Abstract
Background
The pathophysiologic mechanisms behind proliferation of fibroblasts and deposition of dense collagen matrix in idiopathic frozen shoulder remain unclear. Accumulation of advanced glycation end products (AGEs) with cross-linking and stabilization of collagen has been hypothesized to contribute to this pathophysiologic process. This study investigated whether the immunoreactivity of AGEs is higher in patients with idiopathic frozen shoulder than in the control groups.
Methods
Shoulder capsule samples were collected from 8 patients with idiopathic frozen shoulder, 6 with unstable shoulders (control 1), and 8 with rotator cuff tears (control 2). The samples were hematoxylin and eosin stained and analyzed by immunohistochemistry using antibodies against AGEs. Immunoreactivities were rated in a blinded fashion from none (0) to strong (3). Immunohistochemical distribution within the capsule was noted.
Results
Frozen shoulder patients had greater frequency and severity of self-reported pain (P = .02) than rotator cuff tear patients and more restricted range of motion in all planes (P < .05) than patients of the instability and rotator cuff tear groups. Hematoxylin and eosin–stained capsular tissue from frozen shoulder showed fibroblastic proliferation, increased numbers of adipocytes, and increased subsynovial vascularity. Immunoreactivity of AGEs was stronger in frozen shoulder capsules (2.8) than in instability (0.3; P = .0001) and rotator cuff tear (1.1; P = .016) capsules.
Conclusion
This study highlights a potential role for AGEs in the pathogenesis of frozen shoulder. The overexpression of AGEs may explain the fibroblastic proliferation and deposition of collagen matrix in idiopathic frozen shoulder.
Level of evidence
Basic Science Study; Histology
Keywords: Frozen shoulder, advanced glycation end products, immunoreactivity, stiff shoulder, fibroblastic proliferation, adhesive capsulitis
Frozen shoulder, also known as adhesive capsulitis, is a common shoulder condition of unknown etiology characterized by severe pain, stiffness, and restricted active and passive shoulder motion. Estimates of the incidence of frozen shoulder reported from shoulder clinics range from 2% to 5%.4,25 Women aged between 40 and 60 years are the most commonly affected group.
The disease typically lasts between 2 and 3 years and can be divided into 3 phases: a painful inflammatory phase with progressive stiffness (10-36 weeks); a stiff phase when the pain gradually decreases but the range of motion in all planes becomes severely restricted (4-12 months); and a recovery phase, which involves the gradual spontaneous improvement of shoulder function (5-26 months).22 However, Shaffer et al25 and Hand et al,9 in their long-term (averaging 7 and 4 years, respectively) follow-up studies, found that 40% to 50% of nonoperatively managed frozen shoulder patients did not regain their baseline function and still had ongoing residual pain.
Our current understanding of the underlying etiology and pathogenesis of frozen shoulder is limited. Histologic studies5,23 have demonstrated fibroblastic and myofibroblastic proliferation in dense types I and III collagen matrix in the shoulder capsule. Capsular fibrosis and contracture have been suggested to stiffen the shoulder capsule and to restrict range of motion. The pain of frozen shoulder was suggested to relate to neurogenesis and capsular inflammation, supported by the presence of increased numbers of chronic inflammatory cells with higher levels of inflammatory cytokines and angiogenesis.10,28 However, the etiologic mechanisms behind proliferation of fibroblasts and deposition of dense collagen matrix in shoulder capsules of frozen shoulder patients are still unclear.
Diabetic patients are more likely than nondiabetic patients to develop frozen shoulder. Bridgman4 described the strong association between diabetes and frozen shoulder on observing 11% incidence among 800 diabetic patients compared with 2% incidence among 600 nondiabetic patients. Arkkila et al2 found high prevalence of frozen shoulder in type 1 (10%) and type 2 (22%) diabetic patients. A multivariate regression analysis in a case-control study by Wang et al27 has confirmed that diabetes is an independent predictor of adhesive capsulitis (P = .005; odds ratio, 3.05).
Accumulation of advanced glycation end products (AGEs) with cross-linking and stabilization of collagen has been hypothesized to contribute to the higher incidence of frozen shoulder in diabetic patients.12,15 AGEs are thought to form because of the nonenzymatic reaction of the ketone group on reducing sugars with free amino groups on proteins, resulting in progressive rearrangement, dehydration, and condensation.26 The formation of AGEs progressively increases with normal aging. However, because of the long-standing hyperglycemic state in diabetes mellitus, glucose forms covalent adducts with the plasma proteins through a nonenzymatic process known as glycation and thus accelerates accumulation of AGEs.26 AGEs attract monocytes and macrophages that release inflammatory cytokines that coordinate degradation and removal of senescent material with replacement by new tissue components.
To our knowledge, no previous studies considered the potential contribution of AGEs in frozen shoulder. However, previous studies have identified a contribution of AGEs in the pathogenesis of diabetic retinopathy, diabetic nephropathy, and diabetic cardiomyopathy. Treatment of retinal glial cells with 16% and 32% AGEs resulted in dose- and time-dependent immunocytochemical expression elevations of basic fibroblast growth factor in the culture medium (P < .05).1 This suggested the role of AGEs in promoting fibroblast proliferation by enhancing production of basic fibroblast growth factor in diabetic retinopathy. The accumulation of AGEs on vessels facilitated cross-linking of collagen and increased resistance to proteolysis, which aided in fibrosis of the vessels and reduced arterial compliance in diabetic cardiomyopathy.3 Furthermore, interaction of AGEs with RAGE, the receptor for AGEs, contributed to the development of atherosclerosis by activating adhesion molecules and proinflammatory cytokines and growth factors.24 The expression of extracellular proteins including types I and IV collagens, fibronectin, and laminin as well as expression of profibrotic cytokines and growth factors, including transforming growth factor β1, platelet-derived growth factor B, and connective tissue growth factor, was increased by AGEs in a dose- and time-dependent manner in diabetic nephropathy.8 Furthermore, AGEs decreased the expression of matrix metalloproteinases secreted by mesangial cells and increased expression of tissue metalloproteinase inhibitors in diabetic nephropathy.19,29 Decreased activities of matrix metalloproteinases and increased activities of tissue inhibitors of matrix metalloproteinase leading to disruption of homeostasis between synthesis and degradation of extracellular matrix components also have been suggested as one of the pathogenic mechanisms of capsular fibrosis in frozen shoulder.6,11,17
We hypothesized that immunoreactivity of AGEs in frozen shoulder capsules will be higher compared with the control shoulder capsules.
This study was primarily designed to investigate whether AGEs contribute to the pathogenic mechanism of frozen shoulder by determining AGE immunoreactivity in human specimens and to describe the immunohistochemical distribution of antigens to AGE antibodies in regions of the frozen shoulder capsule at the microscopic level.
Materials and methods
Study design
A prospective case-control study was conducted at the Orthopaedics Research Institute, St. George Hospital, and the National Day Surgery, Sydney, NSW, Australia, during a 9-month period.
Inclusion and exclusion criteria
The study included patients with primary frozen shoulders who were receiving arthroscopic capsular releases. It also included patients with unstable shoulders undergoing arthroscopic stabilization and patients with rotator cuff tears undergoing rotator cuff repair as control groups. Instability and rotator cuff tear patients were chosen as control groups as these patients were thought not to have their primary shoulder disease affecting the shoulder capsule and required glenohumeral arthroscopy, which was necessary to obtain tissue samples.
The diagnosis of frozen shoulder was made using Codman’s criteria4 modified by Zuckerman and Rokito.30 The inclusion criteria for the frozen shoulder population comprised a painful stiff shoulder with insidious onset, restricted elevation to ≤100°, passive external rotation ≤50% of the normal contralateral shoulder, loss of function of the affected arm, pain at night and inability to lie on the affected side, and unremarkable radiographic and ultrasound findings of the glenohumeral joint. The exclusion criteria included history of previous surgery of the involved shoulder and radiologic or arthroscopic signs of fracture, glenohumeral arthritis, and concomitant rotator cuff tear.
Inclusion criteria for the instability group consisted of history of >1 dislocation and a Bankart lesion, a Hill-Sachs lesion, or a superior labral anterior-posterior lesion confirmed at arthroscopy.
Rotator cuff tear patients included patients with full-thickness and partial-thickness tear diagnosed with ultrasound or magnetic resonance imaging and confirmed at arthroscopy without concomitant secondary frozen shoulder.
Pain and stiffness scores
On their first visit to the shoulder clinic, patients completed a modified L’Insalata questionnaire form,16 which recorded subjective experience of pain frequency, pain severity, and stiffness. Patients answered questions about frequency of activity pain, night pain, and extreme pain; severity of resting pain, activity pain, and night pain; and shoulder stiffness. Pain frequency was ranked using Likert scales as never (0), sometimes (1), monthly (2), weekly (3), or daily (4). Pain severity and stiffness were ranked as none (0), mild (1), moderate (2), severe (3), or very severe (4). The patient-ranked pain frequency and severity scores were added to a maximum of 12. The maximum patient-ranked stiffness was 4.
Histology and immunohistochemistry
Shoulder capsule samples were collected during arthroscopic shoulder capsular release, shoulder stabilization, or rotator cuff repair. Tissue specimens were excised from the shoulder capsule by the same surgeon (G.A.C.M) during glenohumeral joint arthroscopy. The tissue was removed by meniscal basket forceps from an area just superomedial to the subscapularis tendon, adjacent to the glenoid labrum. The biopsied tissue was immediately fixed in 10% buffered formalin for 18 to 24 hours, dehydrated, and embedded in paraffin wax. Tissues were sectioned into 5 μm and mounted on slides. One slide was stained with hematoxylin and eosin (H&E). The remaining unstained sections were sent to Glasgow Bio-medical Research Centre, UK, where another investigator (N.L.M) immunostained the tissue sections with AGE (ab23722; Abcam, Cambridge, UK) at 3 μg/mL using the following standard immunohistochemistry method with antigen retrieval.
The unstained tissue sections mounted on slides were dewaxed in an oven at 80°C for 10 minutes. They were then immersed in 2 changes of xylene for 3 minutes each; dehydrated in 100%, 95%, and 70% ethanol for 2 minutes each; and rehydrated in water. They were subsequently immersed in 10 mM sodium citrate for 30 minutes in an 80°C water bath for antigen retrieval, followed by 20 minutes of cooldown to room temperature. Sections were then washed well with Tris-HCl 0.05M buffer (pH 7.4), immersed in 3% hydrogen peroxide made up in methanol for 15 minutes for blocking of endogenous peroxidase activity, followed by washing for 5 minutes in a 0.05M Tris-HCl buffer bath. Non-specific binding sites were blocked with normal horse serum solution (MP-7402; Vector Laboratories, Peterborough, UK) for 20 minutes. After removal of the excess solution by blot drying around sections, primary AGE antibody (ab23722, Abcam) was added at 3 μg/mL (diluted with 1% bovine serum albumin in 0.05M Tris-HCl). The sections were incubated at room temperature for 60 minutes in a humidified tray, then placed in a Tris-HCl buffer bath for 5 minutes, and ImmPRESS polymer anti-rabbit IgG reagent (MP-7401, Vector Laboratories) was added. The sections were returned to the humidified tray and incubated at room temperature for 30 minutes. Negative controls were stained without the polymer anti-rabbit Ig reagent at this step. All tissue sections were washed in Tris-HCl buffer for 5 minutes. A drop of liquid diaminobenzidine was diluted in 1 mL of buffer, added to the sections for 1 minute, and quickly washed with Tris-HCl to stop the reaction. The sections were counterstained in hematoxylin for 15 seconds, washed in water and 0.3% acid alcohol, blued with Scott solution for 2 minutes, and washed in water. The sections were then dehydrated in 70%, 95%, and 100% ethanol for 1 minute each and cleared in 2 changes of xylene for 2 minutes each. Finally the slides were dried, mounted in DPX, and covered with a coverslip.
Microscopic and statistical analyses
H&E-stained sections and immunostained sections were examined under light microscopy at ×100, × 200, and ×400 magnifications and were compared with the negative controls. Representative photomicrographs of the slides in each group were made. An experienced examiner (N.L.M.), blinded to the identity of the stained sections, rated their immunoreactivity.20,21 The immunoreactivity of the slides was rated according to the intensity of the staining as none (0), weak (1), moderate (2), and strong (3). In each field, the number of positively and negatively stained cells was counted; the percentage of positive cells was calculated, giving the following semiquantitative grading: grade 0, no staining; grade 1, mild, ≤10% cells stained positive; grade 2, moderate, 10% to 20% cells stained positive; and grade 3, strong, ≥20% cells stained positive.20
The numbers of adipocytes in the H&E-stained sections were manually counted in 10 randomly selected views containing capsular tissue at ×100 magnification (2.5 mm2/view). The number of adipocytes per 10 mm2 was calculated.
Data were analyzed statistically using Microsoft Excel (Microsoft Corp, Bellevue, WA, USA), GraphPad Prism 6 (GraphPad Software, San Diego, CA, USA), and IBM SPSS 22 (IBM, Armonk, NY, USA). Quantitative data were analyzed by 1-way analysis of variance, univariate analysis of covariance, and nonparametric Kruskal-Wallis tests.
Results
Patient demographics
The study included 8 patients (6 nondiabetic and 2 diabetic patients) with frozen shoulder as the disease population. Three male and 5 female frozen shoulder patients had a mean age of 52 years (range, 41-60). Six shoulder instability patients (5 male and 1 female) had a mean age of 23 years (range, 16-40). Eight rotator cuff tear patients (5 male and 3 female) had a mean age of 58 years (range, 52-71) in the control populations. The mean duration of symptoms before shoulder surgeries was 5 months in the frozen shoulder group, 20 months in the instability group, and 8 months in the rotator cuff tear group. Instability patients were significantly younger than rotator cuff tear and frozen shoulder patients (P < .001) (Table I).
Table I.
Patient demographics
| Frozen shoulder | Instability | Rotator cuff tear | |
|---|---|---|---|
| No. of patients | 8 | 6 | 8 |
| Age (years), mean (range) | 52 (41-60) | 23 (16-40) | 58 (52-71) |
| Gender (M:F) | 3:5 | 5:1 | 5:3 |
| Affected shoulder (R:L) | 5:3 | 3:3 | 5:3 |
| Duration of symptoms (months), mean (range) | 5 (2-7) | 20 (2-36.5) | 8 (1.5-41) |
| Weight (kg), mean (range) | 75.4 (55-90) | 77.2 (52-95) | 85.5 (56-105) |
| Diabetic patients | 2/8 | 0/6 | 0/8 |
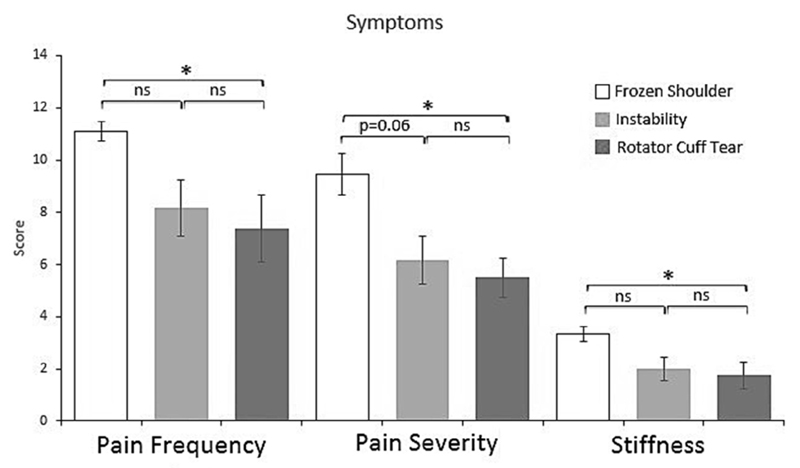
Patient-ranked pain frequency (P = .02), pain severity (P = .02), and stiffness (P = .04) were significantly higher in the frozen shoulder group compared with the rotator cuff tear group (Fig. 1). Patient-reported pain severity approached significant difference (P = .06) between the frozen shoulder group and the shoulder instability group (Fig. 1). Shoulder motion was also significantly more restricted in external rotation, internal rotation, forward flexion, and abduction in frozen shoulder patients compared with instability and rotator cuff tear groups (Fig. 2). There were no significant differences for pain, stiffness, or range of motion between instability and rotator cuff tear patients.
Figure 1.
Patient-ranked shoulder pain frequency, pain severity, and stiffness scores in patients with frozen shoulder (n = 8) compared with those with instability (n = 6) and those with rotator cuff tear (n = 8). Data show mean with the standard error of the mean (error bars). *P < .05; ns, P > .05; Kruskal-Wallis—Dunn’s multiple comparisons test.
Figure 2.
Range of passive shoulder motion in patients with frozen shoulder (n = 8) compared with those with instability (n = 6) and those with rotator cuff tear (n = 8). Mean data with the standard error of the mean (error bars). *P < .05; **P < .01; ****P < .0001; 1-way analysis of variance—Tukey’s multiple comparisons test (Kruskal-Wallis—Dunn’s multiple comparisons test used for internal rotation).
Histologic appearance of the shoulder capsule
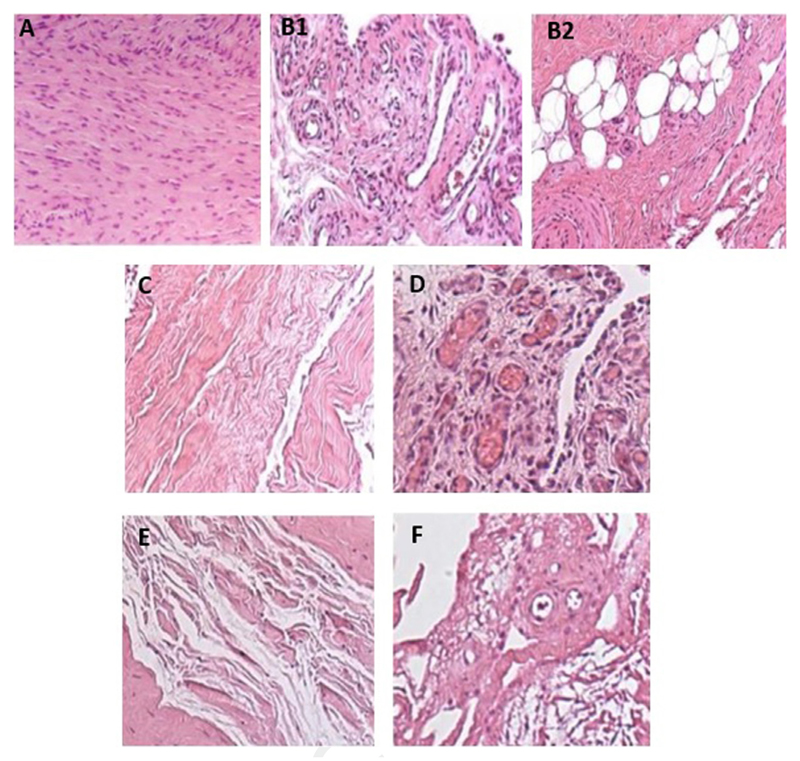
H&E-stained shoulder capsules (Fig. 3) mainly consisted of 2 areas: fibrous areas with fibroblasts embedded in a collagen matrix and subsynovial areas with blood vessels and adipocytes.
Figure 3.
Hematoxylin and eosin (H&E) staining of shoulder capsule from patient samples. (A) Frozen shoulder capsule with fibroblastic proliferation in a fibrocollagenous stroma. (B1) Subsynovial region 1 of frozen shoulder capsule with abundant vascular channels. (B2) Subsynovial region 2 of frozen shoulder capsule with fibrosis and entrapped adipocytes. (C) Fibrous region of shoulder instability capsule with fibrinoid degeneration of collagen. (D) Subsynovial vascular congestion in shoulder instability capsule. (E) Fibrous region of rotator cuff tear capsule with fibrinoid degeneration of collagen. (F) Subsynovial region of rotator cuff tear capsule with fibrinoid degeneration. (All micrographs are represented at magnification ×140.)
The fibrous stroma of the capsules of rotator cuff tear patients contained loosely packed collagen fibers (Fig. 3, E). There were relatively small numbers of capillaries and venules in the subsynovium (Fig. 3, F).
Capsule specimens from 4 instability patients also exhibited few capillaries and venules in the subsynovium (Fig. 3, C), whereas 2 had increased vascularity (Fig. 3, D). Fibroblastic proliferation was noted in 1 of 6 instability capsules.
The histologic appearance of the H&E-stained frozen shoulder capsule specimens showed densely packed collagen fibers and fibroblastic proliferation within the fibrous stroma compared with the controls (Fig. 3, A). There were large numbers of capillaries and venules dilated with erythrocytes in the subsynovium of frozen shoulder samples compared with the controls (Fig. 3, B1). Clusters of adipocytes were observed close to capillaries (Fig. 3, B2).
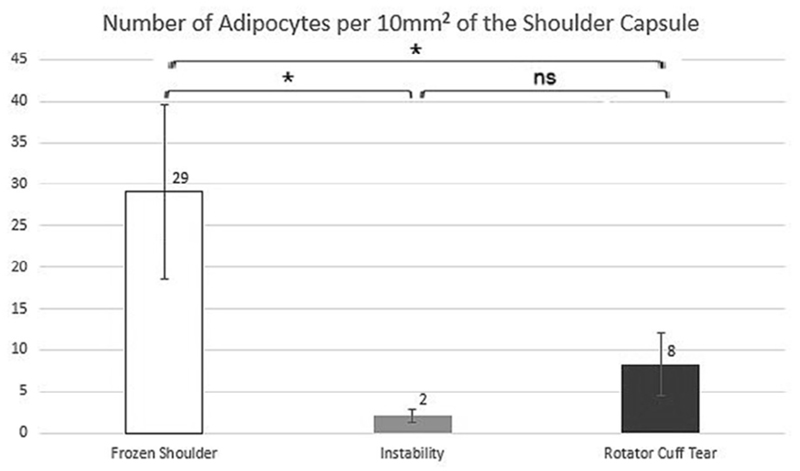
The number of adipocytes counted manually was significantly higher in frozen shoulder capsules with a mean of 29 adipocytes per 10 mm2 compared with 2 adipocytes per 10 mm2 of the capsules of instability patients (P = .034) or 8 adipocytes per 10 mm2 of the capsules of rotator cuff tear patients (P = .036) (Fig. 4). Control groups lacked significant difference in adipocyte numbers (P = .35). Also, adipocyte numbers did not correlate with demographic factors such as age (Pearson correlation = .03; P = .9) or weight (Pearson correlation = .04; P = .8) in all groups.
Figure 4.
Number of adipocytes per 10 mm2 of the shoulder capsule. Data show mean with the standard error of the mean (error bars). *P < .05; ns, P > .05; univariate analysis of covariance with patient age as a covariant.
Immunoreactivity
AGE immunoreactivity
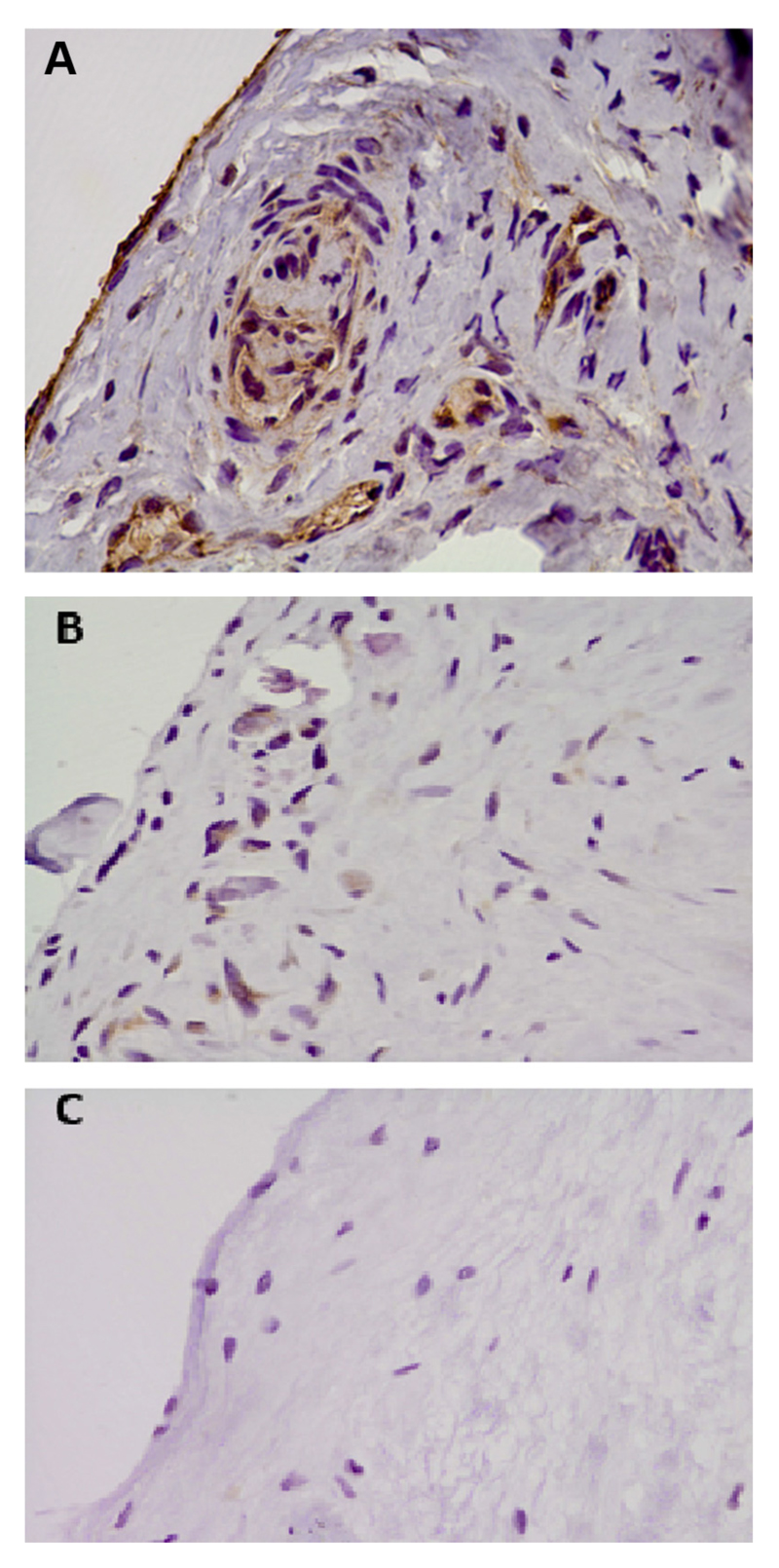
The immunoreactivity of AGEs in the capsular tissue of frozen shoulder patients was mainly observed in immune cells located around vascular areas (CD34) within the subsynovial tissue and in synoviocytes within the fibrous stroma (Fig. 5). AGE immunoreactivity in frozen shoulder capsule (2.8) was significantly higher (P = .0001) than in capsular tissues of instability patients (0.3; Table II). It was also significantly higher (P = .016) than in capsular tissues of rotator cuff tear patients (1.1). There was no difference in immunoreactivities of AGEs between the 2 control groups (P = .38). Additional analyses were performed excluding diabetic patients’ results. There were still significantly higher immunoreactivities of AGEs in frozen shoulder capsule (2.7) compared with both the shoulder capsules of the instability group (0.3; P = .0003) and the rotator cuff tear group (1.1; P = .036).
Figure 5.
Photomicrographs of shoulder capsules immunostained for AGEs in shoulder patient samples. (A) AGEs with positive immunostaining in frozen shoulder capsule. (B) Weak nonspecific staining for AGEs in unstable shoulder capsule. (C) Negative staining for AGEs in capsule from a rotator cuff tear patient. (All micrographs are represented at magnification ×190.)
Table II.
Immunoreactivity of AGEs analyzed with 1-way analysis of variance
| Antigens/proteins | Frozen shoulder | Instability: control 1 | RCT: control 2 |
P values for differences between groups |
||
|---|---|---|---|---|---|---|
| Frozen vs. instability | Frozen vs. RCT | Instability vs. RCT | ||||
| AGEs | 2.8 | 0.3 | 1.1 | .0001*** | .016* | .38 |
| AGEs (nondiabetic) | 2.7 | 0.3 | 1.1 | .0003*** | .036* | .28 |
AGEs, advanced glycation end products; RCT, rotator cuff tear.
* P < .05, *** P < .001.
Discussion
This study has found increased immunoreactivity of AGEs in frozen shoulder capsules compared with control capsules. Histologic features of all H&E-stained frozen shoulder capsule slides included densely packed collagen fibers, fibroblastic hypercellularity, and increased vascularization. In addition, increased numbers of adipocytes were noted in frozen shoulder capsules compared with control capsules.
Our study used tissue from shoulder conditions other than frozen shoulder because it would have been difficult and unethical to obtain capsular tissues from people with normal shoulders that do not need a glenohumeral arthroscopy. The rotator cuff tear group served as a better control of the 2 control groups. The age range was more similar between the rotator cuff tear group and the frozen shoulder group. H&E staining of capsular samples from the unstable shoulder revealed evidence of increased vascularity in the subsynovial area of the capsule in 2 of 6 patients as well as hypercellularity in the fibrous area of the capsule in 1 of the 6 patients. This was consistent with the findings described by McFarland et al.18 These findings probably reflected damage to the shoulder capsule from traumatic dislocations.
Our study is apparently the first to investigate immunoreactivity of AGEs in frozen shoulder capsule. Accumulation of AGEs and the consequent cross-linking of collagen have been proposed to contribute to an increased incidence of frozen shoulder in diabetic patients. This study found increase in AGE immunoreactivity not only in diabetic frozen shoulder patients but also in nondiabetic frozen shoulder patients. This could suggest that accumulation of AGEs potentially contributes to the pathogenic process of frozen shoulder regardless of diabetic status. However, the small diabetic population in this study makes it difficult to draw conclusions in comparing them with frozen shoulders in nondiabetics.
Because of the nature of the study design, it was impossible to determine whether this accumulation of AGEs triggered the pathogenic process of frozen shoulder or whether the pathogenesis of frozen shoulder led to higher immunoreactivity of AGEs found in this study. Nonetheless, previous studies showing the roles of AGE in fibroblastic proliferation and collagen cross-linking and deposition in diabetic retinopathy, diabetic cardiomyopathy, and diabetic nephropathy point toward a potential contribution of AGEs in the pathogenic process of frozen shoulder. A long-term prospective cohort study would be able to answer this question by measuring AGE levels in a population of normal patients and correlating these levels with the incidence of frozen shoulder.
Increased numbers of adipocytes were noted in most frozen shoulder capsular specimens. Recent studies7,13,14 suggested potential contributions of adipokines including adiponectin in inflammatory arthropathies such as osteoarthritis and rheumatoid arthritis. Frozen shoulder is also a joint disease involving inflammatory processes. Thus, the increased number of adipocytes found in this study could suggest that there may be potential roles of inflammatory adipokines such as leptin and resistin that could contribute toward the inflammatory or fibrotic processes of frozen shoulder. Alternatively, this may merely reflect entrapment of normal subsynovial adipocytes in the fibrosing process.
This study had several strengths and limitations. In terms of limitations, first our sample size (8 frozen shoulder patients and 14 control patients) was small. Immunohistochemical analysis was less effective in terms of quantifying the reactivity because of the subjective nature of the rating. The slides were stained, analyzed, and rated by a single experienced examiner (N.L.M). The examiner was blinded for the immunohistochemical analysis of the slides, and the results were statistically analyzed by a different examiner (K.R.H) to increase the reliability of the study. Tissue samples were biopsied by a single surgeon (G.A.C.M.) from the same location of all the patients’ shoulders. This ensured the uniformity of the tissue type collected. In addition, immunocytochemical staining was a good method to determine how the reactivity is localized.
Conclusion.
This study highlights a potential role for AGEs in the pathogenesis of frozen shoulder. As far as we can determine, this paper is the first to consider whether AGEs are involved in the frozen shoulders of patients regardless of their diabetic status. In addition, we have also found increased numbers of adipocytes in the capsular tissues of frozen shoulder patients compared with capsular tissues of rotator cuff tears and shoulder instability patients. The overexpression of AGEs may explain the fibroblastic proliferation and deposition of collagen matrix in idiopathic frozen shoulder.
Disclaimer.
The authors, their immediate families, and any research foundation with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
The study was conducted in accordance with ethics approval from the Human Research Ethics Committee—Central Network, South East Health (HREC/96/55, HREC/14/130).
References
- 1.Ai J, Liu Y, Sun JH. Advanced glycation end-products stimulate basic fibroblast growth factor expression in cultured Muller cells. Mol Med Rep. 2013;7:16–20. doi: 10.3892/mmr.2012.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arkkila PE, Kantola IM, Viikari JS, Ronnemaa T. Shoulder capsulitis in type I and II diabetic patients: association with diabetic complications and related diseases. Ann Rheum Dis. 1996;55:907–14. doi: 10.1136/ard.55.12.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asbun J, Villarreal FJ. The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. J Am Coll Cardiol. 2006;47:693–700. doi: 10.1016/j.jacc.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 4.Bridgman JF. Periarthritis of the shoulder and diabetes mellitus. Ann Rheum Dis. 1972;31:69–71. doi: 10.1136/ard.31.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunker TD, Anthony PP. The pathology of frozen shoulder. A Dupuytren-like disease. J Bone Joint Surg Br. 1995;77:677–83. [PubMed] [Google Scholar]
- 6.Bunker TD, Reilly J, Baird KS, Hamblen DL. Expression of growth factors, cytokines and matrix metalloproteinases in frozen shoulder. J Bone Joint Surg Br. 2000;82:768–73. doi: 10.1302/0301-620x.82b5.9888. [DOI] [PubMed] [Google Scholar]
- 7.Ehling A, Schäffler A, Herfarth H, Tarner IH, Anders S, Distler O, et al. The potential of adiponectin in driving arthritis. J Immunol. 2006;176:4468–78. doi: 10.4049/jimmunol.176.7.4468. [DOI] [PubMed] [Google Scholar]
- 8.Forbes JM, Cooper ME, Oldfield MD, Thomas MC. Role of advanced glycation end products in diabetic nephropathy. J Am Soc Nephrol. 2003;14(Suppl 3):S254–8. doi: 10.1097/01.asn.0000077413.41276.17. [DOI] [PubMed] [Google Scholar]
- 9.Hand C, Clipsham K, Rees JL, Carr AJ. Long-term outcome of frozen shoulder. J Shoulder Elbow Surg. 2008;17:231–6. doi: 10.1016/j.jse.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Hand GCR, Athanasou NA, Matthews T, Carr AJ. The pathology of frozen shoulder. J Bone Joint Surg Br. 2007;89:928–32. doi: 10.1302/0301-620X.89B7.19097. [DOI] [PubMed] [Google Scholar]
- 11.Hutchinson JW, Tierney GM, Parsons SL, Davis TRC. Dupuytren’s disease and frozen shoulder induced by treatment with a matrix metalloproteinase inhibitor. J Bone Joint Surg Br. 1998;80:907–8. doi: 10.1302/0301-620x.80b5.8464. [DOI] [PubMed] [Google Scholar]
- 12.Kohn RR, Hensse S. Abnormal collagen in cultures of fibroblasts from human beings with diabetes mellitus. Biochem Biophys Res Commun. 1977;76:365–71. doi: 10.1016/0006-291x(77)91566-2. [DOI] [PubMed] [Google Scholar]
- 13.Koskinen A, Juslin S, Nieminen R, Moilanen T, Vuolteenaho K, Moilanen E. Adiponectin associates with markers of cartilage degradation in osteoarthritis and induces production of proinflammatory and catabolic factors through mitogen-activated protein kinase pathways. Arthritis Res Ther. 2011;13:R184. doi: 10.1186/ar3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lago R, Gomez R, Otero M, Lago F, Gallego R, Dieguez C, et al. A new player in cartilage homeostasis: adiponectin induces nitric oxide synthase type II and pro-inflammatory cytokines in chondrocytes. Osteoarthritis Cartilage. 2008;16:1101–9. doi: 10.1016/j.joca.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Lebiedz-Odrobina D, Kay J. Rheumatic manifestations of diabetes mellitus. Rheum Dis Clin North Am. 2010;36:681–99. doi: 10.1016/j.rdc.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 16.L’Insalata JC, Warren RF, Cohen SB, Altchek DW, Peterson MG. A self-administered questionnaire for assessment of symptoms and function of the shoulder. J Bone Joint Surg Am. 1997;79:738–48. [PubMed] [Google Scholar]
- 17.Lubis AM, Lubis VK. Matrix metalloproteinase, tissue inhibitor of metalloproteinase and transforming growth factor-beta 1 in frozen shoulder, and their changes as response to intensive stretching and supervised neglect exercise. J Orthop Sci. 2013;18:519–27. doi: 10.1007/s00776-013-0387-0. [DOI] [PubMed] [Google Scholar]
- 18.McFarland EG, Kim TK, Banchasuek P, McCarthy EF. Histologic evaluation of the shoulder capsule in normal shoulders, unstable shoulders, and after failed thermal capsulorrhaphy. Am J Sports Med. 2002;30:636–42. doi: 10.1177/03635465020300050201. [DOI] [PubMed] [Google Scholar]
- 19.McLennan SV, Martell SK, Yue DK. Effects of mesangium glycation on matrix metalloproteinase activities: possible role in diabetic nephropathy. Diabetes. 2002;51:2612–8. doi: 10.2337/diabetes.51.8.2612. [DOI] [PubMed] [Google Scholar]
- 20.Millar NL, Hueber AJ, Reilly JH, Xu Y, Fazzi UG, Murrell GA, et al. Inflammation is present in early human tendinopathy. Am J Sports Med. 2010;38:2085–91. doi: 10.1177/0363546510372613. [DOI] [PubMed] [Google Scholar]
- 21.Millar NL, Wei AQ, Molloy TJ, Bonar F, Murrell GA. Cytokines and apoptosis in supraspinatus tendinopathy. J Bone Joint Surg Br. 2009;91:417–24. doi: 10.1302/0301-620X.91B3.21652. [DOI] [PubMed] [Google Scholar]
- 22.Reeves B. The natural history of the frozen shoulder syndrome. Scand J Rheumatol. 1975;4:193–6. doi: 10.3109/03009747509165255. [DOI] [PubMed] [Google Scholar]
- 23.Rodeo SA, Hannafin JA, Tom J, Warren RF, Wickiewicz TL. Immunolocalization of cytokines and their receptors in adhesive capsulitis of the shoulder. J Orthop Res. 1997;15:427–36. doi: 10.1002/jor.1100150316. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt AM, Yan SD, Wautier JL, Stern D. Activation of receptor for advanced glycation end products: a mechanism for chronic vascular dysfunction in diabetic vasculopathy and atherosclerosis. Circ Res. 1999;84:489–97. doi: 10.1161/01.res.84.5.489. [DOI] [PubMed] [Google Scholar]
- 25.Shaffer B, Tibone JE, Kerlan RK. Frozen shoulder. A long-term follow-up. J Bone Joint Surg Am. 1992;74:738–46. [PubMed] [Google Scholar]
- 26.Singh VP, Bali A, Singh N, Jaggi AS. Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol. 2014;18:1–14. doi: 10.4196/kjpp.2014.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang K, Ho V, Hunter-Smith DJ, Beh PS, Smith KM, Weber AB. Risk factors in idiopathic adhesive capsulitis: a case control study. J Shoulder Elbow Surg. 2013;22:e24–9. doi: 10.1016/j.jse.2012.10.049. [DOI] [PubMed] [Google Scholar]
- 28.Xu Y, Bonar F, Murrell GAC. Enhanced expression of neuronal proteins in idiopathic frozen shoulder. J Shoulder Elbow Surg. 2012;21:1391–7. doi: 10.1016/j.jse.2011.08.046. [DOI] [PubMed] [Google Scholar]
- 29.Zaoui P, Cantin JF, Alimardani-Bessette M, Monier F, Halimi S, Morel F, et al. Role of metalloproteases and inhibitors in the occurrence and progression of diabetic renal lesions. Diabetes Metab. 2000;26(Suppl 4):25–9. [PubMed] [Google Scholar]
- 30.Zuckerman JD, Rokito A. Frozen shoulder: a consensus definition. J Shoulder Elbow Surg. 2011;20:322–5. doi: 10.1016/j.jse.2010.07.008. [DOI] [PubMed] [Google Scholar]