Abstract
Purpose
Leisure-screen-time, including TV viewing, is associated with increased mortality risk. We estimated the all-cause mortality risk reductions associated with substituting leisure-screen-time with different discretionary physical activity types, and the change in mortality incidence associated with different substitution scenarios.
Methods
423,659 UK Biobank participants, without stroke, myocardial infarction or cancer history, were followed for 7.6 (1.4) (median (IQR)) years. They reported leisure-screen-time (TV watching and home computer use) and leisure/home activities, categorised as daily-life activities (walking for pleasure; light DIY; heavy DIY) and structured exercise (strenuous sports; other exercises). Iso-temporal substitution modelling in Cox regression provided hazard ratios (95% confidence intervals) for all-cause mortality when substituting screen-time (30 minutes/day) with different discretionary activity types of the same duration. Potential impact fractions (PIFs) estimated the proportional change in mortality incidence associated with different substitution scenarios.
Results
During 3,202,105 person-years of follow-up, 8,928 participants died. Each 30 minute/day difference in screen-time was associated with lower mortality hazard when modelling substitution of screen-time by an equal amount of daily-life activities (0.95 (0.94-0.97)), as well as structured exercise (0.87 (0.84-0.90)). Re-allocations from screen-time into specific activity subtypes suggested different reductions in mortality hazard (walking for pleasure (0.95 (0.92-0.98)), light DIY (0.97 (0.94-1.00)), heavy DIY (0.93 (0.90-0.96)), strenuous sports (0.87 (0.79-0.95)), other exercises (0.88 (0.84-0.91))). The lowest hazard estimates were found when modelling replacement of TV viewing. PIFs ranged from 4.3% (30 minute/day substitution of screen-time into light DIY) to 14.9% (TV viewing into strenuous sports).
Conclusion
Substantial public health benefits could be gained by replacing small amounts of screen-time with daily-life activities and structured exercise. Daily-life activities may provide feasible screen-time alternatives, if structured exercise is initially too ambitious.
Keywords: TV viewing, physical activity, Cox regression, iso-temporal substitution, potential impact fraction, adult
Introduction
Physical activity is well-established as a lifestyle component contributing to longevity (22). Sedentary behaviour (i.e. awake time sitting/reclining with low energy expenditure (31)) is increasingly recognised as an additional risk factor for chronic disease (39). TV viewing in particular has been most strongly and consistently associated with incident diabetes, cardiovascular disease, specific cancers and premature mortality from a multitude of causes in adults, irrespective of levels of moderate-to-vigorous intensity physical activity (MVPA) (10, 20, 21, 29, 38). Consequently, separate public health guidelines focusing on TV viewing in adults have been advocated (20, 35, 38). Early intervention work focusing on recreational sedentary screen-time has shown promising results (27). Nevertheless, at a population-level, this behaviour still amounts to a substantial proportion of people’s time. English adults, for example, on average watch TV for 3 hours/day, making it the single most prevalent leisure-time pursuit (similar to the US (8)); and although total sedentary time has shown a slight decrease in recent years, TV viewing has not declined (30). This high prevalence together with reasonably strong associations with premature mortality has resulted in sizeable estimates of public health impact (14, 21, 35, 38).
Recommendations on reducing screen-time should involve replacement by non-sitting rather than other sitting activities. The most feasible behavioural change options likely involve replacement of screen-time by non-sitting activities in the home and leisure-time domain (i.e. discretionary time), rather than by non-sitting activities at work or during transportation (i.e. non-discretionary time). It is however largely unknown which types of such discretionary activities would be healthy alternatives. This is partially because current estimates for mortality risk have only considered the potential additive impact of screen-time reductions per se, i.e. while keeping all other activities constant. Total discretionary time is however fairly constant in this age-group and mortality risk reductions from decreasing screen-time depend on the activity that displaces screen-time (25). Higher intensity physical activities are associated with greater longevity but are generally less amenable to change (19, 28). Activities which can be easily incorporated into daily life (e.g. home maintenance and improvement activities) are perceived as more attractive compared to activities which require more organisation and costs (e.g. sports) (19).
Iso-temporal substitution modelling allows the estimation of the mortality benefits of replacing screen-time with another specific type of activity for the same duration, while keeping other activities constant (25). This approach, therefore, provides a more realistic insight into the potential impact of behavioural change using observational data (39), which is of interest given the lack of intervention studies with mortality as an outcome. We therefore aimed to estimate the differential mortality risk reductions associated with substituting leisure-screen-time with different discretionary physical activity types, in a large sample of UK middle-aged adults, by means of iso-temporal substitution modelling. To further inform public health guidance, we also estimated the proportional reduction in all-cause mortality incidence associated with each of these behavioural change scenarios.
Methods
Participants
UK Biobank is a large-scale prospective cohort study of half a million middle-aged UK adults, established with a main aim to determine the aetiological role of various genetic and lifestyle factors in the development of chronic disease (1, 37). Eligible individuals aged 40-69 years and living within a convenient distance (up to ≈25 miles) from one of 22 assessment centres located throughout the UK were identified from NHS registers and invited to participate in a baseline assessment visit (2006-2010) (1, 34). Those who reported a history of stroke, myocardial infarction or cancer at baseline were excluded from the current analyses (n=55,401), as well as those with missing data for any of the covariates (n=26,960). As a result, 423,659 participants were included in the present analysis (45.3 % men). The UK Biobank was approved by the North West Research Ethics Committee and is monitored by the UK Biobank Ethics and Governance Council. All procedures performed were in accordance with the 1964 Helsinki declaration and its later amendments. Informed consent was obtained from all individual participants included in the study.
Measurement methods
Mortality ascertainment
All UK Biobank participants were followed up for vital status by linkage to national datasets (NHS Information Centre and Scottish Morbidity Record) until September 2016. This resulted in a median (IQR) follow-up time of included participants of 7.6 (1.4) years.
Screen-time and discretionary physical activity
As part of an electronic touchscreen questionnaire, participants were asked to indicate how many hours they spend watching TV and subsequently how many hours they spend using the computer (not including occupational computer use) on a typical day. The sum of both estimates was calculated to estimate average daily screen-time (hours/day). Questions were open-ended and screen time was truncated at 9 hour/day.
Participants were also asked about their participation in the last four weeks in five different types of activities in their leisure-time or at home. Activities included walking for pleasure (not as a means of transport), light do-it-yourself (DIY, i.e. home maintenance and improvement and gardening) activities (e.g. pruning) and heavy DIY (e.g. digging, carpentry), strenuous sports that make you sweat or breathe hard, and other exercises (e.g. swimming). Average time (minutes/day) spent in each of these activities was calculated by multiplying the reported frequency and average session duration. All frequency and duration questions were categorical. Strenuous sports and other exercises were combined into an indicator of structured exercise, and time spent in walking for pleasure, light and heavy DIY were combined into an indicator of daily-life activities, indicating the greater ease of embedding these activities into daily life. A screenshot with the exact wording for each of the screen time and discretionary activity questions can be found online (e.g. TV viewing: http://biobank.ctsu.ox.ac.uk/crystal/refer.cgi?id=100277).
Covariates
The Townsend deprivation index, an indicator of material deprivation, was included as a proxy for socio-economic status. It was calculated at recruitment, based on the location of participants’ postcode relative to the national census output areas, with higher scores indicating higher levels of deprivation. Ethnicity (White; Mixed; Asian; Black; Chinese; other) and employment status (unemployed; in paid employment or self-employed) were self-reported through the electronic questionnaire.
Participants also reported their alcohol intake (never; former; current <3 times/week; current ≥3 times/week), smoking status (never; former; current), salt adding behaviour (never/rarely; sometimes; usually; always), oily fish consumption (never; <1 time/week; 1 time/week; >1 time/week), fruit and vegetable intake (score ranging from 0-4 based on fresh and dried fruit intake and raw and cooked vegetable intake), processed and red meat intake (n days/week), and sleep duration (categorised as <7 hours/night; 7-8 hours/night; >8 hours/night). Sleep time was not included in the iso-temporal framework due to non-linearity of the association between sleep time and all-cause mortality; this is similar to the approach taken by others (17).
They were also asked about their chronic disease status at baseline. This included doctor diagnosis of stroke (yes; no), myocardial infarction (yes; no) and cancer (yes; no) and intake of anti-hypertensive (yes; no) and lipid-lowering medication (yes; no). They were considered to have diabetes (yes; no) if they reported a physician confirmed diagnosis and/or treatment with insulin. Finally, parental history of cardiovascular disease or diabetes (yes; no) was defined as self-reported paternal or maternal history of heart disease, stroke, hypertension or diabetes. A similar definition was employed for parental history of cancer (yes; no) based on bowel, lung, paternal prostate or maternal breast cancer.
Participants with baseline history of stroke, myocardial infarction or cancer were excluded from analyses (as described above) and all other covariates were included as confounders in the models, chosen a priori based on the relevant literature.
Statistical analysis
Baseline characteristics were summarised by vital status, and by screen-time and discretionary physical activity tertiles. Cox regression with age as the underlying timescale was used to estimate the association between screen-time, activity types and all-cause mortality. Linearity of the associations between all exposures and all-cause mortality was assessed by fitting cubic spline regression models with 5 knots. As no substantial deviations from linearity were suggested, all exposures were modelled linearly as continuous variables and hazard ratios and 95% confidence intervals (HR (95% CIs)) were estimated for 30 minute/day increments, deemed to be feasible changes for both sedentary and activity behaviours. The proportional hazards assumption for each covariate was examined using Schoenfeld residuals and graphical checks, and found to be appropriate. Partition models were fitted first, which estimate the additive effect of screen-time and each type of activity on all-cause mortality risk, holding all other exposures constant (25). Multiplicative interactions between sex and screen-time and physical activity were tested by including the relevant parameters in the models.
We then estimated the effect of substituting screen-time by each of the physical activity types using iso-temporal substitution models. The resulting HR (95%CIs) for each physical activity type from this model provides an estimate of the potential effect on mortality of increasing that type of activity by 30 minutes/day while decreasing screen-time by the same duration, and holding other activity types constant (25).
To examine the potential public health impact of these substitutional effect estimates on all-cause mortality, we then calculated potential impact fractions (PIFs) using the “distribution shift” method described by Barendregt et al. (3), based on a normal distribution for screen-time. The 95% CIs were derived from bootstrap analysis with 1,000 replications. Each PIF represents the proportionate change in the incidence of mortality in the population if average screen-time in that population decreased by 30 minutes/day, while average physical activity (of the type being examined) increased by the same amount. For calculation of the PIF, the mean and SD of the screen-time variables was estimated from the UK Biobank sample. In a sensitivity analysis, prevalence estimates of TV viewing time from the nationally representative Health Survey for England 2012 (HSE 2012 (30)) were used, to overcome any potential selection bias of the UK Biobank sample. Questions for television viewing time in HSE 2012 data closely resembled those asked in UK Biobank, with the only difference that HSE 2012 questions were interview-based with a reference frame of the last 4 weeks, and asked for week- and weekend days separately. We calculated average daily television viewing estimates from the separate estimates for week- and weekend days (30 minutes/day).
To examine the possibility of reverse causality (i.e. when participants are on the chronic disease pathway but not yet diagnosed at baseline and therefore show or report high levels of screen time and/or low levels of discretionary activity) influencing the estimated HRs (95%CIs), we performed a second sensitivity analysis excluding those who died in the first 2 years of follow-up (in addition to exclusion of those who reported baseline history of stroke, myocardial infarction or cancer). All analyses were performed using Stata version 14 (Stata Statistical Software. College Station, TX: StataCorp LP. 2015).
Results
Descriptive characteristics
During 3,202,105 person-years of follow-up, 8,928 participants died (3,466 women; 5,462 men), a crude mortality rate of 27.9 per 10,000 person-years. Baseline descriptive characteristics by vital status and by screen-time and discretionary activity tertiles are shown in Tables 1 and 2, respectively.
Table 1.
Descriptive characteristics at baseline by vital status in 423,659 UK Biobank participants, 2006-2016
| Characteristics | Alive (n=414,731) | Deceased (n=8,928) |
|---|---|---|
| Male sex, n (%) | 187,264 (45.0) | 4,786 (61.4) |
| Age (yrs) | 56.0 (8.1) | 61.0 (6.7) |
| Ethnicity, n (%) | ||
| White | 392,736 (94.7) | 8,655 (96.9) |
| Mixed | 2,390 (0.6) | 39 (0.4) |
| Asian | 7,953 (1.9) | 102 (1.1) |
| Black | 6,617 (1.6) | 60 (0.7) |
| Chinese | 1,340 (0.3) | 15 (0.2) |
| Other | 3,695 (0.9) | 57 (0.6) |
| Townsend index | -1.39 (3.0) | -0.8 (3.4) |
| Unemployed, n (%) | 162,616 (39.2) | 5,694 (63.8) |
| Alcohol status, n (%) | ||
| Never | 17,471 (4.2) | 378 (4.2) |
| Previous | 13,499 (3.3) | 551 (6.2) |
| Current (<3 times/week) | 200,838 (48.4) | 4,038 (45.2) |
| Current (≥3 times/week) | 182,923 (44.1) | 3,961 (44.4) |
| Smoking status, n (%) | ||
| Never | 233,713 (56.4) | 3,472 (38.9) |
| Previous | 139,544 (33.7) | 3,585 (40.2) |
| Current | 41,474 (10.0) | 1,871 (21.0) |
| Fruit and vegetable intake, n (%) | ||
| Low | 80,658 (19.5) | 2,061 (23.1) |
| Moderately low | 124,324 (30.0) | 2,774 (31.1) |
| Moderate | 113,359 (27.3) | 2,261 (25.3) |
| Moderately high | 69,709 (16.8) | 1,356 (15.2) |
| High | 26,681 (6.4) | 476 (5.3) |
| Processed and red meat intake (N days/week) | 0.9 (0.6) | 1.0 (0.6) |
| Salt adding, n (%) | ||
| Never/rarely | 231,473 (55.8) | 4,514 (50.6) |
| Sometimes | 116,434 (28.1) | 2,517 (28.2) |
| Usually | 47,690 (11.5) | 1,251 (14.0) |
| Always | 19,134 (4.6) | 646 (7.2) |
| Oily fish intake, n (%) | ||
| Never | 45,474 (11.0) | 1,083 (12.1) |
| <1 time/week | 139,811 (33.7) | 2,813 (31.5) |
| 1 time/week | 156,693 (37.8) | 3,306 (37.0) |
| > 1 time/week | 72,753 (17.5) | 1,726 (19.3) |
| Sleep duration, n (%) | ||
| <7 hrs/day | 100,894 (24.3) | 2,350 (26.3) |
| 7-8 hrs/day | 284,281 (68.6) | 5,572 (62.4) |
| >8 hrs/day | 29,556 (7.1) | 1,006 (11.3) |
| Antihypertensive drug intake, n (%) | 75,938 (18.3) | 2,854 (32.0) |
| Lipid lowering drug intake, n (%) | 59,905 (14.4) | 2,280 (25.5) |
| History of diabetes, n (%) | 18,650 (4.5) | 993 (11.1) |
| Parental CVD/diabetes history, n (%) | 307,191 (74.1) | 6,271 (70.2) |
| Parental cancer history, n (%) | 126,502 (30.5) | 2,788 (31.2) |
| Body mass index (kg/m2) | 27.3 (4.7) | 28.0 (5.3) |
| Screen time (hrs/day) | 3.8 (1.9) | 4.2 (2.1) |
| TV time (hrs/day) | 2.7 (1.5) | 3.2 (1.8) |
| Computer use time (hrs/day, median (IQR)) | 1.0 (0.0-1.0) | 0.5 (0.0-1.0) |
| Daily-life activities (min/day, median (IQR)) | 15.8 (4.0-37.5) | 13.9 (2.3-39.9) |
| Walking for pleasure (min/day, median (IQR)) | 6.4 (0.0-16.1) | 4.0 (0.0-16.1) |
| Light DIY (min/day, median (IQR)) | 0.0 (0.0-8.0) | 0.0 (0.0-6.7) |
| Heavy DIY (min/day, median (IQR)) | 0.0 (0.0-4.0) | 0.0 (0.0-3.2) |
| Structured exercise (min/day, median (IQR)) | 0.8 (0.0-16.1) | 0.0 (0.0-6.7) |
| Strenuous sports (min/day, median (IQR)) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) |
| Other exercises (min/day, median (IQR)) | 0.0 (0.0-13.4) | 0.0 (0.0-6.4) |
Results are means (SD) unless otherwise indicated. Parental CVD/diabetes history includes a maternal or paternal history of heart disease, stroke, hypertension or diabetes. Parental cancer history includes history of bowel, lung, maternal breast or paternal prostate cancer.
Table 2.
Descriptive characteristics at baseline by tertiles of screen time and physical activity in 423,659 UK Biobank participants, 2006-2016
| Characteristics | Screen time tertiles |
Discretionary physical activity tertiles |
||||
|---|---|---|---|---|---|---|
| Lowest n=125,679 |
Middle n=164,728 |
Highest n=133,252 |
Lowest n=141,584 |
Middle n=141,203 |
Highest n=140,872 |
|
| Median ((IQR), hrs/day) | 2.0 (1.5-2.0) | 3.5 (3.0-4.0) | 6.0 (5.0-7.0) | 0.1 (0.0-0.2) | 0.4 (0.3-0.5) | 1.3 (0.9-1.8) |
| Male sex, n (%) | 49,750 (39.6) | 75,051 (45.6) | 67,248 (50.5) | 56,869 (40.2) | 62,014 (43.9) | 73,166 (51.9) |
| Age (yrs) | 54.4 (8.0) | 56.4 (8.0) | 57.3 (8.0) | 55.7 (8.1) | 55.7 (8.1) | 56.9 (8.1) |
| Ethnicity, n (%) | ||||||
| White | 118,985 (94.7) | 157,067 (95.4) | 125,339 (94.1) | 129,560 (91.5) | 135,242 (95.8) | 136,589 (97.0) |
| Mixed | 701 (0.6) | 886 (0.5) | 842 (0.6) | 1,031 (0.7) | 724 (0.5) | 674 (0.5) |
| Asian | 2,869 (2.3) | 2,867 (1.7) | 2,319 (1.7) | 4,440 (3.1) | 2,185 (1.6) | 1,430 (1.0) |
| Black | 1,572 (1.3) | 2,156 (1.3) | 2,949 (2.2) | 3,857 (2.7) | 1,661 (1.2) | 1,159 (0.8) |
| Chinese | 399 (0.3) | 476 (0.3) | 480 (0.4) | 715 (0.5) | 365 (0.3) | 275 (0.2) |
| Other | 1,153 (0.9) | 1,276 (0.8) | 1,323 (1.0) | 1,981 (1.4) | 1,026 (0.7) | 745 (0.5) |
| Townsend index | -1.5 (3.0) | -1.5 (3.0) | -1.1 (3.2) | -0.8 (3.3) | -1.6 (2.9) | -1.8 (2.8) |
| Unemployed, n (%) | 33,844 (26.9) | 64,604 (39.2) | 69,862 (52.4) | 51,506 (36.4) | 50,179 (35.5) | 66,625 (47.3) |
| Alcohol status, n (%) | ||||||
| Never | 5,844 (4.7) | 6,511 (4.0) | 5,494 (4.1) | 8,652 (6.1) | 4,830 (3.4) | 4,367 (3.1) |
| Previous | 3,801 (3.0) | 4,943 (3.0) | 5,306 (4.0) | 6,048 (4.3) | 3,947 (2.8) | 4,055 (2.9) |
| Current (<3 times/week) | 57,817 (46.0) | 80,030 (48.6) | 67,029 (50.3) | 75,220 (53.1) | 67,615 (47.9) | 62,041 (44.0) |
| Current (≥3 times/week) | 58,217 (46.3) | 73,244 (44.5) | 55,423 (41.6) | 51,664 (36.5) | 64,811 (45.9) | 70,409 (50.0) |
| Smoking status, n (%) | ||||||
| Never | 77,555 (61.7) | 92,392 (56.1) | 67,238 (50.5) | 77,217 (54.5) | 81,342 (57.6) | 78,626 (55.8) |
| Previous | 37,575 (29.9) | 56,013 (34.0) | 49,541 (37.2) | 45,506 (32.1) | 47,480 (33.6) | 50,143 (35.6) |
| Current | 10,549 (8.4) | 16,323 (9.9) | 16,473 (12.4) | 18,861 (13.3) | 12,381 (8.8) | 12,103 (8.6) |
| Fruit and vegetable intake, n (%) | ||||||
| Low | 20,943 (16.7) | 32,290 (19.6) | 29,486 (22.1) | 36,683 (25.9) | 25,625 (18.2) | 20,411 (14.5) |
| Moderately low | 36,630 (29.2) | 49,549 (30.1) | 40,919 (30.7) | 45,389 (32.1) | 43,457 (30.8) | 38,252 (27.2) |
| Moderate | 35,532 (28.3) | 44,903 (27.3) | 35,185 (26.4) | 35,064 (24.8) | 39,684 (28.1) | 40,872 (29.0) |
| Moderately high | 23,115 (18.4) | 27,656 (16.8) | 20,294 (15.2) | 18,448 (13.0) | 23,746 (16.8) | 28,871 (20.5) |
| High | 9,459 (7.5) | 10,330 (6.3) | 7,368 (5.5) | 6,000 (4.2) | 8,691 (6.2) | 12,466 (8.9) |
| Processed and red meat intake (N days/week) | 0.8 (0.5) | 0.9 (0.5) | 1.0 (0.6) | 0.9 (0.6) | 0.9 (0.5) | 0.9 (0.6) |
| Salt adding, No (%) | ||||||
| Never/rarely | 75,118 (59.8) | 91,857 (55.8) | 69,012 (51.8) | 74,063 (52.3) | 80,437 (57.0) | 81,487 (57.8) |
| Sometimes | 33,823 (26.9) | 46,647 (28.3) | 38,481 (28.9) | 40,955 (28.9) | 39,659 (28.1) | 38,337 (27.2) |
| Usually | 12,453 (9.9) | 18,889 (11.5) | 17,599 (13.2) | 17,788 (12.6) | 15,680 (11.1) | 15,473 (11.0) |
| Always | 4,285 (3.4) | 7,335 (4.5) | 8,160 (6.1) | 8,778 (6.2) | 5,427 (3.8) | 5,575 (4.0) |
| Oily fish intake, n (%) | ||||||
| Never | 12,450 (9.9) | 17,481 (10.6) | 16,626 (12.5) | 19,923 (14.1) | 13,871 (9.8) | 12,763 (9.1) |
| <1 time/week | 41,268 (32.8) | 54,828 (33.3) | 46,528 (34.9) | 52,663 (37.2) | 48,011 (34.0) | 41,950 (29.8) |
| 1 time/week | 48,921 (38.9) | 63,050 (38.3) | 48,028 (36.0) | 49,073 (34.7) | 55,175 (39.1) | 55,751 (39.6) |
| > 1 time/week | 23,040 (18.3) | 29,369 (17.8) | 22,070 (16.6) | 19,925 (14.1) | 24,146 (17.1) | 30,408 (21.6) |
| Sleep duration, n (%) | ||||||
| <7 hrs/day | 28,332 (22.5) | 39,497 (24.0) | 35,415 (26.6) | 39,795 (28.1) | 32,364 (22.9) | 31,085 (22.1) |
| 7-8 hrs/day | 90,375 (71.9) | 114,072 (69.3) | 85,406 (64.1) | 90,278 (63.8) | 99,697 (70.6) | 99,878 (70.9) |
| >8 hrs/day | 6,972 (5.6) | 11,159 (6.8) | 12,431 (9.3) | 11,511 (8.1) | 9,142 (6.5) | 9,909 (7.0) |
| Antihypertensive drugs, n (%) | 16,398 (13.1) | 30,788 (18.7) | 31,606 (23.7) | 30,724 (21.7) | 24,208 (17.1) | 23,860 (16.9) |
| Lipid lowering drugs, n (%) | 11,825 (9.4) | 24,110 (14.6) | 26,250 (19.7) | 23,674 (16.7) | 18,894 (13.4) | 19,617 (13.9) |
| History of diabetes, n (%) | 3,574 (2.8) | 7,062 (4.3) | 9,007 (6.8) | 9,009 (6.4) | 5,519 (3.9) | 5,115 (3.6) |
| Parental CVD/diabetes history, n (%) | 92,595 (73.7) | 122,121 (74.1) | 98,746 (74.1) | 104,913 (74.1) | 105,153 (74.5) | 103,396 (73.4) |
| Parental cancer history, n (%) | 37,482 (29.8) | 50,487 (30.6) | 41,321 (31.0) | 42,265 (29.9) | 43,184 (30.6) | 43,841 (31.1) |
| Body mass index (kg/m2) | 26.0 (4.3) | 27.4 (4.6) | 28.5 (5.0) | 28.3 (5.3) | 27.1 (4.5) | 26.6 (4.1) |
Results are means (SD) unless indicated otherwise. Parental CVD/diabetes history includes a maternal or paternal history of heart disease, stroke, hypertension or diabetes. Parental cancer history includes history of bowel, lung, maternal breast or paternal prostate cancer.
Associations with all-cause mortality
Partition models
As shown in Table 3, each 30 minute/day difference in screen-time was associated with a 1% higher hazard of all-cause mortality, independent of time spent in activity and all other confounding variables included in the model. Furthermore, each 30 minute/day difference in daily-life activities and in structured exercise was independently associated with a 4% and 12% lower hazard for all-cause mortality. When examining the two screen-time variables separately, associations for TV viewing time were the strongest, showing a 2% higher mortality hazard for each 30 minute/day difference, whereas associations for computer use were non-significant. A significant interaction between computer use and sex was identified (p=0.001). Examining the independent associations with all-cause mortality for computer use separately by sex, there was a positive association (1.015 (1.001-1.031)) in women, whereas the association was negative (0.986 (0.975-0.996)) in men. Therefore, iso-temporal substitution for computer use was modelled in men and women separately.
Table 3.
Hazard ratios (95% confidence intervals) for all-cause mortality from partition models for screen time and different types of discretionary physical activity (all expressed in 30 minute/day units) in 423,659 UK men and women, UK Biobank, 2006-2016
| Model 1 | Screen time | Daily-life activities | Structured exercise | |
|---|---|---|---|---|
| 1.01 (1.01-1.02)* | 0.96 (0.95-0.98)* | 0.88 (0.85-0.91)* | ||
| Model 2 | TV viewing time | Computer use | Daily-life activities | Structured exercise |
| 1.02 (1.01-1.03)* | 1.00 (0.99-1.01) | 0.96 (0.95-0.98)* | 0.88 (0.85-0.92)* | |
Models are mutually adjusted for all screen time and physical activity variables mentioned, as well as for sex, ethnicity, socio-economic status, employment status, smoking status, alcohol, fruit and vegetable, processed and red meat, salt and oily fish intake, sleep duration, blood pressure lowering medication, dyslipidaemia medication, personal diabetes history and parental history of CVD/diabetes and cancer.
p<0.001
Iso-temporal substitution models
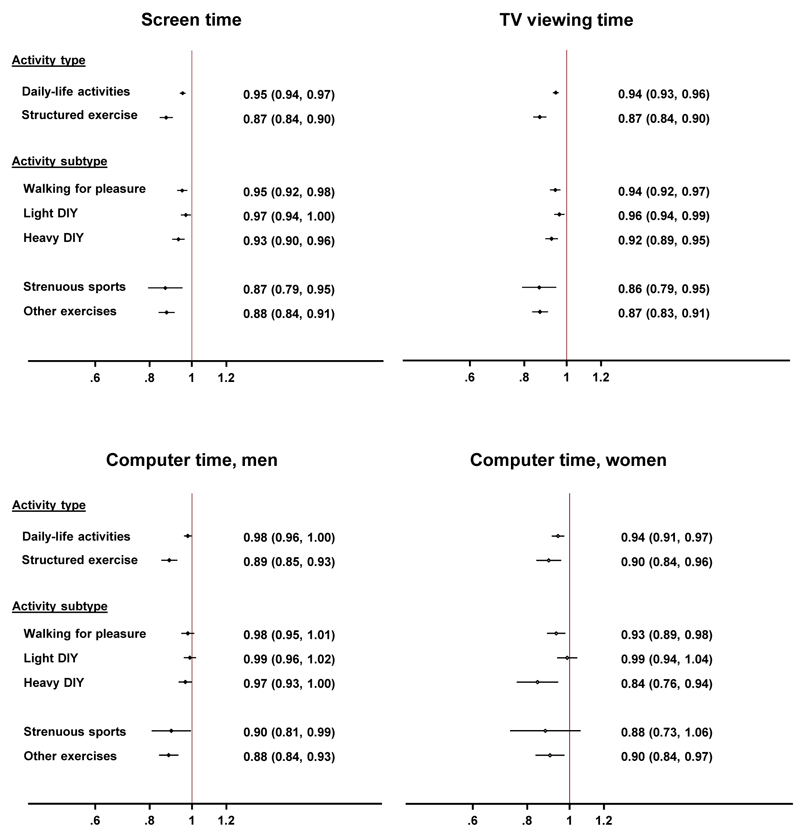
As shown in Figure 1, each 30 minute/day difference in screen-time was associated with a lower mortality hazard when modelling substitution of screen-time by an equal amount of daily-life activities as well as by an equal amount of structured exercise. Although modelling 30 minute/day replacements of screen-time by structured exercise resulted in the lowest mortality hazard (0.87 (0.84-0.90)), modelling substitution by daily-life activities also suggested a relevant protective effect (0.95 (0.94-0.97)). When looking at more specific subtypes of daily-life activities, the lowest mortality hazards were found when modelling 30 minute/day re-allocations from screen-time into walking for pleasure (0.95 (0.92-0.98)) and into heavy DIY (0.93 (0.90-0.96)); whereas potential mortality benefits were more limited when modelling re-allocation into light DIY (0.97 (0.94-1.00)). Confidence intervals did however overlap between estimates for these activity subtypes. As expected, the modelled effects of replacing 30 minutes of screen-time with an equivalent duration of strenuous sports (0.87 (0.79-0.95)) and other types of exercise (0.88 (0.84-0.91)) were stronger.
Figure 1.
Hazard ratios (95% confidence intervals) of all-cause mortality when modelling 30 minute/day substitutions of screen time (total screen time, TV viewing time or computer time) by equivalent durations of different types of discretionary physical activity in 423,659 UK men and women, UK Biobank, 2006-2016. Models have omitted the sedentary behaviour component under study, and are adjusted for total discretionary time, sex, ethnicity, socio-economic status, employment status, smoking status, alcohol, fruit and vegetable, processed and red meat, salt and oily fish intake, sleep duration, blood pressure lowering medication, dyslipidaemia medication, personal diabetes history and parental history of CVD or diabetes and cancer. Model for TV viewing substitution is adjusted for computer time and vice versa.
Results when modelling substitution of TV viewing time were similar to those when modelling substitution of screen-time (Figure 1). The modelled effects of substitution of computer time by different activity types were less consistent, with generally weaker effect estimates than for screen and TV viewing time. In both men and women a potential protective effect was found when modelling substitution of computer time by daily-life activities (men: 0.98 (0.96-1.00); women: 0.94 (0.91-0.97)) and structured exercise (men: 0.89 (0.85-0.93); women: 0.90 (0.84-0.96)). For daily-life activities in women, this was mainly driven by walking for pleasure (0.93 (0.89-0.98)) and heavy DIY (0.84 (0.76-0.94)). Modelling re-allocation of computer time into other exercises showed a potential protective effect in men (0.88 (0.84-0.93)) and women (0.90 (0.84-0.97)), whereas results for re-allocation into strenuous sports only reached significance in men (0.90 (0.81-0.99)).
Results were similar when those who died within the first 2 years of follow-up were excluded. [see Table, Supplemental Digital Content 1, Hazard ratios (95% confidence intervals) for all-cause mortality from partition models for screen time and different types of discretionary activity; see Table, Supplemental Digital Content 2, Hazard ratios (95% confidence intervals) for all-cause mortality when modelling 30 minute/day substitutions of screen time.]
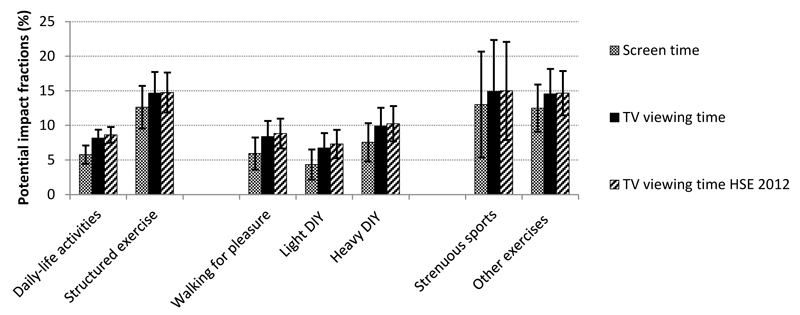
Potential impact fractions
Figure 2 displays PIFs for time-re-allocation of overall screen and TV viewing time (i.e. the sedentary behaviours which consistently showed associations with mortality) into the different discretionary activity types. For example, if UK Biobank participants were to decrease their screen-time by an average of 30 minutes/day in exchange for a daily 30 minute walk, the incidence of all-cause mortality would decrease by 5.9%, assuming causality. The estimated PIFs were highest when replacing TV viewing time by either strenuous sports or other exercises (14.9% and 14.6%, respectively) and lowest when replacing screen-time with light DIY (4.3%). Sensitivity analysis using the weighted HSE 2012 distribution for TV viewing time (restricting the age range to that of UK Biobank participants, i.e. 40-69 years) gave similar PIF estimates to those obtained using the UK Biobank distribution (Figure 2).
Figure 2.
Potential impact fractions (%; 95% confidence intervals) representing the proportionate decrease in incidence of all-cause mortality in the population if average screen time were to decrease by 30 minutes/day, while average time spent on the respective discretionary physical activity type increased by the same amount, assuming causality. Grey and black bars are based on distribution estimates from UK Biobank for screen time and TV viewing time respectively, whereas striped bars are based on the weighted TV viewing time distribution from the Health Survey for England 2012.
Discussion
The findings of this study provide novel insights into the potential beneficial effects on mortality risk of substituting recreational screen-time by different types of discretionary active alternatives in a large population-based sample of UK middle-aged adults. They suggest that replacing small amounts of screen-time (i.e. 30 minutes/day) by everyday activities such as DIY and walking, which are generally more easily adopted (19), results in important mortality benefits. Replacement by sports and other exercise provides additional benefits. The substantial differences in effect estimates arising from different substitution scenarios highlight the importance of using iso-temporal substitution modelling to more fully inform public health guidance on effective behaviour change to increase longevity.
The direction and strength of the associations from partition models found for screen-time (32) and TV viewing time (14) confirm those found previously; as well as the stronger and more consistent associations for TV viewing compared to computer time (2, 4, 26). Previous meta-analytical work has also indicated protective mortality effects for exercise/sports as well as daily-life activities, with stronger effect estimates for the former (28). However, none of these studies examined the substitutional effects between screen-time and these different discretionary activity types. Indeed, the few studies that have employed an iso-temporal substitution approach in relation to mortality have focused on non-domain specific sedentary time and/or non-domain specific physical activity (11, 23, 33). We chose to specifically focus on leisure-screen-time, given its high prevalence across all age groups, stronger associations with health compared to other sedentary behaviours, and responsiveness to change through intervention (8, 20, 21, 27, 29, 30, 35, 38)). We specifically focused on discretionary activity types in the leisure and home domain, as these are likely more realistic and feasible alternatives for replacing leisure-screen-time in a middle-aged population compared to activities in the occupational and transportation domain.
Our findings suggested that both replacement by lower (i.e. walking for pleasure and light DIY) and higher intensity activities (i.e. heavy DIY, strenuous sports and other exercise) was found to be beneficial; however, the latter conferred the largest benefit. This is in line with findings for intermediate health risk factors. Some intermediate cardio-metabolic risk factors (such as adiposity) require substitution of sedentary time by higher intensity activities, whereas others (such as glucose and lipid metabolism markers) may respond beneficially from substitution into both lower (i.e. as low as standing) and higher intensity activity (7, 17). For risk factors reliant on energy balance, this may be due to the higher energy cost associated with higher-intensity activities for the same duration. Other risk factors may be influenced via other protective pathways related to features such as more fragmented accumulation patterns or an upright posture, which may be applicable to both low and high intensity activities. Enzymes regulating glucose and lipid metabolism, for example, may be up-regulated with muscle activity related to standing and non-sedentary activities and with higher activity fragmentation (13, 15). In terms of mental health as an intermediate risk factor, replacements of sitting time into both lower and higher intensity activity have been shown to be beneficial, which is likely also due to differential pathways, such as increased socialisation and increased β-endorphines (6, 24).
We have estimated that 4.3% to 14.9% of premature deaths in the UK could be avoided through substitution of 30 minutes/day of total screen or TV viewing time by discretionary active alternatives, with the highest potential shift in mortality cases to be gained from substituting TV viewing by sports and exercise. Lee et al. estimated that physical inactivity (i.e. not achieving 150 minutes/week of MVPA) causes 9% of premature mortality globally (an effect on par with smoking and obesity (22)). The latter is an estimate of the excess proportion of deaths that could be avoided through the increase in MVPA to prescribed levels, keeping all other activities constant. A direct comparison of study results is challenging due to differences in populations and methodology. However, most of our PIF estimates for discretionary activities, which would be classified as moderate-to-vigorous in terms of intensity, exceeded 9% (e.g. 30 minute/day reallocation of TV viewing into heavy DIY (10%), strenuous sports (15%) or other exercises (15%)). Although caution is needed when comparing these study results, this may be partially due to our PIF estimates reflecting the combined effect of reducing screen-time and increasing activity levels. The latter provide more realistic estimates of the potential public health impact of behavioural change, as reducing one type of activity necessarily results in increased engagement in another type of activity (39). Future studies should, therefore, aim to also incorporate such estimates.
There are several strengths of this study. A wide range of potential confounding variables was controlled for. These included several dietary variables, confounding the associations for physical activity. These dietary covariates may, however, be on the causal pathway between screen-time, especially TV viewing time, and premature mortality (12, 16), potentially resulting in over-adjustment and, therefore, underestimation of the effect estimates for screen-time. Inclusion of a large population-based sample of UK adults enabled us to exclude all those with baseline chronic conditions, and in sensitivity analyses additionally those who died in the first 2 years of follow-up. This helped us to minimise the risk of reverse causality influencing our estimates, and potentially also the risk of severe recall bias by those with chronic disease influencing our findings. We minimised the potential risk of selection bias influencing the PIF estimates, by recalculating these estimates in a separate population-representative sample of English adults (30). Finally, we examined the substitution effects for both types of screen-time separately. However, there were also limitations to the study. Iso-temporal substitution modelling estimates are based on statistical modelling rather than actual behavioural change. It is also unlikely that the self-report instrument captured all screen-time and activity behaviours in the domestic and leisure domain. Screen-time and physical activity were self-reported through questions which were categorical and have not been directly examined for criterion validity, and social desirability bias may have caused over-reporting of physical activity and underreporting of screen time. However, most self-report instruments have similar validity (18) and effect estimates were comparable to those found previously in comparable populations using similar adjustment strategies (14, 28, 32). We examined potential bias associated with differences in education level, by adjusting for education level instead of Townsend index, which resulted in very similar effect estimates (results not shown). The questionnaire did not distinguish between week- and weekend days, and the time frame of reference for screen-time (“typical day”) was different to that for physical activity (“last 4 weeks”), although it is unlikely the latter would have substantially impacted on results (9). Objective activity measures are currently unable to classify activities by type and domain, which makes subjective measures particularly valuable for these types of research questions (23, 33). Finally, while we aimed to increase internal validity by excluding those with baseline conditions, this may have enhanced the healthy cohort effect on observed associations. Replication in populations with different health status, age, ethnicity, and lifestyle profiles is needed. Ideally these studies would also incorporate repeated objective measures for screen-time and physical activity, allowing consideration of the patterns of sitting and activity, and have longer follow up.
In conclusion, replacing small amounts of screen-time (i.e. 30 minutes/day) by everyday activities such as DIY or walking could result in considerable public health benefits. These may be important targets for adults for whom taking up more structured activities or higher intensity sports (which show stronger mortality benefits) to replace screen-time initially is less feasible or compromised through ill health. Given the ubiquitous nature of screen-time, the achievability of the examined behavioural change options and the substantial mortality benefits estimated, specific guidelines on reductions in screen-time, so far mainly implemented for pediatric populations (36), could be considered for adult age groups, to complement emerging guidelines on occupational sitting (5). These could not only recommend reductions in screen-time but also substitution by alternative healthy activities which can take place in the home and leisure domain.
Supplementary Material
Acknowledgements
This work was conducted using the UK Biobank resource and was supported by the British Heart Foundation (Intermediate Basic Science Research Fellowship grant FS/12/58/29709) and the Medical Research Council (Unit Programme numbers MC_UU_12015/1 and MC_UU_12015/3). The results of this study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Footnotes
Conflict of Interest
The results of the present study do not constitute endorsement by the American College of Sports Medicine. The authors declare that they have no conflict of interest.
References
- 1.Allen N, Sudlow C, Downey P, et al. UK Biobank: Current status and what it means for epidemiology. Health Policy and Technology. 2012;1(3):123–6. [Google Scholar]
- 2.Altenburg TM, de Kroon ML, Renders CM, Hirasing R, Chinapaw MJ. TV time but not computer time is associated with cardiometabolic risk in Dutch young adults. PLoS One. 2013;8:e57749. doi: 10.1371/journal.pone.0057749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barendregt JJ, Veerman JL. Categorical versus continuous risk factors, and the calculation of potential impact fractions. J Epidemiol Comm Health. 2010;64(3):209–12. doi: 10.1136/jech.2009.090274. [DOI] [PubMed] [Google Scholar]
- 4.Basterra-Gortari FJ, Bes-Rastrollo M, Gea A, Nunez-Cordoba JM, Toledo E, Martinez-Gonzalez MA. Television viewing, computer use, time driving and all-cause mortality: the SUN cohort. J Am Heart Assoc. 2014;3(3):e000864. doi: 10.1161/JAHA.114.000864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckley JP, Hedge A, Yates T, et al. The sedentary office: an expert statement on the growing case for change towards better health and productivity. Br J Sports Med. 2015;49(21):1357–62. doi: 10.1136/bjsports-2015-094618. [DOI] [PubMed] [Google Scholar]
- 6.Buman MP, Hekler EB, Haskell WL, et al. Objective light-intensity physical activity associations with rated health in older adults. Am J Epidemiol. 2010;172(10):1155–65. doi: 10.1093/aje/kwq249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buman MP, Winkler EA, Kurka JM, et al. Reallocating time to sleep, sedentary behaviors, or active behaviors: associations with cardiovascular disease risk biomarkers, NHANES 2005-2006. Am J Epidemiol. 2014;179(3):323–4. doi: 10.1093/aje/kwt292. [DOI] [PubMed] [Google Scholar]
- 8.Bureau of Labor and Statistics. [Accessed 2016 Oct 04];American Time Use Survey 2014. 2015 Available from: http://www.bls.gov/news.release/atus.nr0.htm.
- 9.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 10.Ekelund U, Steene-Johannessen J, Brown WJ, et al. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet. 2016;388(10051):1301–10. doi: 10.1016/S0140-6736(16)30370-1. [DOI] [PubMed] [Google Scholar]
- 11.Fishman EI, Steeves JA, Zipunnikov V, et al. Association between objectively measured physical activity and mortality in NHANES. Med Sci Sports Exerc. 2016;48(7):1303–11. doi: 10.1249/MSS.0000000000000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster JA, Gore SA, West DS. Altering TV viewing habits: An unexplored strategy for adult obesity intervention? Am J Health Behav. 2006;30(1):3–14. doi: 10.5555/ajhb.2006.30.1.3. [DOI] [PubMed] [Google Scholar]
- 13.Gay J, Buchner D, Schmidt M. Dose-response association of physical activity with HbA1c: Intensity and bout length. Prev Med. 2016;86:58–63. doi: 10.1016/j.ypmed.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Grontved A, Hu FB. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a meta-analysis. JAMA. 2011;305(23):2448–55. doi: 10.1001/jama.2011.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton MT, Hamilton DG, Zderic TW. Exercise physiology versus inactivity physiology: An essential concept for understanding lipoprotein lipase regulation. Exerc Sport Sci Rev. 2004;32(4):161–6. doi: 10.1097/00003677-200410000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris JL, Bargh JA, Brownell KD. Priming effects of television food advertising on eating behavior. Health Psychol. 2009;28(4):404–13. doi: 10.1037/a0014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Healy GN, Winkler EA, Owen N, Anuradha S, Dunstan DW. Replacing sitting time with standing or stepping: associations with cardio-metabolic risk biomarkers. Eur Heart J. 2015;36(39):2643–9. doi: 10.1093/eurheartj/ehv308. [DOI] [PubMed] [Google Scholar]
- 18.Helmerhorst HJ, Brage S, Warren J, Besson H, Ekelund U. A systematic review of reliability and objective criterion-related validity of physical activity questionnaires. Int J Behav Nutr Phys Act. 2012;9:103. doi: 10.1186/1479-5868-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hillsdon M, Thorogood M, Anstiss T, Morris J. Randomised controlled trials of physical activity promotion in free living populations: a review. J Epidemiol Community Health. 1995;49(5):448–53. doi: 10.1136/jech.49.5.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keadle SK, Arem H, Moore SC, Sampson JN, Matthews CE. Impact of changes in television viewing time and physical activity on longevity: a prospective cohort study. Int J Behav Nutr Phys Act. 2015;12:156. doi: 10.1186/s12966-015-0315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keadle SK, Moore SC, Sampson JN, Xiao Q, Albanes D, Matthews CE. Causes of death associated with prolonged TV viewing: NIH-AARP Diet and Health Study. Am J Prev Med. 2015;49(6):811–21. doi: 10.1016/j.amepre.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380(9839):219–29. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matthews CE, Moore SC, Sampson J, et al. Mortality benefits for replacing sitting time with different physical activities. Med Sci Sports Exerc. 2015;47(9):1833–40. doi: 10.1249/MSS.0000000000000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mekary RA, Lucas M, Pan A, et al. Isotemporal substitution analysis for physical activity, television watching, and risk of depression. Am J Epidemiol. 2013;178(3):474–83. doi: 10.1093/aje/kws590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mekary RA, Willett WC, Hu FB, Ding EL. Isotemporal substitution paradigm for physical activity epidemiology and weight change. Am J Epidemiol. 2009;170(4):519–27. doi: 10.1093/aje/kwp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nang EE, Salim A, Wu Y, Tai ES, Lee J, Van Dam RM. Television screen time, but not computer use and reading time, is associated with cardio-metabolic biomarkers in a multiethnic Asian population: a cross-sectional study. Int J Behav Nutr Phys Act. 2013;10:70. doi: 10.1186/1479-5868-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramsey BL, Rooks-Peck CR, Finnie RK, et al. Reducing recreational sedentary screen time: a community guide systematic review. Am J Prev Med. 2016;50(3):402–15. doi: 10.1016/j.amepre.2015.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samitz G, Egger M, Zwahlen M. Domains of physical activity and all-cause mortality: systematic review and dose-response meta-analysis of cohort studies. Int J Epidemiol. 2011;40(5):1382–1400. doi: 10.1093/ije/dyr112. [DOI] [PubMed] [Google Scholar]
- 29.Schmid D, Leitzmann MF. Television viewing and time spent sedentary in relation to cancer risk: a meta-analysis. J Natl Cancer Inst. 2014;106(7) doi: 10.1093/jnci/dju098. pii:dju098. [DOI] [PubMed] [Google Scholar]
- 30.Scholes S, Mindell J. [Accessed 2016 Oct 04];Health Survey for England 2012 - Volume 1 Chapter 2 Physical activity in adults. 2013 Available from: http://www.hscic.gov.uk/catalogue/PUB13218/HSE2012-Ch2-Phys-act-adults.pdf. [Google Scholar]
- 31.Sedentary Behaviour Research Network. Standardized use of the terms "sedentary" and "sedentary behaviours". Appl Physiol Nutr Metab. 2012;37(3):540–42. doi: 10.1139/h2012-024. [DOI] [PubMed] [Google Scholar]
- 32.Stamatakis E, Hamer M, Dunstan DW. Screen-based entertainment time, all-cause mortality, and cardiovascular events population-based study with ongoing mortality and hospital events follow-up. J Am Coll Cardiol. 2011;57(3):292–9. doi: 10.1016/j.jacc.2010.05.065. [DOI] [PubMed] [Google Scholar]
- 33.Stamatakis E, Rogers K, Ding D, et al. All-cause mortality effects of replacing sedentary time with physical activity and sleeping using an isotemporal substitution model: a prospective study of 201,129 mid-aged and older adults. Int J Behav Nutr Phys Act. 2015;12:121. doi: 10.1186/s12966-015-0280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sudlow C, Gallacher J, Allen N, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun JW, Zhao LG, Yang Y, Ma X, Wang YY, Xiang YB. Association between television viewing time and all-cause mortality: a meta-analysis of cohort studies. Am J Epidemiol. 2015;182(11):908–16. doi: 10.1093/aje/kwv164. [DOI] [PubMed] [Google Scholar]
- 36.Tremblay MS, Leblanc AG, Janssen I, et al. Canadian sedentary behaviour guidelines for children and youth. Appl Physiol Nutr Metab. 2011;36(1):59–64. doi: 10.1139/H11-012. [DOI] [PubMed] [Google Scholar]
- 37.UK Biobank. Protocol for a large-scale prospective epidemiological resource. [Accessed 2016 Oct 04];2007 Available from: http://www.ukbiobank.ac.uk/wp-content/uploads/2011/11/UK-Biobank-Protocol.pdf.
- 38.Wijndaele K, Brage S, Besson H, et al. Television viewing time independently predicts all-cause and cardiovascular mortality: the EPIC Norfolk Study. Int J Epidemiol. 2011;40(1):150–9. doi: 10.1093/ije/dyq105. [DOI] [PubMed] [Google Scholar]
- 39.Wijndaele K, Healy GN. Sitting and chronic disease: where do we go from here? Diabetologia. 2016;59(4):688–91. doi: 10.1007/s00125-016-3886-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.