This assay is sensitive because it detects low levels of DNA damage (13). It is also a simple method compared with other tests that detect DNA damage (14). The alkaline comet assay is being widely used as a standard test method since it detects DNA damage including single strand breaks, double strand breaks and akali labile site (15). The comet assay has been used widely to evaluate testicular and sperm toxicity including measurement of ROS, antioxidant enzymes and apoptosis (16–21).
Hydroxyurea (HU) is known as a ribonucleotide reductase enzyme that limits DNA biosynthesis by inhibiting the conversion of ribonucleotides into deoxyribonucleotides. HU requires antineoplastic and chemotherapeutic agents (22,23). A previous study showed that HU altered sperm chromatin structure and resulted in abnormal sperm head morphology in mice (24). Another study reported that testis and epididymis weights of transgenic sickle cell mice were reduced after being administered HU (25). HU also decreased sperm density and testosterone concentration (25).
The aim of the present study is to develop a new alternative test method to evaluate reproductive toxicity using mSSCs and identify cytotoxicity mechanisms with HU treatment.
MATERIALS AND METHODS
Materials
StemPro34 media, StemPro nutrient supplement, N2 supplement, fetal bovine serum (embryonic stem cell qualified, FBS), MEM Vitamin, L-glutamine, D-(+)-glucose, pyruvic acid, bovine serum albumin (BSA), minimal essential medium (MEM) non-essential amino acids, MEM sodium pyruvate, β-mercaptoethanol and Dulbecco’s phosphate buffered saline (DPBS) were purchased from Invitrogen (Carlsbad, CA, USA). Penicillin/streptomycin was purchased from Welgene (Daegu, Korea). Recombinant human glial-derived neurotrophic factor (GDNF), recombinant human fibroblast growth factor-basic (bFGF) and recombinant human epidermal growth factor (EGF) were purchased from Peprotech (Rocky Hill, NJ, USA). Recombinant mouse leukemia inhibitory factor (LIF) was purchased from Pospec (East Brunswick, NJ, USA). Matrigel was purchased from Corning Life Science (Corning, NY, USA). Comet slide, lysis solution, and SYBR gold were purchased from Trevigen (Gaithersburg, MD, USA). 2′, 7′-dichlorodihydrofluorescein diacetate (DCFH-DA) assay kit was purchased from Abcam (Cambridge, UK). Annexin V-Cy3 apoptosis detection kit, Apo-BrdU-Red in situ DNA fragmentation assay kit and Apo-BrdU-IHC in situ DNA Fragmentation Assay kit were purchased from Biovision (Mountain Veiw, CA, USA). Sperm counting chamber slide was purchased from Leja (Nieuw Vennep, Netherlands). All other materials were purchased from Sigma Chemical Co. (St. Louis, MO, USA) HU was dissolved in culture medium.
Cell culture
mSSCs provided by Dr. Ko (KonKuk University, Seoul, Korea) were used for the experiments. mSSCs were established from Oct4-GFP and Oct4-GFP/LacZ transgenic mice (C57BL/6 background) in the previous studies (6,8). mSSCs were maintained on feeder-free (Matrigel-coated) plates and passaged every five days. Cells were replated (5 × 105 cells/well) in 12-well plates. mSSCs were cultured in StemPro-34 SFM (Grand Island, NY, USA) supplemented with StemPro nutrient supplement, 1× N2 supplement, 1% FBS, 1× penicillin/streptomycin, 1× MEM vitamin, 2 mM L-glutamine, 6 mg/mL D-(+)-glucose, 30 mg/mL pyruvic acid, 1 μL/mL DL-lactic acid, 5 mg/mL BSA, 50 μM β-mercaptoethanol, 1× MEM non-essential amino acids, 30 ng/mL β-estradiol, 60 ng/mL progesterone, 20 ng/mL EGF, 20 ng/mL bFGF, 20 ng/mL GDNF, and 100 μg/mL LIF based on established protocols (6,26). mSSCs were maintained at 37°C in a humidified atmosphere containing 5% CO2.
Cell viability
Cell viability was determined by MTT assay, which measures the reduction in plating efficiency in treatment groups relative to the controls. The cells were seeded in 96-well plates at a density of 8 × 104 cells/well, and incubated in medium in the presence of different concentrations of HU for 24 h. Cells were also treated with only medium as a vehicle control. Following the incubation period, 10 μL of 5 mg/mL thiazolyl blue tetrazolium bromide (MTT) solution, diluted DPBS were added into each well and the mixture was incubated for a further 3 h at 37°C. The medium was aspirated and formazan crystal was dissolved by adding 100 μL of isopropanol in to each well. The mixture was incubated at room temperature for 30 min. Thereafter, the absorbance at 570 nm was measured using an enzyme-linked immunosorbent assay microplate reader (SpectraMax, Molecular Devices, CA, USA). Cell viability was calculated relative to that of the vehicle control. For the determination of 50% inhibitory concentration (IC50) dose-response graphs were constructed using series of different concentrations of HU.
Measurement of reactive oxygen species (ROS) in mSSCs
To determine intracellular ROS production, DCFH-DA assay was conducted using a DCFH-DA assay kit according to the manufacturer’s instructions. Cells were seeded in luminometer 96-well plates at a density of 8 × 104 cells/well, and incubated in medium in the presence of different concentrations of HU for 24 h. Cells were also treated with only medium as a vehicle control. Here, 25 μM DCFH-DA was put into each well and incubated for 45 min at 37°C. The cells were then washed with DPBS and 100 μL buffer was added into each sample. Intracellular ROS was measure by a Victor 3 fluorescence plate reader (Perkin Elmer, Waltham, MA, USA) at Ex 485 nm/Em 535 nm. ROS values of each sample were divided by cell viability. Finally, relative DCF fluorescence (RFU) was calculated relative to that of the vehicle control.
Analysis of DNA damage of mSSCs by Comet assay
Cells were seeded in 24-well plates at a density of 4.5 × 105 cells/well, and incubated in medium in the presence of different concentrations of HU for 24 h.
The alkaline comet assay performed according to the modified OECD TG 489 protocol (5). Triplicate slides were prepared per treatment. Incubated cells were harvested, centrifuged and gently resuspended in 1 mL of DPBS. Cells were then centrifuged at 12,000 rpm for 5 min and the cell pellet was mixed with 180 μL of 0.5% (w/v) low melting agarose and 55 μL was immediately pipette onto the comet slide. These slides were refrigerated (4°C) for more than 20 min. The slides were immersed in 250 mL chilled lysis solution with 10 mM dithiothreitol (DTT) for 1 h in a refrigerator. After lysis, the slides were washed twice with distilled water (DW) for 10 min. The slides were then incubated in alkaline buffer (0.3 M NaCl, 1 mM EDTA) for 20 min in the dark at 4°C. Electrophoresis as performed at 21 V and 300 mA for 20 min. After that, the slides were washed twice with DW for 5 min. The slides were then immersed for 5 min in ethanol. Slides were air-dried at room temperature in the dark. Immediately before scoring, slides were stained with 55 μL SYBR green (1 : 10,000 dilution of liquid concentrate). Slides were analyzed using a fluorescence microscope (Leica Microsystems, Wetzlar, Germany) at 40× magnification. For each sample, 50 comets per slide were analyzed, with three slides scored per sample. The percentage of tail DNA and Olive tail moment (OTM) was measured according to the DNA damage degree using computer software (Komet 5.5, Kinetic Imaging, Liverpool, UK) with a fluorescence microscope (DM-4000B, Leica Microsystems).
Annexin V, PI staining and TUNEL assay
Annexin V measured with Annexin V-Cy3 apoptosis detection kit to examine apoptosis. After treatment of HU for 24 h, cells were washed with DPBS and centrifuged at 1,300 rpm for 5 min. This process was repeated two times. After supernatant was removed, 500 μL binding buffer was added. Cells were then stained with 5 μL Annexin V-Cy3. The fluorescent intensity of the cells (10,000 events/sample) was measured using FACSCalibur (BD biosciences, Mountain View, CA, USA).
After treatment of HU for 24 h, cells were washed with DPBS and centrifuged at 1,300 rpm for 5 min. This process was repeated two times. Cells were then stained with 500 μL RNase (0.1 mg/mL) and 500 μL PI (2 mg/mL) for 20 min. The fluorescent intensity of the cells (10,000 events/sample) was measured by using FACSCalibur (BD biosciences).
Apoptotic cells were measured by Apo-BrdU-Red in situ DNA fragmentation assay kit. TUNEL assay was based on the ability of terminal deoxynucleotidyl transferase to label the ends of broken double-stranded DNA to detect apoptotic cells with DNA degradation during apoptosis. After 24 h of HU treatment, cells were washed with DPBS and centrifuged at 300 ×g for 5 min. This process was repeated two times. 1 mL wash buffer added and cells were centrifuged at 300 ×g for 5 min. After supernatant was removed, 50 μL DNA labeling solution was added and cells were incubated for 60 min at 37°C. After supernatant was removed again, 1 mL rinse buffer was added and cells were centrifuged at 300 ×g for 5 min. After supernatant was removed, 100 μL antibody solution was added and cells were incubated for 30 min at room temperature. Then, 500 μL 7AAD/RNase A solution was added and cells were incubated for 30 min at room temperature. Finally, the fluorescent intensity of the cells (10,000 events/sample) was measured by using FACSCalibur (BD biosciences).
Animals
Male C57BL/6 mice (7 weeks old) were obtained from Koatech (Pyeongtaek, Korea). The animals were kept in a Ministry of Food and Drug Administration (Certification Number: 1501MFDS07; Korea) animal facility in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International Animal Care Policies (Accredited Unit, KFDA: Unit No. 000996). The animals were given access to a solid diet and sterilized water ad libitum. The mice were housed in a pathogen-free condition at 21 ± 3°C, a relative humidity of 55 ± 15% and a 12 : 12 hr light/dark cycle per day (Lights were on at 08:00 and off at 20:00). The mice were acclimated for a period of one week prior to the commencement of the experiments. The mice showed weight variation of ± 20% of the mean weight and no abnormalities were observed. The mice were randomly allocated to each test group (3 mice per group) and the average body weight of each group was similar.
Dose selection, chemical preparation and animal treatment
To assess sperm toxicity in mice, the dose of HU (25, 50, 100 and 200 mg/kg/day) was selected on the basis of studies conducted by previous study (25). HU was dissolved in saline solution (10 mL/kg/day) and injected orally (p.o.) in to the mice once a day for two weeks. The animals were weighed daily to adjust the gavage volume.
Body and organ weight
At the end of the two week experimental period, all animals were euthanized by cervical dislocation. The epididymis and testes were quickly removed, the attached tissues were trimmed away, and the organs were weighed. The relative weights of the testis and epididymis were expressed as mg of tissue/g of body weight.
Sperm motility
After the mouse was sacrificed, epididymis was removed and placed in a 1.5 mL tube containing 300 μL of Hank’s balanced salt solution (HBSS) medium containing 0.5% bovine serum albumin (BSA) at 37°C. The epididymis was cut into small portions to allow the sperm to swim out. Sperm were incubated for 5 min at 37°C. After the incubation, supernatant was taken and was then diluted 1 : 1 to 1 : 5 in HBSS medium containing 0.5% BSA. Sperm samples (30 μL) were placed in Leja counting chambers, and sperm motility was evaluated by using the IVOS sperm analyzer (Hamilton Throne Research, MA, USA). Total sperm motility was measured in the same conditions (Table 1). At least five fields were recorded for each sample analyzed, covering the entire viewable area of the chamber without overlapping successive fields. Sperm motility was calculated relative to that of the vehicle control.
Table 1.
Software settings of Tox-IVOS used in the study
| Parameters | Value |
|---|---|
| Chamber type | Leja |
| Temperature of analysis (°C) | 37.0 |
| Frame acquired | 30 |
| Frame rate (Hz) | 60 |
| Minimum static contrast | 15 |
| Minimum cell size (pixels) | 4 |
| Straightness (STR), thresholds (%) | 50.0 |
| VAP cut-off (μm/s) | 10.0 |
| Progressive minimum VAP (μm/s) | 50.0 |
| VSL cut-off (μm/s) | 0.0 |
| Cell intensity | 75 |
| Magnification | 0.50 |
Measurement of reactive oxygen species in mouse sperm
For determining intracellular ROS production, the DCFH-DA assay was conducted using the DCFH-DA assay kit according to the manufacturer’s instructions. Intracellular ROS was measured using a Victor 3 fluorescence plate reader (Perkin Elmer) at Ex 485 nm/Em 535 nm. ROS values of each sample divided by sperm counts. Finally, RFU was calculated relative to that of the vehicle control.
Analysis of DNA damage of sperm by Comet assay
The alkaline comet assay performed according to the modified OECD TG 489 protocol. Triplicate slides were prepared per treatment. Sperm sample (20 μL) containing 5 × 105 sperm per mL were suspended in 180 μL of 0.5% (w/v) low melting agarose and 55 μL was immediately pipetted onto a comet slide. These slides were incubated for 30 min. The slides were immersed in 250 mL chilled lysis solution with 10 mM DTT for 1 h in a refrigerator. After lysis, the slides were washed twice with DW for 10 min. The slides were then incubated in alkaline buffer (0.3 M NaCl, 1 mM EDTA) for 20 min in the dark at 4°C. Electrophoresis as performed at 21 V and 300 mA for 20 min. After that, the slides were washed twice with DW for 5 min. The slides were then immersed for 5 min in ethanol. Slides were airdried at room temperature in the dark. Immediately before scoring, slides were stained with 55 μL SYBR green (1 : 10,000 dilution of liquid concentrate). Slides were analyzed using a fluorescence microscope (DM-4000B, Leica Microsystems) at 40× magnification. For each sample, 50 comets per slide were analyzed, with three slides scored per sample. The percentage of tail DNA and OTM were measured according to the DNA damage degree using computer software (Komet 5.5, Kinetic Imaging Liverpool) with a fluorescence microscope (DM-4000B, Leica Microsystems).
Histopathological observation of testes using H&E staining
The testes were fixed overnight in 4% formaldehyde, dehydrated in ethanol (70, 80, 90, 95 and 100%) and then embedded in paraffin. Tissue sections (5 μm) were mounted onto glass slides and dried at the room temperature for 1 h. Tissue sections were stained with hematoxylin and eosin (H&E) and then observed under a Nikon Eclipse Ni microscope (Melville, NY, USA).
TUNEL assay
TUNEL assay was performed using an Apo-BrdU-IHC in situ DNA Fragmentation Assay kit. Paraffin-embedded tissue sections were deparaffinized and rehydrated using xylene and ethanol. Tissue sections were stored in 1× phosphate buffered saline. After permeabilization, endogenous peroxidase was inactivated using hydrogen peroxide. DNA labeling solution was added to tissue sections to stain DNA for 90 min. After that, Anti-BrdU-Biotin antibody added for 90 min in the dark. Stained tissue sections (5 μm) were mounted with mounting solution and then observed under a Nikon Eclipse Ni microscope.
Statistical analyses
Data are expressed as means ± standard deviation (SD). All statistical analyses were performed using the SPSS 20.0 (Statistical Package for Social Science, SPSS Inc., Chicago, IL, USA) software. Statistical differences in the experimental data were determined using one-way ANOVA followed by two-sided Dunnett’s test. A level of p < 0.001 was used as the criterion for statistical significance.
RESULTS
Effect of HU on mSSCs’ viability, intracellular ROS formation and DNA damage in mSSCs
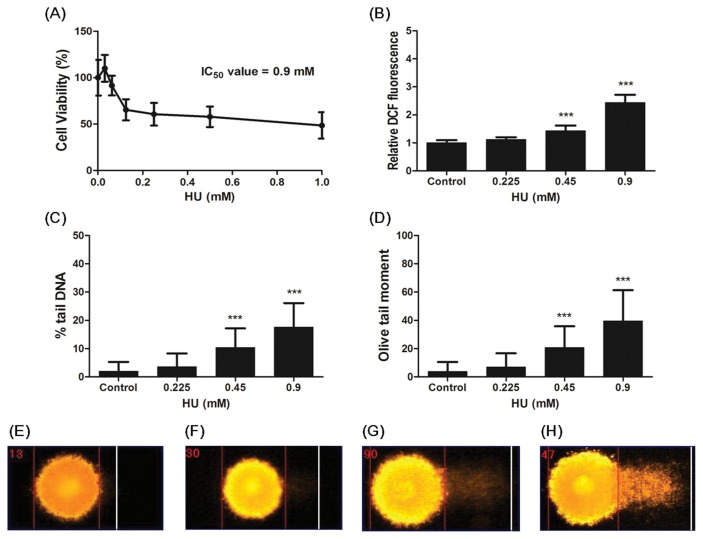
In order to determine 50% inhibitory concentration (IC50), the MTT assay conducted. mSSCs were incubated in medium in the presence of different concentrations (0~1 mM) of HU for 24 h. As shown in Fig. 1, 0.125~1 mM HU treatment significantly decreased in cell viability (p < 0.001). Based on the dose-response curve (Fig. 1A), the IC50 of HU was determined to 0.9 mM.
Fig. 1.
Effect of HU treatment on Cell viability, ROS formation and Comet parameters (% tail DNA and OTM) in mSSCs. (A) mSSCs were exposed to various concentrations of HU for 24 h. Cell viability using MTT assay was expressed as the mean percentage of absorbance values relative to the vehicle control. (B) After mSSCs were treated with 0~0.9mM HU for 24 h, ROS formation using DCFH-DA assay was measured. Result was expressed as the mean of the DCF fluorescence units relative to the vehicle control. (C) % tail DNA and (D) OTM were measured by comet assay. Representative photomicrographs of comet assays showing DNA migration pattern in the mSSCs from (E) the control group, (F) 0.225mM HU group, (G) 0.45mM HU group, (H) 0.9mM HU group. All data represent mean ± SD. ***p < 0.001 versus vehicle control.
The production of ROS was determined by DCFH-DA assay. As shown in Fig. 1B, high levels of ROS were significantly produced depending on 0.45 mM and 0.9 mM HU when compared with the control (p < 0.001). Also, HU increased intracellular ROS formation in a dose-dependent manner.
To determine the effect of HU on DNA damage in mSSCs, we conducted comet assay using the mSSCs exposed to 0~0.9 mM HU for 24 h. As shown in Fig. 1C and 1D, % tail DNA and OTM of 0.45 and 0.9 mM HU treatment significantly increased in comparison with the control (p < 0.001). HU increased comet parameters (% tail DNA and OTM) in a dose-dependent manner. Morerover, we observed using photomicrographs that intensity of tail DNA in the mSSCs increased according to HU concentration (Fig. 1E–1H). These results showed HU led to DNA damage in the mSSCs.
Effect of HU on apoptosis in mSSCs
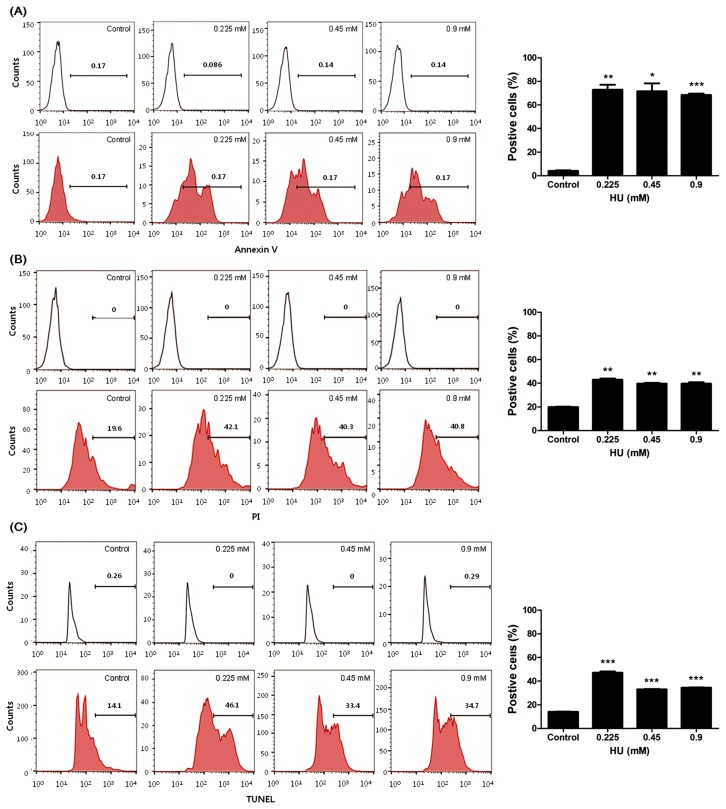
To examsine the effect of HU on apoptosis in mSSCs, Annexin V, PI staining and TUNEL assay was performed after 0~0.9 mM HU treatment in mSSCs for 24 h. As shown in Fig. 2A, Annexin-V-Cy3-positive fluorescent intensity levels of HU-treated mSSCs were significantly increased than that that of control (p < 0.05, 0.01 or 0.001). PI-positive fluorescent cells were significantly increased in mSSCs exposed to all concentration of HU (p < 0.01, Fig. 2B). Furthermore, levels of TUNEL-positive fluorescent intensity significantly increased in mSSCs by HU exposure (p < 0.001, Fig. 2C). These results showed HU induced mSSCs’ apoptosis.
Fig. 2.
Effect of HU on apoptosis in mSSCs. After HU (0, 0.225, 0.45 and 0.9mM) treatment for 24 h, mSSCs that stained (A) Annexin V, (B) PI and (C) TUNEL were analyzed using FACS. Note upper lines; isotype controls, the broad peak of Annexin-V-Cy3-positive, PI-positive or TUNEL-positive fluorescent cells induced apoptosis. All data represent mean ± SD. *p < 0.05, **p < 0.01 or ***p < 0.001 versus vehicle control.
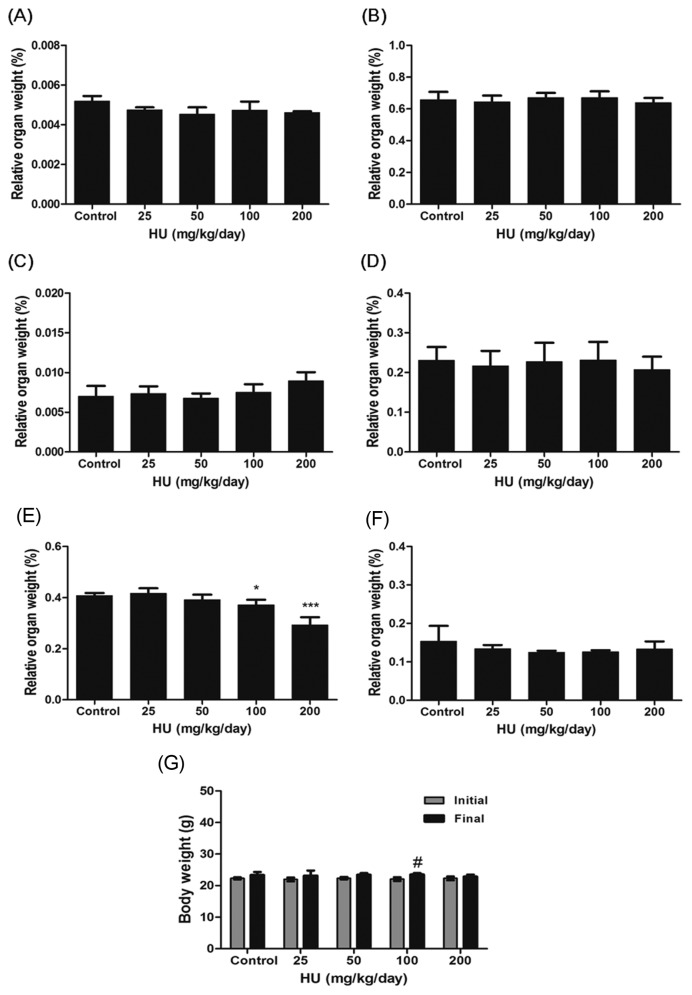
Effect of HU on organ and body weight in mice
Liver, kidney, adrenal and spleen weights in HU group were not significantly different compared with those of control (Fig. 3A–3D). Administration of 200 mg/kg/day HU to mice resulted in a significant reduction in the relative testicular weight (p < 0.001, Fig. 3E). The final body weights of mice receiving HU administration were similar to those of control (Fig. 3G). No differences in body weight loss and epididymis weight were found between experimental and control groups (Fig. 3F, 3G).
Fig. 3.
Effect of HU on relative organ weights and body weights in C57BL/6 mice. C57BL/6 mice were administered with saline (vehicle control) or HU (25, 50, 100 and 200 mg/kg/day) for a period of 14 days. The relative organ weights of the are expressed as mg tissue/g of body weight ((A) liver, (B) kidney, (C) adrenal, (D) spleen, (E) testis and (F) epididymis). (G) Body weights (initial weight and final weight) are expressed. All data represent mean ± SD. *p < 0.05 or ***p < 0.001 versus vehicle control. #p < 0.05 versus initial body weights.
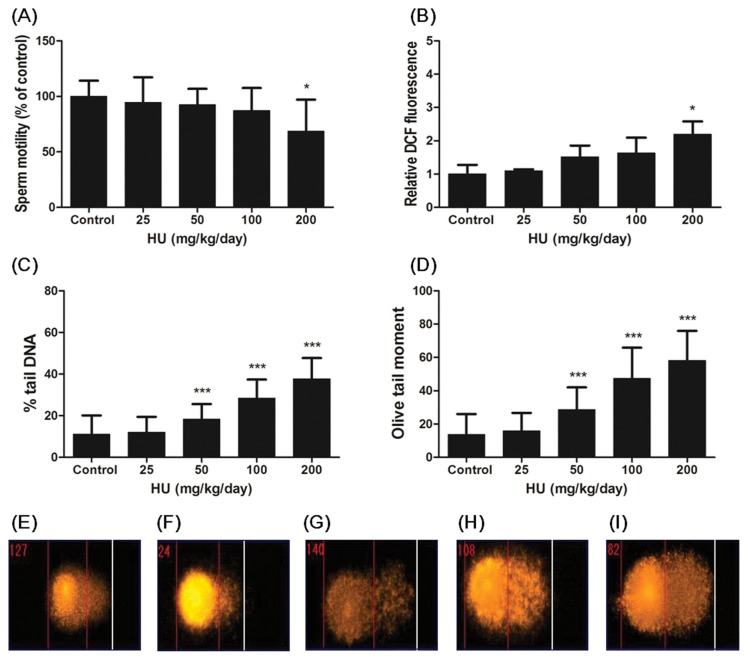
Effect of HU on motility, intracellular ROS formation and DNA damage in sperm of C57BL/6 mice
The effect of HU on the sperm motility, a commonly used evaluation of sperm quality, is presented in Fig. 4A. Administration of 200 mg/kg/day HU to mice induced a significant reduction in relative testicular weight (p < 0.001). This result showed that the administration of HU led to toxicity in the sperm of mice.
Fig. 4.
Effect of HU on motility, ROS formation and DNA damage in sperm. C57BL/6 mice were administered saline (vehicle control) or HU (25, 50, 100 and 200 mg/kg/day) for a period of 14 days. (A) Sperm motility values are expressed as the mean percentage relative to the vehicle control. (B) ROS formation using DCFH-DA assay was measured. Results are expressed as mean of the DCF fluorescent units relative to the vehicle control. (C) % tail DNA and (D) OTM were measured by comet assay. Representative photomicrographs of comet assay showing DNA migration pattern in the sperm of C57BL/6 mice from the (E) control group, (F) 25 mg/kg HU group, (G) 50 mg/kg HU group, (H) 100 mg/kg HU group, (I) 200 mg/kg HU group. All data represent mean ± SD. *p < 0.05 or ***p < 0.001 versus vehicle control.
Excessive ROS levels adversely affected to sperm function (27,28). We measured ROS generation in sperm by DCFH-DA assay. Intracellular ROS levels of sperm were significantly increased in 200 mg/kg/day HU-administered mice compared with the control group (p < 0.001, Fig. 4B).
To determine the effect of HU on DNA damage in sperm of C57BL/6 mice, the mice were exposed to 25, 50, 100 and 200 mg/kg/day HU for 14 days by gavage. As shown in Fig. 4C and 4D, parameters reflecting DNA damage like % tail DNA and OTM of the HU administration group (50, 100 and 200 mg/kg/day) were significantly increased in a dose-dependent manner compared with control group (p < 0.001). Also we observed using photomicrographs that intensity of tail DNA in the sperm increased according to HU dose (Fig. 4E–4I). We confirmed that HU caused DNA damage in the sperm.
Effect of HU on histopathological observation and apoptosis in testes of C57BL/6 mice
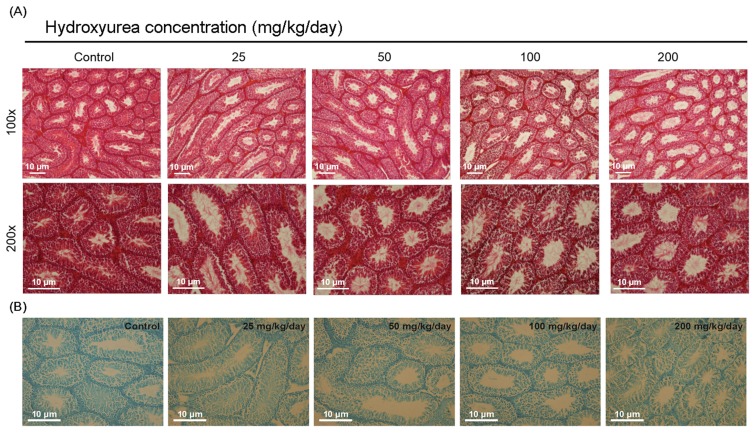
H&E staining is test method to be identify spermatogenesis in the seminiferous tubules of testis. We observed morphological alterations of the testes using H&E staining before TUNEL assay. The control group showed normal morphology (Fig. 5A). However, the HU administration group showed remarkably reduced numbers of germ cells compared to control.
Fig. 5.
Effect of HU on histological changes and apoptosis in testes of C57BL/6 mice. Representative photographs of sperm in testis from male C57BL/6 mice after oral administration of saline (vehicle control) or HU (25, 50, 100 and 200mg/kg/day) for a period of 14 days. (A) Sections were stained with hematoxylin and eosin (Original magnification, 100×, 200×). (B) Sections were stained with TUNEL assay (Original magnification, 200×).
To investigate the effect of HU on testicular apoptosis, we performed TUNEL assay in testes following HU administration. We found that the number of testicular apoptotic cells of HU-administered mice increased compared with that of control (Fig. 5B).
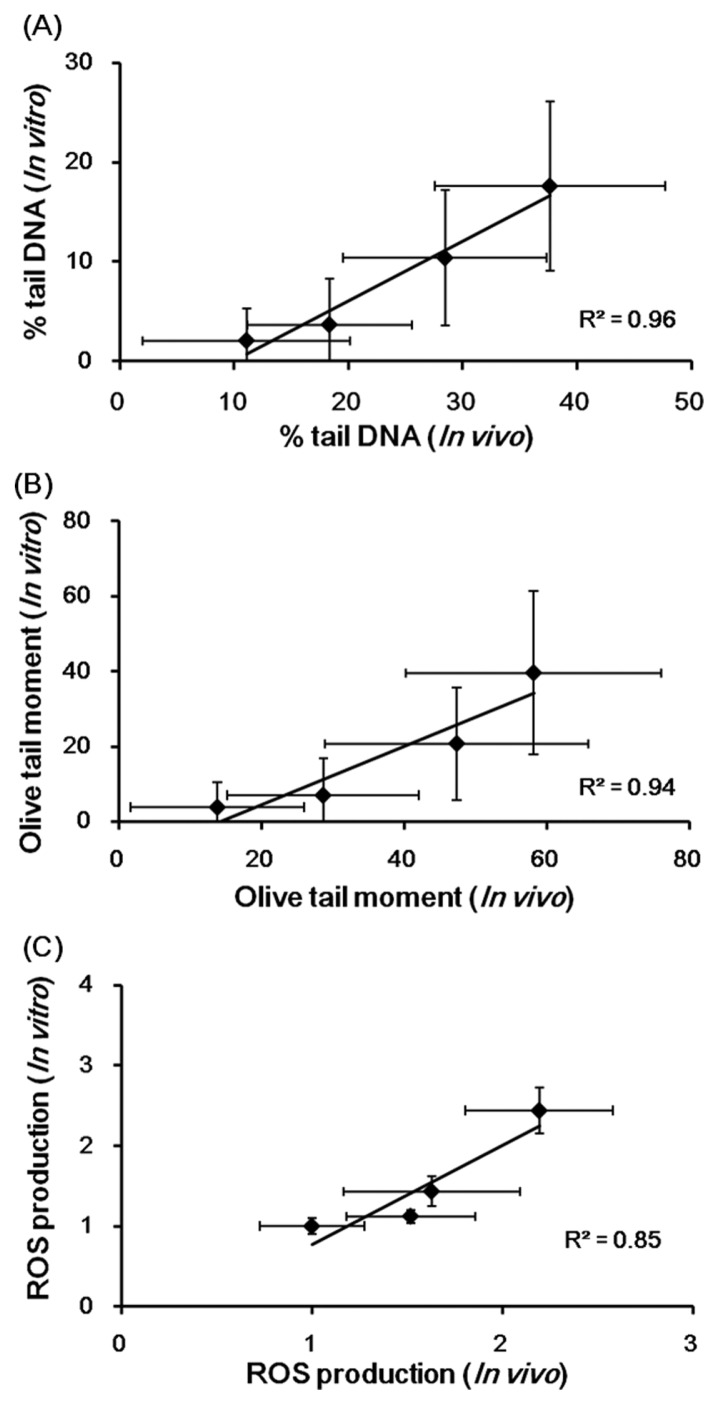
Correlation analysis between in vitro and in vivo study
Linear regression analysis between in vitro and in vivo results are presented in Fig. 6. These results showed a strong positive correlation between results of in vitro comet assays (% tail DNA and OTM) and ROS production using mSSCs and those of in vivo comet assays using sperm in male C57BL/6 mice of HU (R2 = 0.96, 0.94, and 0.85, respectively).
Fig. 6.
Linear regression analysis showed the correlation of in vitro comet assay and in vivo comet assay (A) % tail DNA, (B) OTM, and (C) ROS production in HU. mSSCs were treated with HU (0, 0.225, 0.45 and 0.9mM) for 24 h. C57BL/6 mice were administered saline (vehicle control) or HU (25, 50, 100 and 200 mg/kg/day) for a period of 14 days. All the values are expressed as mean ± SD.
DISCUSSION
The existing reproductive and developmental toxicity tests require laboratory animals and costs more than other in vivo toxicity tests in the OECD Test Guidelines. Alternative test methods based on the 3R principles are being developed. However, the development of alternative reproductive toxicity test methods is still at an early stage. Further, a long-term germ cell culture method has not been developed yet. For these reasons, this study was designed to develop an alternative test method using mSSCs instead of sperm to predict male reproductive toxicity.
In the in vitro comet assay using mSSCs, HU damaged DNA in mSSCs because % tail DNA and OTM in the HU-treated groups showed a significant increase compared with the control. Further, ROS production of the HU-treated groups grew in a dose-dependent manner in comparison with the control. A previous study reported that HU caused DNA damage through the formation of hydrogen peroxide and nitric oxide (29). Based on this previous study, we believed that HU could cause DNA damage in mSSCs by increasing intracellular ROS production. By measuring apoptosis using the Annexin V, PI staining and TUNEL assay, our current results show values of the HU-treated groups were consistently higher than that of the control. A recent study found out that Toll like receptor 3/interferon regulatory factor 3 facilitated Fas ligand expression and induced apoptosis in spermatogonial stem cells (30). In another study, testicular germ cells of DBA/C57BL mice administered HU significantly increased expression of Fas, Fas ligand, Caspase-3, Caspase-8 and Caspase-9 related to apoptosis (31). Consequently, HU-induced apoptosis in mSSCs may be caused by Fas ligand as is the case in germ cells.
In a previous study, lesions similarly occurred in animals treated for two weeks and four weeks. It showed that effects of pharmaceuticals on male reproductive organs could be detected with treatment for two weeks (32). According to another study, it was concluded that treatment for two weeks is enough to evaluate drug-induced effects on male reproductive organs (33). Oral administration of HU for two weeks significantly lowered testicular weight more than the control group. This was similar to a decrease in testicular weights in the groups treated with HU for 28 days and 56 days (25). Moreover, HU administration resulted in significant inhibition of sperm motility and a change in testis morphology. Parameters for DNA damage, such as % tail DNA and OTM, in the 50, 100 and 200 mg/kg/day HU administration groups significantly grew in comparison to those with the control mice. HU has been already reported to cause sperm chromatin structure alternation and an increase in abnormal morphology of the sperm head in male C57B/6J × C3H/HeJ F1 mice (24). Further, a previous study reported that male mice (B6C3/F1/BOM M) showed decreased testicular weight and abnormal sperm chromatin structure on days 27 and 33 after five consecutive days of HU administration (34). HU leads to spermatogenesis delay by changing spermatid stages. A number of previous studies indicated that HU induced reproductive toxicity in male mice. Our data showed that HU caused sperm toxicity as in the previous studies. One of the current means of evaluating male factor infertility, sperm head morphological evaluation test, has a strong positive correlation with the sperm comet assay (35). Thus, our experiment results indicated that the sperm comet assay could evaluate sperm toxicity in HU-administered mice.
ROS balance with anti-oxidants in normal metabolism. However, if imbalanced, the ROS are overproduced and thus, may damage cellular DNA, proteins, and lipids. Sperm are sensitive to ROS because they contain a number of unsaturated fatty acids in the plasma membrane and few antioxidant enzymes in the cytoplasm. An increase in the ROS could damage the sperm membrane and reduce motility and sperm-oocyte fusion. In addition, ROS could be the cause of male infertility because it directly damages sperms’ DNA (17). In our study, ROS production significantly increased in the groups receiving 200 mg/kg/day of HU compared with the control group.
Apoptosis is the process of programmed cell death that plays a crucial role in development and homeostasis (36). However, if excessive apoptosis occurs, it induces defective spermatogenesis, a decrease in sperm motility, sperm DNA fragmentation, testicular torsion, varicocele and immunological infertility (37,38). Following our TUNEL assay result, we observed that HU induced apoptotic DNA fragmentations in testes.
Finally, we performed linear regression analysis of the in vitro and in vivo tests. The analysis results demonstrated a strong positive correlation between results of the in vitro tests (% tail DNA, OTM and ROS production) using mSSCs and those of the in vivo tests using sperm in male C57BL/6 mice of HU (R2 = 0.96, 0.94, and 0.85, respectively). In conclusion, the present study shows for the first time that HU induces DNA damage, ROS formation and apoptosis in mSSCs. Further, the results of the current study suggest that mSSCs could be a useful model to predict male reproductive toxicity.
ACKNOWLEDGMENTS
This research was supported by a grant (14181MFDS451) from Ministry of Food and Drug Safety in 2015. We thank Ku Eun-kyung revising the manuscript.
REFERENCES
- 1.Rovida C, Hartung T. Re-evaluation of animal numbers and costs for in vivo tests to accomplish REACH legislation requirements for chemicals - a report by the transatlantic think tank for toxicology (t(4)) ALTEX. 2009;26:187–208. doi: 10.14573/altex.2009.3.187. [DOI] [PubMed] [Google Scholar]
- 2.Schulte RT, Ohl DA, Sigman M, Smith GD. Sperm DNA damage in male infertility: etiologies, assays, and outcomes. J Assist Reprod Genet. 2010;27:3–12. doi: 10.1007/s10815-009-9359-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makker K, Agarwal A, Sharma R. Oxidative stress & male infertility. Indian J Med Res. 2009;129:357–367. [PubMed] [Google Scholar]
- 4.Adler S, Basketter D, Creton S, Pelkonen O, van Benthem J, Zuang V, Andersen KE, Angers-Loustau A, Aptula A, Bal-Price A, Benfenati E, Bernauer U, Bessems J, Bois FY, Boobis A, Brandon E, Bremer S, Broschard T, Casati S, Coecke S, Corvi R, Cronin M, Daston G, Dekant W, Felter S, Grignard E, Gundert-Remy U, Heinonen T, Kimber I, Kleinjans J, Komulainen H, Kreiling R, Kreysa J, Leite SB, Loizou G, Maxwell G, Mazzatorta P, Munn S, Pfuhler S, Phrakonkham P, Piersma A, Poth A, Prieto P, Repetto G, Rogiers V, Schoeters G, Schwarz M, Serafimova R, Tähti H, Testai E, van Delft J, van Loveren H, Vinken M, Worth A, Zaldivar JM. Alternative (non-animal) methods for cosmetics testing: current status and future prospects-2010. Arch Toxicol. 2011;85:367–485. doi: 10.1007/s00204-011-0693-2. [DOI] [PubMed] [Google Scholar]
- 5.Tegelenbosch RA, de Rooij DG. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat Res. 1993;290:193–200. doi: 10.1016/0027-5107(93)90159-D. [DOI] [PubMed] [Google Scholar]
- 6.Ko K, Arauzo-Bravo MJ, Kim J, Stehling M, Scholer RH. Conversion of adult mouse unipotent germ-line stem cells into pluripotent stem cells. Nat Protoc. 2010;5:921–928. doi: 10.1038/nprot.2010.44. [DOI] [PubMed] [Google Scholar]
- 7.Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, Shinohara T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- 8.Ko K, Tapia N, Wu G, Kim JB, Arauzo Bravo MJ, Sasse P, Glaser T, Ruau D, Han DW, Greber B, Hausdorfer K, Sebastiano V, Stehling M, Fleischmann BK, Brustle O, Zenke M, Scholer HR. Induction of pluripotency in adult unipotent germline stem cells. Cell Stem Cell. 2009;5:87–96. doi: 10.1016/j.stem.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 9.Meng YU, Long W, Yue H, Zhi-min L, Jin-lian H. ALK family inhibitor A83–01 promotes the proliferation of mouse male germline stem cells (mGSCs) under serum-and feeder-free conditions. J Integr Agric. 2013;12:1839–1846. doi: 10.1016/S2095-3119(13)60413-X. [DOI] [Google Scholar]
- 10.Lei Z, Qi-sheng Z, Dong L, Chao L, Ahmed KE, Beibei T, Jiu-zhou S, Ya-ni Z, Bi-chun L. Study on the role of JAK/STAT signaling pathway during chicken spermatogonial stem cells generation based on RNA-Seq. J Integr Agric. 2015;14:939–948. doi: 10.1016/S2095-3119(14)60938-2. [DOI] [Google Scholar]
- 11.Mahaldashtian M, Naghdi M, Ghorbanian MT, Makoolati Z, Movahedin M, Mohamadi SM. In vitro effects of date palm (Phoenix dactylifera L.) pollen on colonization of neonate mouse spermatogonial stem cells. J Ethnopharmacol. 2016;186:362–368. doi: 10.1016/j.jep.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Hashemi E, Akhavan O, Shamsara M, Daliri M, Dashtizad M, Farmacy A. Synthesis and cytogenotoxicity evaluation of graphene on mice spermatogonial stem cells. Colloids Surf B Biointerfaces. 2016;146:770–776. doi: 10.1016/j.colsurfb.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 13.Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YF. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen. 2000;35:206–221. doi: 10.1002/(SICI)1098-2280(2000)35:3<206::AID-EM8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 14.Lewis SE, John Aitken R, Conner SJ, Iuliis GD, Evenson DP, Henkel R, Giwercman A, Gharagozloo P. The imopact of sperm DNA damage in assisted conception and beyond: recent advances in diagnosis and treatment. Reprod Biomed Online. 2013;27:325–327. doi: 10.1016/j.rbmo.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 15.OECD. Test No. 489: In vivo Mammalian alkaline Comet assay. OECD Publishing; Paris: 2014. pp. 1–25. [Google Scholar]
- 16.Mohamed HM, Mohamed MA. Effect of different doses of nandrolone decanoate on lipid peroxidation, DNA fragmentation, sperm abnormality and histopathology of testes of male Wister rats. Exp Toxicol Pathol. 2014;67:1–11. doi: 10.1016/j.etp.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Ceribasi AO, Sakin F, Turk G, Sonmez M, Atessahin A. Impact of ellagic acid on adriamycin-induced testicular histopathological lesions, apoptosis, lipid peroxidation and sperm damages. Exp Toxicol Pathol. 2012;64:717–724. doi: 10.1016/j.etp.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 18.La Maestra S, De Flora S, Micale RT. Effect of cigarette smoke on DNA damage, oxidative stress, and morphological alterations in mouse testis and spermatozoa. Int J Hyg Environ Health. 2015;218:117–122. doi: 10.1016/j.ijheh.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Mojica-Villegas MA, Izquierdo-Vega JA, Chamorro-Cevallos G, Sanchez-Gutierrez M. Protective effect of resveratrol on biomarkers of oxidative stress induced by iron/ascorbate in mouse spermatozoa. Nutrients. 2014;6:489–503. doi: 10.3390/nu6020489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan S, Ahmad T, Parekh CV, Trivedi PP, Kushwaha S, Jena G. Investigation on sodium valproate induced germ cell damage, oxidative stress and genotoxicity in male Swiss mice. Reprod Toxicol. 2011;32:385–394. doi: 10.1016/j.reprotox.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Rahimipour M, Talebi AR, Anvari M, Sarcheshmeh AA, Omidi M. Effects of different doses of ethanol on sperm parameters, chromatin structure and apoptosis in adult mice. Eur J Obstet Gynecol Reprod Biol. 2013;170:423–428. doi: 10.1016/j.ejogrb.2013.06.038. [DOI] [PubMed] [Google Scholar]
- 22.Yarbro JW. Mechanism of action of hydroxyurea. Semin Oncol. 1992;19:1–10. [PubMed] [Google Scholar]
- 23.Donehower RC. An overview of the clinical experience with hydroxyurea. Semin Oncol. 1992;19:11–19. [PubMed] [Google Scholar]
- 24.Evenson DP, Jost LK. Hydroxyurea exposure alters mouse testicular kinetics and sperm chromatin structure. Cell Prolif. 1993;26:147–159. doi: 10.1111/j.1365-2184.1993.tb00015.x. [DOI] [PubMed] [Google Scholar]
- 25.Jones KM, Niaz MS, Brooks CM, Roberson SI, Aguinaga MP, Hills ER, Rice VM, Bourne P, Bruce D, Archibong AE. Adverse effects of a clinically relevant dose of hydroxyurea used for the treatment of sickle cell disease on male fertility endpoints. Int J Environ Res Public Health. 2009;6:1124–1144. doi: 10.3390/ijerph6031124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi NY, Park YS, Ryu JS, Lee HJ, Arauzo-Bravo MJ, Ko K, Han DW, Scholer HR, Ko K. A novel feeder-free culture system for expansion of mouse spermatogonial stem cells. Mol Cells. 2014;37:473–479. doi: 10.14348/molcells.2014.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guthrie HD, Welch GR. Effects of reactive oxygen species on sperm function. Theriogenology. 2012;78:1700–1708. doi: 10.1016/j.theriogenology.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Sanocka D, Kurpisz M. Reactive oxygen species and sperm cells. Reprod Biol Endocrinol. 2004;2:12. doi: 10.1186/1477-7827-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakano K, Oikawa S, Hasegawa K, Kawanishi S. Hydroxyurea induces site-specific DNA damage via formation of hydrogen peroxide and nitric oxide. Jpn J Cancer Res. 2001;92:1166–1174. doi: 10.1111/j.1349-7006.2001.tb02136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu J, Song D, Luo G, Xu S, Cao Y, Sun Z. Activation of Toll like receptor 3 induces spermatogonial stem cell apoptosis. Cell Biochem Funct. 2015;33:415–420. doi: 10.1002/cbf.3133. [DOI] [PubMed] [Google Scholar]
- 31.Zhou L, Wu CQ, Luo YW, Liao MY, Sun ZY. Studies on the characteristics and mechanisms of testicular toxicity induced by Hydroxyurea. Toxicol Mech Methods. 2015;25:396–401. doi: 10.3109/15376516.2015.1045657. [DOI] [PubMed] [Google Scholar]
- 32.Sakai T, Takahashi M, Mitsumori K, Yasuhara K, Kawashima K, Mayahara H, Ohno Y. Collaborative work to evaluate toxicity on male reproductive organs by repeated dose studies in rats. J Toxicol Sci. 2000;25:1–21. doi: 10.2131/jts.25.SpecialIssue_1. [DOI] [PubMed] [Google Scholar]
- 33.Ozawa S, Yokoi R, Kitamura T, Kuriyama K, Kobayashi K, Shibata N. Collaborative work to evaluate toxicity on male reproductive organs by repeated dose studies in rats 15) two-week and 4-week administration study of methyl methanesulfonate (MMS) J Toxicol Sci. 2000;25:155–162. doi: 10.2131/jts.25.SpecialIssue_155. [DOI] [PubMed] [Google Scholar]
- 34.Wiger R, Hongslo JK, Evenson DP, De Angelis P, Schwarze PE, Holme JA. Effects of acetaminophen and hydroxyurea on spermatogenesis and sperm chromatin structure in laboratory mice. Reprod Toxicol. 1995;9:21–33. doi: 10.1016/0890-6238(94)00052-X. [DOI] [PubMed] [Google Scholar]
- 35.Trivedi PP, Kushwaha S, Tripathi DN, Jena GB. Evaluation of male germ cell toxicity in rats: correlation between sperm head morphology and sperm comet assay. Mutat Res. 2010;703:115–121. doi: 10.1016/j.mrgentox.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Steller H. Mechanisms and genes of cellular suicide. Science. 1995;267:1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- 37.Bejarano I, Espino J, Paredes SD, Ortiz Á, Lozano G, Pariente JA, Rodríguez AB. In: Apoptosis, ROS and calcium signaling in Human Spermatozoa: Relationship to Infertility. Bashamboo A, editor. InTech; Croatia: 2012. pp. 51–76. [Google Scholar]
- 38.Moline JM, Golden AL, Bar-Chama N, Smith E, Rauch ME, Chapin RE, Perreault SD, Schrader SM, Suk WA, Landrigan PJ. Exposure to hazardous substances and male reproductive health: a research framework. Environ Health Perspect. 2000;108:803–813. doi: 10.1289/ehp.00108803. [DOI] [PMC free article] [PubMed] [Google Scholar]