Abstract
Heme has been characterized as potent trigger of inflammation. In hemostasis, although heme has been shown to both induce and inhibit different compartments of hemostasis, its net effect on the hemostatic balance, and the biological relevance of these effects remain to be determined. Herein we evaluated the effect of heme on hemostasis using a global assay able to generate clinically relevant data in several other complex hemostatic diseases. Citrated whole blood samples from healthy participants were stimulated by heme or vehicle and incubated for 4h at 37°C. Rotational thromboelastometry was immediately performed. The participation of tissue factor in coagulation activation was evaluated using inhibitory antibody. Heme was able of inducing ex vivo coagulation activation in whole blood, affecting predominantly parameters associated with the initial phases of clot formation. This activation effect was at least partially dependent on hematopoietic tissue factor, since the effects of heme were partially abrogated by the inhibition of human tissue factor. In conclusion, using a global hemostasis assay, our study confirmed that heme is able to activate coagulation in whole blood, in a tissue factor-dependent way. These findings could explain the disturbance in hemostatic balance observed in conditions associated with the release of heme such as sickle cell disease.
Introduction
Clinical and experimental data indicate that hemolytic anemias are associated with a hypercoagulable state [1–4], and that coagulation activation could be a critical element in the pathogenesis of sickle cell disease (SCD) [5,6]. Recently, the release of heme from its intracellular compartment emerged as one of the potential candidates for mediating coagulation activation in these conditions. In addition to its direct toxic effects on cell membranes, heme can act as a danger associated molecular pattern through the activation of TLR4 receptors [7,8], thereby inducing vasocclusion in animal models of SCD [9]. Heme is also able of potently stimulating tissue factor expression in endothelial cells [10] and monocytes [11]. In SCD patients, high levels of free heme have been associated with the risk of acute chest syndrome and vasocclusion [12]. On the other hand, heme has also been shown to have anticoagulant effects in patients with porphyria [13], and to inhibit the activity of specific coagulation factors in in vitro studies [14,15]. Accordingly, it was recently hypothesized that heme could trigger coagulation initiation, while also limiting its propagation [16]. A potential explanation for these discrepancies is the well-known limitation of the study of discrete compartments of hemostasis in providing a full appreciation of the hemostatic balance.
Thromboelastometry is a global hemostasis assay that evaluates the interplay between different compartments of hemostasis, as well as the overall kinetics and dynamic of thrombin generation. As such, it could provide novel information about the net effect of heme on whole blood coagulation activation, thus contributing to the understanding of the effects of heme on hemostasis. The biological relevance of this assay has been validated in several clinical scenarios, and it has been previously used to characterize hypercoagulability in hemolytic diseases [2,17]. Here we investigated the effect of heme on coagulation activation using rotational thromboelastometry (Rotem).
Methods
Blood sample collection and processing
The study was performed in accordance with the Declaration of Helsinki and approved by the Ethics Committee of State University of Campinas (CEP Unicamp, CAAE 46853115.4.0000.5404). Each participant provided written informed consent form before sample collection. This study was performed with 20 healthy volunteers, homozygotes AA for hemoglobin genotype, without a history of thromboembolic disease or use of drugs that modulated hemostasis. Heme was diluted to an initial working concentration of 700μM in NaOH 0.1M. The pH of this solution was adjusted to 7.4. This solution was filtrated though a 0.22μm filter and immediately used to stimulate whole blood samples, as detailed below. Ultra-filtered water was used for all dilutions. Blood samples were drawn in anticoagulant sodium citrate plastic vacuum tubes (BD Vacutainer Coagulation Tube, 2.7 ml, 0.109 M buffered sodium citrate). Whole blood was immediately incubated with the freshly prepared heme to a final concentration of 30μM (Ref H651-9; Frontier Scientific, USA) for 4h at 37°C, based on the time-course of tissue factor procoagulant activity increase in monocytes and endothelial cells [10,18]. After that, samples were gently mixed and immediately used for Rotem test. This final concentration of heme was used because it mimics the levels of free heme observed in sickle cell disease patients [19,20].
Rotational thromboelastometry (Rotem) procedures
Rotem was performed using a ROTEM®instrument (InnovationsGmbH, Munich, Germany), which evaluates the viscoelastic properties of blood and generate parameters associated with coagulation initiation, kinetics, stability and susceptibility to fibrinolysis. Alll ssays were initiated using a non-activated thromboelastometry (Natem) procedure. Natem is a Rotem-based assay used to assess whole blood clot formation in which no specific activator is used, and only calcium is added to plasma [21,22]. Standard parameters of different stages of clotting were determined and included in the statistical analysis. Clotting time (CT) in seconds (sec) is the time from test start until curve generated by the instrument reaches an amplitude of 2 mm. It is a measure of the initiation phase of coagulation, and is mainly affected by the enzymatic activity of coagulation factors, the concentration of anticoagulants, and by tissue factor expression on circulating cells [22]. Clot formation time (CFT) in sec is defined as the time necessary for the Rotem curve amplitude to increase from 2 to 20 mm. It is mainly dependent on thrombin generation, platelet count and function, and fibrin polymerization [22], and measures reactions that are part of the amplification and propagation phases of coagulation. Maximum clot firmness (MCF) corresponds to the maximum amplitude of the Rotem curve and reflects the mechanically strength of the clot. It depends mainly on platelet count and function, fibrin polymerization and factor XIII activity. In addition to these standard parameters, we also analyzed the effect of heme on parameters derived from the first derivative of the Rotem curve, which corresponds to the velocity signal of the coagulation process. These parameters have been shown to improve the resolution of smaller differences in samples activated by minimal concentrations of tissue factor [23]. These additional parameters are routinely reported by the Rotem software as “research parameters”, and have been extensively used in studies using procoagulant agents such as recombinant factor VIIa, recombinant factor XIII, as well as in other contexts [24–29]. The time to maximum velocity (MAXV-t) is the time from test start until the maximal amplitude of the velocity signal is achieved, and reflects the velocity to maximal clot firmness measured by the method. The area under the velocity curve (AUC) represents the area under this 1st derivative curve (velocity signal) until the point when the maximal amplitude of the Rotem curve (maximum clot firmness) is reached, and indirectly reflects the thrombin generation potential [23,28,30]. All assays were peformed using paired citrated whole blood previously incubated with heme (30μM, pH = 7.4) or vehicle (NaOH 0.1M, pH = 7.4), as described above. Briefly, 300μL of whole blood samples were mixed with 20μL of CaCl2 (0.2M, pH = 7.4) in a pre-heated cup at 37°C, and the reaction was started in no more than 17 seconds. Neutralizing anti-tissue factor antibody (Ref 4509, Sekisui Diagnostics, USA) was used at final concentration of 10μg/ml to assess the participation of tissue factor in heme-induced coagulation activation. Non-immune IgG1 isotype (Control IgG mouse, SC-2025, Santa Cruz Biotechnology) was used as negative control at the same concentration.
Statistical analysis
Statistical analysis was performed using GraphPadPrism 6 (GraphPadPrism Software Inc. San Diego, Califórnia, USA). Wilcoxon two-side test was used to compare quantitative parameters obtained from the same samples exposed to vehicle or heme, with or without inhibition. Results are presented as mean (±SEM). The p-value of < 0.05 was considered significant.
Results
Heme induces ex vivo coagulation activation in whole blood samples
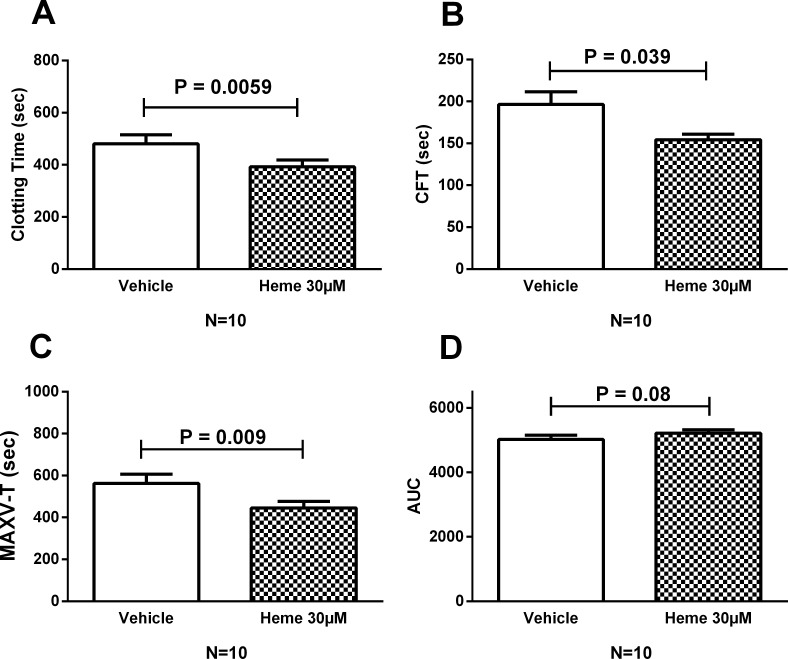
In non-activated thromboelastometry (Natem), heme (30μM) was able of inducing coagulation activation in whole blood samples from healthy participants. After 4h of incubation, heme significantly reduced the CT (Fig 1A) and CFT (Fig 1B) when compared to vehicle. Similar results were obtained with a different preparation of heme (Sigma, Ref 5280). Heme also reduced the MAXV-t (Fig 1C). While a trend towards a higher AUC could be observed in heme-incubated samples (Fig 1D), no difference was observed in maximal clot firmness, MCF (P = 0.054).
Fig 1. Activation of coagulation and clot formation induced by heme.
Comparisons were made for heme vs vehicle after 4h of incubation. Histograms were obtained for selected Rotem parameters and show mean (±SEM). Heme induced a significant reduction of clotting time (A), clot formation time (B) and time for maximal velocity (C). No significant change was observed for the area under the curve (D). Two tailed Wilcoxon test was performed and p-values < 0.05 were considered significant. CFT: clot formation time; MAXV-T: time of maximal velocity; AUC: area under curve.
Coagulation activation of whole blood induced by heme is dependent on tissue factor
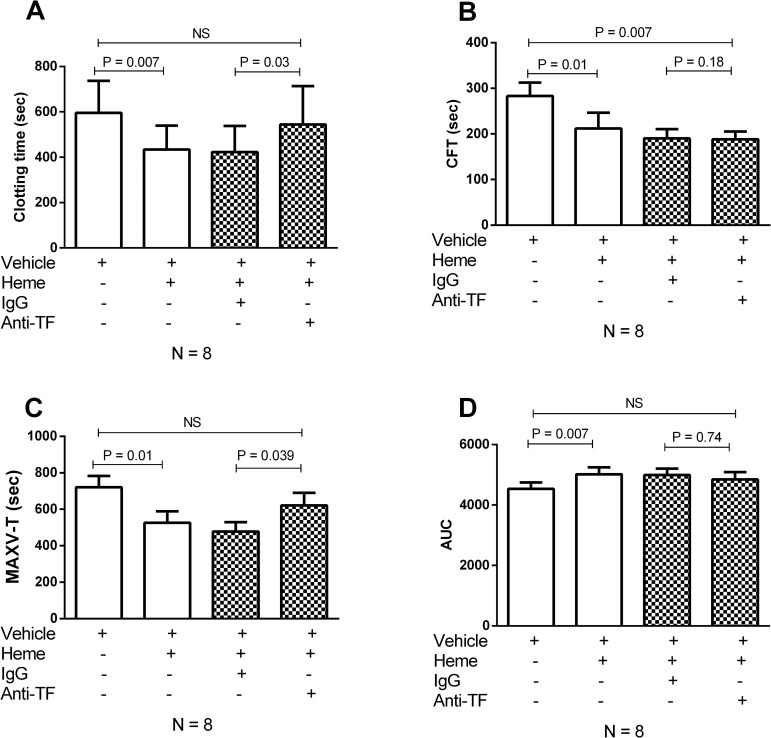
Since heme has been previously shown to induce tissue factor expression in endothelial cells [31] and monocytes [32], we explored whether the observed alterations induced by heme were dependent on tissue factor expression in our system, by using an inhibitory antibody against tissue factor. When compared to an isotype control, the use of an anti-tissue factor inhibitory antibody abrogated the effect of heme in coagulation activation, resulting in increase in CT (Fig 2A) and in a decrease in MAXV-t (Fig 2C), which represent the two most significant effects of heme compared to vehicle. Inhibition had no effect on CFT (Fig 2B) or in the AUC (Fig 2D). Of note, the effects of heme compared to vehicle were similar to those obtained in the experiment presented in Fig 1, except that in these experiments the trend towards a higher AUC in heme-incubated samples reached statistical significance (Fig 2D).
Fig 2. Heme triggered-coagulation activation is tissue factor-dependent.
A human inhibitory anti-tissue factor antibody (Ref 4509 Sekisui) significantly delayed the coagulation initiation, prolonging the clotting time (A) and the time to maximal velocity of clot formation (C). No change was observed in the clot formation time (B) or area under curve (D). Two tailed Wilcoxon test was performed and p-values < 0.05 were considered significant. CFT: clot formation time; MAXV-T: time of maximal velocity; AUC: area under curve; Anti-TF: Tissue factor inhibiting antibody.
Discussion
Increased thrombotic risk is a hallmark of several conditions associated with the extracellular release of heme such as SCD [32] paroxysmal nocturnal hemoglobinuria [33,34], sepsis [35] and intracerebral hemorrhage [36]. Although the mechanisms that underlie the disruption of hemostatic balance in these conditions are not known, the observation that heme can trigger the expression of the physiologic initiator of hemostasis in both endothelial cells [10] and monocytes [11] raises the possibility that it could be involved in the pathogenesis of hypercoagulability in these conditions. Accordingly, the main finding of our study is that heme was able to induce coagulation activation in whole blood, by pathways that are at least partially dependent on tissue factor.
Activation of hemostasis is currently considered a part of innate immunity, participating in the host response to both pathogens, and to sterile danger signals [37]. Heme is an ubiquitous molecule in organisms from all kingdoms of life and in most cells which, over the last decade, has been shown to activate several pathways of innate immunity [7,38–40]. Thus, it is fair to speculate that heme could also represent a mediator of coagulation activation in conditions associated with increased extracellular heme levels [8]. Accordingly, heme has been shown to induce the expression of tissue factor, the physiologic initiator of this process, in both endothelial cells and monocytes [10,11]. Moreover, the biological relevance of this observation was suggested in by the demonstration of tissue factor-dependent coagulation activation in mice treated with heme [32]. In contrast, intravenous hematin (which is very similar to heme preparations used in the former experiments) used in patients with porphyria is not associated with a systemic hypercoagulable state, and has been previously associated with prolongation of coagulation times and to reduced levels of coagulation factors V and VIII [41,42]. Moreover, heme presents complex effects on different compartments of hemostasis that include platelet activation [43] and interactions with VIII [15] and fibrinogen [44] of yet unknown physiological significance. Together, these results illustrate the challenges of addressing the effects of heme on hemostasis, and highlight the importance of methods able to explore its net global effect on this process.
Thromboelastometry is a global hemostasis assay capable to demonstrate signs of hypo- and hypercoagulability in patients with complex alterations of hemostasis such as sepsis, trauma and SCD [45,46]. The use of whole blood samples allows the evaluation of hemostasis in the presence of critical elements to the hemostatic balance such as red blood cells, platelets and leukocytes, in addition to plasma proteins and platelets [17]. Of note, a recent study in SCD demonstrated that relevant parameters of coagulation activation could only be demonstrated in whole blood, but not in plasma [47]. Using this method we demonstrated that heme was able to induce coagulation activation in whole blood samples, as evidenced by shortenings of the CT, CFT and MAXV-t. The first two parameters reflect the initiation and amplification phases of coagulation respectively, while the latter reflects the velocity to reaching maximum clot firmness as measured by the Rotem method (refs Sorensen 2003 e Gorlinger 2016). No significant change was observed in the MCF, which reflects clot strength, and has been shown to be the only Rotem parameter to correlate with the endogenous thrombin potential [48]. These results suggest that the tissue factor-dependent effects of heme could be more relevant to the initial phases of clot formation, with a lower impact on the overall thrombin potential and on clot stability. Interestingly, a similar pattern was observed when the thrombin generation assay was used in a cohort of 92 sickle cell disease patients [49]. Accordingly, we speculate that the fact that TF inhibition only reversed the effects of heme on CT and MAXV-t possibly reflect the higher dependence of these parameters on the initial phases of clot formation when compared to CFT and AUC, which are more dependent on fibrin polymerization and clot stabilization.
In mice, heme-induced coagulation activation was dependent on tissue factor of both endothelial and hematopoietic tissue factor [32]. Using an inhibitory antibody that has been widely used in studies addressing the role of tissue factor, we demonstrated that in human whole blood samples, heme-induced coagulation activation is at least partially dependent on the expression and/or activation of tissue factor. Since in our experimental conditions coagulation activation is dependent solely on circulating blood cells, our results confirm that hematopoietic tissue factor is involved in this process. Accordingly, hematopoietic tissue factor is believed to be the most relevant source for coagulation activation in pathological conditions such as sepsis [50] and SCD [51].
Our system does not intend to model the in vivo putative effects of heme in coagulation activation, but only to address its isolated effect on a clinically relevant global hemostasis assay, and to confirm the participation of hematopoietic tissue factor in this process. The effect of free heme on the hemostatic balance in vivo is a much more complex scientific question, influenced by variables such as the wide availability of heme scavengers such as hemopexin and albumin in plasma [52], the effects of heme on other discrete compartments of hemostasis [16], and an extremely complex network of cellular pathways that participate in the pathogenesis of hemolytic anemias such as sickle cell disease [53]. Moreover, our results do not exclude the participation of other pathways such as leukocytes, platelets and factor XII in heme-induced coagulation activation in vivo.
In conclusion, using a global hemostasis assay capable to detect clinically relevant changes in hemostasis we demonstrated that heme is able to induce coagulation activation of whole blood in a tissue factor-dependent way. These results support the concept that free extracellular heme could play a relevant role in coagulation activation in SCD patients, and in other pathological conditions. Further studies are necessary to elucidate the mechanisms by which heme induces the activation of tissue factor, as well as the in vivo relevance of these findings.
Acknowledgments
This study was financially supported by Fapesp grants 2013/09319-0, 2014/00984-3 and 2015/24666-3; and CNPq-Brazil grant 2014/457780. The Hematology and Hemotherapy Center—Hemocentro UNICAMP, forms part of the National Institute of Science and Technology of Blood, Brazil (INCT do SangueCNPq / MCT / FAPESP).
Data Availability
All relevant data are within the paper.
Funding Statement
This study was financially supported by Fapesp grants 2013/09319-0, 2014/00984-3 and 2015/24666-3; and CNPq-Brazil grant 2014/457780. The Hematology and Hemotherapy Center—Hemocentro UNICAMP, forms part of the National Institute of Science and Technology of Blood, Brazil (INCT do SangueCNPq / MCT / FAPESP). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Colella MP, De Paula E V, Conran N, Machado-Neto JA, Annicchino-Bizzacchi JM, Costa FF, et al. Hydroxyurea is associated with reductions in hypercoagulability markers in sickle cell anemia. J Thromb Haemost. 2012;10: 1967–70. doi: 10.1111/j.1538-7836.2012.04861.x [DOI] [PubMed] [Google Scholar]
- 2.Lim MY, Ataga KI, Key NS. Hemostatic abnormalities in sickle cell disease. Curr Opin Hematol. 2013;20: 472–7. doi: 10.1097/MOH.0b013e328363442f [DOI] [PubMed] [Google Scholar]
- 3.Colella MP, de Paula E V, Machado-Neto JA, Conran N, Annicchino-Bizzacchi JM, Costa FF, et al. Elevated hypercoagulability markers in hemoglobin SC disease. Haematologica. 2015;100: 466–71. doi: 10.3324/haematol.2014.114587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naik RP, Streiff MB, Haywood C Jr., Segal JB, Lanzkron S. Venous thromboembolism incidence in the Cooperative Study of Sickle Cell Disease. J Thromb Haemost. 2014;12: 2010–2016. doi: 10.1111/jth.12744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sparkenbaugh E, Pawlinski R. Interplay between coagulation and vascular inflammation in sickle cell disease. British Journal of Haematology. 2013. pp. 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arumugam PI, Mullins ES, Shanmukhappa SK, Monia BP, Loberg A, Shaw MA, et al. Genetic diminution of circulating prothrombin ameliorates multiorgan pathologies in sickle cell disease mice. Blood. 2015;126: 1844–55. doi: 10.1182/blood-2015-01-625707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Figueiredo RT, Fernandez PL, Mourao-Sa DS, Porto BN, Dutra FF, Alves LS, et al. Characterization of heme as activator of Toll-like receptor 4. J Biol Chem. 2007;282: 20221–9. doi: 10.1074/jbc.M610737200 [DOI] [PubMed] [Google Scholar]
- 8.Soares MP, Bozza MT. Red alert: labile heme is an alarmin. Curr Opin Immunol. 2016;38: 94–100. doi: 10.1016/j.coi.2015.11.006 [DOI] [PubMed] [Google Scholar]
- 9.Belcher JD, Chen C, Nguyen J, Milbauer L, Abdulla F, Alayash AI, et al. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood. 2014;123: 377–90. doi: 10.1182/blood-2013-04-495887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Setty BNY, Betal SG, Zhang J, Stuart MJ. Heme induces endothelial tissue factor expression: potential role in hemostatic activation in patients with hemolytic anemia. J Thromb Haemost. 2008;6: 2202–9. doi: 10.1111/j.1538-7836.2008.03177.x [DOI] [PubMed] [Google Scholar]
- 11.Rehani T, Mathson K, Belcher JD, Vercellotti GM. Heme Potently Stimulates Tissue Factor Expression By Peripheral Blood Monocytes: A Novel Mechanism For Thrombosis In Intravascular Hemolytic Diseases. Blood. 2013;122: 2215 Available: http://www.bloodjournal.org/content/122/21/2215.abstract [Google Scholar]
- 12.Adisa OA, Hu Y, Ghosh S, Aryee D, Osunkwo I, Ofori-Acquah SF. Association between plasma free haem and incidence of vaso-occlusive episodes and acute chest syndrome in children with sickle cell disease. Br J Haematol. 2013;162: 702–5. doi: 10.1111/bjh.12445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McColl KE, Moore MR, Thompson GG, Goldberg A. Treatment with haematin in acute hepatic porphyria. Q J Med. 1981;50: 161–174. [PubMed] [Google Scholar]
- 14.Green D, Reynolds N, Klein J, Kohl H, Ts’ao CH. The inactivation of hemostatic factors by hematin. J Lab Clin Med. 1983;102: 361–369. [PubMed] [Google Scholar]
- 15.Repessé Y, Dimitrov JD, Peyron I, Farrokhi Moshai E, Kiger L, Dasgupta S, et al. Heme binds to factor VIII and inhibits its interaction with activated factor IX. J Thromb Haemost. 2012;10: 1062–71. doi: 10.1111/j.1538-7836.2012.04724.x [DOI] [PubMed] [Google Scholar]
- 16.Roumenina LT, Rayes J, Lacroix-Desmazes S, Dimitrov JD. Heme: Modulator of Plasma Systems in Hemolytic Diseases. Trends in Molecular Medicine. 2016. pp. 200–213. doi: 10.1016/j.molmed.2016.01.004 [DOI] [PubMed] [Google Scholar]
- 17.Van Geffen M, Van Heerde WL. Global haemostasis assays, from bench to bedside. Thrombosis Research. 2012. pp. 681–687. doi: 10.1016/j.thromres.2011.12.006 [DOI] [PubMed] [Google Scholar]
- 18.Meszaros K, Aberle S, Dedrick R, Machovich R, Horwitz A, Birr C, et al. Monocyte Tissue Factor Induction by Lipopolysaccharide (LPS): Dependence on LPS-Binding Protein and CD14, and Inhibition by a Recombinant Fragment of Bactericidal/Permeability-Increasing Protein. Available: http://www.bloodjournal.org/content/bloodjournal/83/9/2516.full.pdf [PubMed]
- 19.Oh JY, Hamm J, Xu X, Genschmer K, Zhong M, Lebensburger J, et al. Absorbance and redox based approaches for measuring free heme and free hemoglobin in biological matrices. Redox Biol. 2016;9: 167–177. doi: 10.1016/j.redox.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muller-Eberhard U, Javid J, Liem HH, Hanstein A, Hanna M. Plasma concentrations of hemopexin, haptoglobin and heme in patients with various hemolytic diseases. Blood. 1968;32: 811–815. [PubMed] [Google Scholar]
- 21.Schoergenhofer C, Buchtele N, Schwameis M, Bartko J, Jilma B, Jilma-Stohlawetz P. The use of frozen plasma samples in thromboelastometry. Clin Exp Med. Springer International Publishing; 2017; 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Görlinger K, Dirkmann D, Hanke AA. Rotational Thromboelastometry (ROTEM®). Trauma Induced Coagulopathy. Cham: Springer International Publishing; 2016. pp. 267–298. [Google Scholar]
- 23.Sørensen B, Johansen P, Christiansen K, Woelke M, Ingerslev J. Whole blood coagulation thrombelastographic profiles employing minimal tissue factor activation. J Thromb Haemost. 2003;1: 551–8. [DOI] [PubMed] [Google Scholar]
- 24.Sørensen B, Persson E, Ingerslev J. Factor VIIa analogue (V158D/E296V/M298Q-FVIIa) normalises clot formation in whole blood from patients with severe haemophilia A. Br J Haematol. 2007;137: 158–165. doi: 10.1111/j.1365-2141.2007.06534.x [DOI] [PubMed] [Google Scholar]
- 25.Tran HTT, Sørensen B, Bjørnsen S, Pripp AH, Tjønnfjord GE, Andre Holme P. Monitoring bypassing agent therapy—a prospective crossover study comparing thromboelastometry and thrombin generation assay. Haemophilia. 2015;21: 275–283. doi: 10.1111/hae.12570 [DOI] [PubMed] [Google Scholar]
- 26.Rea CJ, Foley JH, Okaisabor O, Sørensen B. FXIII: mechanisms of action in the treatment of hemophilia A. J Thromb Haemost. 2014;12: 159–68. doi: 10.1111/jth.12478 [DOI] [PubMed] [Google Scholar]
- 27.REA CJ, FOLEY JH, INGERSLEV J, SØRENSEN B. Factor XIII combined with recombinant factor VIIa: a new means of treating severe hemophilia A. J Thromb Haemost. Blackwell Publishing Ltd; 2011;9: 510–516. doi: 10.1111/j.1538-7836.2010.04171.x [DOI] [PubMed] [Google Scholar]
- 28.Tarzia V, Bortolussi G, Buratto E, Paolini C, Dal Lin C, Rizzoli G, et al. Single vs double antiplatelet therapy in acute coronary syndrome: Predictors of bleeding after coronary artery bypass grafting. World J Cardiol. Baishideng Publishing Group Inc; 2015;7: 571–8. doi: 10.4330/wjc.v7.i9.571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whiting D, DiNardo JA. TEG and ROTEM: Technology and clinical applications. Am J Hematol. 2014;89: 228–232. doi: 10.1002/ajh.23599 [DOI] [PubMed] [Google Scholar]
- 30.Whiting D, Dinardo JA. TEG and ROTEM: Technology and clinical applications. Am J Hematol. 2014;89: 228–232. doi: 10.1002/ajh.23599 [DOI] [PubMed] [Google Scholar]
- 31.Setty BNY, Betal SG, Zhang J, Stuart MJ. Heme induces endothelial tissue factor expression: potential role in hemostatic activation in patients with hemolytic anemia. J Thromb Haemost. 2008;6: 2202–2209. doi: 10.1111/j.1538-7836.2008.03177.x [DOI] [PubMed] [Google Scholar]
- 32.Sparkenbaugh EM, Chantrathammachart P, Wang S, Jonas W, Kirchhofer D, Gailani D, et al. Excess of heme induces tissue factor-dependent activation of coagulation in mice. Haematologica. 2015;100: 308–313. doi: 10.3324/haematol.2014.114728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hillmen P, Lewis SM, Bessler M, Luzzatto L, Dacie J V. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995;333: 1253–8. doi: 10.1056/NEJM199511093331904 [DOI] [PubMed] [Google Scholar]
- 34.Noubouossie D, Key NS, Ataga KI. Coagulation abnormalities of sickle cell disease: Relationship with clinical outcomes and the effect of disease modifying therapies. Blood Reviews. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaplan D, Casper TC, Elliott CG, Men S, Pendleton RC, Kraiss LW, et al. VTE Incidence and Risk Factors in Patients With Severe Sepsis and Septic Shock. Chest. American College of Chest Physicians; 2015;148: 1224–30. doi: 10.1378/chest.15-0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skaf E, Stein PD, Beemath A, Sanchez J, Bustamante MA, Olson RE, et al. Venous thromboembolism in patients with ischemic and hemorrhagic stroke. Am J Cardiol. Elsevier; 2005;96: 1731–3. [DOI] [PubMed] [Google Scholar]
- 37.Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13: 34–45. doi: 10.1038/nri3345 [DOI] [PubMed] [Google Scholar]
- 38.Dutra FF, Alves LS, Rodrigues D, Fernandez PL, de Oliveira RB, Golenbock DT, et al. Hemolysis-induced lethality involves inflammasome activation by heme. Proc Natl Acad Sci U S A. 2014;111: E4110–8. doi: 10.1073/pnas.1405023111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernandez PL, Dutra FF, Alves L, Figueiredo RT, Mourão-Sa D, Fortes GB, et al. Heme amplifies the innate immune response to microbial molecules through spleen tyrosine kinase (Syk)-dependent reactive oxygen species generation. J Biol Chem. 2010;285: 32844–51. doi: 10.1074/jbc.M110.146076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vasconcellos LRC, Dutra FF, Siqueira MS, Paula-Neto HA, Dahan J, Kiarely E, et al. Protein aggregation as a cellular response to oxidative stress induced by heme and iron. Proc Natl Acad Sci. 2016; 201608928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morris DL, Dudley MD, Pearson RD. Coagulopathy associated with hematin treatment for acute intermittent porphyria. Ann Intern Med. 1981;95: 700–1. [DOI] [PubMed] [Google Scholar]
- 42.Glueck R, Green D, Cohen I, Ts’ao CH. Hematin: unique effects of hemostasis. Blood. 1983;61: 243–9. [PubMed] [Google Scholar]
- 43.Neely SM, Gardner D V., Reynolds N, Green D, Tšao C. Mechanism and characteristics of platelet activation by haematin. Br J Haematol. Blackwell Publishing Ltd; 1984;58: 305–316. [DOI] [PubMed] [Google Scholar]
- 44.Nielsen VG, Cohen JB, Malayaman SN, Nowak M, Vosseller K. Fibrinogen is a heme-associated, carbon monoxide sensing molecule: a preliminary report. Blood Coagul Fibrinolysis. 2011;22: 443–7. doi: 10.1097/MBC.0b013e328345c069 [DOI] [PubMed] [Google Scholar]
- 45.Yee DL, Edwards RM, Mueller BU, Teruya J. Thromboelastographic and hemostatic characteristics in pediatric patients with sickle cell disease. Arch Pathol Lab Med. 2005;129: 760–5. doi: 10.1043/1543-2165(2005)129[760:TAHCIP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 46.Nogami K. The utility of thromboelastography in inherited and acquired bleeding disorders. Br J Haematol. 2016;174: 503–514. doi: 10.1111/bjh.14148 [DOI] [PubMed] [Google Scholar]
- 47.Whelihan MF, Lim MY, Mooberry MJ, Piegore MG, Ilich A, Wogu A, et al. Thrombin generation and cell-dependent hypercoagulability in sickle cell disease. J Thromb Haemost. 2016;14: 1941–1952. doi: 10.1111/jth.13416 [DOI] [PubMed] [Google Scholar]
- 48.Schneider T, Siegemund T, Siegemund R, Petros S. Thrombin generation and rotational thromboelastometry in the healthy adult population. Hamostaseologie. Schattauer Publishers; 2014;35: 181–186. doi: 10.5482/HAMO-14-08-0033 [DOI] [PubMed] [Google Scholar]
- 49.Gerotziafas GT, Van Dreden P, Chaari M, Galea V, Khaterchi A, Lionnet F, et al. The acceleration of the propagation phase of thrombin generation in patients with steady-state sickle cell disease is associated with circulating erythrocyte-derived microparticles. Thromb Haemost. 2012;107: 1044–52. doi: 10.1160/TH11-10-0689 [DOI] [PubMed] [Google Scholar]
- 50.Pawlinski R, Wang J-G, Owens AP, Williams J, Antoniak S, Tencati M, et al. Hematopoietic and nonhematopoietic cell tissue factor activates the coagulation cascade in endotoxemic mice. Blood. 2010;116: 806–14. doi: 10.1182/blood-2009-12-259267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chantrathammachart P, Mackman N, Sparkenbaugh E, Wang J-G, Parise L V, Kirchhofer D, et al. Tissue factor promotes activation of coagulation and inflammation in a mouse model of sickle cell disease. Blood. 2012;120: 636–46. doi: 10.1182/blood-2012-04-424143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tolosano E, Fagoonee S, Morello N, Vinchi F, Fiorito V. Heme scavenging and the other facets of hemopexin. Antioxid Redox Signal. 2010;12: 305–20. doi: 10.1089/ars.2009.2787 [DOI] [PubMed] [Google Scholar]
- 53.Hounkpe BW, Fiusa MML, Colella MP, da Costa LNG, Benatti R de O, Saad STO, et al. Role of innate immunity-triggered pathways in the pathogenesis of Sickle Cell Disease: a meta-analysis of gene expression studies. Sci Rep. 2015;5: 17822 doi: 10.1038/srep17822 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.