Abstract
ERBB2/HER2 has long been recognized as an oncogenic driver in some breast and gastro-esophageal cancers, in which amplification of this gene confers sensitivity to treatment with ERBB2 directed agents. More recently, somatic mutations in ERBB2 have been reported in 1–2% of patients with lung adenocarcinoma. Previous case series have suggested clinical tumor responses using anti-ERBB2 small molecules and antibody therapies. Here we report the outcomes of nine patients with metastatic lung adenocarcinoma with ERBB2 mutations being treated with ERBB2 targeted therapies. Four of the nine patients had response to targeted therapies, with durations of response ranging from three to 10 months. We also explored potential resistance mechanisms upon progression on targeted therapies in a subset of patients, and identified a de novo PIK3CA mutation and ERBB2 copy number gain as potential resistance mechanisms.
Introduction
Treatment of patients with metastatic non-small cell lung cancers (NSCLC) historically relied upon choosing the most efficacious chemotherapy regimen based on histologic subtypes. With the discovery of “driver mutations” such as the epidermal growth factor receptor (EGFR) and the development of targeted tyrosine kinase inhibitor (TKI) therapies against these specific mutations, the treatment paradigm has shifted. There are now FDA approved targeted therapies for lung cancers with mutations in epidermal growth factor receptor (EGFR, also known as ERBB1/HER1), and gene rearrangements in anaplastic lymphoma kinase (ALK), and ROS-1. Several other recurrent genomic alterations have been identified and can also be targeted with off label use of targeted therapies. NSCLC with ERBB2/HER2 mutations appears to be one such example.
Earlier studies on ERBB2 aberrations in lung cancers focused on ERBB2 protein overexpression analyzed by immunohistochemistry (IHC) and gene amplification as detected by fluorescence in situ (FISH). A decade ago, trials of targeted therapies in ERBB2-amplified NSCLC yielded disappointing results. In a study including patients with tumors that had ERBB2 overexpression by IHC, treatment with trastuzumab plus cisplatin and gemcitabine was well tolerated, but there was no clear relationship between the intensity of ERBB2 overexpression and the likelihood of response [1]. A randomized phase II trial of cisplatin-gemcitabine with or without trastuzumab for ERBB2-positive NSCLC (overexpression defined by IHC 2+/3+, or amplification by FISH, or elevated circulating serum ERBB2 extracellular domain by enzyme-linked immunosorbent assay ELISA) also showed no clinical benefit [2].
ERBB2 kinase domain mutations were first reported in 2004 to be present in approximately 4% of unselected lung tumors, and in 10% of the adenocarcinoma subtype of lung cancer [3]. Subsequently, Shigematsu et al. reported ERBB2 mutations in only 1.5% of NSCLC cases, but with higher prevalence in women and non-smokers [4]. There is less genetic diversity of ERBB2 mutations compared with EGFR mutations, as 96% are exon 20 insertions, and 83% of those are a recurrent 12 base-pair insertion causing duplication of amino acids YVMA at codon 775 [5].
In 2006, Cappuzzo et al. reported a case of a patient with a ERBB2 exon 20 mutation (G776L) as well as an EGFR exon 21 mutation (A859T) that responded to weekly trastuzumab and paclitaxel[6]. Subsequently De Grève et al. reported on three patients with ERBB2 exon 20 mutation responding to afatinib [7]. In 2013, Mazières et al [8] identified 65 (1.7%) out of 3800 patients tested to have ERBB2 mutant NSCLC, and of the 65 patients, 16 received ERBB2 targeted treatments after conventional chemotherapy. The authors confirmed the higher prevalence of ERBB2 mutations among women and never smokers. In addition, they observed a disease control rate (DCR) of 93% for trastuzumab-based therapies, and a DCR of 100% for afatinib, but no response to other targeted drugs such as lapatinib and masatinib (a TKI with activity against c-Kit, PDGFR and FGFR3). A larger series was recently published by Mazières et al [9], in which the authors identified 101 patients from 38 centers. They again noted predominance in females (62.4%) and non-smokers (60.4%). Sixty-five patients received ERBB2-targeted therapies and overall response rate was 50.9%. Response rate (RR), DCR, and progression free survival (PFS) were 50%, 75%, and 5.1 months, respectively, for trastuzumab in combination with chemotherapy. For afatinib, the RR, DCR, and PFS were 18.2%, 63.7%, and 3.9 months, respectively.
In the current study, we identified a total of nine patients between 2 institutions with tumors harboring the ERBB2 exon 20 insertion mutation who received ERBB2 targeted therapies and report their outcomes. As there is currently limited data available on ERBB2 mechanisms of acquired resistance to targeted therapies, we also postulated potential resistance mechanisms after progression on targeted therapies. We identified a de novo PIK3CA mutation as well as ERBB2 copy number gain as potential mechanisms for resistance.
Methods
Patient selection
Our cohort included patients with advanced NSCLC with molecular testing demonstrating ERBB2 mutations at Stanford University in Stanford, CA, USA (patients 1–7) between April 2013 to May 2016 and at Sun-yat-sen University Cancer Center, China from August 2014 to Octorber 2015 (patients 8 and 9) that were treated with ERBB2 targeted therapies. The cohort was generated with assistance from the Stanford Cancer Institute Research Database (SCIRDB) group under an IRB-approved retrospective review protocol. The institutional review board of the Stanford University School of Medicine approved of this study. This research was also approved by the Sun Yat-sen University CancerCenter Research Ethics Board.
Targeted therapy
Docetaxel was dosed at 35 mg/m2 on Day 1 and 8 of 21-day cycle initially for patient #2, then reduced to 25 mg/m2. Paclitaxel was dosed at 60mg/m2 weekly. Vinorelbine was dosed as 25 mg/m2 weekly with trastuzumab 2 mg/kg weekly (following a 4 mg/kg loading dose) or 6 mg/kg every 3 weeks. Afatinib was dosed at 40mg/day. Adotrastuzumab emtansine was dosed at 3.6 mg/kg IV every 3 weeks.
Molecular testing in tissue and plasma
We performed several different methods of molecular testing on biopsy samples. ERBB2 mutations in tissue were identified using a Stanford PCR-based ERBB2 sizing assay, the NGS based methods Stanford Solid Tumor Actionable Mutation Panel (STAMP) assay [10], NGS-based Foundation One assay [11], or Geneseeq assay Technology Inc [12]. Plasma samples were collected and circulating tumor DNA (ctDNA) was analyzed using NGS-based CAPP-Seq with non-invasive copy number calling for EGFR and ERBB2 as previously described [13–15].
Response assessment
Response to therapy was measured using RECIST v1.1 criteria. RR is defined as the percentage of patients whose cancer shows partial response (there was no complete response) per RECIST v1.1 criteria. PFS is defined as the length of time during and after the treatment with ERBB2 targeted therapy that a patient lives with the disease and it does not get worse.
Case Reports of Responders
Patient #1
The first patient was a 64-year-old Caucasian woman with minimal smoking history who was diagnosed with stage IIIA lung adenocarcinoma in early 2011. She underwent neoadjuvant cisplatin and pemetrexed treatment for 2 months, followed by surgical resection. Pathology was consistent with T1N2 poorly differentiated adenocarcinoma of lung primary.
She then developed recurrent metastatic disease mainly in the liver after completing definitive chemoradiotherapy. She was treated with carboplatin, gemcitabine, and bevacizumab followed by gemcitabine and bevacizumab maintenance as part of a clinical trial and was able to achieve stable disease for six months before progression. She was next treated with docetaxel for 1 month with rapid progression, followed by pemetrexed alone for 2.5 months with slight progression, then with irinotecan for 2 months with significant progression with near replacement of liver with tumor. At that time she was found to have ERBB2 exon 20 insertion mutation (A775_G776 insSVMA) by NGS testing, and was started on vinorelbine and trastuzumab. She tolerated the treatment well. She achieved radiographic stable disease for 6 months and significant clinical improvement before progression of disease in all sites, with a subsequent decision to pursue hospice.
Patient #2
The second patient is a 67-year-old Asian woman, never smoker, who was diagnosed with T3N1M1a stage IV lung adenocarcinoma with biopsy-proven bilateral lung involvement. Mutational studies revealed in frame insertion in exon 20 of the ERBB2 gene (A775_G776 insYVMA).
She received carboplatin and pemetrexed chemotherapy for 3 months, followed by 6 months of pemetrexed maintenance. She then developed progressive disease and was started on trastuzumab and docetaxel, with a partial response as her best response. She progressed after 8 months, at which point her therapy was changed to afatinib. The patient took afatinib for one month and her CT showed response, but the patient had to change therapy due to insurance denial and was changed back to trastuzumab based therapy.
Patient #3
The third patient was a 61-year-old Caucasian woman with a 10-pack-year smoking history. She was diagnosed with stage IV lung carcinoma when she presented with extensive bone disease including a lytic lesion in the right femur and a mass in the left lower lung. She underwent urgent stabilization of the right femur. Pathology from the surgery was consistent with metastatic carcinoma of lung primary. She was found to have a ERBB2 exon 20 insertion mutation (A775_G776 insYVMA).
She was treated with carboplatin, paclitaxel, bevacizumab, and an investigational anti-MET therapy with initial mild decrease in lung mass and nodules after one month, then mild progression for 14 months. She was subsequently treated with vinorelbine and trastuzumab for 7 months, then docetaxel and trastuzumab with focal palliative radiation to control lesion bone metastases. She achieved stable disease as her best response for 5 additional months. At progression she was treated with single agent pemetrexed, after which time she progressed and transitioned to hospice care.
Patient #4
The fourth patient was a 36-year-old Asian woman, non-smoker, who was diagnosed with stage IV lung adenocarcinoma when she presented with a large left-sided pleural effusion and lung masses. CT-guided fine needle aspiration showed adenocarcinoma consistent with lung primary. She was found to have a ERBB2 exon 20 insertion mutation (unknown exact sequence).
She was treated with carboplatin, pemetrexed, and bevacizumab first followed by pemetrexed and bevacizumab maintenance, with initial mild improvement then progression after 4.5 months. She was then treated with erlotinib on the control arm of a clinical trial with rapid progression within 1 month. She started afatinib 40 mg daily based on the ERBB2 mutation, and had improved disease after 2 months with best response of 21% reduction, then progression after 3 more months (5 months total of clinical benefit). She was then started on vinorelbine and trastuzumab, and had a partial response to therapy after 4 months. Her disease progressed after 6 months of therapy, and she was switched to docetaxel plus trastuzumab. She achieved stable disease after 1 month, and then progressed systemically in lungs and adrenal metastasis after 3 months. She then started ado-trastuzumab emtansine, but repeat imaging showed progressive disease after 2 months. She required three separate rounds of stereotactic radiation for brain lesions during the above treatments. She then received nivolumab for 1 month and continued to have progression. She next enrolled in a clinical trial with a CNS-active chemotherapy for 2 months, but continued to show progression with multiple new brain metastases and progression in her chest. The patient received whole brain radiation at that point. She was then re-challenged with afatinib daily, in combination with bevacizumab IV every 3 weeks, and tolerated the therapy well with mixed response at 6 weeks, but eventual progression in the liver after 3 months.
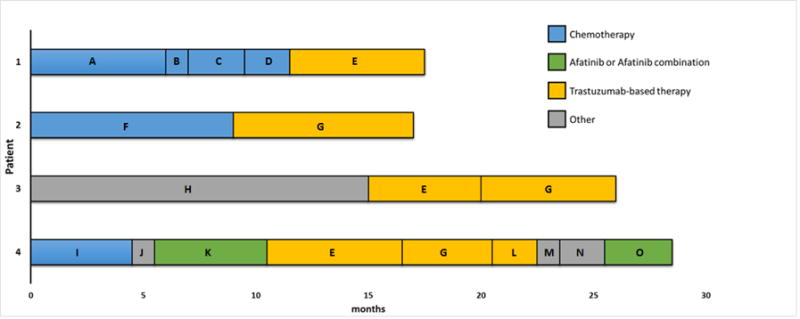
The demographic characteristics, treatments and responses for the above four patients are summarized in Table 1. Representative CT imaging of the best responses is shown in Figure 1. A swimmer plot summarizing the treatment courses of the 4 patients treated with ERBB2 targeted therapy is shown in Figure 2.
Table 1.
Summary of Responders
| Patient | Age | Race/Gender | Smoking status | Histology | HER2 alteration | Testing Method | Other alterations | Systemic Therapy*# | Best Responseˆ | PFS |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| #1 | 62 | C/F | <10 pack year | Adeno | Exon 20 ins (A775_G77 6 insSVMA) | Foundation One NGS | After recurrence: | |||
| carbo/gem/bev, then gem/bev maintenance X6 mo docetaxel X1 mo | SD PD |
6 mo | ||||||||
| Pem X2.5 mo | PD | |||||||||
| Irinotecan X2 mo | PD | |||||||||
| Tras/vinorelbine X6 mo | PR (−32%) | 6 mo | ||||||||
|
| ||||||||||
| #2 | 67 | A/F | Never | Adeno | A775_G77 6 insYVMA | STAMP NGS | RB1 R320L, ASTN1 R214W, IFLTD1 L71P, DDX1 V429I, FAM5C D431V | Carbo/pem, then pem maintenance X9 mo | PR | 9 mo |
| Tras/docetaxel X 8 mo | PR (−33%) | 8 mo | ||||||||
| Afatinib X 1 mo | SD (+2%) | 1 mo | ||||||||
|
| ||||||||||
| #3 | 61 | C/F | 10 pack year | Carcinoma | A775_G77 6 insYVMA | STAMP NGS | PIK3CA R88Q | Carbo/docetaxel/bev + investigational anti-Met therapy X15 mo | PR | 1 mo |
| Tras/vinorelbine X7 mo | SD (+2%) | 5 mo | ||||||||
| Tras/docetaxel X5mo | SD (+9%) | 5 mo | ||||||||
|
| ||||||||||
| #4 | 36 | A/F | Never | Adeno | Exon 20 ins (exact sequence unknown) | PCR amplification | HER2 amplification | Carbo/pem/bev, then pem/bev maintenance X 4.5mo | PR | 4.5 mo |
| Erlotinib X 1 mo | PD | |||||||||
| Afatinib X 6.5 mo | PR (−43%) | 5 mo | ||||||||
| Tras/vinorelbine X 6 mo | PR (−35%) | 6 mo | ||||||||
| Tras/docetaxel X4 mo | SD (−3%) | 4 mo | ||||||||
| Ado-trastuzumab X2 mo | PD | |||||||||
| Nivolumab X 1mo etirinotecan pegol (clinical trial) X 2mo | PD PD |
|||||||||
| Afatinib/bev X 3 months | Mixed response, with SD (−20%) in lung and PD in liver | n/a | ||||||||
HER2 targeted therapies in bold
number indicates number of cycles unless otherwise specified
as determined by RECIST v1.1 criteria
C: Caucasian
A: Asian
M: male
F: female
Adeno: adenocarcinoma
mo: month
PFS: progression free survival
NGS: next generation sequencing
STAMP: Stanford solid tumor actionable mutation panel
SD: stable disease
PD: progressive disease
PR: partial response
Tras: trastuzumab
Gem: gemcitabine
Bev: bevacizumab
Carbo: carboplatin
Pem: pemetrexed
Figure 1. Examples of best responses on HER2 directed therapies.
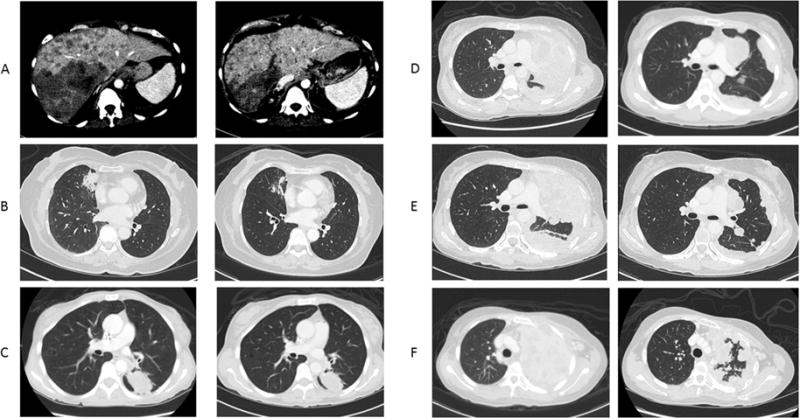
A. Patient #1 at beginning of therapy (Left) and 5 months after starting vinorelbine and trastuzumab (Right)
B. Patient #2 at beginning of therapy (Left) and 3.5 months after starting vinorelbine and trastuzumab (Right)
C. Patient #3 at beginning of therapy (Left) and 2 months after starting vinorelbine and trastuzumab (Right)
D. Patient #4 at beginning of therapy (Left) and 2 months after starting afatinib (Right)
E. Patient #4 at beginning of therapy (Left) and 3.5 months after starting vinorelbine and trastuzumab (Right)
F. Patient #4 at beginning of therapy (Left) and 1 month after starting afatinib and bevacizumab (Right)
Figure 2. Swimmer Plot of Responses to Various Therapies in the Responders.
A. carboplatin/gemcitabine/bevacizumab followed by gemcitabine/bevacizumab maintenance
B. Docetaxel
C. Pemetrexed
D. Irinotecan
E. Trastuzumab/vinorelbine
F. Carboplatin/pemetrexed followed by pemetrexed maintenance
G. Trastuzumab/docetaxel
H. Carboplatin/docetaxel/bevacizumab + investigational anti-MET agent
I. Carboplatin/pemetrexed/bevacizumab followed by pemetrexed/bevacizumab maintenance
J. Erlotinib
K. Afatinib
L. Ado-trastuzumab
M. Nivolumab
N. Etirinotecan pegol
O. Afatinib/bevacizumab
Potential Resistance Mechanisms
In order to further understand potential resistance mechanisms upon progression on targeted therapies, we performed NGS-based genotyping on post-progression tumor biopsies and/or CAPP-Seq on post-progression plasma specimens available from these patients.
Patient #2 had a repeat biopsy of a left lung nodule after progression on trastuzumab/docetaxel and 1 month of afatinib treatment. In addition to ERBB2 exon 20 insertion mutation, she was also found to have several variants of unknown significance including RB1 R320L, ASTN1 R214W, IFLTD1 L71P, DDX1 V429I, and FAM5C D431V in her repeat biopsy by NGS. However, since these variants were not included in the genotyping assay preformed on the pre-treatment biopsy, it is unclear whether these were pre-existing or acquired mutations.
For patient #3, the initial femur biopsy leading to the diagnosis of lung carcinoma was re-tested using NGS. In addition to the previously identified ERBB2 mutation, we also detected PIK3CA R88Q mutation in the femur biopsy sample. In the plasma sample obtained upon progression on trastuzumab plus chemotherapy, we observed a denovo mutation that was not present in the tumor biopsy: PIK3CA R425L. The allele frequency of this mutation was 0.42%. We did not observe the ERBB2 exon 20 insertion, which was most likely due to the low ctDNA concentration. Based on a binomial model the expected probability of detecting the mutation given the median sequencing depth at the position (2,047) and a ctDNA concentration of 0.42% is only 58%. The PIK3CA R88Q mutation observed in the tumor was not re-assessed by the ctDNA NGS panel. These mutations may thus represent putative resistance mechanisms.
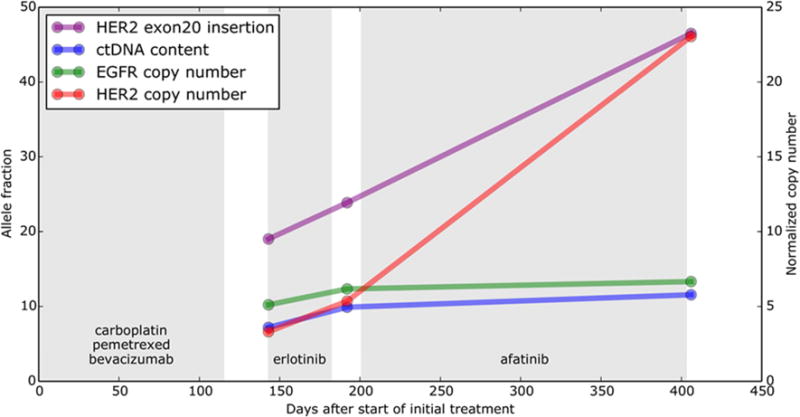
For patient #4, plasma samples were obtained upon progression on her initial chemotherapy regimen (carboplatin, pemetrexed, and bevacizumab), upon progression on erlotinib, and upon progression on afatinib. These were tested using CAPP-Seq. The ERBB2 exon20 insertion was identified in all plasma samples with increasing allele fraction over time. Furthermore, ERBB2 copy number gain was present in all samples and the copy number level normalized to ctDNA content increased over time. There was a dramatic increase in ERBB2 copy number at the time of progression on afatinib, suggesting that this represents a potential resistance mechanism (Fig. 3).
Figure 3.
ERBB2 copy number gain as a putative resistance mechanism to afatinib. Serial ctDNA measurements from patient #4. The panel displays ctDNA content, the ERBB2 exon 20 insertion allele fraction, and the copy number of ERBB2 and EGFR normalized by % ctDNA (based on a heterozygous mutation in PCDH10 observed in all samples).
Panel displays alterations in EGFR detected in plasma. ND, not detected.
Summary of Non-Responders
We treated five patients (patients 5 through 9) with afatinib and/or trastuzumab-based therapies who did not respond to ERBB2 targeted therapies. Their clinical, pathological, and treatment characteristics are summarized in Table 2.
Table 2.
Summary of Non-responders
| Patient | Age | Race/Gender | Smoking status | Histology | HER2 alteration | Testing Method | Other alterations | Systemic therapies*# | Best Responseˆ | |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| #5 | 57 | A/M | Never | Adeno | A775_G776 insYVMA + amplification | Foundation One NGS | TERT promoter, TP53 C135F, NFKB1A amplification, NKX2-1 amplification | Carbo/pem, then pem maintenance X 6 mo | PR | |
| Afatinib X1.5 months | PD | |||||||||
| Nivolumab X 2 mo | PD | |||||||||
| Tras/Docetaxel X3 mo | Mixed response systemically, development of leptomeningeal disease | |||||||||
|
| ||||||||||
| #6 | 79 | A/M | <10 pack year | Adeno | A775_G776 insYVMA | Foundation One NGS | Carbo/pem, then pem maintenance X 3.5 mo | Tolerated therapy poorly, SD | ||
| Docetaxel/ramucirumab X 1 mo | Tolerated therapy poorly, SD | |||||||||
| Afatinib X1 month | Symptomatic progression | |||||||||
|
| ||||||||||
| #7 | 61 | A/F | Never | Adeno | L755A | STAMP NGS | TP53 c892G>T (E298X), RB1 c2101A>T (D701V) | Carbo/pem X2 mo | SD | |
| Paclitaxel X 3mo (therapy interrupted by WBRT) | SD | |||||||||
| Afatinib X1.5 months | PD | |||||||||
| Nivolumab (with 2 cycles of paclitaxel in between) X2 mo | PD | |||||||||
| Paclitaxel (re-challenge 2 month after last dose) X 1mo | PD | |||||||||
|
| ||||||||||
| #8 | 65 | A/M | <10 pack year | Adeno | V777delinsVG SP | Geneseeq Technology Inc NGS | JAK1 exon7 p.V310I (c.G928A) mutation, MED12 exon24 p.R1138G (c.C3412G) mutation, MET exon2 p.S204Y (c.C611A) mutation, TP53 p.P72R (c.C215G) mutation | Carbo/pem X 2 mo | PD | |
| Cis/Docetaxel X3 mo | SD | |||||||||
| Erlotinib X 4 mo | PD | |||||||||
| Gemcitabine X 2 mo | PD | |||||||||
| Afatinib X 2 mo | PD | |||||||||
|
| ||||||||||
| #9 | 43 | A/F | Never | Adeno | V776delinsVC | Geneseeq Technology Inc NGS | BLM p.S144X (c.C431A) mutation, BRCA1 exon4 p.S59X (c.C176A) mutation | Cis/pem, then cis/pem/bev X 4.5mo | Initial PD after cis/pem, then SD after addition of bev | |
| Tras/Paclitaxel X2 mo | PD | |||||||||
| Erlotinib X 1 month | PD | |||||||||
HER2 targeted therapies in bold
number indicates number of cycles unless otherwise specified
as determined by RECIST v1.1 criteria
A: Asian
M: male
F: female
Adeno: adenocarcinoma
NGS: next generation sequencing
STAMP: Stanford solid tumor actionable mutation panel
SD: stable disease
WBRT: whole brain radiotherapy
Tras: trastuzumab
Bev: bevacizumab
Carbo: carboplatin
Cis: cisplatin
Pem: pemetrexed
Discussion
Consistent with prior reports, our experience with patients with ERBB2-mutated NSCLC demonstrates that ERBB2 is a targetable driver mutation. In addition, we explored the potential resistance mechanisms to ERBB2 targeted therapies. Our patients with ERBB2 mutations tend to be younger with little or no smoking history. Four of the nine patients treated with ERBB2 targeted therapies had response (RR 44%). A total of 6 patients received afatinib, and of these patients, 2 had response (RR 33%). A total of 6 patients received trastuzumab in combination with chemotherapy, and of these patients, 4 had response (RR 67%). From our experience, ERBB2 targeted therapy can provide disease control for patients with metastatic ERBB2-mutated NSCLC that has progressed on previous therapies. The PFS times on each therapy range from 3–10 months. Our results are consistent with those reported by Mazières et al [8, 9] and Costa et al [16].
To the best of our best knowledge, this is the first study describing putative resistance mechanisms to ERBB2 targeted therapies in patients with lung cancer. Previously, one preclinical study shows the G776 YVMA insertion in ERBB2 is expected to induce allosteric effects that may influence inhibitor binding [17]. In this study, we identified potential resistance mechanisms using CAPP-Seq ctDNA analysis on plasma samples collected at the time of progression. We identified in patient #3 a novel mutation—PIK3CA R425L—that may be a resistance mechanism after treatment with trastuzumab. Interestingly, acquired PIK3CA mutations have been rarely reported as a mechanism of resistance to EGFR TKI therapy in EGFR mutated NSCLC [18, 19]. For patient #4, target amplification by increasing ERBB2 copy number is a plausible resistance mechanism to afatinib. Resistance to ERBB2 targeted therapies has also been observed in other ERBB2 driven cancers. In the case of breast cancer, putative resistance mechanisms include PIK3CA mutation, Src activation, insulin-like growth factor-I receptor signaling, and c-Met overexpression [20]. Activation of PI3K pathway has also been found to contribute to trastuzumab resistance in breast cancer [21, 22]. In gastric cancer, a recent preclinical study showed that in gastric cancer cells, MACC1 promoted the Warburg effect mainly through the PI3K/AKT signaling pathway, which enhanced trastuzumab resistance [23]. Understanding resistance mechanisms, through repeat tumor biopsies and analysis of ctDNA, is critical to the design of more potent therapies that overcome recurrent escape mechanisms.
There are issues that need to be considered when administering ERBB2 targeted therapies. Tolerability of these medications can be a challenge for patients. In our experience, trastuzumab (at 2 mg/kg, consistent with a previous report [6]) plus chemotherapy was generally well tolerated. On the other hand, afatinib toxicities can be challenging for some patients as diarrhea and mucositis can be dose limiting. Afatinib was dosed at 50 mg/day in the series by De Greve et al. [7], and we chose to dose afatinib at a slightly lower dose of 40 mg/day. Recently, a pulse dosing of afatinib at 280-mg weekly dose was reported to have promising results and apparent tolerability— with less severe diarrhea than might have been anticipated—and this dosing regimen is worth further investigation [16]. Additionally, patients with ERBB2-mutated NSCLC often develop brain metastases, and trastuzumab is not known to cross the blood brain barrier, and therefore likely would not impact CNS metastases. Therefore, monitoring and treatment of CNS metastases may need to be considered separately.
Potential therapeutic roles for other ERBB2 targeted agents are worthy of further investigation. Lapatinib is another ERBB2 targeted TKI, but patients treated with lapatinib did not respond in two separate series by Mazières et al [8, 9]. Dacomitinib has shown activity in ERBB2-mutated NSCLC [24], as has neratinib [25], with more trials of this agent still ongoing alone and in combination with other agents (NCT01827267, NCT01953926, NCT02593708). Another ERBB2 TKI, AP32788, shows promise in preclinical studies involving ERBB2 exon 20 insertion mutant cancer cell lines [26], and a clinical trial is ongoing (NCT02716116). Potential activity of ado-trastuzumab emtansine is also intriguing; while our treated patient did not respond to the medication, there has been one case report showing response to the medication in a patient with ERBB2 exon 20 insertion mutation, overexpression and amplification [27]. Another Fc-modified chimeric monoclonal antibody against ERBB2, margetuximab (MGAH22), is being tested in trials (NCT01148849). Because we identified PIK3CA mutation as a potential resistance mechanism to ERBB2 targeted therapy, there is a rationale for combined treatment. However, to the best of our knowledge there are currently no trials combining a PIK3CA inhibitor with an ERBB2 inhibitor in NSCLC.
Immunotherapies are also worth considering in the treatment of ERBB2-mutated disease. Though our patient did not respond to nivolumab, there is a case report showing response to this medication [28]. The PD-L1 expression status of our patients’ tumors were unknown, and most patients were not treated with these agents because their treatment course preceded the FDA approval of checkpoint inhibitors, but it would have been interesting to observe the response rates to these agents in patients with ERBB2 mutant NSCLC.
One unanswered question is what tumor genotypes are predictive of response. All four patients who responded to targeted therapy had tumors with exon 20 insertion mutations, but patients #5 and #6, also with the most common exon 20 insertion mutations, did not respond to targeted therapies. Patients #7, 8, and 9 had tumors with uncommon ERBB2 mutations, and did not respond to therapy, but we included them in this report to inform other clinicians. There is limited literature on the response to ERBB2 targeted therapies from different mutations. We note in the series by Mazières et al [8], all ERBB2 mutations identified were in-frame insertions of exon 20, with duplication of amino acids YVMA at codon 775. Given the small number of patients in our series, it is not possible to conclude whether a certain mutation predicts response to therapy. In addition, there may be other unidentified driver mutations for the non-responders, and there may be host factors that may affect responses to these therapies.
We note several limitations in our study. The sample size in this study is small and therefore no conclusive statistical associations can be made regarding the effectiveness of targeted therapies, or any factors that can predict response to targeted therapies. In addition, our study focused on examining potential resistance mechanisms upon progression on ERBB2 targeted therapies; we did not examine any potential primary resistance mechanisms in the non-responders. Lastly, we had experiences with afatinib and trastuzumab (in combination with chemotherapy), and only one patient received ado-trastuzumab; our patients did not receive other available or experimental ERBB2 targeted therapies.
Overall, we recommend testing for ERBB2 mutations, in addition to other uncommon driver genomic alterations, for patients whose NSCLC tumors are negative for genomic alterations in EGFR, ALK and ROS1. Small molecule ERBB2 inhibitors or ERBB2 monoclonal antibodies may provide an additional therapy in these patients, but in our series and in previously published data, the response rate and duration are modest compared with TKI’s against other molecular drivers. With ongoing research, additional highly effective and tolerable ERBB2 therapies will hopefully be identified in order to expand treatment options for ERBB2 mutant NSCLC.
Acknowledgments
The Stanford Cancer Institute Research Database (SCIRDB) group performed cohort generation for this work. SCIRDB is a research and development project at Stanford Cancer Institute that developed and manages a modular informatics platform that integrates internal and external data sources and streamlines curation to support cancer research. This publication was supported by the Stanford Cancer Institute Cancer Center Support Grant of the National Institutes of Health under Award Number P30 CA124435. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr. Diehn reports personal fees from Roche, outside the submitted work; In addition, Dr. Diehn has a patent related to ctDNA analysis that is pending.
Dr. Wakelee reports grants from AstraZeneca, during the conduct of the study; personal fees from Peregrine, grants from Novartis, personal fees from ACEA, grants and personal fees from Pfizer, grants from BMS, grants from XCovery, grants from Celegene, grants and other from Roche/Genentech, grants from MedImmune, grants from Lilly, grants from Gilead, grants from Pharmacyclics, personal fees from Helsinn, outside the submitted work.
Dr. Neal reports personal fees from Clovis, personal fees from CARET/Physicians Resource Mgmt., grants and personal fees from Nektar, grants and personal fees from BI, grants from Genentech/Roche, grants from MERCK, grants from Arqule, grants from Exelixis, outside the submitted work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
Other authors have nothing to disclose.
References
- 1.Zinner RG, et al. Trastuzumab in combination with cisplatin and gemcitabine in patients with Her2-overexpressing, untreated, advanced non-small cell lung cancer: report of a phase II trial and findings regarding optimal identification of patients with Her2-overexpressing disease. Lung Cancer. 2004;44(1):99–110. doi: 10.1016/j.lungcan.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 2.Gatzemeier U, et al. Randomized phase II trial of gemcitabine-cisplatin with or without trastuzumab in HER2-positive non-small-cell lung cancer. Ann Oncol. 2004;15(1):19–27. doi: 10.1093/annonc/mdh031. [DOI] [PubMed] [Google Scholar]
- 3.Stephens P, et al. Lung cancer: intragenic ERBB2 kinase mutations in tumours. Nature. 2004;431(7008):525–6. doi: 10.1038/431525b. [DOI] [PubMed] [Google Scholar]
- 4.Shigematsu H, et al. Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer Res. 2005;65(5):1642–6. doi: 10.1158/0008-5472.CAN-04-4235. [DOI] [PubMed] [Google Scholar]
- 5.Arcila ME, et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin Cancer Res. 2012;18(18):4910–8. doi: 10.1158/1078-0432.CCR-12-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cappuzzo F, Bemis L, Varella-Garcia M. HER2 mutation and response to trastuzumab therapy in non-small-cell lung cancer. N Engl J Med. 2006;354(24):2619–21. doi: 10.1056/NEJMc060020. [DOI] [PubMed] [Google Scholar]
- 7.De Greve J, et al. Clinical activity of afatinib (BIBW 2992) in patients with lung adenocarcinoma with mutations in the kinase domain of HER2/neu. Lung Cancer. 2012;76(1):123–7. doi: 10.1016/j.lungcan.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Mazieres J, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol. 2013;31(16):1997–2003. doi: 10.1200/JCO.2012.45.6095. [DOI] [PubMed] [Google Scholar]
- 9.Mazieres J, et al. Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targeted drugs: results from the European EUHER2 cohort. Ann Oncol. 2016;27(2):281–6. doi: 10.1093/annonc/mdv573. [DOI] [PubMed] [Google Scholar]
- 10.http://www.stanfordlab.com/esoteric/test-stanford-solid-tumor-actionable-mutation-panel.html.
- 11.Frampton GM, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31(11):1023–31. doi: 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie J, et al. Capture-based next-generation sequencing reveals multiple actionable mutations in cancer patients failed in traditional testing. Mol Genet Genomic Med. 2016;4(3):262–72. doi: 10.1002/mgg3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newman AM, et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med. 2014;20(5):548–54. doi: 10.1038/nm.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newman AM, et al. Integrated digital error suppression for improved detection of circulating tumor DNA. Nat Biotechnol. 2016;34(5):547–55. doi: 10.1038/nbt.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chabon JJ, et al. Circulating tumour DNA profiling reveals heterogeneity of EGFR inhibitor resistance mechanisms in lung cancer patients. Nat Commun. 2016;7:11815. doi: 10.1038/ncomms11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costa DB, et al. Pulse Afatinib for ERBB2 Exon 20 Insertion-Mutated Lung Adenocarcinomas. J Thorac Oncol. 2016;11(6):918–23. doi: 10.1016/j.jtho.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen X, et al. A systematic analysis of the resistance and sensitivity of HER2YVMA receptor tyrosine kinase mutant to tyrosine kinase inhibitors in HER2-positive lung cancer. J Recept Signal Transduct Res. 2016;36(1):89–97. doi: 10.3109/10799893.2015.1049361. [DOI] [PubMed] [Google Scholar]
- 18.Ji W, et al. Mechanisms of acquired resistance to EGFR-tyrosine kinase inhibitor in Korean patients with lung cancer. BMC Cancer. 2013;13:606. doi: 10.1186/1471-2407-13-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu SG, et al. The Role of PIK3CA Mutations among Lung Adenocarcinoma Patients with Primary and Acquired Resistance to EGFR Tyrosine Kinase Inhibition. Sci Rep. 2016;6:35249. doi: 10.1038/srep35249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gagliato DM, et al. Mechanisms of resistance and sensitivity to anti-HER2 therapies in HER2+ breast cancer. Oncotarget. 2016 doi: 10.18632/oncotarget.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berns K, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12(4):395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 22.Chakrabarty A, et al. Trastuzumab-resistant cells rely on a HER2-PI3K-FoxO-survivin axis and are sensitive to PI3K inhibitors. Cancer Res. 2013;73(3):1190–200. doi: 10.1158/0008-5472.CAN-12-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, et al. A new mechanism of trastuzumab resistance in gastric cancer: MACC1 promotes the Warburg effect via activation of the PI3K/AKT signaling pathway. J Hematol Oncol. 2016;9(1):76. doi: 10.1186/s13045-016-0302-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kris MG, et al. Targeting HER2 aberrations as actionable drivers in lung cancers: phase II trial of the pan-HER tyrosine kinase inhibitor dacomitinib in patients with HER2-mutant or amplified tumors. Ann Oncol. 2015;26(7):1421–7. doi: 10.1093/annonc/mdv186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gandhi L, et al. Phase I study of neratinib in combination with temsirolimus in patients with human epidermal growth factor receptor 2-dependent and other solid tumors. J Clin Oncol. 2014;32(2):68–75. doi: 10.1200/JCO.2012.47.2787. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalvez F, et al. AP32788, a potent, selective inhibitor of EGFR and HER2 oncogenic mutants, including exon 20 insertions, in preclinical models. AACR Annual Meeting. 2016:216. Abstract #2644. [Google Scholar]
- 27.Weiler D, et al. Rapid response to trastuzumab emtansine in a patient with HER2-driven lung cancer. J Thorac Oncol. 2015;10(4):e16–7. doi: 10.1097/JTO.0000000000000424. [DOI] [PubMed] [Google Scholar]
- 28.Catania C, et al. Dramatic Antitumor Activity of Nivolumab in Advanced HER2-Positive Lung Cancer. Clin Lung Cancer. 2016 doi: 10.1016/j.cllc.2016.05.004. [DOI] [PubMed] [Google Scholar]


