Abstract
Background
Means to reduce future risk for cardiovascular disease in subjects with type 2 diabetes are urgently needed.
Methods
Thirty-two patients with type 2 diabetes (age 59±8 years) followed a Paleolithic diet for 12 weeks. Participants were randomized to either standard care exercise recommendations (PD) or 1-h supervised exercise sessions (aerobic exercise and resistance training) three times per week (PD-EX).
Results
For the within group analyses, fat mass decreased by 5.7 kg (IQR: −6.6, −4.1; p<0.001) in the PD group and by 6.7 kg (−8.2, −5.3; p<0.001) in the PD-EX group. Insulin sensitivity (HOMA-IR) improved by 45% in the PD (p<0.001) and PD-EX (p<0.001) groups. HbA1c decreased by 0.9% (−1.2, −0.6; p<0.001) in the PD group and 1.1% (−1.7, −0.7; p<0.01) in the PD-EX group. Leptin decreased by 62 % (p<0.001) in the PD group and 42 % (p<0.001) in the PD-EX group. Maximum oxygen uptake increased by 0.2 L/min (0.0, 0.3) in the PD-EX group, and remained unchanged in the PD group (p<0.01 for the difference between intervention groups). Male participants decreased lean mass by 2.6 kg (−3.6, −1.3) in the PD group and by 1.2 kg (−1.3, 1.0) in the PD-EX group (p<0.05 for the difference between intervention groups).
Conclusions
A Paleolithic diet improves fat mass and metabolic balance including insulin sensitivity, glycemic control, and leptin in subjects with type 2 diabetes. Supervised exercise training may not enhance the effects on these outcomes, but preserves lean mass in men and increases cardiovascular fitness.
Keywords: Type 2 diabetes, Paleolithic diet, Diet intervention, Exercise, Glycosylated hemoglobin A, Insulin sensitivity, leptin
Introduction
Among patients with diabetes, cardiovascular disease is the primary cause of death [1]. Thus, in this population, it is imperative to counteract cardiovascular risk factors, such as hyperglycemia, high blood pressure, and dyslipidemia through diet, exercise, and drug treatment [2]. Earlier studies suggested a Paleolithic diet had powerful beneficial metabolic effects on obesity, as well as in type 2 diabetes [3, 4]. This diet emphasizes a high intake of vegetables, fruit, nuts, eggs, fish, and lean meat, while excluding refined sugar, salt, legumes, dairy products, and grains.
Additional metabolic effects beyond diet may be achieved with structured exercise interventions [5, 6]. The combination of diet interventions with energy restrictions and resistance training or aerobic exercise is beneficial for body composition in non-diabetic subjects [7]. In subjects with type 2 diabetes, the combination of aerobic exercise with resistance training lowers HbA1c levels more than either exercise modality separately [8]. To the best of our knowledge, studies on Paleolithic diet combined with resistance training and aerobic exercise have not been performed in subjects with type 2 diabetes.
In the present study, subjects with type 2 diabetes consumed a Paleolithic diet for 12 weeks, with or without supervised aerobic exercise and resistance training. Our hypothesis was that exercise training would improve the beneficial effects of a Paleolithic diet on fat mass and metabolic balance including insulin sensitivity, glycemic control, and leptin.
Materials and methods
Study design
We conducted a randomized controlled trial with two arms: Paleolithic diet and standard care exercise recommendations (PD) and Paleolithic diet with 1-h supervised exercise sessions three times per week (PD-EX). In a secondary analysis, we included a non-randomized observational group as a reference.
Participants of the randomized controlled trial
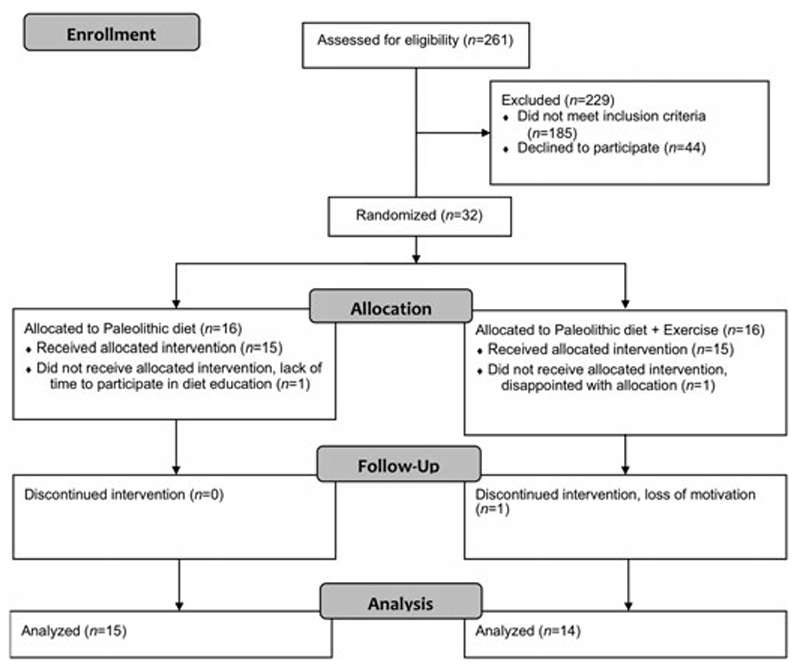
Subjects were recruited from the greater Umeå area of Northern Sweden through advertisements in local newspapers and posters at Umeå University Hospital. Recruitment began in 2012, and the study was completed in June 2014. We included individuals diagnosed with type 2 diabetes within the past 10 years, who had a BMI of 25–40 kg/m2 and were weight stable (i.e. <5% weight loss) for 6 months before study inclusion. Eligible males were 30–70 years old, while women were included after menopause and up to 70 years of age. All participants had HbA1c values between 6.5% and 10.8% (47–94 mmol/mol), and were using lifestyle modification and/or metformin for diabetes treatment. Exclusion criteria were treatment with anti-diabetic drugs other than metformin, use of beta-blockers, blood pressure > 160/100 mmHg, macroalbuminuria, heart disease, and being a smoker. Since we aimed to study the effect of exercise on sedentary individuals, we excluded those who reported more than 30 min of moderate physical activity 5 days per week or resistance training more than once every other week during the past 6 months. Of 261 volunteers who were interested in participating, 32 met the inclusion criteria and were randomized into the PD or PD-EX groups (Fig. 1). All participants provided written informed consent. The study protocol was in accordance with the Helsinki declaration, and was approved by the Regional Ethical Review Board, Umeå, Sweden.
Fig. 1.
CONSORT flow diagram.
Randomization and blinding
Participants were assigned to the PD and PD-EX groups using biased coin minimization with an allocation ratio of 1:1 [9]. To minimize marginal imbalance based on the prognostic factors (sex and BMI above/below 30), participants were sequentially allocated using a base probability of 0.9. The computer program MinimPy was used for treatment allocation [10]. Randomization was conducted after the baseline examinations. The study was single-blinded, such that group allocation was unknown to all staff that performed examinations and dietary counseling. Additionally, the statistician who randomized the participants and the research assistant who informed the participants of the randomization outcome were not involved in data collection or data analysis. The study was unblinded after the analysis of results.
Diet intervention
Both randomized groups (PD and PD-EX) were introduced to the Paleolithic diet after baseline examinations, and were instructed to follow the diet until all study measurements were completed. The diet was based on consuming lean meat, fish, seafood, eggs, vegetables, fruits, berries, and nuts. Cereals, dairy products, legumes, refined fats, refined sugars, and salt were excluded with the exception of canned fish and cold cuts like ham. The diet was consumed ad libitum, with restrictions of the following: eggs (1–2/day but a maximum of 5/week), potatoes (1 medium sized/day), dried fruit (130 g/day), and nuts (60 g/day). Rapeseed or olive oil (maximum 15 g/day) and small amounts of honey and vinegar were allowed as flavoring in cooking. Participants were instructed to drink mainly still water. Coffee and tea were restricted to a maximum of 300 g/day, and red wine to a maximum of one glass/week.
Each group participated separately in five group sessions held by a trained dietician at the Department of Food and Nutrition, Umeå University, Sweden. The first two meetings were held during the first 2 weeks, and the following meetings took place once a month. The participants received information about the diet and cooked food and were given recipes. Between the meetings, the participants could contact the dietician, who held the meetings by e-mail or phone.
Exercise intervention
Prior to randomization, both intervention groups received exercise recommendations based on the current guidelines for patients with type 2 diabetes. Thus, all study participants were advised to perform moderate exercise (e.g. brisk walking) for at least 30 min every day. The PD-EX group underwent a program comprising a combination of aerobic exercise and resistance training in 1-h sessions three times weekly at the Sports Medicine unit at Umeå University. The exercise sessions were performed on weekdays, with at least 1 day of rest between sessions. They were supervised by experienced personal trainers with bachelor’s degrees in Sports Medicine. The training protocol had a progressive design in accordance with the guidelines of the American College of Sports Medicine [11].
All exercise sessions started with aerobic exercise. The first session of each week consisted of low-intensity aerobic training at 70% of the maximum heart rate on a cross-trainer (Monark Prime, XT 50, Vansbro, Sweden). The second session of the week consisted of ten high-intensity sprint intervals at 100% of the maximal workload on a cycle-ergometer (Monark, Ergomedic 839E, Vansbro, Sweden), with low-intensity cycling between the sprints. The third session of each week comprised six moderate-intensity 5-min intervals between 45 and 60% of maximal workload on a cycle-ergometer. The duration/workload of the intervals increased every other week. When necessary, the intensity of the aerobic exercise sessions was adjusted in accordance with the participant’s performance.
After the aerobic exercise, the sessions progressed to resistance training with both upper and lower body exercises, including leg presses, seated leg extensions, leg curls, hip raises, flat and incline bench presses, seated rows, dumbbell rows, lat pull-downs, shoulder raises, back extensions, burpees, sit-ups, step-ups, and wall ball shots. At each training session, the participant performed 3–5 of the aforementioned resistance exercises, with 10–15 repetitions and 2–4 sets. Once participants could complete all repetitions, the workload was increased for the following session.
Measurements
At baseline and at 12 weeks, dietary intake was assessed using a 4-day self-reported weighed food record. Each 4-day food record period included 1 or 2 weekend days. Participants were instructed to weigh all food, beverages, and leftovers. Any uncertainties regarding the food records were clarified during meetings, via e-mail, or by phone. A trained dietician converted the reported food intake into estimated energy and nutrient intake using the nutritional analysis software Dietist XP version 3.2 (Kost och Näringsdata AB, Bromma, Sweden), based on the Swedish National Food Administration’s food database.
Participants were examined at baseline and after 12 weeks of the intervention by experienced physicians and nurses at the Clinical Research Center at Umeå University Hospital, Umeå, Sweden. Resting energy expenditure was measured using indirect calorimetry (Datex-Ohmeda Deltatrac II; Datex-Ohmeda Inc., Madison, WI, USA) and adjusted by subtracting 5% during 8 h of sleep. Daily physical activity energy expenditure over a 7-day period was estimated using data from a combined accelerometer and heart rate monitor (Actiheart®; CamNtech Ltd., Cambridge, UK) [12], modeled as described previously [13–15]. Diet-induced thermogenesis was fixed at 10% of the total energy expenditure. Total energy expenditure was calculated as the sum of the resting energy expenditure and physical activity expenditure plus 10%.
Fat mass (i.e. the primary outcome) and lean mass were analyzed by dual-energy X-ray absorptiometry (Lunar Prodigy X-ray Tube Housing Assembly, Brand BX-1L, Model 8743; GE Medical Systems, Madison, WI, USA). The participants were weighed on a digital calibrated scale, wearing light clothing. Height was measured with a calibrated height-measuring gauge. Waist circumference was assessed with a measuring tape placed midway between the lowest rib and iliac crest during gentle exhalation. The abdomen height was measured at the umbilicus level with the participant lying down with straight legs.
An automated blood pressure meter (Boso Medicus, Bosch, Germany) was used to measure systolic and diastolic blood pressure from the right arm with the participant in a sitting position. Measurements were made twice at 2-min intervals, after 5 min of rest. Fasting venous blood samples were collected from patients in the intervention groups for analysis of HbA1c, serum insulin, serum cholesterol, high density lipoprotein (HDL), serum triglycerides, and plasma high-sensitivity C-reactive protein at the Department for Clinical Chemistry, Umeå University Hospital. We analyzed fasting glucose from a capillary sample (HemoCue 201 RT; Radiometer Medical Aps, Brønshøj, Denmark). Aliquots of plasma were immediately stored at -80°C for analysis of non-esterified fatty acids (NEFAs), adiponectin, and leptin after study completion. The NEFAs were analyzed with NEFA-HR2 (Wako Chemicals, Neuss, Germany), adiponectin with the Human Adiponectin ELISA Kit, and leptin with the Human Leptin ELISA Kit, both from Merck Millipore (Darmstadt, Germany). Insulin sensitivity was calculated as follows: homeostatic model assessment of insulin resistance (HOMA-IR) = (fasting glucose × fasting insulin)/22.5 and the revised quantitative insulin sensitivity check index (Revised QUICKI) = 1/(log fasting glucose + log fasting insulin + log NEFA) [16, 17]. The low density lipoprotein (LDL) was calculated as follows: serum cholesterol – serum HDL – serum triglycerides)/2.2. The maximal oxygen uptake (VO2max) and maximal workload were measured via cardiopulmonary exercise test at the Department of Clinical Physiology, Umeå University Hospital, Umeå, Sweden.
Observational group
For a secondary analysis, we recruited an observational group by advertisement in local newspapers and among those who were excluded from the intervention because of a lack of time, beta-blocker use, and cardiovascular disease. Nine individuals were included in the observational group, one of whom could not attend the assessments at the end of the intervention period due to illness. Fasting glucose, fasting insulin, HbA1c, leptin, adiponectin, and blood lipids were analyzed from venous blood samples. Body composition, weight, blood pressure, dietary intake, and physical activity energy expenditure were examined as described above.
Sample size and statistical analysis
The primary outcome in this study was the change in fat mass. Based on previous results from a similar study [18], we calculated that 13 individuals in each intervention group would be sufficient to detect a significant difference (p<0.05) with 80% power. Since several variables had a skewed distribution, the Wilcoxon rank-sum test was used to compare groups. All data were reported as medians with the interquartile range. The primary analysis compared treatment effects (change from 0 to 12 weeks) between the PD and PD-EX groups. The change over time within each intervention group was determined using the Wilcoxon signed-rank test. The secondary analysis compared the treatment effect (change from 0 to 12 weeks) in each intervention group with the observational group. A two-sided p value of <0.05 was considered statistically significant. All statistical analyses were performed using R version 3.1.1, a language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria).
Results
Subject characteristics
The participants’ baseline characteristics are presented in Tables 1 and 2. The randomized groups did not differ in age, sex, BMI, or diabetes duration. The PD-EX group had higher fasting glucose and HDL levels than the PD group. During the course of the study, one participant in the PD group stopped his metformin treatment, two participants in the PD group stopped their blood pressure medication, and one participant in the PD group started antihypertensive treatment.
Table 1.
Participants’ baseline characteristics
| Paleolithic diet (n=15) | Paleolithic diet + Exercise (n=14) | |
|---|---|---|
| Age (years) | 60 (53–64) | 61 (58–66) |
| Men/women (n) | 10/5 | 9/5 |
| Diabetes duration (years) | 3 (1–5) | 5.5 (1–8) |
| BMI (kg/m2) | 31.4 (29.4–33.1) | 31.7 (29.2–35.4) |
| Diabetes treatment (n) | ||
| Diet only | 5 | 4 |
| Metformin | 10 | 10 |
| Other treatment (n) | ||
| ACEI/ARB | 9 | 10 |
| Diuretic | 6 | 5 |
| Calcium-channel blocker | 4 | 5 |
| Statin | 6 | 8 |
| Antiplatelet drug | 2 | 3 |
| Other | 8 | 2 |
Data are reported as the median (interquartile range). Abbreviations: ACEI, ACE inhibitor; ARB, angiotensin receptor blocker.
Table 2.
Energy balance, body composition, and cardiovascular risk factors during 12 weeks of intervention
| Paleolithic diet (n=15) | Paleolithic diet + Exercise (n=14) | |
|---|---|---|
| Energy balance | ||
| Energy intake (kcal/day) | ||
| Baseline | 2022 (1583–2268) | 1595 (1428–2257) |
| Change 0–12 weeks | –291 (–587, –66)## | –530 (–863, –157)### |
| Physical activity energy expenditure (kcal/day) | ||
| Baseline | 1022 (904–1319) | 997 (806–1568) |
| Change 0–12 weeks | –28 (–208, 30) | –18 (–368, 340) |
| Physical activity energy expenditure (kcal/kg/day) | ||
| Baseline | 11.6 (10.6–13.0) | 10.1 (9.2–16.6) |
| Change 0–12 weeks | 0.1 (–1.5, 2.1) | 0.6 (–2.9, 4.8) |
| Resting energy expenditure (kcal/day) | ||
| Baseline | 1620 (1463–1719) | 1709 (1319–1883) |
| Change 0–12 weeks | –120 (–157, –71)### | –89 (–164, –44)## |
| Resting energy expenditure (kcal/kg/day) | ||
| Baseline | 17.0 (16.4–17.6) | 16.7 (15.7–18.6) |
| Change 0–12 weeks | 0.0 (–0.4, 0.8) | 0.5 (–0.3, 1.4) |
| Total energy expenditure (kcal/day) | ||
| Baseline | 2995 (2754–3356) | 2960 (2433–3855) |
| Change 0–12 weeks | –227 (–307, –91)# | –312 (–562, 122) |
| Weight (kg) | ||
| Baseline | 90.0 (83.3–100.8) | 97.3 (83.9–110.3) |
| Change 0–12 weeks | –7.1 (–9.7, –6.3)### | –7.1 (–8.7, –6.2)### |
| Body composition | ||
| Body fat (%) | ||
| Baseline | 37.8 (33.1–40.8) | 37.7 (34.7–43.1) |
| Change 0–12 weeks | –3.5 (–4.4, –2.6)### | –4.1 (–5.8, –3.4)### |
| Lean mass (kg) | ||
| Baseline | 56.5 (49.0–63.8) | 61.0 (44.1–66.8) |
| Change 0–12 weeks | –1.4 (–3.3, –1.2)### | –1.2 (–1.4, –0.2) |
| Waist circumference (cm) | ||
| Baseline | 111 (105–116) | 108 (104–115) |
| Change 0–12 weeks | –9 (–12, –7)### | –8 (–10, –7)### |
| Abdominal height (cm) | ||
| Baseline | 27.2 (25.0–29.0) | 26.5 (23.0–29.8) |
| Change 0–12 weeks | –3.6 (–4.7, –2.5)### | –3.0 (–4.3, –1.1)### |
| Glucose metabolism | ||
| HbA1c (%) | ||
| Baseline | 7.1 (6.5–7.3) | 7.3 (6.8–7.6) |
| Change 0–12 weeks | −0.9 (−1.2, −0.6)### | −1.1 (−1.7, −0.7)## |
| HbA1c (mmol/mol) | ||
| Baseline | 54 (48–57) | 57 (51–60) |
| Change 0–12 weeks | −10 (−13, −6)### | −12 (−19, −8)## |
| Fasting glucose (mmol/L) | ||
| Baseline | 8.0 (7.2–8.4) | 8.9 (7.9–10.5)* |
| Change 0–12 weeks | −0.9 (−1.7, −0.2)# | −2.0 (−3.2, −1.1)## |
| Fasting insulin (mIU/L) | ||
| Baseline | 23 (15–30) | 16 (11–20) |
| Change 0–12 weeks | −8 (−16, −3)## | −4 (−8, −2)### |
| Revised QUICKI | ||
| Baseline | 0.223 (0.192–0.227) | 0.207 (0.196–0.224) |
| Change 0–12 weeks | 0.027 (0.004, 0.054)## | 0.041 (0.031, 0.054)### |
| Cardiovascular fitness | ||
| VO2max (mL/kg/min) | ||
| Baseline | 23.4 (21.5–27.0) | 22.5 (21.0–25.2) |
| Change 0–12 weeks | 1.9 (0.6, 2.9)### | 3.3 (2.7, 6.2)###* |
| Resting heart rate (bpm) | ||
| Baseline | 70 (65–78) | 72 (66–77) |
| Change 0–12 weeks | −3 (−8, 1) | −11 (−12, −7)#* |
| Blood pressure | ||
| Systolic (mmHg) | ||
| Baseline | 135 (127–148) | 132 (122–143) |
| Change 0–12 weeks | −17 (−24, 0)## | −11 (−14, −7)### |
| Diastolic (mmHg) | ||
| Baseline | 86 (82–94) | 82 (74–91) |
| Change 0–12 weeks | −9 (−15, −6)### | −10 (−13, −7)### |
| Blood lipids | ||
| Total cholesterol (mmol/L) | ||
| Baseline | 4.2 (3.4–4.7) | 4.3 (4.1–4.9) |
| Change 0–12 weeks | −0.3 (−0.6, 0.1) | −0.6 (−0.6, −0.4)## |
| Triglycerides (mmol/L) | ||
| Baseline | 2.1 (1.4–2.9) | 1.7 (1.1–2.4) |
| Change 0–12 weeks | −0.6 (−1.5, −0.2)## | −0.5 (−1.0, −0.2)### |
| HDL (mmol/L) | ||
| Baseline | 0.85 (0.81–0.99) | 1.09 (0.98–1.21)** |
| Change 0–12 weeks | –0.01 (–0.08, 0.05) | 0.01 (–0.03, 0.07) |
| LDL (mmol/L) | ||
| Baseline | 2.1 (1.8–2.7) | 2.4 (2.0–3.0) |
| Change 0–12 weeks | –0.1 (–0.4, 0.2) | –0.1 (–0.5, 0.1) |
| NEFA (μmol/L) | ||
| Baseline | 599 (560–756) | 826 (670–922) |
| Change 0–12 weeks | 26 (–12, 173) | –56 (–128, 117) |
| Adipokines | ||
| Leptin (ng/mL) | ||
| Baseline | 13.8 (6.4–26.5) | 13.3 (7.2–16.7) |
| Change 0–12 weeks | –8.5 (–12.2, –2.6)### | –5.6 (–9.4, –3.5)### |
| Adiponectin (ng/mL) | ||
| Baseline | 4685 (2942–5939) | 4864 (4004–6268) |
| Change 0–12 weeks | 379 (213, 717)## | 5 (–256, 282)* |
| High-sensitivity CRP (mg/L) | ||
| Baseline | 1.2 (0.7–1.9) | 1.5 (0.7–2.5) |
| Change 0–12 weeks | –0.4 (–1.1, 0.0) | –0.4 (–0.9, 0.0)## |
Data are reported as the median (interquartile range); *p<0.05, **p<0.01, ***p<0.001 between the Paleolithic diet group and the Paleolithic diet + Exercise group; #p<0.05, ##p<0.01, ###p<0.001 for the change over time from baseline to 12 weeks within the group. Abbreviation: CRP, C-reactive protein.
Compliance with the diet and supervised exercise program
Dietary intake did not differ between the groups at baseline and 12 weeks, except for a higher fiber intake in the PD-group at 12 weeks (Table 3). Both groups increased their relative intake of protein and their intake of monounsaturated and polyunsaturated fatty acids. Both groups lowered their intake of carbohydrates and saturated fatty acids. The reduction of sodium intake was only significant in the PD-EX group. Nine of the 14 participants in the PD-EX group completed the 36 exercise sessions according to the study protocol. The remaining five participants completed between 27 and 35 workouts during the study period. The participants in the PD-EX group increased the cumulative weight load (weight × repetitions × sets) with the leg press during one exercise session from 1350 kg (900−1800) to 3000 kg (2700−4000) after 12 weeks.
Table 3.
Dietary intake
| Paleolithic diet (n=14) | Paleolithic diet + Exercise (n=13) | |
|---|---|---|
| Protein (g/day) | ||
| Baseline | 83 (72–99) | 77 (67–106) |
| 12 weeks | 96 (80–111) | 79 (58–100) |
| Carbohydrate (g/day) | ||
| Baseline | 200 (160–262) | 169 (152–197) |
| 12 weeks | 127 (93–158)### | 77 (71–102)### |
| Total fat (g/day) | ||
| Baseline | 88 (66–102) | 67 (48–94) |
| 12 weeks | 71 (56–97) | 61 (47–79) |
| Protein (E%) | ||
| Baseline | 17 (15–19) | 18 (17–20) |
| 12 weeks | 24 (19–27)### | 26 (22–29)### |
| Carbohydrate (E%) | ||
| Baseline | 41 (37–45) | 42 (33–48) |
| 12 weeks | 31 (24–39)## | 27 (24–29)### |
| Total fat (E%) | ||
| Baseline | 39 (37–40) | 34 (31–41) |
| 12 weeks | 42 (37–48) | 45 (37–47)# |
| Saturated fatty acids (E%) | ||
| Baseline | 15.3 (13.4–16.8) | 13.3 (12.1–17.1) |
| 12 weeks | 9.6 (7.9–11.6)### | 8.8 (8.7–10.7)### |
| Monounsaturated fatty acids (E%) | ||
| Baseline | 14 (14–17) | 12 (11–15) |
| 12 weeks | 20 (16–24)# | 23 (20–24)## |
| Polyunsaturated fatty acids (E%) | ||
| Baseline | 5.0 (4.8–6.4) | 5.4 (4.0–6.3) |
| 12 weeks | 7.9 (6.6–8.7)# | 8.4 (6.8–9.7)## |
| Saturated fatty acids (g/day) | ||
| Baseline | 34 (24–44) | 27 (20–35) |
| 12 weeks | 15 (12–23)### | 14 (10–17)### |
| Monounsaturated fatty acids (g/day) | ||
| Baseline | 31 (26–38) | 27 (17–36) |
| 12 weeks | 36 (25–52) | 28 (23–40) |
| Polyunsaturated fatty acids (g/day) | ||
| Baseline | 12 (10–13) | 10 (7–14) |
| 12 weeks | 15 (10–19) | 13 (7–16) |
| Omega-3 fatty acids (g/day) | ||
| Baseline | 2.3 (2.0–3.1) | 2.2 (1.4–2.9) |
| 12 weeks | 2.4 (1.3–4.6) | 2.7 (1.3–2.9) |
| Omega-6 fatty acids (g/day) | ||
| Baseline | 10.9 (8.6–12.0) | 8.0 (6.7–10.8) |
| 12 weeks | 11.8 (7.5–16.1) | 10.6 (6.1–13.8) |
| Dietary cholesterol (mg/day) | ||
| Baseline | 315 (213–364) | 324 (206–526) |
| 12 weeks | 531 (390–686)## | 510 (399–632) |
| Dietary fiber (g/day) | ||
| Baseline | 21 (18–26) | 20 (18–22) |
| 12 weeks | 23 (15–30)* | 14 (13–17)##* |
| Sodium (mg/day) | ||
| Baseline | 3051 (2610–3863) | 3003 (2449–4097) |
| 12 weeks | 2119 (1745–2843) | 1789 (1223–2786)# |
Data are reported as the median (interquartile range); *p<0.05 between the Paleolithic diet group and the Paleolithic diet + Exercise group; #p<0.05, ##p<0.01, ###p<0.001 for the change over time from baseline to 12 weeks within the group.
Energy balance
At baseline, energy intake (kcal/day) and total energy expenditure (kcal/day) did not differ between groups (Table 2). Baseline energy intake in the PD group was 1112 kcal/day (−1434, −609) less than the total energy expenditure. In the PD-EX group, baseline energy intake was 1340 kcal/day (−1909, −778) less than the total energy expenditure. Energy intake decreased in both groups during the intervention (Table 2). Furthermore, total energy expenditure decreased in the PD group (p<0.05), but remained stable in the PD-EX-group (p=0.17, Table 2). This was caused by a decrease in the resting energy expenditure, while the physical activity energy expenditure was unchanged. At the end of the study, the PD group reported an energy intake that was 1245 kcal/day (−1480, −905) less than the total energy expenditure; while, the energy intake of the PD-EX group was 1657 kcal/day (−2533, −881) less than total energy expenditure.
Body composition
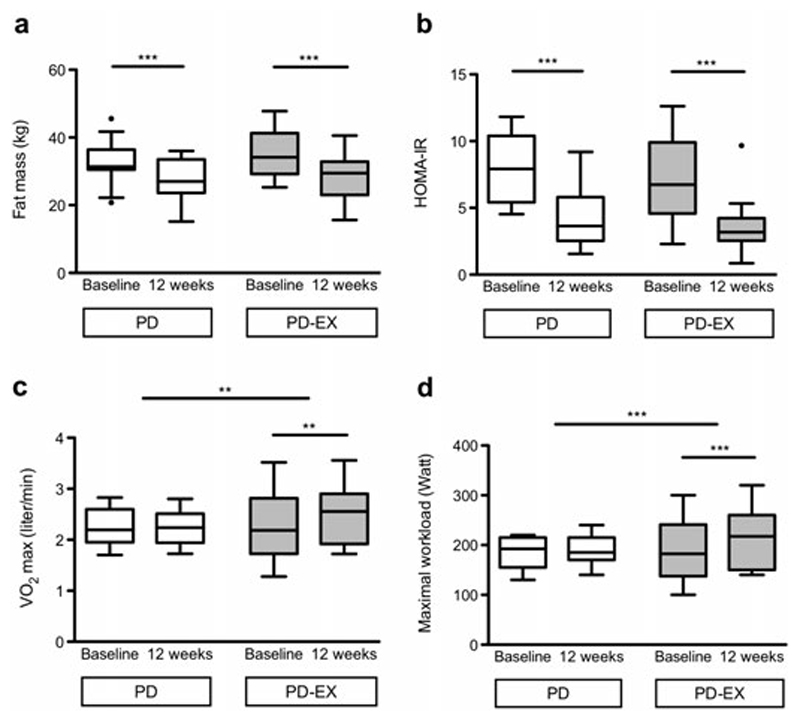
Fat mass decreased during the study in both the PD and PD-EX groups (Fig. 2). Both groups also showed decreases in body weight, abdominal height, and waist circumference, without differences between intervention groups (Table 2). Male participants decreased their waist circumference more in the PD-group compared to the PD-EX group (p<0.05, Supplementary Table 2). Males in the PD-EX group retained more lean mass than males in the PD-group (p<0.05, Supplementary Table 2).
Fig. 2.
Fat mass (a), insulin sensitivity (b), and cardiovascular fitness (c and d) during 12 weeks following either a Paleolithic diet with a supervised exercise program (PD-EX) or a Paleolithic diet combined with general exercise recommendations (PD). Boxes represent medians and IQRs, whiskers represent the most extreme values besides outliers, and filled circles represent outliers (>1.5 IQR); **p<0.01, ***p<0.001.
Glucose metabolism
Insulin sensitivity and glycemic control improved in both groups, without a difference between groups. The HOMA-IR and revised QUICKI improved in both intervention groups (Fig. 2, Table 2), and the HbA1c decreased during the study in both the PD group (19%) and the PD-EX group (20%, Table 2).
Cardiovascular fitness
Resting heart rate decreased more in the PD-EX group than the PD group (Table 2). The VO2max and the ergometer cycling workload increased during the study in the PD-EX group, but not in the PD group (Fig. 2).
Blood pressure and blood lipids
Blood pressure decreased during the study in both intervention groups without any group difference: systolic, 13% in PD and 8% in PD-EX; diastolic, 10% in PD and 12% in PD-EX (Table 2). Triglycerides decreased in both study groups between baseline and 12 weeks; while, the HDL, LDL, and NEFA levels remained unchanged throughout the intervention (Table 2).
Adipokines
Leptin decreased in both the PD group (62%) and the PD-EX group (42%) (Table 2). Adiponectin increased in the PD group (8%) compared with the PD-EX group (Table 2).
Intervention groups (PD and PD-EX) versus the observational group
There were no significant differences in baseline characteristics between the observational group and the PD and PD-EX intervention groups (Supplementary Table 1). Compared with the observational group, the PD and PD-EX groups decreased their total energy intakes and intakes of carbohydrates and saturated fatty acids, but increased their relative intake of protein and monounsaturated fatty acids during the 12 weeks of intervention (Supplementary Tables 2 and 3). The intervention groups improved fat mass (p<0.001), HOMA-IR, fasting insulin, HbA1c, systolic and diastolic blood pressure, and leptin compared with the observational group (Supplementary Table 2). Triglycerides did not decrease in the intervention group compared with the observational group (Supplementary Table 2).
Discussion
Twelve weeks on a Paleolithic diet improved fat mass and metabolic balance including insulin sensitivity, glycemic control and leptin among individuals with type 2 diabetes. The addition of resistance training and aerobic exercise under observation increased cardiovascular fitness, without further improvements in fat mass or glycemic control. The observed effects of the Paleolithic diet were substantial. The lowering of HbA1c by 0.9% units was an effect size similar to that reported with metformin in type 2 diabetes [19]. A previous study demonstrated that the Paleolithic diet reduced HbA1c by 0.4% units more than a conventional diabetes diet [4]. The UK prospective diabetes study (UKPDS) stated that a 1% unit improvement of HbA1c reduces microvascular complications by 37% and reduces diabetes-related death by 21% [20]. Thus, if sustained over time, the improvement in glycemic control will provide large benefits in terms of morbidity and mortality.
These powerful observed effects of the Paleolithic diet may be explained by altered dietary patterns. The participants reported reduced intakes of carbohydrates and saturated fatty acids, with relatively higher intakes of protein, as well as monounsaturated and polyunsaturated fatty acids. The reduction of carbohydrates with a high glycemic index may be an important part of the beneficial effects of this diet [21]. Furthermore, increased intake of monounsaturated fat may reduce postprandial hyperglycemia [22].
Supervised training with aerobic exercise in combination with resistance training did not improve glycemic control and insulin sensitivity beyond the improvements observed with the Paleolithic diet alone. This was unexpected, as exercise training has previously been shown to improve glycemic control substantially, particularly when combining resistance training with aerobic exercise for more than 150 min per week [6, 23, 24]. Among individuals with type 2 diabetes, structured exercise interventions reduce HbA1c levels by about 0.6% units without weight changes [6]. In contrast, the addition of resistance or aerobic exercise to short-term (16 weeks) dietary interventions with a calorie-restricted high-protein diet or a very low-calorie diet had limited additive effects on insulin sensitivity and glycemic control in patients with type 2 diabetes [18, 25]. Notably, our diet recommendations were given ad libitum without any restrictions of caloric intake. However, it is possible that the catabolic state caused by decreased energy intake may have masked any potential effects of exercise on glycemic control and insulin sensitivity. Moreover, several study participants were using metformin or statins on a daily basis. These drugs may blunt the positive effects of exercise [26–28].
Importantly, cardiovascular fitness improved significantly in the PD-EX group compared with the PD group. Low cardiorespiratory fitness is a strong risk factor for all-cause mortality, independent of glycemic status and other cardiovascular risk factors [29, 30]. A large cohort study showed that an increase of 1.44 mL/kg/min in VO2max (equivalent to a 1-min increase in the Balke protocol treadmill time) corresponded to a 7.9% reduction in overall mortality [31]. Using these data, the presently observed increase of 3.3 mL/kg/min in VO2max would lead to an 18% reduction in all-cause mortality if changes can be sustained over time. Furthermore, it is of major interest to study if tissue-specific insulin sensitivity is influenced differently between groups. We demonstrated that weight reduction by a Paleolithic diet had a profound effect on liver insulin sensitivity, while peripheral insulin sensitivity was unaltered [32]. The exercise intervention would be expected to add an increased muscular (peripheral) sensitivity. This is of interest because insulin resistance in skeletal muscle can play a key role in the development of metabolic complications in obesity-related disorders, including type 2 diabetes [33].
The combination of aerobic and resistance training is known to preserve or even increase lean mass during diet intervention [34]. In our study male participants in the PD-EX group lost less lean mass compared to males in the PD group. This difference was not significant if men and women were analyzed together. Notably, a recent study showed that 12 weeks of exercise increased lean mass in obese males, but not in women [35].
In line with earlier studies, another beneficial effect of the Paleolithic diet is a major reduction in blood pressure [3, 4, 32]. The combination of weight reduction and reduced sodium intake may be important for this effect. The reduced triglyceride levels, in both study groups, are also consistent with earlier studies. Previous studies showed that a Paleolithic diet decreased triglycerides even more than a consensus diet [3, 4], but exercise under supervision did not significantly improved blood lipids [6].
Leptin levels decreased 62% in the PD group and 42% in the PD-EX group. Compared to other diet interventions is this a powerful reduction relative to the weight loss of 7.1 kg [36, 37]. This is in line with a study of individuals with ischemic heart disease where a Paleolithic diet for 12 weeks reduced leptin more relative to the amount of weight loss than a Mediterranean-like diet [38]. These beneficial effects on leptin levels are of major importance because hyperleptinemia increases inflammation [39, 40] and is an independent risk factor for cardiovascular events [41–43].
The increased adiponectin levels in the PD group may relate to weight loss, with increased protein intake as a contributing factor [44, 45]. The unaltered hormone levels in the PD-EX group may indicate the increased plasma volume because of physical activity [46].
In a secondary analysis, we compared the non-randomized observational group with the intervention groups. The intervention groups improved their anthropometric status and metabolic balance versus the observational group, except for triglycerides, which improved in the observational group. Notably, the observational group was not randomized and therefore, we cannot guarantee equal distribution of confounding factors between the intervention groups and the observational group. Furthermore, some participants in the observational group suffered from cardiovascular disease, used beta-blockers and did not have the time to participate in the interventions.
A strength of the present study is that energy intake was validated with objectively measured total energy expenditure. Differences between reported energy intake and measured total energy expenditure at baseline might be due to undereating, underreporting, overestimation of physical activity energy expenditure, and/or increased physical activity during the measurement period. Participants may have started changing their dietary intake and physical activity during the baseline-measuring period, even though they were not introduced to the intervention part of the study until baseline measurements were finished. Despite the validation of energy intake, it is a weakness of the study that it is not known to what degree the participants actually followed the Paleolithic diet.
Based on our results, we conclude that the Paleolithic diet is a powerful tool to improve fat mass and metabolic balance including insulin sensitivity, glycemic control, and leptin in individuals with type 2 diabetes. Supervised exercise training did not provide additional effects on these outcomes, but preserved lean mass in men and increased cardiovascular fitness. Detailed analyses of tissue-specific effects of these interventions, including putative effects on hepatic versus muscle insulin sensitivity, are of further interest.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the study participants. We thank the research nurses Inger Arnesjö, Katarina Iselid, Lena Uddståhl, Camilla Ring, and Liv-Helene Bergman for their skillful technical assistance. We thank Marie Eriksson of the Department of Statistics, Umeå University, Sweden, for the randomization and Magnus Hedström of the Heart Centre, Umeå University Hospital, Sweden for planning and interpretation of the cardiopulmonary exercise test. We thank laboratory technician Kristina Eriksson for adiponectin and leptin analysis and Kate Westgate (MRC Epidemiology Unit, University of Cambridge, UK) for assistance with physical activity data processing.
Funding - This study was supported by grants from the Swedish Heart and Lung Foundation (20120450), King Gustav V and Queen Victoria’s Foundation, The Swedish Diabetes Research Foundation (2014-096), the County Council of Västerbotten (VLL-460481), and Umeå University, Sweden.
Abbreviations
- LDL
low density lipoprotein
- HDL
high density lipoprotein
- HOMA-IR
homeostatic model assessment of insulin resistance
- NEFAs
Non-esterified fatty acids
- PD
Paleolithic diet and general exercise recommendations
- PD-EX
Paleolithic diet with 3 h supervised exercise training per week
- QUICKI
quantitative insulin sensitivity check index
- VO2max
Maximal oxygen uptake
Footnotes
Author contributions
JO and AS designed the study, recruited participants, collected the data, performed the statistical analysis, and wrote the manuscript. MW designed the study, implemented the dietary intervention, and analyzed the data. AI conducted the exercise intervention and analyzed the data. AT conducted the dietary intervention and analyzed the data. LLO and MS designed the study and interpreted the data. SB analyzed and interpreted the Actiheart data. MR designed the study, recruited participants, collected the data, and edited the manuscript. TO designed the study, interpreted the data, and wrote the manuscript. All authors actively participated in revising the paper and approved the final version. JO is the guarantor of this work and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of interest
The authors declare that there is no duality of interest associated with this manuscript.
Clinical trial registration number
References
- 1.Gregg EW, Cheng YJ, Saydah S, Cowie C, Garfield S, Geiss L, et al. Trends in death rates among U.S. adults with and without diabetes between 1997 and 2006: findings from the National Health Interview Survey. Diabetes Care. 2012;35(6):1252–7. doi: 10.2337/dc11-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Authors/Task Force M. Ryden L, Grant PJ, Anker SD, Berne C, Cosentino F, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD) Eur Heart J. 2013;34(39):3035–87. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 3.Mellberg C, Sandberg S, Ryberg M, Eriksson M, Brage S, Larsson C, et al. Long-term effects of a Palaeolithic-type diet in obese postmenopausal women: a 2-year randomized trial. Eur J Clin Nutr. 2014;68:350–7. doi: 10.1038/ejcn.2013.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jonsson T, Granfeldt Y, Ahren B, Branell UC, Palsson G, Hansson A, et al. Beneficial effects of a Paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol. 2009;8:35. doi: 10.1186/1475-2840-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snowling NJ, Hopkins WG. Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients: a meta-analysis. Diabetes Care. 2006;29(11):2518–27. doi: 10.2337/dc06-1317. [DOI] [PubMed] [Google Scholar]
- 6.Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2006;(3) doi: 10.1002/14651858.CD002968.pub2. CD002968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Layman DK, Evans E, Baum JI, Seyler J, Erickson DJ, Boileau RA. Dietary protein and exercise have additive effects on body composition during weight loss in adult women. J Nutr. 2005;135(8):1903–10. doi: 10.1093/jn/135.8.1903. [DOI] [PubMed] [Google Scholar]
- 8.Church TS, Blair SN, Cocreham S, Johannsen N, Johnson W, Kramer K, et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2010;304(20):2253–62. doi: 10.1001/jama.2010.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–15. [PubMed] [Google Scholar]
- 10.Saghaei M, Saghaei S. Implementation of an open-source customizable minimization program for allocation of patients to parallel groups in clinical trials. J Biomedical Science and Engineering. 2011;4:734–9. [Google Scholar]
- 11.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–59. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 12.Brage S, Brage N, Franks PW, Ekelund U, Wareham NJ. Reliability and validity of the combined heart rate and movement sensor Actiheart. Eur J Clin Nutr. 2005;59(4):561–70. doi: 10.1038/sj.ejcn.1602118. [DOI] [PubMed] [Google Scholar]
- 13.Brage S, Ekelund U, Brage N, Hennings MA, Froberg K, Franks PW, et al. Hierarchy of individual calibration levels for heart rate and accelerometry to measure physical activity. J Appl Physiol (1985) 2007;103(2):682–92. doi: 10.1152/japplphysiol.00092.2006. [DOI] [PubMed] [Google Scholar]
- 14.Stegle O, Fallert SV, MacKay DJ, Brage S. Gaussian process robust regression for noisy heart rate data. IEEE Trans Biomed Eng. 2008;55(9):2143–51. doi: 10.1109/TBME.2008.923118. [DOI] [PubMed] [Google Scholar]
- 15.Brage S, Westgate K, Wijndaele K, Godinho J, Griffin S, Wareham N. Evaluation Of A Method For Minimizing Diurnal Information Bias In Objective Sensor Data; In 3rd International Conference on Ambulatory Monitoring of Physical Activity and Movement; Amherst, Massachusetts, USA: 2013. [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 17.Perseghin G, Caumo A, Caloni M, Testolin G, Luzi L. Incorporation of the fasting plasma FFA concentration into QUICKI improves its association with insulin sensitivity in nonobese individuals. J Clin Endocrinol Metab. 2001;86(10):4776–81. doi: 10.1210/jcem.86.10.7902. [DOI] [PubMed] [Google Scholar]
- 18.Wycherley TP, Noakes M, Clifton PM, Cleanthous X, Keogh JB, Brinkworth GD. A high-protein diet with resistance exercise training improves weight loss and body composition in overweight and obese patients with type 2 diabetes. Diabetes Care. 2010;33(5):969–76. doi: 10.2337/dc09-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell IW, Howlett HC. Worldwide experience of metformin as an effective glucose-lowering agent: a meta-analysis. Diabetes Metab Rev. 1995;11(Suppl 1):S57–62. doi: 10.1002/dmr.5610110509. [DOI] [PubMed] [Google Scholar]
- 20.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–12. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manheimer EW, van Zuuren EJ, Fedorowicz Z, Pijl H. Paleolithic nutrition for metabolic syndrome: systematic review and meta-analysis. Am J Clin Nutr. 2015;102(4):922–32. doi: 10.3945/ajcn.115.113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Keefe JH, Gheewala NM, O'Keefe JO. Dietary strategies for improving post-prandial glucose, lipids, inflammation, and cardiovascular health. J Am Coll Cardiol. 2008;51(3):249–55. doi: 10.1016/j.jacc.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 23.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C. Physical activity/exercise and type 2 diabetes. Diabetes Care. 2004;27(10):2518–39. doi: 10.2337/diacare.27.10.2518. [DOI] [PubMed] [Google Scholar]
- 24.Sigal RJ, Kenny GP, Boule NG, Wells GA, Prud'homme D, Fortier M, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147(6):357–69. doi: 10.7326/0003-4819-147-6-200709180-00005. [DOI] [PubMed] [Google Scholar]
- 25.Snel M, Gastaldelli A, Ouwens DM, Hesselink MK, Schaart G, Buzzigoli E, et al. Effects of adding exercise to a 16-week very low-calorie diet in obese, insulin-dependent type 2 diabetes mellitus patients. J Clin Endocrinol Metab. 2012;97(7):2512–20. doi: 10.1210/jc.2011-3178. [DOI] [PubMed] [Google Scholar]
- 26.Malin SK, Gerber R, Chipkin SR, Braun B. Independent and combined effects of exercise training and metformin on insulin sensitivity in individuals with prediabetes. Diabetes Care. 2012;35(1):131–6. doi: 10.2337/dc11-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharoff CG, Hagobian TA, Malin SK, Chipkin SR, Yu H, Hirshman MF, et al. Combining short-term metformin treatment and one bout of exercise does not increase insulin action in insulin-resistant individuals. Am J Physiol Endocrinol Metab. 2010;298(4):E815–23. doi: 10.1152/ajpendo.00517.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mikus CR, Boyle LJ, Borengasser SJ, Oberlin DJ, Naples SP, Fletcher J, et al. Simvastatin impairs exercise training adaptations. J Am Coll Cardiol. 2013;62(8):709–14. doi: 10.1016/j.jacc.2013.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohl HW, Gordon NF, Villegas JA, Blair SN. Cardiorespiratory fitness, glycemic status, and mortality risk in men. Diabetes Care. 1992;15(2):184–92. doi: 10.2337/diacare.15.2.184. [DOI] [PubMed] [Google Scholar]
- 30.Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med. 2000;132(8):605–11. doi: 10.7326/0003-4819-132-8-200004180-00002. [DOI] [PubMed] [Google Scholar]
- 31.Blair SN, Kohl HW, 3rd, Barlow CE, Paffenbarger RS, Jr, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA. 1995;273(14):1093–8. [PubMed] [Google Scholar]
- 32.Ryberg M, Sandberg S, Mellberg C, Stegle O, Lindahl B, Larsson C, et al. A Palaeolithic-type diet causes strong tissue-specific effects on ectopic fat deposition in obese postmenopausal women. J Intern Med. 2013;274(1):67–76. doi: 10.1111/joim.12048. [DOI] [PubMed] [Google Scholar]
- 33.Shulman GI. Ectopic Fat in Insulin Resistance, Dyslipidemia, and Cardiometabolic Disease. N Engl J Med. 2014;371(12):1131–41. doi: 10.1056/NEJMra1011035. [DOI] [PubMed] [Google Scholar]
- 34.Ghroubi S, Elleuch H, Chikh T, Kaffel N, Abid M, Elleuch MH. Physical training combined with dietary measures in the treatment of adult obesity. A comparison of two protocols. Ann Phys Rehabil Med. 2009;52(5):394–413. doi: 10.1016/j.rehab.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 35.Sanal E, Ardic F, Kirac S. Effects of aerobic or combined aerobic resistance exercise on body composition in overweight and obese adults: gender differences. A randomized intervention study. Eur J Phys Rehabil Med. 2013;49(1):1–11. [PubMed] [Google Scholar]
- 36.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346(21):1623–30. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 37.Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597–604. doi: 10.1056/NEJMoa1105816. [DOI] [PubMed] [Google Scholar]
- 38.Jonsson T, Granfeldt Y, Erlanson-Albertsson C, Ahren B, Lindeberg S. A paleolithic diet is more satiating per calorie than a mediterranean-like diet in individuals with ischemic heart disease. Nutr Metab (Lond) 2010;7:85. doi: 10.1186/1743-7075-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, et al. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12(1):57–65. [PubMed] [Google Scholar]
- 40.Yamagishi SI, Edelstein D, Du XL, Kaneda Y, Guzman M, Brownlee M. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J Biol Chem. 2001;276(27):25096–100. doi: 10.1074/jbc.M007383200. [DOI] [PubMed] [Google Scholar]
- 41.Soderberg S, Ahren B, Stegmayr B, Johnson O, Wiklund PG, Weinehall L, et al. Leptin is a risk marker for first-ever hemorrhagic stroke in a population-based cohort. Stroke. 1999;30(2):328–37. doi: 10.1161/01.str.30.2.328. [DOI] [PubMed] [Google Scholar]
- 42.Soderberg S, Stegmayr B, Stenlund H, Sjostrom LG, Agren A, Johansson L, et al. Leptin, but not adiponectin, predicts stroke in males. J Intern Med. 2004;256(2):128–36. doi: 10.1111/j.1365-2796.2004.01351.x. [DOI] [PubMed] [Google Scholar]
- 43.Soderberg S, Ahren B, Jansson JH, Johnson O, Hallmans G, Asplund K, et al. Leptin is associated with increased risk of myocardial infarction. J Intern Med. 1999;246(4):409–18. doi: 10.1046/j.1365-2796.1999.00571.x. [DOI] [PubMed] [Google Scholar]
- 44.Belalcazar LM, Lang W, Haffner SM, Hoogeveen RC, Pi-Sunyer FX, Schwenke DC, et al. Adiponectin and the mediation of HDL-cholesterol change with improved lifestyle: the Look AHEAD Study. J Lipid Res. 2012;53(12):2726–33. doi: 10.1194/jlr.M030213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kitabchi AE, McDaniel KA, Wan JY, Tylavsky FA, Jacovino CA, Sands CW, et al. Effects of High-Protein Versus High-Carbohydrate Diets on Markers of beta-Cell Function, Oxidative Stress, Lipid Peroxidation, Proinflammatory Cytokines, and Adipokines in Obese, Premenopausal Women Without Diabetes: A randomized controlled trial. Diabetes Care. 2013;36(7):1919–25. doi: 10.2337/dc12-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Convertino VA. Blood volume response to physical activity and inactivity. Am J Med Sci. 2007;334(1):72–9. doi: 10.1097/MAJ.0b013e318063c6e4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.