Abstract
Soluble beta-amyloid (Aβ) oligomer is believed to be the most important toxic species in the brain of Alzheimer’s disease (AD) patients. Thus, it is critical to develop a simple method for the selective detection of Aβ oligomer with low cost and high sensitivity. In this paper, we report an electrochemical method for the detection of Aβ oligomer with a peptide as the bioreceptor and silver nanoparticle (AgNP) aggregates as the redox reporters. This strategy is based on the conversion of AgNP-based colorimetric assay into electrochemical analysis. Specifically, the peptide immobilized on the electrode surface and presented in solution triggered together the in situ formation of AgNP aggregates, which produced a well-defined electrochemical signal. However, the specific binding of Aβ oligomer to the immobilized peptide prevented the in situ assembly of AgNPs. As a result, a poor electrochemical signal was observed. The detection limit of the method was found to be 6 pM. Furthermore, the amenability of this method for the analysis of Aβ oligomer in serum and artificial cerebrospinal fluid (aCSF) samples was demonstrated.
Keywords: electrochemical biosensors, Alzheimer’s disease, beta-amyloid oligomer, peptide, silver nanoparticles
Introduction
Alzheimer’s disease (AD), the most common neurodegenerative disorder, will affect ~66 million people globally by the year 2030.1 A hallmark of AD is the deposition of the beta-amyloid (Aβ) peptide in the brain.2,3 Aβ monomer, typically comprising 39−43 amino acid residues, results from proteolytic cleavage of amyloid precursor protein (APP) by β- and γ-secretase.4 Furthermore, the monomers can coalesce to form small, soluble oligomeric species and then assemble into higher molecular weight fibrils. Thus, Aβ monomer and its aggregates have been considered not only as a therapeutic target but also as a diagnostic marker.5–9 There are many methods for the detection of Aβ monomer with high sensitivity, such as electrochemical immunosensors, colorimetric assays, resonance light scattering and surface plasmon resonance.10–18 However, assay of Aβ monomer only might be unable to discriminate between AD patients and healthy controls or other types of dementia because the levels of Aβ monomer may differ by gender and age.19 Soluble Aβ oligomer comprising 50–100 Aβ monomers is believed to be neurotoxic and responsible for neuronal death in preclinical AD.20,21 In addition, elevated levels of Aβ oligomer have been detected in the cerebrospinal fluid (CSF) of AD patients.22,23 Therefore, the direct detection of Aβ oligomer level would be more reliable for AD diagnosis than assay of its monomer.24,25
Recently, a few novel biosensors have been developed for the detection of Aβ oligomer, including electrochemistry,26–29 surface plasma resonance (SPR),30 localized surface plasmon resonance (LSPR),24,31 fluorescence,32,33 nuclear magnetic resonance,34 and surface-enhanced Raman spectroscopy.35 These methods are feasible, but they require the use of special instruments and/or relatively expensive and variable antibodies for the capture and recognition of Aβ oligomer. Moreover, the reported antibody of Aβ oligomer also recognizes Aβ monomer and other Aβ aggregates and metabolites to some extent.36 Alternatively, the organic dye-based fluorescence assays (eg, thioflavin T [ThT]) have been commonly used for monitoring the formation of Aβ aggregates in laboratory investigation.37,38 However, most of the dyes cannot be used to discriminate Aβ oligomer from other β-sheets of Aβ aggregates,37 thus limiting their applications for the routine test of Aβ oligomer for early diagnosis of AD.
Cellular prion protein (PrPC) is a membrane-bound glycoprotein present in the central nervous system. There is increasing evidence demonstrating that PrPC may be a high-affinity receptor for Aβ oligomer.39–44 The core region of PrPC to bind with Aβ oligomer is PrP95–110, which is located within the unstructured N-terminal region of PrPC with an amino acid sequence of THSQWNKPSKPKTNMK (PrP95–110).39,42–45 The dissociation constant (Kd) for the Aβ oligomer/PrP95–110 interaction is in the subnanomolar range, and the interaction is highly specific for Aβ oligomer, but not for its monomer and fibril.42,43,46 These results provide researchers a hint that PrP95–110 would be a good receptor for the design of novel biosensors for Aβ oligomer detection.
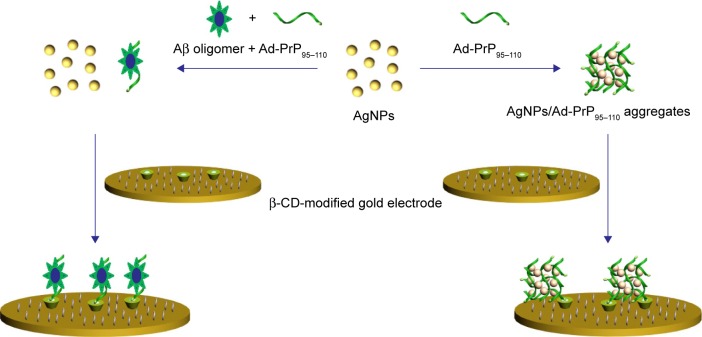
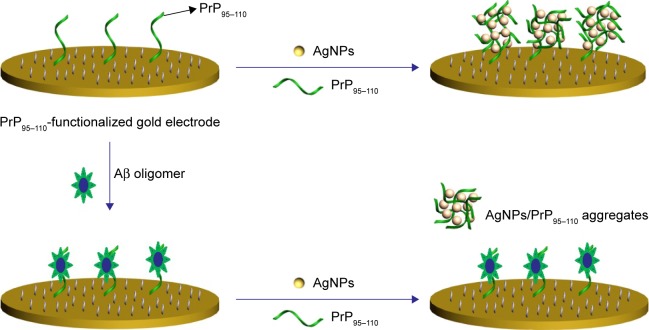
In recent years, metal nanoparticles (MNPs) have been widely used for creating effective recognition and transduction processes in chem/biosensing due to their unique physicochemical attributes.47–59 In particular, silver nanoparticles (AgNPs) offer clear advantages for the design of electrochemical (bio) sensors, such as a simple preparation procedure, a size-dependent optical property, facile surface modification, a high surface area and a low oxidation potential.55–59 Based on the specific Aβ oligomer/PrP95–110 interaction and the well-defined and signal-amplified electrochemical signal of AgNP aggregates, Xia et al59 have developed an electrochemical biosensor for the determination of Aβ oligomer by using adamantine (Ad)-labeled PrP95–110 (Ad-PrP95–110) as the receptor and AgNP aggregates as the redox reporters. In this work, the network architecture of Ad-PrP95–110/AgNP nanocomposites produced in solution was introduced onto the β-cyclodextrin (β-CD)-modified electrode surface through the host–guest interaction (Scheme 1). The specific Aβ oligomer/PrP95–110 interaction made the Ad-PrP95–110 in solution to lose its capability to trigger the formation of AgNPs-based network architecture. This work presented a concept for converting the AgNPs-based colorimetric assay into a sensitive electrochemical analysis by simply incorporating the colorimetric principle into the electrochemical platform. The method is simple and does not require the modification of analyte-binding molecules onto the surface of nanoparticles. However, it requires the modification of both electrode and peptide probe. More importantly, the unmodified method showed poor anti-interference ability to high concentration of salts and other components in body fluids, thus failing to determine Aβ oligomer in biological samples. In the present study, we reported an innovative electrochemical method for the detection of Aβ oligomer based on the in situ formation of AgNP aggregate tags. As shown in Scheme 2, PrP95–110 immobilized on the electrode surface and presented in solution triggered together the in situ formation of AgNP aggregates, which produced a well-defined electrochemical signal. Once the electrode was covered with Aβ oligomer, PrP95–110 on the electrode surface would lose its ability to trigger the in situ formation of AgNPs-based network architecture. To avoid the absorption of other components onto the surface of unmodified AgNPs in the real sample analysis, the competitive assay was performed by a two-step procedure: incubation of the sensing electrode with Aβ oligomer sample first and follow-up incubation with AgNPs/PrP95–110. The proposed strategy not only features simple manipulation principle similar to that of colorimetric assay but also shows high sensitivity and specificity of electrochemical biosensor.
Scheme 1.
Schematic illustration of the previous electrochemical strategies for the detection of Aβ oligomer with PrP95–110 as the receptor and AgNP aggregates as the redox reporters.
Abbreviations: Aβ, beta-amyloid; PrP, prion protein; AgNP, silver nanoparticle; Ad, adamantine; β-CD, β-cyclodextrin.
Scheme 2.
Schematic illustration of the present electrochemical strategies for the detection of Aβ oligomer with PrP95–110 as the receptor and AgNP aggregates as the redox reporters.
Abbreviations: Aβ, beta-amyloid; PrP, prion protein; AgNP, silver nanoparticle.
Experimental section
Chemicals and materials
Peptides with the sequences of CTHSQWNKPSKPKTNMK and THSQWNKPSKPKTNMK (PrP95–110) were synthesized and purified by Synpeptide Co., Ltd (Shanghai, China). The Aβ peptide with 42 amino acid residues (Aβ1–42), 6-mercapto-1-hexanol (MCH), tris(2-carboxyethyl)phosphine (TCEP), bovine serum albumin (BSA), immunoglobin G (IgG), lysozyme, thrombin, serum and 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). All other chemicals were of analytical grade and provided by Beijing Chemical Reagent Co. Ltd (Beijing, China).
Citrate-stabilized AgNPs and soluble Aβ oligomer were prepared as in our previous report.59 Artificial cerebrospinal fluid (aCSF) used in the determination of the samples was prepared by 150 mM NaCl, 3 mM KCl, 1.4 mM CaCl2, 1 mM phosphate and 0.8 mM MgCl2.29,60
Instruments
The ultraviolet (UV)/visible (Vis) spectra were collected on a Cary 60 spectrophotometer using a 1-cm quartz spectrophotometer cell. The atomic force microscopy (AFM) images were taken using a Dimension Edge microscope (Bruker Nano Inc., Santa Barbara, CA, USA) equipped with a tapping mode. The transmission electron microscope (TEM) images were taken using an FEI Tecnai G2 T20 TEM (Hillsboro, OR, USA). The electrochemical experiments were carried out using a CHI-660E (CH Instruments, Shanghai, China) electrochemical workstation. Platinum wire was used as the auxiliary electrode. The reference electrode was Ag/AgCl.
Stability of AgNPs
To examine the inhibition of Aβ oligomer on the PrP95–110-triggered assembly of AgNPs, PrP95–110 was mixed with Aβ oligomer for 10 min. Then, AgNPs suspension was added to the PrP95–110 solution. After incubation for 5 min, color change was observed with the naked eye and the photograph was taken by a digital camera. UV/Vis absorption spectra were collected using the spectrophotometer.
Electrochemical detection of Aβ oligomer
The cleaned gold disk electrode with a diameter of 2 mm was placed in a 100 μL phosphate-buffered saline (PBS) solution (10 mM, pH 7.2) containing 10 μM thiolated PrP95–110 (CTHSQWNKPSKPKTNMK) and 50 μM TCEP overnight. After the formation of peptide self-assembled monolayers (SAMs), the electrode was washed with water and then soaked in a 1 mM MCH solution for 30 min. For the detection of Aβ oligomer, the PrP95–110-functionalized electrode was first immersed in a 20 μL PBS solution containing a given concentration of Aβ oligomer for 10 min, and the electrode was then rinsed thoroughly with water and exposed to 20 μL of AgNPs suspension in an opened plastic tube. This step was followed by the addition of 20 μL of PrP95–110 to incubation for 10 min. After being rinsed with water, the electrode was placed in a 1 M KCl solution for linear sweep voltammetry (LSV) measurement.
Results and discussion
PrP95–110-triggered AgNPs aggregation
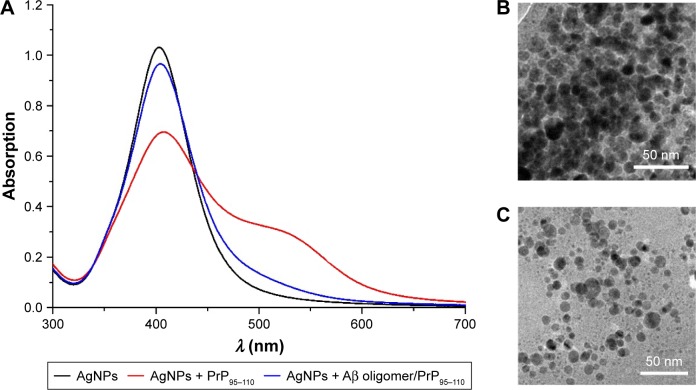
As shown in Figure 1A, the AgNPs solution showed an absorption peak at 404 nm (black curve), which is ascribed to the surface plasmon resonance of AgNPs. With the addition of PrP95–110, the original absorbance of AgNPs at 404 nm decreased, while a new absorbance peak at 525 nm appeared (red curve). The red-shifted band demonstrated that PrP95–110 triggered the aggregation of AgNPs. The aggregation is attributed to the electrostatic interaction between the negatively charged citrate-capped AgNPs and the positively charged lysine residues in PrP95–110.59 We also found that the absorption intensity of AgNPs at 525 nm increased and reached a plateau value within 7 min, indicating the achievement of the PrP95–110-triggered AgNPs assembly. When PrP95–110 was first mixed with Aβ oligomer, only one absorption peak at 404 nm was observed (blue curve) with the addition of the mixed solution to AgNPs suspension. It is indicative of a good dispersion of AgNPs in the presence of the Aβ oligomer–PrP95–110 complex. Furthermore, these results were confirmed by the TEM observations: aggregated AgNPs in the presence of PrP95–110 only (Figure 1B) and dispersed AgNPs in the presence of Aβ oligomer/PrP95–110 (Figure 1C). We also found that Aβ monomer and fibril did not inhibit the PrP95–110-triggered red shift of AgNPs absorbance, which agrees with the previous report.59 These results confirmed that only Aβ oligomer inhibited the PrP95–110-induced assembly of AgNPs, which is contributed to the strict dependence of the recognition of PrP95–110 on the secondary structure of Aβ.
Figure 1.
Characterization of AgNPs in the presence PrP95–110 or Aβ oligomer/PrP95–110.
Notes: (A) UV–Vis absorption spectra of AgNPs in the absence and presence of PrP95–110 or Aβ oligomer/PrP95–110. TEM images of AgNPs in the presence of PrP95–110 (B) or Aβ oligomer/PrP95–110 (C). The concentrations of AgNPs, PrP95–110 and Aβ sample (equivalent monomer) were 2.4 nM, 0.1 μM and 2 μM, respectively.
Abbreviations: UV, ultraviolet; Vis, visible; AgNP, silver nanoparticle; PrP, prion protein; Aβ, beta-amyloid; TEM, transmission electron microscope.
Electrochemical analysis
Herein, we suggested that PrP95–110 both on electrode and in solution could trigger the in situ formation of AgNP aggregates on the electrode surface. When PrP95–110 immobilized on the electrode surface interacted with Aβ oligomer, it lost the ability to trigger the in situ formation of AgNPs-based network architecture. To demonstrate the feasibility of our design, LSV was used to measure the oxidation current of AgNPs. As shown in Figure 2, incubation of the PrP95–110-functionalized electrode with AgNPs/PrP95–110 resulted in the appearance of a well-defined oxidation peak at ~65 mV (black curve), which is attributed to the solid-state Ag/AgCl reaction from AgNPs. However, no oxidation peak was observed when the functionalized electrode was incubated with PrP95–110 itself (red curve), and only a small oxidation peak was observed when the electrode was incubated with AgNPs only (blue curve). These results demonstrated that the strong oxidation peak in the black curve should be attributed to the formation of the AgNPs/PrP95–110 network architecture. When the electrode was incubated with Aβ oligomer, followed by incubation with PrP95–110/AgNPs (green curve), the current dropped almost to the background level. This indicated that the binding of PrP95–110 to Aβ oligomer inhibited the in situ formation of AgNPs/PrP95–110 network architecture on the electrode surface. Additionally, we found that a slight decrease in the current was observed (magenta curve) when the sensor electrode was incubated with the mixed solution comprising AgNPs, PrP95–110 and Aβ oligomer (one-step method). Thus, the two-step method performed by incubation of the sensor electrode with Aβ oligomer first and follow-up incubation with AgNPs/PrP95–110 (green curve) is more sensitive than the one-step method. The result is understandable since large amount of PrP95–110 in solution would preferentially bind to Aβ oligomer, thus hampering the formation of Aβ oligomer/PrP95–110 on the electrode surface and facilitating the in situ assembly of AgNPs. Furthermore, other components in biological samples may absorb on the surface of unmodified AgNPs to reduce the selectivity of biosensor.59 Therefore, the competitive assay was performed by the two-step procedure.
Figure 2.
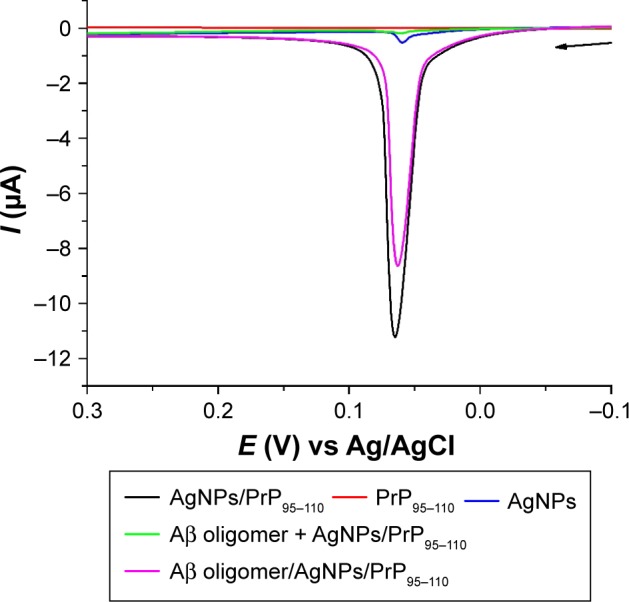
The LSV responses of the PrP95–110-functionalized electrodes after incubation with AgNPs/PrP95–110 (black curve), PrP95–110 (red curve), AgNPs (blue curve), Aβ oligomer and AgNPs/PrP95–110 (green curve) and the mixture of Aβ oligomer/PrP95–110/AgNPs (magenta curve).
Notes: The arrow indicates the scan direction. The concentrations of AgNPs, PrP95–110 and Aβ sample were 2.4 nM, 0.1 μM and 2 μM, respectively.
Abbreviations: LSV, linear sweep voltammetry; PrP, prion protein; AgNP, silver nanoparticle; Aβ, beta-amyloid.
Optimization of experimental conditions
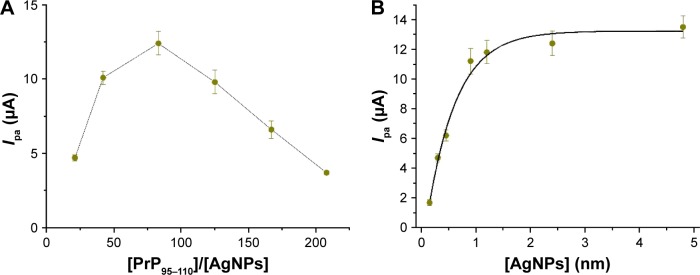
A higher concentration of PrP95–110 can make the aggregation of AgNPs more powerful. However, a higher concentration of PrP95–110 in solution would compete with the anchored PrP95–110 on the electrode surface to bind with AgNPs, thus hampering the in situ formation of the AgNPs/PrP95–110 network architecture. Thus, we first investigated the effect of the concentration ratio of PrP95–110 to AgNPs ([PrP95–110]/[AgNPs]) on the oxidation current (Ipa). It was found that Ipa initially increased with the increasing [PrP95–110]/[AgNPs] ratio until the maximal value appeared at 83:1 (Figure 3A). Furthermore, the dependence of Ipa on the AgNPs concentration was examined. It was found that Ipa increased upon increasing concentrations of AgNPs and began to level off beyond 1.2 nM (Figure 3B). Thus, in the following quantitative assays of Aβ oligomer, the concentrations of AgNPs and PrP95–110 were kept at 1.2 and 100 nM, respectively.
Figure 3.
Dependence of the current on the concentration ratio of PrP95–110 to AgNPs (A) and the AgNPs concentration (B).
Notes: In (A), the AgNPs concentration was kept at 2.4 nM and the concentration of PrP95–110 was increased from 0.05 to 0.5 μM (0.05, 0.1, 0.2, 0.3, 0.4 and 0.5 μM). In (B), the concentration ratio of PrP95–110 to AgNPs was kept at 83:1 and the AgNPs concentration was increased from 0.15 to 4.8 nM (0.15, 0.3, 0.45, 0.9, 1.2, 2.4 and 4.8 nM). Ipa, oxidation current.
Abbreviations: PrP, prion protein; AgNP, silver nanoparticle; Aβ, beta-amyloid.
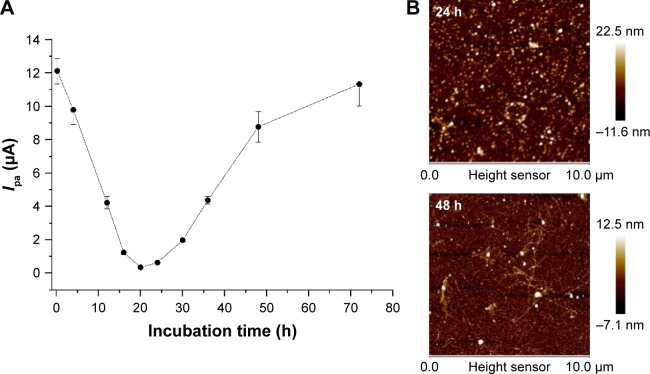
With the increase in incubation time, Aβ monomers can assembly spontaneously into oligomeric and fibrous species. We also studied the influence of Aβ incubation time on the formation of Aβ oligomer and the inhibition of PrP95–110-triggered assembly of AgNPs. As shown in Figure 4, the lowest points of the currents are in the range of 16–24 h, indicating the optimal incubation time for the formation and detection of Aβ oligomer. In the following quantitative assays, 20 h was set as the optimized time for oligomer preparation.
Figure 4.
Influence of Aβ incubation time on the formation of Aβ oligomer and the current.
Notes: (A) Dependence of the current on the incubation time for the preparation of Aβ oligomer. The final concentrations of AgNPs, PrP95–110 and Aβ sample were kept at 1.2 nM, 100 nM and 1 μM, respectively. (B) AFM images of the mica substrate after incubation with Aβ samples pre-incubated for 24 and 48 h. Ipa, oxidation current.
Abbreviations: Aβ, beta-amyloid; AgNP, silver nanoparticle; PrP, prion protein; AFM, atomic force microscopy.
Sensitivity and selectivity
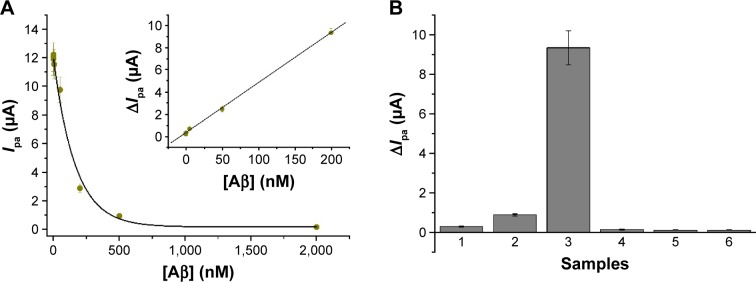
Under the optimized experimental conditions, the quantitative detection of Aβ oligomer was performed. As shown in Figure 5A, Ipa decreased with increasing Aβ oligomer concentration ([Aβ], equivalent monomer) varying from 0 to 2 μM. The relative standard deviations (RSDs) are all <13% for assay of the same Aβ oligomer sample at three different electrodes in parallel. The acceptable reproducibility demonstrated that multiple electrodes can be prepared concurrently for the analysis of many different samples. Herein, the current change ΔIpa (Ipa − Ipa′, where Ipa and Ipa′ represent the current in the absence and presence of Aβ oligomer, respectively), was used to evaluate the sensor performances. As shown in the inset, ΔIpa is proportional to [Aβ] in a linear range of 0.01–200 nM. The regression equation was found to be ΔIpa =0.289+0.045 [Aβ] (nM). The detection limit was estimated to be 6 pM by measuring the sensor response to a dilution series and determining the target smallest concentration at which the sensor response is clearly distinguishable from the response to a blank solution. This value is comparable to that achieved by the AgNPs- or AuNPs-based LSPR techniques (0.1 or 1.5 pM),24,31 and is significantly lower than that achieved by other methods, including molecular beacon (MB; 3.57 nM)-based,6 graphene oxide (1 nM)-based and CdTe quantum dots (QDs)-based fluorescent assays;46 square wave voltammetry (48 pM);27 electrochemical impedance spectroscopy (100 pM);45 magnetic bead-droplet immunoassay (2.22 mM)61 and surface-enhanced Raman spectroscopy (0.1 μM).35 Moreover, our method required very simple sample handling procedure and obviated the modification of nanoparticles and the utilization of expensive and variable antibodies for the capture and recognition of Aβ oligomer. The physiological content of Aβ in a normal human CSF is in the range of nanomolar, and a higher concentration of Aβ oligomer is present in AD patients.2 Thus, the proposed method is promising to detect Aβ oligomer in body fluids.
Figure 5.
Sensitivity and selectivity.
Notes: (A) Dependence of the current on the concentration of Aβ sample (0.01, 0.2, 5, 50, 200, 500 and 2,000 nM). The inset shows the linear dependence of the current change on the concentration of the Aβ sample. (B) Selectivity of the sensing protocol (bar 1, Aβ monomer; bar 2, Aβ fibril; bar 3, Aβ oligomer; bar 4, BSA; bar 5, IgG and bar 6, thrombin). The concentration of the Aβ sample was 200 nM and that of BSA, IgG and thrombin was 1 μM. Ipa, oxidation current.
Abbreviations: Aβ, beta-amyloid; BSA, bovine serum albumin; IgG, immunoglobin G.
To explore the specificity of our method, Aβ monomer, Aβ fibril and three interfering proteins (BSA, IgG and thrombin,) were tested. As shown in Figure 5B, compared to the control, only the fibril control caused a significant change in the current. This is probably due to the existence of a small amount of unfibrillar oligomer in the solution. The other four interferences did not cause significant change in the current. The result demonstrated that the tested interferences did not prevent the assembly of AgNPs/PrP95–110 on the sensor surface. Therefore, the proposed electrochemical method showed extraordinary selectivity toward the detection of Aβ oligomer. The high selectivity could be principally attributed to the strong and specific binding capacity of PrP95–110 to Aβ oligomer.
Assay of Aβ oligomer in serum and aCSF
To demonstrate the viability of our method for real sample assay, the content of Aβ oligomer in aCSF and 20% serum was determined by the standard addition method. The accuracy of the assay was evaluated by determining the recovery for the spiked sample. As shown in Table 1, the recoveries for assays of three different concentrations of Aβ oligomer varied from 86% to 109%. The acceptable values implied that the proposed method could provide a potential platform for the detection of Aβ oligomer in CSF and serum samples of AD patients.
Table 1.
Results of the proposed method for the detection of Aβ oligomer in aCSF and serum
| Sample | Added (nM) | Found (nM) | Recovery (%) |
|---|---|---|---|
| 1 (aCSF) | 1 | 0.89 | 89 |
| 2 (aCSF) | 20 | 21.8 | 109 |
| 3 (aCSF) | 50 | 52.4 | 104.8 |
| 4 (serum) | 1 | 0.86 | 86 |
| 5 (serum) | 20 | 17.9 | 89.5 |
| 6 (serum) | 50 | 43.7 | 91.4 |
Abbreviations: Aβ, beta-amyloid; aCSF, artificial cerebrospinal fluid.
Conclusion
This work presented an innovative electrochemical method for the detection of Aβ oligomer by inhibiting the in situ formation of AgNPs-based network architecture on the electrode surface. The Aβ oligomer-binding peptide was used as the recognition element. The proposed electrochemical method not only features simple manipulation principle and easy detection procedure similar to that of colorimetric assay but also shows high sensitivity and specificity. The detection limit of this method for Aβ oligomer detection is 6 pM, which is comparable to or lower than that achieved by the previously reported methods. However, our method is rapid (<30 min) and label free, obviates the modification of nanoparticles for signal amplification and does not require the utilization of expensive and variable antibodies and enzymes for the capture and recognition of Aβ oligomer. In view of the high toxicity of soluble Aβ oligomer in the brains of AD patients, the proposed biosensor could potentially serve as a viable alternative for facile clinical diagnosis of AD. The result also demonstrated that the bare AgNPs-based colorimetric assay can be converted into an electrochemical analysis with improving specificity. Moreover, this proposed detection principle should be valuable for developing label-free optical platforms with multiplexed aptameric peptide microarrays.
Acknowledgments
Partial support of this work by the National Natural Science Foundation of China (nos 61661014 and 61301038) and the Natural Science Foundation of Guangxi Province (no 2015GXNSFBA139041) is gratefully acknowledged.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wortmann M. Dementia: a global health priority-highlights from an ADI and world health organization report. Alzheimers Res Ther. 2012;4(5):40. doi: 10.1186/alzrt143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamley IW. The amyloid beta peptide: a chemist’s perspective. Role in Alzheimer’s and fibrillization. Chem Rev. 2012;112:5147–5192. doi: 10.1021/cr3000994. [DOI] [PubMed] [Google Scholar]
- 3.Heo CH, Kim KH, Kim HJ, et al. A two-photon fluorescent probe for amyloid-beta plaques in living mice. Chem Commun. 2013;49:1303–1305. doi: 10.1039/c2cc38570h. [DOI] [PubMed] [Google Scholar]
- 4.Xia N, Zhang YJ, Guan PP, Hao YQ, Liu L. A simple and label-free electrochemical method for detection of beta-site amyloid precursor protein cleaving enzyme and screening of its inhibitor. Sens Actuat B Chem. 2015;213:111–115. [Google Scholar]
- 5.de la Escosura-Muñiza A, Plichtab Z, Horákb D, Merkoçia A. Alzheimer’s disease biomarkers detection in human samples by efficient capturing through porous magnetic microspheres and labelling with electrocatalytic gold nanoparticles. Biosens Bioelectron. 2015;67:162–169. doi: 10.1016/j.bios.2014.07.086. [DOI] [PubMed] [Google Scholar]
- 6.Zhu LL, Zhang JY, Wang FY, et al. Selective amyloid β oligomer assay based on a basic site-containing molecular beacon and enzyme-free amplification. Biosens Bioelectron. 2016;78:206–212. doi: 10.1016/j.bios.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 7.Vieira DB, Gamarra LF. Getting into the brain: liposome-based strategies for effective drug delivery across the blood–brain barrier. Int J Nanomed. 2016;11:5381–5414. doi: 10.2147/IJN.S117210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaman M, Ahmad E, Qadeer A, Rabbani G, Khan RH. Nanoparticles in relation to peptide and protein aggregation. Int J Nanomed. 2014;9:899–912. doi: 10.2147/IJN.S54171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitatha S, Moongfangklang N, Jalil MA, et al. Proposal for Alzheimer’s diagnosis using molecular buffer and bus network. Int J Nanomed. 2011;6:1209–1216. doi: 10.2147/IJN.S22165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rama EC, Gonzalez-Garcia MB, Costa-Garcia A. Competitive electrochemical immunosensor for amyloid-beta 1–42 detection based on gold nanostructurated screen-printed carbon electrodes. Sens Actuat B Chem. 2014;201:567–571. [Google Scholar]
- 11.Carneiro P, Loureiro J, Delerue-Matos C, Morais S, Pereira MD. Alzheimer’s disease: development of a sensitive label-free electrochemical immunosensor for detection of amyloid beta peptide. Sens Actuat B Chem. 2017;239:157–165. [Google Scholar]
- 12.Hu T, Chen CX, Huang GM, Yang XR. Antibody modified-silver nanoparticles for colorimetric immuno sensing of A beta((1–40/1–42)) based on the interaction between beta-amyloid and Cu2+ Sens Actuat B Chem. 2016;234:63–69. [Google Scholar]
- 13.Wang C, Liu D, Wang Z. Resonance light scattering as a powerful tool for sensitive detection of β-amyloid peptide by gold nanoparticle probes. Chem Commun. 2011;47:9339–9341. doi: 10.1039/c1cc12939b. [DOI] [PubMed] [Google Scholar]
- 14.Xia N, Liu L, Harrington MG, Wang J, Zhou F. Regenerable and simultaneous surface plasmon resonance detection of Aβ(1–40) and Aβ(1–42) peptides in cerebrospinal fluids with signal amplification by streptavidin conjugated to an N-terminus-specific antibody. Anal Chem. 2010;82:10151–10157. doi: 10.1021/ac102257m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L, He Q, Zhao F, et al. Competitive electrochemical immunoassay for detection of β-amyloid (1–42) and total β-amyloid peptides using p-aminophenol redox cycling. Biosens Bioelectron. 2014;51:208–212. doi: 10.1016/j.bios.2013.07.047. [DOI] [PubMed] [Google Scholar]
- 16.Liu L, Zhao F, Ma F, et al. Electrochemical detection of β-amyloid peptides on electrode covered with N-terminus-specific antibody based on electrocatalytic O2 reduction by Aβ(1–16)-heme-modified gold nanoparticles. Biosens Bioelectron. 2013;49:231–235. doi: 10.1016/j.bios.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 17.Yu Y, Zhang L, Li C, et al. A method for evaluating the level of soluble β-amyloid (1–40/1–42) in Alzheimer’s disease based on the binding of gelsolin to β-amyloid peptides. Angew Chem. 2014;126:13046–13049. doi: 10.1002/anie.201405001. [DOI] [PubMed] [Google Scholar]
- 18.Han SH, Chang YJ, Jung ES, Kim JW, Na DL, Mook-Jung I. Effective screen for amyloid β aggregation inhibitor using amyloid β-conjugated gold nanoparticles. Int J Nanomedicine. 2011;6:1–12. doi: 10.2147/IJN.S15278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golde TE, Eckman CB, Younkin SG. Biochemical detection of A beta isoforms: implications for pathogenesis, diagnosis, and treatment of Alzheimer’s disease. Biochim Biophy Acta. 2000;1502:172–187. doi: 10.1016/s0925-4439(00)00043-0. [DOI] [PubMed] [Google Scholar]
- 20.Glabe CG. Structural classification of toxic amyloid oligomers. J Biol Chem. 2008;283(44):29639–29643. doi: 10.1074/jbc.R800016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walsh DM, Klyubin I, Fadeeva JV, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416(6880):535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 22.Fukumoto H, Tokuda T, Kasai T, et al. High-molecular-weight β-amyloid oligomers are elevated in cerebrospinal fluid of Alzheimer patients. FASEB J. 2010;24:2716–2726. doi: 10.1096/fj.09-150359. [DOI] [PubMed] [Google Scholar]
- 23.Hölttä M, Hansson O, Andreasson U, et al. Evaluating amyloid-β oligomers in cerebrospinal fluid as a biomarker for Alzheimer’s disease. PLoS One. 2013;8:e66381. doi: 10.1371/journal.pone.0066381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haes AJ, Chang L, Klein WL, Duyne RPV. Detection of a biomarker for Alzheimer’s disease from synthetic and clinical samples using a nanoscale optical biosensor. J Am Chem Soc. 2005;127:2264–2271. doi: 10.1021/ja044087q. [DOI] [PubMed] [Google Scholar]
- 25.Arosio P, Cukalevski R, Frohm B, Knowles TPJ, Linse S. Quantification of the concentration of Aβ42 propagons during the lag phase by an amyloid chain reaction assay. J Am Chem Soc. 2014;136(1):219–225. doi: 10.1021/ja408765u. [DOI] [PubMed] [Google Scholar]
- 26.Veloso AJ, Chow AM, Ganesh HVS, et al. Electrochemical immunosensors for effective evaluation of amyloid-beta modulators on oligomeric and fibrillar aggregation processes. Anal Chem. 2014;86(10):4901–4909. doi: 10.1021/ac500424t. [DOI] [PubMed] [Google Scholar]
- 27.Li H, Xie H, Cao Y, Ding X, Yin Y, Li G. A general way to assay protein by coupling peptide with signal reporter via supermolecule formation. Anal Chem. 2013;85(2):1047–1052. doi: 10.1021/ac302906c. [DOI] [PubMed] [Google Scholar]
- 28.Zhou YL, Liu LT, Hao YQ, Xu MT. Detection of Aβ monomers and oligomers: early diagnosis of Alzheimer’s disease. Chem Asian J. 2016;11:805–817. doi: 10.1002/asia.201501355. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Y, Zhang H, Liu L, et al. Fabrication of an antibody-aptamer sandwich assay for electrochemical evaluation of levels of β-amyloid oligomers. Sci Rep. 2016;6:35186. doi: 10.1038/srep35186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yi XY, Feng CT, Hu SQ, Li HF, Wang JX. Surface plasmon resonance biosensors for simultaneous monitoring of amyloid-beta oligomers and fibrils and screening of select modulators. Analyst. 2015;141(1):331–336. doi: 10.1039/c5an01864a. [DOI] [PubMed] [Google Scholar]
- 31.Kang MK, Lee J, Nguyen AH, Sim SJ. Label-free detection of ApoE4-mediated β-amyloid aggregation on single nanoparticle uncovering Alzheimer’s disease. Biosens Bioelectron. 2015;72:197–204. doi: 10.1016/j.bios.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 32.Park MC, Kim M, Lim GT, et al. Droplet-based magnetic bead immunoassay using microchannel connected multiwell plates (μ CHAMPs) for the detection of amyloid beta oligomers. Lap Chip. 2016;16:2245–2253. doi: 10.1039/c6lc00013d. [DOI] [PubMed] [Google Scholar]
- 33.Esparza TJ, Zhao H, Cirrito JR, et al. Amyloid-beta oligomerization in Alzheimer dementia vs. high pathology controls. Ann Neurol. 2013;73:104–119. doi: 10.1002/ana.23748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Espargaró A, Busquets MA, Estelrich J, Sabate R. Amyloids in solid-state nuclear magnetic resonance: potential causes of the usually low resolution. Int J Nanomedicine. 2015;10:6975–6983. doi: 10.2147/IJN.S89385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guerrini L, Arenal R, Mannini B, et al. SERS detection of amyloid oligomers on metallorganic-decorated plasmonic beads. ACS Appl Mater Interfaces. 2015;7(18):9420–9428. doi: 10.1021/acsami.5b01056. [DOI] [PubMed] [Google Scholar]
- 36.Tsukakoshi K, Abe K, Sode K, Ikebukuro K. Selection of DNA aptamers that recognize α-synuclein oligomers using a competitive screening method. Anal Chem. 2012;84:5542–5547. doi: 10.1021/ac300330g. [DOI] [PubMed] [Google Scholar]
- 37.Teoh CL, Su DD, Sahu S, et al. Chemical fluorescent probe for detection of Aβ oligomers. J Am Chem Soc. 2015;137(42):13503–13509. doi: 10.1021/jacs.5b06190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matveeva EG, Rudolph A, Moll JR, Thompson RB. Structure-selective anisotropy assay for amyloid beta oligomers. ACS Chem Neurosci. 2012;3(11):982–987. doi: 10.1021/cn3001262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balducci C, Beeg M, Stravalaci M, et al. Synthetic amyloid-β oligomers impair long-term memory independently of cellular prion protein. Proc Natl Acad Sci U S A. 2010;107:2295–2300. doi: 10.1073/pnas.0911829107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freir DB, Nicoll AJ, Klyubin I, et al. Interaction between prion protein and toxic amyloid β assemblies can be therapeutically targeted at multiple sites. Nat Commun. 2011;2:1–9. doi: 10.1038/ncomms1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-β oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen S, Yadav SP, Surewicz WK. Interaction between human prion protein and amyloid-β (Aβ) oligomers. J Biol Chem. 2010;285:26377–26383. doi: 10.1074/jbc.M110.145516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fluharty BR, Biasini E, Stravalaci M, et al. An N-terminal fragment of the prion protein binds to amyloid-β oligomers and inhibits their neurotoxicity in vivo. J Biol Chem. 2013;288(11):7857–7866. doi: 10.1074/jbc.M112.423954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guillot-Sestier MV, Sunyach C, Ferreira ST, et al. α-Secretase-derived fragment of cellular prion, N1, protects against monomeric and oligomeric amyloid β (Aβ)-associated cell death. J Biol Chem. 2012;287(7):5021–5032. doi: 10.1074/jbc.M111.323626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rushworth JV, Ahmed A, Griffiths HH, et al. A label-free electrical impedimetric biosensor for the specific detection of Alzheimer’s amyloid-beta oligomers. Biosens Bioelectron. 2014;56:83–90. doi: 10.1016/j.bios.2013.12.036. [DOI] [PubMed] [Google Scholar]
- 46.Xia N, Zhou B, Huang N, Jiang M, Zhang J, Liu L. Visual and fluorescent assays for selective detection of beta-amyloid oligomers based on the inner filter effect of gold nanoparticles on the fluorescence of CdTe quantum dots. Biosens Bioelectron. 2016;85:625–632. doi: 10.1016/j.bios.2016.05.066. [DOI] [PubMed] [Google Scholar]
- 47.Li F, Pei H, Wang LH, et al. Nanomaterial-based fluorescent DNA analysis: a comparative study of the quenching effects of graphene oxide, carbon nanotubes, and gold nanoparticles. Adv Funct Mater. 2013;23:4140–4148. [Google Scholar]
- 48.Liu L, Sun T, Ren H. Electrochemical detection of hydrogen peroxide by inhibiting the p-benzenediboronic acid-triggered assembly of citrate-capped Au/Ag nanoparticles on electrode surface. Materials. 2017;10:40. doi: 10.3390/ma10010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pérez-López B, Merkoçi A. Nanoparticles for the development of improved (bio) sensing systems. Anal Bioanal Chem. 2011;399(4):1577–1590. doi: 10.1007/s00216-010-4566-y. [DOI] [PubMed] [Google Scholar]
- 50.Xia N, Wang X, Wang X, Zhou B. Gold nanoparticle-based colorimetric and electrochemical methods for dipeptidyl peptidase-IV activity assay and inhibitor screening. Materials. 2016;9:857. doi: 10.3390/ma9100857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu JJ, Zhao WW, Song SP, Fan CH, Chen HY. Functional nanoprobes for ultrasensitive detection of biomolecules: an update. Chem Soc Rev. 2014;43(5):1601–1611. doi: 10.1039/c3cs60277j. [DOI] [PubMed] [Google Scholar]
- 52.Zhu CZ, Yang GH, Li H, Du D, Lin YH. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal Chem. 2015;87:230–249. doi: 10.1021/ac5039863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gan N, Jin HJ, Li TH, Zheng L. Fe3O4/Au magnetic nanoparticle amplification strategies for ultrasensitive electrochemical immunoassay of alfa-fetoprotein. Int J Nanomed. 2011;6:3259–3269. doi: 10.2147/IJN.S26212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feng XB, Gan N, Zhang HR, et al. A novel “dual-potential” electrochemiluminescence aptasensor array using CdS quantum dots and luminolgold nanoparticles as labels for simultaneous detection of malachite green and chloramphenicol. Biosens Bioelectron. 2015;74:587–593. doi: 10.1016/j.bios.2015.06.048. [DOI] [PubMed] [Google Scholar]
- 55.Li H, Sun Z, Zhong W, Hao N, Xu D, Chen HY. Ultrasensitive electrochemical detection for DNA arrays based on silver nanoparticle aggregates. Anal Chem. 2010;82(13):5477–5483. doi: 10.1021/ac101193e. [DOI] [PubMed] [Google Scholar]
- 56.Miao P, Meng F, Wang B, Zhu X, Tang Y. Highly sensitive microRNA quantification with zero background signal from silver nanoparticles. Electrochem Commun. 2015;51:89–92. [Google Scholar]
- 57.Wei T, Dong T, Wang Z, Bao J, Tu W, Dai Z. Aggregation of individual sensing units for signal accumulation: conversion of liquid-phase colorimetric assay into enhanced surface-tethered electrochemical analysis. J Am Chem Soc. 2015;137(28):8880–8883. doi: 10.1021/jacs.5b04348. [DOI] [PubMed] [Google Scholar]
- 58.Xia N, Liu L, Chang Y, Hao Y, Wang X. 4-Mercaptophenylboronic acid-induced in situ formation of silver nanoparticle aggregates as labels on an electrode surface. Electrochem Commun. 2017;74:28–32. [Google Scholar]
- 59.Xia N, Wang X, Zhou B, Wu Y, Mao W, Liu L. Electrochemical detection of amyloid-β oligomers based on the signal amplification of a network of silver nanoparticles. ACS Appl Mater Interfaces. 2016;8(30):19303–19311. doi: 10.1021/acsami.6b05423. [DOI] [PubMed] [Google Scholar]
- 60.Hegnerova K, Bockova M, Vaisocherova H, et al. Surface plasmon resonance biosensors for detection of Alzheimer disease biomarker. Sens Actuat B Chem. 2009;139:69–73. [Google Scholar]
- 61.Kim JA, Kim M, Kang SM, et al. Magnetic bead droplet immunoassay of oligomer amyloid beta for the diagnosis of Alzheimer’s disease using micro-pillars to enhance the stability of the oil-water interface. Biosens Bioelectron. 2015;67:724–732. doi: 10.1016/j.bios.2014.10.042. [DOI] [PubMed] [Google Scholar]