Abstract
Drosophila leg morphogenesis occurs under the control of a relatively well-known genetic cascade, which mobilizes both cell signaling pathways and tissue-specific transcription factors. However, their cross-regulatory interactions, deployed to refine leg patterning, remain poorly characterized at the gene expression level. Within the genetically interacting landscape that governs limb development, the bric-à-brac2 (bab2) gene is required for distal leg segmentation. We have previously shown that the Distal-less (Dll) homeodomain and Rotund (Rn) zinc-finger activating transcription factors control limb-specific bab2 expression by binding directly a single critical leg/antennal enhancer (LAE) within the bric-à-brac locus. By genetic and molecular analyses, we show here that the EGFR-responsive C15 homeodomain and the Notch-regulated Bowl zinc-finger transcription factors also interact directly with the LAE enhancer as a repressive duo. The appendage patterning gene bab2 is the first identified direct target of the Bowl repressor, an Odd-skipped/Osr family member. Moreover, we show that C15 acts on LAE activity independently of its regular partner, the Aristaless homeoprotein. Instead, we find that C15 interacts physically with the Dll activator through contacts between their homeodomain and binds competitively with Dll to adjacent cognate sites on LAE, adding potential new layers of regulation by C15. Lastly, we show that C15 and Bowl activities regulate also rn expression. Our findings shed light on how the concerted action of two transcriptional repressors, in response to cell signaling inputs, shapes and refines gene expression along the limb proximo-distal axis in a timely manner.
Author summary
Limb morphogenesis is controlled by a well-known genetic cascade, mobilizing both cell signaling and tissue-specific transcription factors (TFs). However, how their concerted action refines gene expression remains to be deciphered. It is thus crucial to understand how cell signaling inputs are integrated by transcriptional “enhancers”. The Drosophila leg provides a good paradigm to dissect the molecular mechanisms underlying gene regulation. Here, we used the bric-a-brac2 (bab2) gene as a model to study the integrated regulation of patterning genes implicated in tarsal segmentation. bab2 expression in the leg primordium is dynamic and complex, going from initial broad distal expression to precisely positioned tarsal rings. By genetic and molecular analyses, we show here that the cell signaling-responding TFs C15 and Bowl interact directly with the limb-specific bab2 enhancer as a repressive duo. Moreover, C15 acts independently of its partner Aristaless through physical interaction with the Dll activator. We propose that Dll induces early circular bab2 expression pattern, then EGFR signaling-induced C15 in the distalmost cells competes with Dll for LAE binding and resolves bab2 pattern as a ring. Taken together our data shed light on how the concerted action of a quartet of transcription factors reshapes gene expression during limb proximo-distal axis development.
Introduction
In developing arthropod and vertebrate appendages, morphogen gradients play critical roles in instructing the spatially-restricted expression of patterning genes that mostly encode transcription factors (TFs) [1, 2]. However, how their expression domains are set up precisely and how their cross-regulation refines pattern layouts, remain to be deciphered, particularly by characterizing the cis-regulatory modules within the gene regulatory networks governing limb development.
The Drosophila leg provides a paradigm with which to tackle the issue of the molecular mechanisms underlying proximo-distal limb development [2–4]. The primordia of the adult appendages, the leg imaginal discs, originate from clusters of roughly 20–30 embryonic ectodermal cells which proliferate during the three larval stages. During the first and second instar larval stages, in response to antagonistic wingless (wg) and decapentaplegic (dpp) cell signaling pathways, the TF-encoding Distal-less (Dll), dachshund (dac) and homothorax (hth) genes are activated in broad concentric domains within the leg disc, that prefigure the distal, medial and proximal subdivisions of the adult appendage, respectively [3]. By the end of the third instar larval (L3) stage, the leg disc is highly folded presaging movements towards a tri-dimensional structure.
The distal portion of the adult leg comprises the tarsus (ts), which is divided into five segments (ts1-5, from proximal to distal), and the pretarsus (pt), which is characterized by a pair of terminal claws. Around the early L3 stage, ~72–80 hours (h) after egg laying (AEL), the combinatorial activities of the leg “gap” genes Dll, dac and hth induce the expression of the tarsal-specific rotund (rn), bric-à-brac (bab) complex (bab1 and bab2) and Bar complex (BarH1 and BarH2) genes in distal circular domains [5–8]. At 80-82h AEL, secreted Wg and Dpp molecules jointly activate the epidermal growth factor receptor (EGFR) signaling pathway in distalmost pretarsal cells [5, 7]. In turn, EGFR cell signaling activates the expression of pretarsal patterning genes, including the homeobox genes aristaless (al), dlim1 and C15 (also known as clawless), and spatially extinguishes expression of tarsal patterning genes, including bab and Bar complex loci [5, 7–10]. As for leg gap genes, most pretarsal and tarsal patterning genes encode DNA-binding transcriptional regulators, expressed either in the very center of the leg disc (e.g., C15/clawless and al) or in concentric rings (e.g., bab2, BarH1 and rn) [2, 4]. Lastly, at mid-late L3 stage, activation of the Notch (N) cell signaling pathway through the restricted expression of the Delta (Dl) and Serrate (Ser) ligands (in response to leg gap combinatorial activities) is required for formation of the flexible joint connecting each leg segment, through induction of a set of target genes, including the related genes odd-skipped (odd), drumstick (drm), bowl and sister-of-bowl (sob) [4, 11, 12].
Dl and Ser expression boundaries are maintained by a negative feedback loop between N signaling and the Lines/Bowl pathway [13]. In Dl expressing cells, the Lines protein is nuclear, leading to specific degradation of the Odd-family zinc-finger (ZF) protein Bowl [13–15]. In the adjacent joint cells, N signaling induces the expression of redundant Odd-family ZF proteins (Odd, Sob and Drm), which specifically re-localize Lines to the cytoplasm, thus preventing Bowl destabilization; consequently, Bowl TF accumulates in the nuclear compartment where it down-regulates Dl expression [13]. This feedback mechanism reinforces a Dl+/Dl− boundary, ensuring maintenance of N signaling through joint morphogenesis. Several genes required for distal leg segmentation have altered expression in bowl mutant cells, such as bab2 [16], but none have been shown so far to be under the direct control of the Bowl transcription factor.
The bric-à-brac complex is formed by the duplicated paralogous genes bab1 and bab2, encoding BTB/POZ domain TFs with overlapping functional roles in several developmental processes [17, 18]. While bab1 is expressed similarly to bab2, only the latter is critically required for distal leg and antennal segmentation [18]. The bab2 gene displays dynamic expression in a restricted proximo-distal (P-D) expression pattern in the distal leg and antennal primordia [17–20]. Initially expressed as a broad circle within the Dll-expressing distal domain at early-mid L3 stage, the bab2 pattern in late-L3-stage leg and antennal discs resolves to four or two concentric rings, respectively [20]. Later on, at the pupal and adult stages, a P-D graded pattern is observed for each bab2-expressing ring, which is essential for ts2-5 and antennal (a) a3-5 segment joint formation [18].
While many DNA-binding factors have been identified, little is known about their molecular relationships within the gene regulation landscape governing limb formation along the P-D axis. To this aim, we previously identified within the bric-à-brac locus a single cis-regulatory module that reliably governs bab2 expression in developing appendages, termed LAE for leg/antennal enhancer, and showed that the Distal-less homeodomain and Rotund zinc-finger TFs interact directly with discrete critical sites within the LAE enhancer to activate or up-regulate bab2 in all or specifically in the proximalmost expressing cells, respectively, within the developing distal leg and antenna [19]. Here, we show that the C15 homeodomain and Bowl zinc-finger proteins act jointly by binding directly conserved LAE sequences, to restrict bab2 expression within the developing leg, in response to cell signaling. We show that C15 competes with the Dll activator for LAE binding on neighboring cognate sites. Furthermore, instead of engaging direct partnership with the Aristaless homeoprotein, we find that C15 interacts physically with Dll through contacts between their homeodomain. Taken together with our previous data, this study provides a deeper understanding of how a transcriptional enhancer integrates diverse repressive cell signaling inputs produced along the proximo-distal axis of the developing limb.
Results
LAE enhancer repression along the leg proximo-distal axis through its conserved CR2 sequence
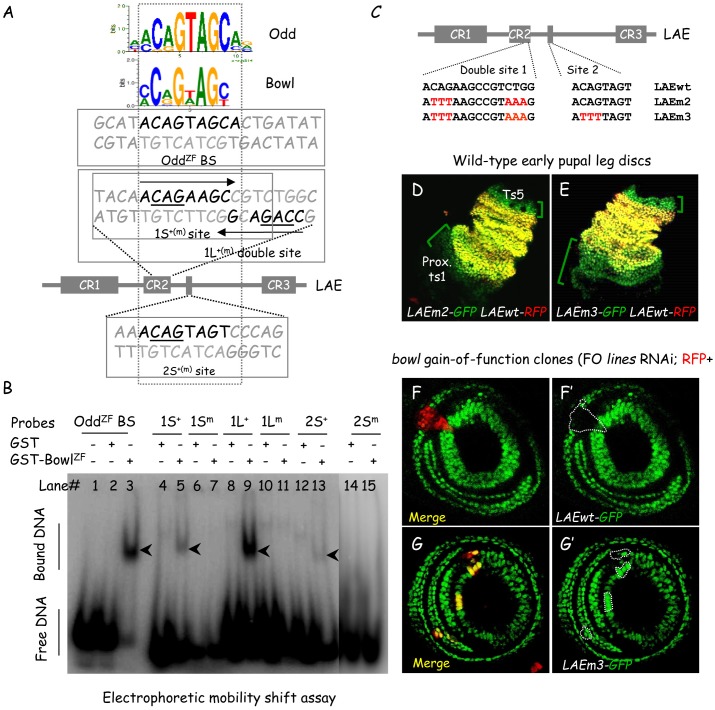
Limb-specific bab2 expression is governed by a single 567-bp cis-regulatory module (CRM), termed LAE [19]. Among the three evolutionarily-conserved regions (CR1-3) comprised in this CRM, CR1 is critical for enhancer activity, notably through Dll activator binding [19]. Here, we have investigated the functional role of the 41-bp CR2 sequence. To this end, expression of an internally-deleted LAE construct (LAEΔ2-GFP) (Fig 1A) was compared to expression of a wild-type (wt) LAE reporter (LAEwt-RFP), faithfully reflecting the endogenous bab2 gene [19]. Prominent ectopic expression from the LAEΔ2-GFP construct was detected at the proximal (ts1) and distal (ts5) edges of the RFP (bab2)-expressing tarsal domain (compare Fig 1B to 1C and 1D, white brackets), indicating a requirement for CR2 in defining bab2 expression limits. However, GFP expression was absent from developing pretarsal cells, indicating that CR2 is dispensable for EGFR-signaling mediated bab2 extinction there. We conclude that within the LAE enhancer, the conserved CR2 sequence is absolutely required for repressing LAE reporter (bab2) expression along the proximo-distal axis of the leg, to delimit precisely the tarsal expression borders.
Fig 1. The Lines/Bowl pathway restricts LAE activity within the developing distal leg.

(A) Scheme of the wild-type (wt) LAE enhancer and of its ΔCR2 derivative. Filled boxes indicate the three regions (CR1 to CR3) conserved among dipterans [19]. (B-D) Wild-type late L3 leg discs expressing LAEwt-RFPZH86Fb with either LAEwt-GFPZH2A (B; medial view) or LAEΔ2-GFPZH2A (C-D; distal and proximal views, respectively). Boxed areas are magnified for each confocal view, in (B’), (C’) and (D'), respectively, with a comparison to RFP fluorescence in isolation, in (B”), (C”) and (D”), respectively. Ectopic GFP expression at the distal (ts5) and proximal (ts1) edges of the bab2-expressing tarsal domain are indicated by white brackets. (E) Bowl expression within a wild-type late L3 leg disc expressing LAE-GFP. Merged Bowl immunostaining (red) and GFP fluorescence (green) is shown, as well as each of the two markers in isolation, in (E’) and (E”), respectively. (F-G) Mosaic late L3 leg discs expressing LAEwt-RFP and harboring either bowl null mutant clones (F) (detected by the loss of GFP) or lines dsRNA-expressing FO clones (G) (specifically expressing GFP). Merged GFP (green) fluorescence, LAE-driven RFP (red) fluorescence and Bab2 immunostaining (cyan) are shown, as well as Bab2 and RFP expression in isolation, in (F’, G’) and (F”, G”), respectively. Mitotic clones are circled with white dashed lines. Note that ectopic bab2 (and LAE-RFP) expression is observed in bowl mutant clones both distally and proximally (F’, F”), and conversely their down-regulation is observed in Bowl-stabilized FO clones deficient for lines (G’, G”), in a cell-autonomous manner.
The Lines/Bowl pathway regulates LAE enhancer activity within the distal leg primordium
The lines/bowl gene cassette has been proposed to regulate bab2 expression along the leg P-D axis [13, 15, 16]. Indeed, tarsal LAE-GFP expression is flanked both proximally and distally by Bowl-expressing domains (Fig 1E). In contrast, LAEΔ2-GFP is up-regulated in Bowl-expressing cells at the ts5-pretarsal boundary (S1 Fig, compare panels A to B). Further, CR2 contains a consensus Bowl binding site (see Fig 2A). We thus tested the functional relationships between bowl and the LAE enhancer in loss- (lof) and gain-of-function (gof) clonal analyses in the leg disc. As for endogenous bab2 [13], LAEwt-RFP expression was cell-autonomously de-repressed in bowl-/- mitotic clones situated both proximally and distally within the developing distal leg (Fig 1F; clones are circled with white dashed lines). Ectopically-expressed Bowl protein accumulates poorly within nuclei of developing tarsal cells due to instability induced by nuclear Lines protein, a dedicated Bowl antagonist specifically expressed there [13, 16]. bowl gain-of-function was therefore obtained through RNA interference (RNAi) coupled to “flip out” (FO) Gal4 expression (see Materials and methods) to achieve clonal down-regulation of lines [13]. Endogenous bab2 [13, 15] and LAEwt-RFP expression were both autonomously repressed in most lines-deficient FO cells (GFP+) generated within the developing leg (Fig 1G; a large clone is circled). Our data indicate that LAE enhancer activity is regulated by the Lines/Bowl pathway, raising the possibility that repressive Bowl transcription factor might bind directly to LAE sequences.
Fig 2. Bowl binds directly to LAE sequences in vitro to regulate LAE activity in vivo.
(A) LOGOs of consensus Odd-family ZF binding sites [21] are shown in the upper part. The dotted line box indicates this consensus in the binding sites under investigation. The double-stranded sequences encompassing a bona fide Odd ZF-binding site [22] as well as the two consensus Bowl ZF-binding sites within the LAE are shown beneath. Corresponding probed double-stranded DNA sequences tested in EMSA are framed. The shared CAG sub-motifs in wild-type (+) sequences, substituted by TTT in each mutant (m) probe, are underlined. For the small (1S) and large (1L) CR2 probes, near palindromic sub-sequences are indicated by converging arrows. (B) EMSA experiments examining Bowl-DNA complexes. Each tested DNA probe, shown in (A), was incubated with either purified non-fused GST (as a negative control) or purified GST-BowlZF. Specific complexes are indicated by black arrowheads. Note that Bowl ZF binding to LAE sequences was fully abolished for each CAG mutant probe. (C) Wild-type as well as mutant LAE sequences encompassing the biochemically-defined Bowl ZF binding sites are shown, with mutated nucleotides in red. (D-E) Wild-type late L3 leg discs co-expressing LAEwt-RFP (red) and either LAEm2-GFP (D) or LAEm3-GFP (E) (green). Bowl ZF cognate binding sites are critical for restraining LAE reporter activity in distal ts5 and in proximal ts1. (F-G) Mosaic late L3 leg discs expressing either LAEwt-GFP (F) or LAEm3-GFP (G) and harboring FO clones (RFP+) expressing lines dsRNA (i.e., ectopically-stabilizing nuclear Bowl). Merged LAE-driven GFP (green) and FO RFP (red) fluorescence are shown, as well as GFP expression in isolation, in (F’) and (G’), respectively. Representative Clones deficient for lines activity are circled with white dashed lines. While ectopically-stabilized nuclear Bowl induced LAEwt-GFP repression (F’), it was unable to down-regulate the mutant LAE reporter (G’) (as revealed by the merged yellow instead of red color obtained in F).
Bowl zinc-finger protein binds directly conserved motifs within the LAE enhancer
Bowl belongs to the Odd family of zinc-finger TFs, and closely-related consensus DNA binding motifs have been recently defined for Odd [5’-(A/C)CAGTAGC] and Bowl [5’-(C/A)C(A/G)G(T/A)AG(C/T)] ZF domains by a bacterial one-hybrid approach [21]. Two sequences matching consensus binding sites (BS) for Odd/Bowl are present within the LAE enhancer (Fig 2A), and both are conserved among 24 available Drosophilidae bric-à-brac locus sequences (S2A and S2B Fig, respectively), one being precisely a part of the repressive CR2 sequence acting along the leg P-D axis (see Fig 1C and 1D). In addition to Drosophilidae species, the CR2 consensus site is conserved also in the Mediterranean fruit fly Ceratitis capitata, the tsetse fly Glossina morsitans and the domestic house fly Musca domestica (S2A Fig). Moreover, the putative Bowl BS in CR2 (5’-ACAGAAGC) is embedded within an imperfect palindromic sequence (5’-ACAGAAGCCGTCTGG; underlined nucleotides may constitute a second Bowl BS in an inverted orientation), which appears, however, to be strictly conserved only among Drosophilidae species (S2A Fig).
Full-length Bowl GST fusion is toxic when expressed in bacteria. To test for direct interaction with LAE sequences in vitro, we therefore expressed a 163 amino-acid long segment of the Bowl protein encompassing its zinc-finger domain (termed BowlZF) as a glutathione S-transferase (GST) fusion protein in E. coli. Purified GST-BowlZF was examined for specific DNA binding in an electrophoretic gel mobility shift assay (EMSA) (Fig 2B). As a positive control, we used a DNA probe (OddZF BS) previously shown to interact with a recombinant GST-OddZF fusion protein [22]. DNA probes encompassing each consensus site within CR2 (1S+) and in the 3’-end neighboring conserved sequence (2S+) (see Fig 2A) interacted with GST-BowlZF, albeit weakly compared to the Odd ZF BS (Fig 2B, compare lanes 5 and 13 with lane 3), but not with unfused GST used as a negative control (lanes 2, 4 and 12). By contrast, GST-BowlZF interacted strongly with a larger DNA probe (1L+) encompassing the entire CR2 near-palindromic sequence (compare panels 5 and 9), and binding was abolished when the common CAG nucleotides (underlined in Fig 2A) present in each probe were mutated to TTT (1Sm, 1Lm and 2Sm; lanes 7, 11 and 15, respectively). The shifted 1S+ and 1L+ DNA-protein complexes (black arrowheads) migrated similarly, suggesting that Bowl ZF fusion protein might interact as a monomer in both cases. Taken together, these data indicate that the Bowl ZF DNA-binding domain interacts specifically with dedicated LAE sequences in vitro, particularly with the near-palindromic site within CR2.
The Lines/Bowl pathway regulates LAE enhancer activity through the repressive CR2 sequence
To evaluate whether the two conserved Bowl ZF-binding sites within the LAE enhancer are critical for bab2 repression in the developing distal leg, we sequentially mutated the common CAG motif of the double site within CR2 and of the single one outside CR2 to TTT, to yield the LAEm2-GFP and then LAEm3-GFP derivatives (Fig 2C). When compared to the normal bab2-expressing tarsal domain (monitored with LAEwt-RFP), LAEm2-GFP displayed strong GFP de-repression in proximal ts1 and distal ts5 (Fig 2D), as previously seen for the CR2-deleted LAEΔ2-GFP reporter (above, Fig 1C and 1D). LAEm3-GFP (mutated for both Odd/Bowl ZF BS) was likewise de-repressed proximally and distally (Fig 2E). However, proximal LAEm3-GFP up-regulation is significantly broader, compared to LAEm2-GFP (compare the extents of the green brackets in Fig 2D and 2E), indicating that both conserved Bowl binding sites are required for full repression of the LAE enhancer in proximal ts1, while only the CR2 site is apparently needed in distal ts5.
To confirm that the Lines/Bowl pathway acts in vivo through the biochemically-defined binding sites within the LAE enhancer, we ectopically-stabilized nuclear Bowl protein in flip-out mitotic clones, by down-regulating lines activity within the developing leg through mosaic RNAi knockdown (above, see Fig 1G) and examining fluorescence patterns driven by thrice CAG-mutated LAEm3-GFP. Whereas the wild-type LAE reporter was repressed in lines-deficient tarsal cells (RFP+), LAEm3-GFP was impervious to repression by stabilized Bowl within the same cells (compare Fig 2F, 2F’, 2G and 2G’; lines-deficient FO clones are circled with white dashed lines). Therefore, we conclude that the Bowl transcription factor acts directly through its evolutionarily-conserved cognate binding sites within the LAE enhancer, to limit bab2 expression in the developing tarsus.
C15/clawless activity is required for bab2 repression in the developing pretarsus
Previous work has shown that EGFR signaling extinguishes bab2 expression within the distalmost subdivision of the developing leg, the pretarsus [5, 8]. The mutant LAE reporter LAEm3-GFP, while resistant to Bowl-mediated P-D repression (Fig 2G) is still repressed in the pretarsal primordium (Fig 2E), as visualized upon co-staining with the C15 specific marker (S1C Fig). Moreover, Bowl protein is expressed in a row of C15-expressing pretarsal cells at the ts5/pretarsal boundary, where LAEm3-GFP expression remains undetected (S1D Fig). Since LAE reporter expression is absent from C15-expressing cells, we asked whether C15 TF activity mediates EGFR signaling-dependent down-regulation of LAE enhancer activity in the developing pretarsus.
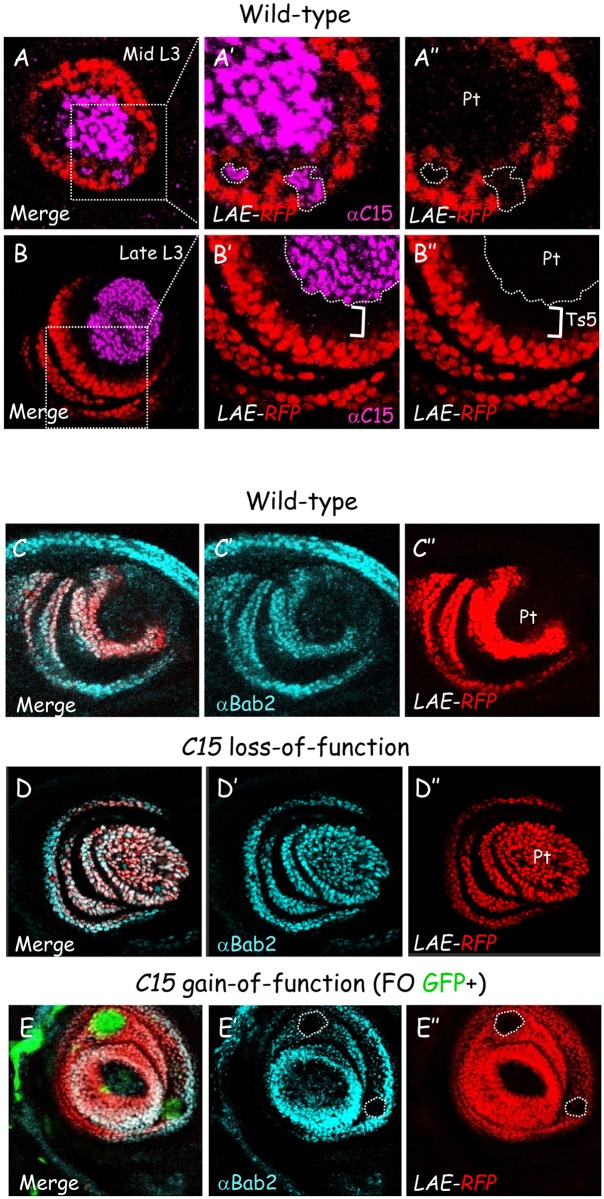
C15 homozygous mutants for a null allele (C152) survive to adulthood with distal leg defects, exemplified by the absence of claws [8]. In early-mid L3, C15/clawless expression is activated in the pretarsal primordium, in response to activation of EGFR signaling at the center of the leg disc, then subsequently maintained until the adult stage [8, 9]. To ask whether C15 regulates LAE enhancer activity, we first compared expression of the bab2 (LAE-RFP reporter) and C15 genes in the leg disc. At ~84-90h AEL, C15 and LAE-RFP are expressed in adjacent territories within the emerging tarsus/pretarsus (Fig 3A). Soon afterwards at mild-late L3 stage C15 and LAE-RFP expression domains become well separated from each other, through gradual restriction of the LAE reporter by repressive Bowl activity in developing distal ts5 cells (Fig 3B; white bracket) [16]. Note that in the mid-L3 leg disc, LAE-RFP reporter expression is never detected in C15+ cells split away from the emerging central C15-expressing domain (Fig 3A, circled areas; n = 20) (see also below), suggesting that C15 TF might be sufficient to cell-autonomously extinguish LAE activity in the developing pretarsus, as suggested by Campbell [8]. Consistent with this possibility, bab2 (LAE-RFP) de-repression was observed in all pretarsal cells in C152 homozygous late-L3 leg discs (Fig 3C and 3D). Surprisingly, in C15 homozygous mutant larvae bab2 (LAE-RFP) expression was likewise de-repressed in developing distal ts5 cells, where repressive Bowl activity should be acting given our previous data (see Fig 2D and 2E). In fact, distal bowl expression is fully lost in C15-/- leg discs (compare S3A and S3B Fig) [8]. Altogether these data support the notion that the C15 transcription factor might mediate directly EGFR-induced bab2 repression in developing pretarsal cells, in addition to allowing indirectly gradual repression in ts5 cells by stabilizing Bowl there through Notch signaling activation.
Fig 3. C15 represses bab2 and LAE enhancer activity in the developing leg.
(A-B) Mid (A) and late (B) L3 leg discs expressing the LAE-RFP reporter. Merged LAE-driven RFP fluorescence (red) and C15 immunostaining (magenta) are shown. Boxed areas are magnified for each stage, in (A’) and (B’), in comparison with LAE-driven RFP expression in isolation, in (A”) and (B”), respectively. At mid L3 stage, bab2 is expressed within a single tarsal cell ring abutting the C15-expressing pretarsal cells. Note that LAE-RFP expression is never detected in isolated peripheral C15+ cells (circled with white dashed lines in A’ and A”). At late L3 stage, C15 and bab2 are no longer expressed in abutting territories but now separated by the ts5 primordium (white brackets in B’ and B”). (C-D) Wild–type (C) or C152 homozygous mutant (D) late L3 leg discs expressing the LAE-RFP reporter. Merged Bab2 immunostaining (blue) and RFP fluorescence (red) are shown, as well as each marker in isolation, in (C’) and (D’) and (C”) and (D”). Note that bab2 and LAE-RFP are both ectopically expressed in the mutant pretarsus, as well as in distal ts5. (E) Bab2 immunostaining (cyan) of a mosaic late L3 leg disc expressing LAE-RFP (red) and harboring FO clones (GFP+; green) ectopically-expressing C15 protein. Merged markers as well as bab2 or LAE-RFP expression in isolation, in (E’) and (E”), are shown. Clones are circled with white dashed lines. A cell-autonomous down-regulation of both bab2 and LAE-RFP is observed in tarsal cells ectopically expressing C15.
To examine whether C15 activity is sufficient for bab2 (LAE-RFP) down-regulation when ectopically expressed in developing tarsal cells, in accordance with a putative direct repression, we used the FO technique to misexpress C15 protein in the leg disc. Ectopic C15 expression led to cell-autonomous bab2 (LAE-RFP) repression in tarsal FO clones (Fig 3E). Both the loss- and gain-of-function genetic experiments indicate that the C15 transcription factor down-regulates bab2 and LAE enhancer activity within the developing pretarsus, and this negative regulation might occur possibly through direct interaction with LAE sequences.
Repressive C15 and activating Dll homeodomain proteins interact with distinct LAE sequences
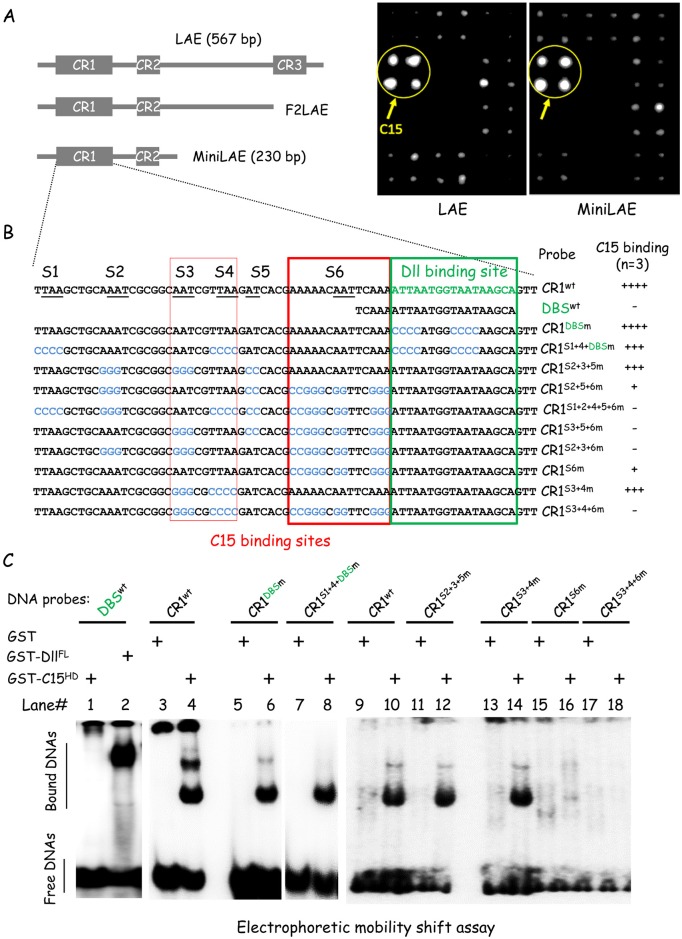
The first indication for direct C15 binding to the LAE enhancer came from its identification as an LAE–interacting protein through a one-hybrid (Y1H) assay in yeast cells. Taking advantage of a nearly-complete D. melanogaster TF prey library (i.e., 692 upon 755 predicted DNA-binding regulators) [23], we performed a Y1H screen using as DNA bait the entire LAE or a 230 base pair (bp) 3’-truncated derivative (minimal CRM or miniLAE; see Fig 4A, left part). Note that miniLAE appeared sufficient (albeit with lower enhancer efficiency) for tissue-specific expression in a reporter assay, notably repression in pretarsal cells [19]. Significantly, from two independent Y1H screens, each done in duplicate, a strong TF-LAE interaction, corresponding to the C15 homeodomain protein (homeoprotein), reproducibly stood out for both constructs (Fig 4A, right part).
Fig 4. C15 homeoprotein interacts directly with LAE sequences.
(A) C15 interacts with LAE in a yeast one-hybrid assay. Scheme of full-length LAE and of two shorter derivatives with slightly less (F2LAE) or much less (miniLAE) enhancer activities, are shown in the left part. The three evolutionarily-conserved LAE elements are indicated as grey-filled boxes. Examples of interactions are shown in the right with 12x4 yeast colonies expressing distinct Drosophila TFs fused to the Gal4 activating domain (AD) [23]. The quadruplicated colonies expressing the C15-Gal4AD fusion are circled, for both the entire and minimal LAE baits. TF-DNA bait interactions were identified based on growth on selective 20 mM 3-amino-1, 2, 4-triazole plates. Note that surrounding colonies are considered negatives and represent overall background growth. (B) Probing C15 HD binding to wild-type and mutant CR1 in EMSA experiments. Sequence of the evolutionarily conserved CR1 region is shown in the upper part. Dll-binding sub-region (DBS) is depicted in green and A/T-only sequence motifs (of at least two nucleotide long, and prone to interact with any homeodomain) are underlined. Mutated CR1 probes are shown beneath, with mutated nucleotides in blue. The Dll (DBS) and C15 binding sites are framed in green and red, respectively. The relative efficiency of C15 HD binding for each tested probes in shown on the right, quantified from 3 experiments. (C) Like Dll homeoprotein, purified C15 HD interacts with CR1 in EMSA. Tested probes are indicated above each lane. Purified GST was used as a negative control. Protein-DNA complexes from lanes #1–2, #3–8 and #9–18, respectively, were analyzed on three distinct native polyacrylamide gels.
Given that Y1H assays reveal direct interactions, we sought to identify C15 binding site(s) [24, 25] within the miniLAE sequence (Fig 4B), that includes both the conserved CR1 region critical for activation, and the Bowl-interacting CR2 [19]. Neither the deletion of CR2 (above, Fig 1C and S1B Fig) nor systematic mutagenesis of CR1 sub-motifs [19] resulted in LAE-driven reporter gene de-repression in developing pretarsal cells. Thus, repressive C15 homeoprotein TF might act through redundant homeodomain (HD) binding sites or, alternatively, compete with activating Distal-less homeoprotein for shared HD cognate sites within CR1 [19]. The 69-bp CR1 sequence is highly enriched in A/T nucleotides (67%; Fig 4B), including the previously-identified Dll-binding sequence (DBS) within its 3’-moiety (a 22-bp region encompassing three canonical TAAT homeodomain binding sites) [19]. Using a purified recombinant GST fusion protein, we asked whether a 101 amino-acid-long C15 fragment encompassing the homeodomain (C15HD) can bind stably the DBS in a gel retardation assay. Contrary to a purified GST-Dll fusion used as a positive control, GST-C15HD did not bind detectably the DBS probe (Fig 4C, lanes 1–2). In striking contrast, GST-C15HD bound strongly a DNA probe encompassing the whole CR1 sequence, forming up to three protein-DNA complexes depending on EMSA conditions (Fig 4C, lanes 3–4). The simplest interpretation is that several GST-C15HD molecules can interact simultaneously with several cognate binding sites distinct from the Dll DBS within CR1. To test this possibility, we mutated the Dll DBS TAAT sites, as reported in [19]. The GST-C15HD fusion readily interacted with mutated CR1 (CR1DBSm), leading to equivalent retarded protein-DNA complexes (Fig 4C, lane 6; quantification from three experiments is shown in Fig 4B, on the right side). This result suggested that C15 homeodomain interacts mainly with sequences outside the DBS. Next, we sought to identify C15HD binding sequences among the numerous A/T-rich motifs (prone to interact with C15 HD) present within CR1 (termed S1 to S6; underlined in Fig 4B). In addition to the TAAT sequences within the DBS, we tested a probe (CR1S1+4+DBSm) also mutated for the non-consensus TAA sequences within the S1 and S4 motifs. The GST-C15HD fusion still interacted with CR1S1+4+DBSm (Fig 4C, lane 8), albeit with slightly lower efficiency (see Fig 4B, right side) and with only one detectable retarded complex (Fig 4C, compare lanes 4 and 8). Second, we tested a series of probes mutated for at least three A/T motifs (formed by at least two consecutive A or T), substituting them by purely G/C sequences (Fig 4B; depicted in blue), leaving intact the DBS. Binding was faint for all probes mutated for S6, or absent when S3 or S4 were also mutated (Fig 4C and S4A Fig), suggesting that S6 and S3-4 are respectively high and low affinity cognate sites. We then tested a second series of probes specifically mutated for S3-4 (CR1S3+4m), S6 (CR1S6m) or both (CR1S3+4+6m) (see Fig 4B for sequences). As expected, GST-C15HD interaction was slightly reduced (CR1S3+4m), strongly reduced (CR1S6m) or abolished (CR1S3+4+6m) (Fig 4C, lanes 14, 16 and 18, respectively; see Fig 4B for quantification). Altogether, these biochemical data indicate that C15 homeodomain interacts strongly with S6 and much less efficiently with S3-4 binding sites within CR1.
We then asked whether the C15 HD binding sites within the critical 69-bp CR1 region contributes to LAE repression in vivo. Given that systematic linker scanning mutagenesis of CR1 never resulted in de-repression [19], transgenic lines expressing LAE-RFP derivatives mutated for both the major (S6) and minor (S3-4) sites were generated. In contrast to a wild-type LAE-RFP construct inserted at a same genomic site, none of the two tested mutated derivatives (miniLAE and F2LAE, a larger form without the TAA-rich CR3 prone to interact with homeoproteins; Fig 4A and [19]) was detectably expressed within the leg disc (S4B Fig). These results suggest that in addition to a role in C15-mediated repression S3-4 and/or S6 CR1 sequences are also required for enhancer activation in the developing leg, which thus preclude from evaluating their implication in distal repression. Consistent with this hypothesis, our previous linker scanning mutagenesis revealed partially-redundant activating regulatory information within CR1, notably for subsequences encompassing the C15 binding sites [19].
Although C15 binding remains to be formally established in vivo, our data suggest that the EGFR-responsive C15 transcriptional repressor extinguishes bab2 in the developing pretarsus by interacting directly with the LAE enhancer, at least through two cognate binding sites within CR1.
C15 represses LAE activity on its own, without functional partnership with aristaless
C15 has been shown to function together with Aristaless (Al), another homeoprotein [9]. Al and C15 proteins are co-expressed in the developing pretarsus and both HD proteins interact cooperatively with a composite DNA binding site [(T/C)TAATTAA(T/A)(T/A)G][26]. However, the assumption of a systematic partnership between C15 and Al is based on the single identified common target locus Bar. Given that C15 HD binds directly LAE sequences distinct from the Bar enhancer (i.e. without TAATTAA core sequence; see Fig 4B), we next asked whether C15 acts independently of Al in repressing LAE activity.
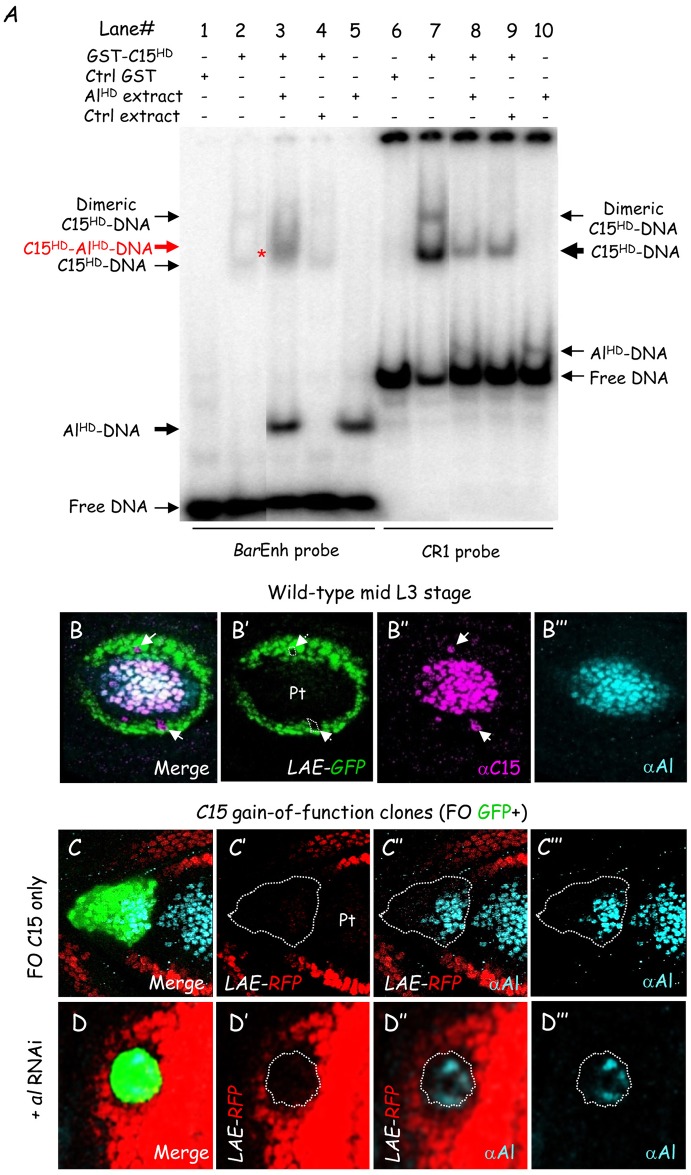
First, we tested whether C15 binds cooperatively with Al to LAE sequences in vitro. Given that C15 and Al interact physically through contacts between their homeodomain [26], we performed EMSA experiments with protein fragments sufficient for intermolecular interaction. Purified GST-C15HD (see above) was combined with in vitro translated Al homeodomain (AlHD; Fig 5A). As a positive control, we used the Bar enhancer probe (BarEnh). In accordance with previous data [9], while GST-C15HD bound poorly to the BarEnh probe, a new retarded complex was clearly observed upon addition of AlHD extracts (Fig 5A, compare lanes 2 and 3). In striking contrast, GST-C15HD bound strongly to the CR1 probe alone and adding AlHD-containing extracts did no yield heterodimeric complex (lanes 7–8). In fact, C15 HD binding was not enhanced, but instead diminished presumably due to the presence of competitors in the reticulocyte extracts (compare lanes 8–9). While Al homeodomain bound strongly to the BarEnh probe, it interacted poorly with the CR1 probe (compare lanes 5 and 10). Altogether these data indicate that C15 HD interacts strongly in vitro with LAE sequences independently of Al.
Fig 5. C15 functions independently of aristaless.
(A) DNA-binding interaction between purified recombinant GST-C15HD and in vitro translated Al HD on Bar and bab2 enhancer sequences was examined in EMSA. In striking contrast to the BarEnh (compare lanes 2 and 3), upon addition of AlHD no heterodimeric C15-Al DNA complex (red asterisk in lane 3) could be detected with the CR1 probe (compare lanes 7 and 8). Note that adding control reticulocyte extracts led to the same diminished GST-C15HD binding to the CR1 probe. (B) Wild-type mid L3 leg disc expressing LAE-GFP. Merged GFP fluorescence (green) together with C15 (magenta) and Al (blue) immunostainings is shown, as well as each markers in isolation in (B’), (B”) and (B”‘), respectively. Note that isolated C15+ cells neither expressed LAE-RFP nor Al. (C-D) Mosaic Late L3 leg discs expressing the LAE-RFP reporter and harboring FO clones ectopically-expressing C15 either alone (C) or together with al dsRNA (D). Merged GFP fluorescence (green), RFP fluorescence (red) and Al immunostaining (cyan) are shown, as well as RFP and Al together, in (C”) and (D”), and both signals in isolation, in (C’) and (C”‘) and in (D’) and (D”‘), respectively. Despite Al depletion in a majority of C15-misexpressing FO cells, LAE-RFP expression remained fully down-regulated within all the cells of the examined clones (n = 8).
Second, we asked whether C15 homeoprotein is able to down-regulate LAE activity independently of Al in vivo. A first indication came from the observation in early-mid L3 that LAE-RFP expression is never detected in C15+ cells split away from the central domain (above, see Fig 3A), suggesting a cell autonomous C15-mediated repression. We found that those peripheral C15+ cells never co-expressed Al (n = 15) (Fig 5B, see white arrows), indicating that this repression does not require Al activity. A second indication came from C15 mis-expression within the bab2-expressing tarsal field. We previously showed that ectopic C15 expression in FO clones is sufficient to down-regulate LAE activity within the developing tarsus (above, see Fig 3E). C15 ectopic expression in tarsal FO clones has been shown in fact to be sufficient to induce al expression in a cell autonomous manner [8], although not in all the C15-misexpressing cells (Fig 5C). To separate the roles of C15 and Al in LAE-RFP repression, we used the FO technique to couple C15 gain-of-function with RNAi-induced Al knock-down. Although dsRNA treatment was not sufficient for al extinction everywhere, an autonomous LAE-RFP down-regulation was still observed in all C15+ cells, even those without detectable Al protein (Fig 5D). Altogether these data provide evidence that C15 transcription factor represses directly LAE activity in vivo independently of Al.
C15 and Dll homeoproteins compete in vitro and in vivo
Repressive C15 and activating Dll homeoproteins bind strongly adjacent cognate sites within CR1 (above, Fig 4B). We next asked whether they can do so simultaneously. To this end, we tested their binding with a probe encompassing the high-affinity C15 as well as the neighboring Dll DBS sites (corresponding to the 3’ half of CR1; Fig 6A, see upper part). In addition to the GST-C15HD protein described above, we purified a Dll fusion protein (GST-DllHD) comprising only its homeodomain. As expected each homeodomain fusion bound the 3’ CR1 probe (Fig 6A, left lower part), yielding either a strictly monomeric GST-DllHD or a monomeric as well as some detectable dimeric GST-C15HD complexes. To ask whether C15 and Dll homeodomains can interact jointly with their respective CR1 binding sequence, we simultaneously added the same amount of proteins in the DNA retardation assay. Significantly, no heterodimeric complexes could be detected, and respective monomeric complexes were even diminished. Moreover, adding increasing amounts of GST-C15HD resulted in disappearance of GST-DllHD bound probe in favor of bound GST-C15HD (Fig 6A, right lower part). Thus C15 and Dll compete for CR1 binding in vitro.
Fig 6. C15 and Dll interact functionally.
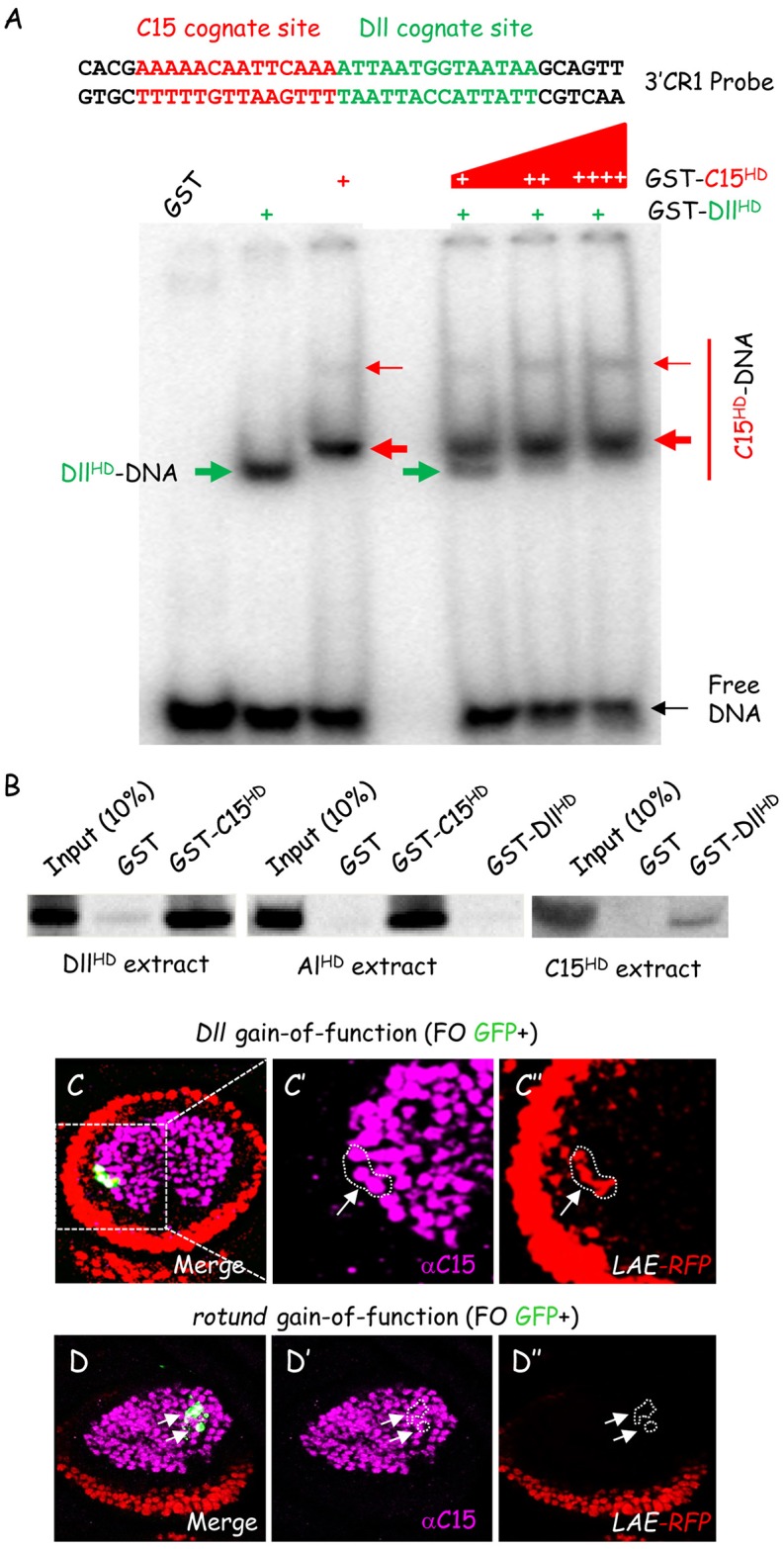
(A) C15 and Dll outcompete for CR1 binding. EMSA was done with a probe encompassing the DBS and the neighboring S6 C15 binding site (CR1 3’ half; upper panel). DllHD and C15HD GST fusions were tested in isolation or together with increasing amount of purified GST-C15HD. No heterodimeric C15-Al DNA complex could be detected. Moreover, GST-DllHD binding was abolished in a dose-dependent manner in favor of GST-C15HD. (B) Pull-down experiments with purified GST-C15HD or GST-DllHD fusions and in vitro translated C15, Dll or Al HD. (C-D) Mosaic late L3 leg discs expressing the LAE-RFP reporter and harboring FO clones (GFP positive) (circled with white dashed lines) misexpressing C15 (C) or Rn (D) proteins. Merged LAE-driven RFP fluorescence (red) and C15 immunostaining (magenta) are shown, as well as each marker in isolation, in (C’–C”) and (D’-D”), respectively. Note that LAE-RFP is specifically de-repressed in Dll overexpressing FO clones without detectable change in C15 expression.
C15 and Al interact physically through contacts between their homeodomain [26]. We therefore asked whether C15 also establishes intermolecular interactions with the Dll HD, by performing pull-down experiments with purified GST-C15HD and GST-DllHD fusions. In vitro translated 35S-labelled Dll homeodomain interacted with GST-C15HD as efficiently as Al HD (Fig 6B, compare middle to left panels). Likewise, in vitro translated 35S-C15 homeodomain bound to GST-DllHD in the reciprocal test (right panel). Significantly, GST-DllHD did not interact with radiolabeled Al homeodomain. Thus, Dll and C15 engage specific protein-protein interaction via their respective DNA-binding domain, providing a possible rationale for their competitive interaction with LAE sequences.
Lastly, we examined the functional antagonism of C15 and Dll in vivo. We postulated that the down-regulation of LAE expression observed on ectopically expressing C15 in developing tarsal cells (above, Fig 3E), involves direct competition with activating Dll. Accordingly, we asked whether Dll up-regulation in pretarsal cells could impede repression by C15. On overexpressing Dll activator in C15+ FO clones (that are likewise Dll+), clear de novo LAE-RFP expression could be detected in all pretarsal clones (n = 12) (Fig 6C, see white arrow), while C15 expression remained apparently unchanged (Fig 6C’). These data indicate that increased Dll protein is able to specifically counteract C15 repressive activity in pretarsal cells. Importantly, LAE-RFP up-regulation was never observed on ectopically expressing Rn protein (Fig 6D and see also below), indicating that the Dll-induced de-repressive effect is not a general effect of bab2 activators. Given that C15 and Rn interact directly with well separated LAE sequences [19], the functional specificity of up-regulated Dll on C15 repressive activity is consistent with their binding site proximity.
Taken together, these data provide evidence for a new layer of bab2 regulation by C15, through direct physical interaction with the Dll activator and competitive binding to neighboring cognate sequences within the LAE. In fact, three non-exclusive distinct mechanisms can be envisioned for C15 repressive activity: (1) active repression due to direct C15 binding to CR1 sequences, (2) steric hindrance toward Dll activator binding to adjacent cognate sites and (3) heterodimerization with Dll changing its binding specificity.
C15 and Bowl proteins act jointly as a repressive TF duo responding to cell signaling
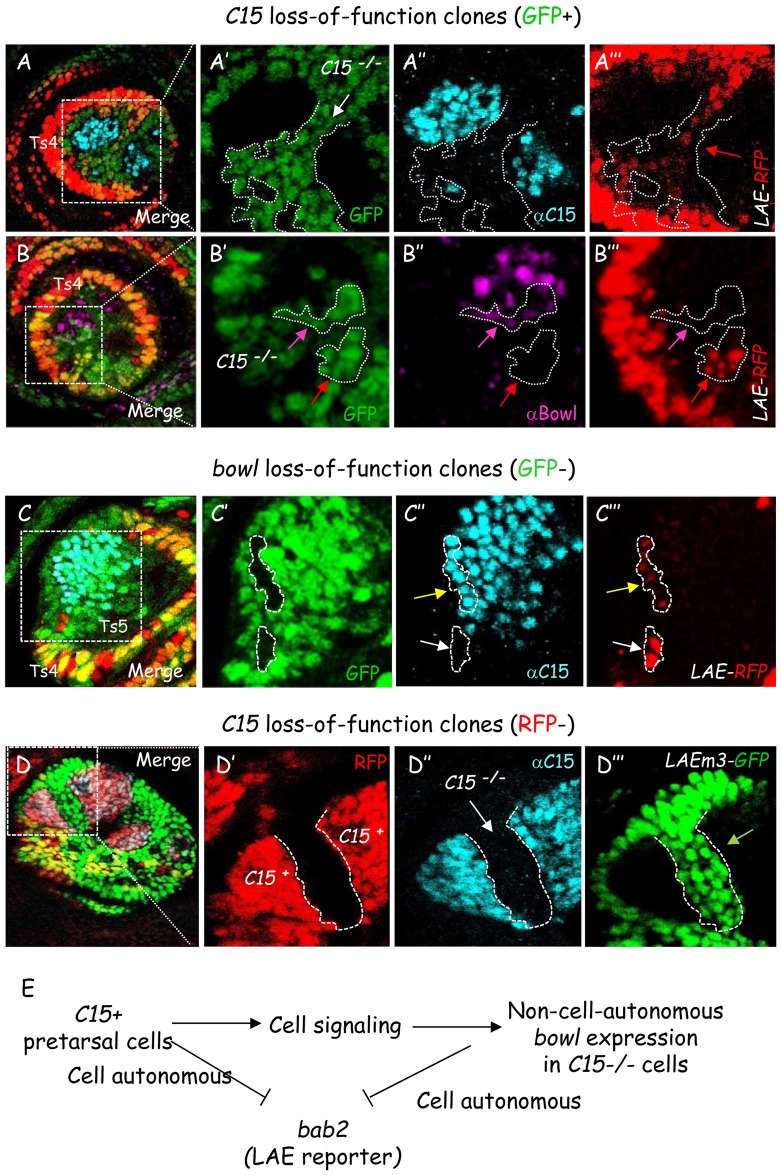
Surprisingly, while in C152 homozygous leg discs we observed a clear ectopic bab2 expression in all cells of the developing mutant pretarsus (above, Fig 3D), only a faint de-repression of LAE-RFP was detected within mutant clones (Fig 7A). Moreover, LAE-RFP de-repression only occurred in mutant cell subsets. This localized slight de-repression (red arrow) corresponded to those mutant cells situated farthest away from remaining C15+ cells (as detected by the presence of C15 antibody staining as well as absence of GFP fluorescence). This observation suggested that in addition to its cell-autonomous repressive activity, C15 could also activate non-autonomously another bab2 repressor. As mentioned above, C15 induces bowl expression in a non-cell autonomous manner through N signaling activation [8]. We therefore asked whether Bowl TF could be responsible for this non-autonomous repressive effect. As shown in Fig 7B, Bowl protein was indeed expressed (as detected by the presence of Bowl antibody staining) in some C15 mutant clones (expressing GFP) surrounding remaining C15+ cells (GFP-). Furthermore, LAE-RFP expression was detected only in C15-/- cells (n>50) that corresponded precisely to those that lacked detectable Bowl protein accumulation (Fig 7B, red arrows). This observation suggested that in C15 mutant clones even low amounts of Bowl protein (magenta arrows) are sufficient to fully extinguish LAE reporter activity. In support of this, in mosaic leg discs harboring bowl loss-of-function clones a faint LAE-RFP de-repression was observed in all bowl-/- clones situated within the C15-expressing pretarsal field (Fig 7C, see yellow arrow). Moreover, the developing pretarsus does not detectably accumulate nuclear Bowl TF except for a single row of cells at the ts5-pt boundary (see S1B Fig).
Fig 7. C15 down-regulates LAE in the developing pretarsus, cell autonomously as well as non-autonomously through Bowl stabilization.
(A-B) Mosaic late L3 leg discs harboring C15 loss-of-function clones (GFP positive) in the presence of the LAEwt-RFP reporter. Merged GFP (green) and LAE-driven RFP (red) fluorescence together with C15 (cyan) (A) or Bowl (magenta) (B) immunostaining are shown. Boxed distalmost areas are magnified for each marker in isolation, in (A’, B’), (A”, B”) and (A”‘, B”‘), respectively. A faint LAEwt-RFP up-regulation (red arrow) is observed in mutant pretarsal cells (white arrow) situated farthest away from C15+ (GFP negative) cells, i.e., black areas in (A’) and (B’). Strongest LAEwt-RFP up-regulation is observed in C15 mutant cells without detectable nuclear Bowl (red arrows). Conversely, LAEwt-RFP expression is not observed in C15-/- cells expressing Bowl protein (magenta arrows). (C) Mosaic late L3 leg disc expressing LAE-RFP and harboring bowl-/- clones (GFP-; i.e. black areas). Merged GFP (green), C15 (cyan) and LAE-driven RFP (red) expression is shown. The boxed distalmost area is magnified for each marker in isolation, in (C’), (C”) and (C’”), respectively. LAEwt-RFP is strongly de-repressed in bowl mutant cells which does not express C15 (white arrow), whereas only a faint up-regulation is observed in pure pretarsal clones. Note that bowl activity is not required for C15 expression there. Taken together, this result indicates that C15 TF activity is not sufficient for full LAE repression in the developing pretarsus. (D) Mosaic late L3 leg disc harboring C15-/- clones (detected by the absence of RFP fluorescence; i.e., black areas are mutant for C15 here; see Materials and methods), in the presence of the LAEm3-GFP reporter. Merged RFP (red), C15 (cyan) and LAE-driven GFP (green) expression is shown. The boxed distalmost area is magnified for each marker in isolation, in (D’), (D”) and (D”‘), respectively. Note that full de-repression of Bowl-unresponsive LAEm3-GFP reporter is now observed (green arrow in D”‘) in most if not all pretarsal cells of a large C15 mutant clone (white arrow in D”), to reach expression levels equivalent to those observed in neighboring tarsal cells. (E) Epistatic relationships between C15 and bowl and their joint repressive activity on bab2 (LAE-RFP) expression through cell autonomous and non-autonomous effects.
To explore the hypothesis of Bowl-mediated C15 dependent repression, we then performed C15 clonal loss-of-function analysis with LAEm3-GFP, specifically refractory to Bowl repressive activity (above). Note that in this experiment, wild-type cells (as detected with C15 immunostaining) are marked with RFP. In this effectively Bowl-inoperative context, LAEm3-GFP expression was fully de-repressed (green arrow) in most if not all C15-/- pretarsal cells (visualized here by the absence of RFP fluorescence) (Fig 7D), indicating that the previously-observed non-autonomous repressive effect induced by C15+ cells is no longer at work. Taken together, we conclude that C15 homeodomain and Bowl zinc-finger proteins act as a repressive TF duo by repressing directly LAE enhancer activity in the developing leg, in response to a cell signaling cascade, to refine the distal bab2 expression border.
Epistatic relationships between bab2 regulators
Together with the Dll and Rn activators, Bowl and C15 repressors constitute a TF quartet binding directly discrete sequences within the LAE enhancer, to ensure dynamic resolution of bab2 expression in the developing distal leg. We next asked whether they engage cross-regulatory interactions. Contrary to Dll, transcriptional enhancers have not been yet characterized for rn, bowl and C15.
First, we sought to examine rn expression. To this end, we generated a polyclonal antibody recognizing Rn (see Materials and methods). While in situ hybridization indicated transient expression limited from early-mid to mid-late L3 stages [10, 27], nuclear Rn could be detected later on. Indeed, at the late L3 stage Rn and LAE-RFP are co-expressed in most tarsal cells (Fig 8A), with Rn extending more proximally other several cell rows, while conversely LAE-RFP expression extends more distally (Fig 8A, see brackets).
Fig 8. Bowl and C15 activities repress jointly rotund expression.
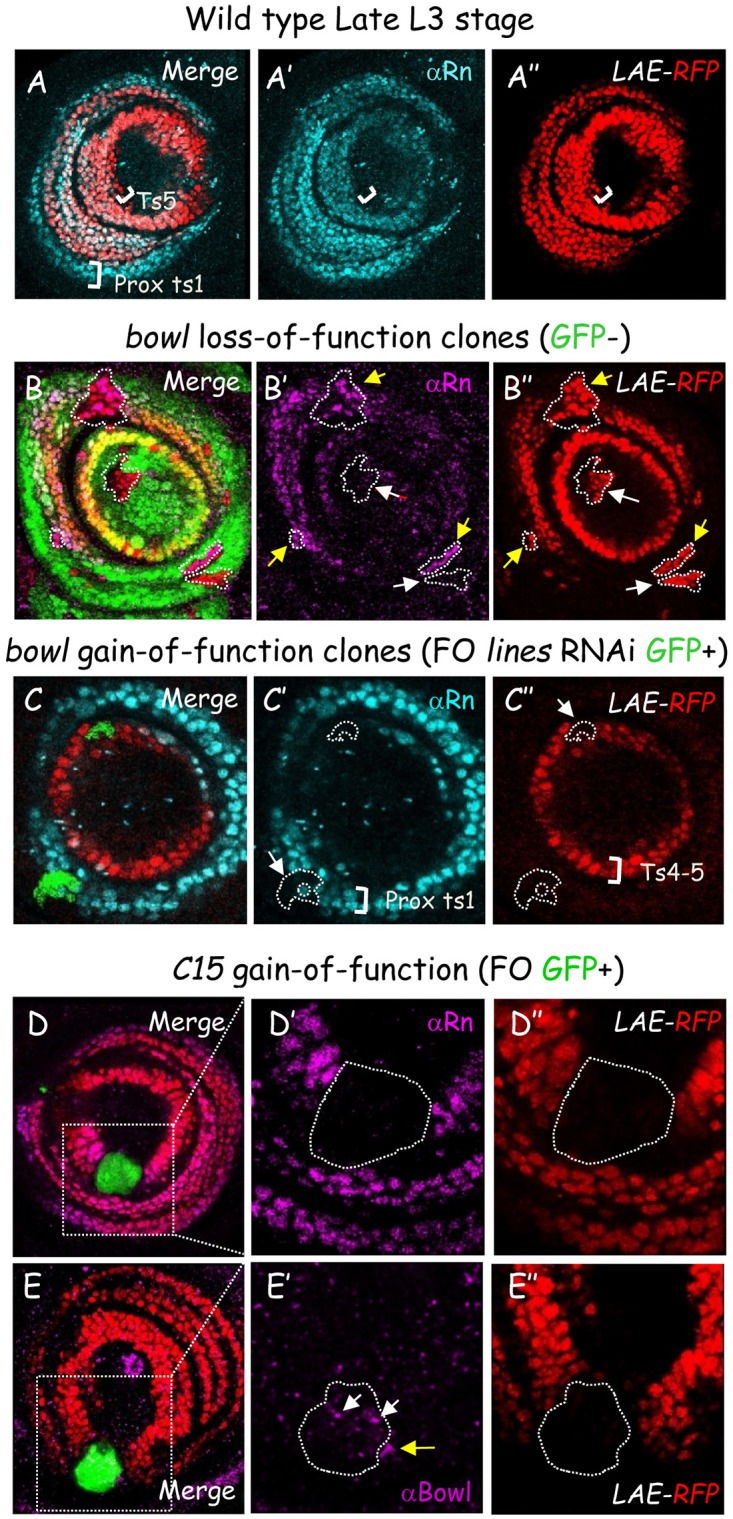
(A) Rn protein is detected lately in the emerging tarsus of a wild-type late L3 leg disc expressing LAE-RFP. Merged Rn immunostaining (cyan) and RFP fluorescence (red) is shown, as well as each marker in isolation, in (A’) and (A”), respectively. Rn and RFP are expressed in overlapping patterns, with proximalmost and distalmost (see also C’) cells expressing only rn or LAE-RFP, respectively, as indicated by white brackets. (B-C) The Lines/Bowl pathway cell-autonomously regulates rn expression in mosaic late L3 leg discs expressing LAE-RFP and harboring either bowl null mutant clones (GFP-) (B) or lines dsRNA-expressing FO clones (GFP+) (C). Merged GFP (green) fluorescence, Rn immunostaining (magenta) and LAE-driven RFP (red) fluorescence are shown, as well as Rn and RFP markers in isolation, in (B’, C’) and (B”, C”), respectively. Mitotic clones are circled with white dashed lines. In striking contrast to LAE-RFP (yellow arrows), rn was neither de-repressed in distalmost (pretarsal) nor proximalmost bowl-/- clones (white arrows). Conversely, Rn and RFP expression were both down-regulated in clones ectopically-stabilizing nuclear Bowl (white arrows). (D-E) Ectopic C15 protein represses rn expression, in a Bowl-independent manner, in mosaic late L3 leg discs expressing LAE-RFP and harboring FO clones (GFP+) ectopically-expressing C15 homeoprotein. Merged GFP (green) fluorescence, Rn (D) or Bowl (E) immunostaining (magenta) and LAE-driven (RFP) fluorescence are shown. Boxed areas are magnified for each marker in isolation, in (D’, E’) and (D”, E”), respectively, with large FO clones circled with white dashed lines. As for LAE-RFP, Rn was fully extinguished in tarsal cells ectopically-expressing C15 protein. Note that nuclear Bowl was autonomously-stabilized in a few C15 misexpressing cells (white arrows), as well as non-autonomously in neighboring GFP negative cells (yellow arrow), as shown in [8].
Second, we asked whether the Bowl/Lines cassette regulates rn in leg discs expressing LAE-RFP as an internal control. As previously observed for bab2 (Figs 1F and 7C), Rn was cell-autonomously up-regulated in some bowl mutant clones (Fig 8B, yellow arrows). Indeed, distalmost and proximalmost mutant clones only up-regulated LAE-RFP (white arrows). Conversely, rn was cell-autonomously repressed in Bowl gain-of-function clones (Fig 8C, yellow arrow), obtained through FO expression of dsRNA directed against the dedicated Bowl antagonist lines. Altogether these data indicate that rn expression in the leg disc is regulated negatively by the Lines/Bowl pathway, as previously shown in the antennal disc [13].
Third, we wondered whether C15 regulates rn expression. Contrary to bab2 which is first expressed as a distal circular domain [20], onset of rn expression occurs at the early-mid L3 stage in the emerging tarsal field in the form of a ring between Dac- and Bar-expressing ring-shaped domains [10]. Rn and C15 proteins are thus never abutting. As previously shown for bab2 (Fig 3E), clonal analyses revealed that rn was autonomously repressed in all C15-misexpressing cells (Fig 8D). As previously described [8], tarsal C15 misexpression stabilized nuclear Bowl in some but not all FO cells (Fig 8E, white arrows), thus ruling out that ectopically-stabilized Bowl mediates Rn extinction in C15+ cells.
Fourth, we asked whether rn regulates Dll, bowl and C15. In mosaic leg discs expressing the LAE-GFP or LAE-RFP construct, no effect on Dll, Bowl and C15 expression could be observed either in rn loss- or gain-of-function (S5 Fig and Fig 6D). As seen in our previous work [19], we did observe cell-autonomous LAE-GFP down-regulation in rn-/- clones within ts1-2, but not ts3-5 (S5 Fig, panels A-C). Conversely, clonal Rn overexpression led to cell-autonomous LAE-RFP up-regulation (S5D Fig, see white arrow), particularly in bab2-expressing ts4-5 cells (n = 12).
Fifth, as previously reported [8], misexpressed C15 protein down-regulated bowl expression distally (S6A Fig), while conversely ectopically-stabilized Bowl protein did not affect C15 expression (S6B Fig). Importantly, bowl gain-of-function clones (through lines down-regulation) leading to LAE-RFP repression (Fig 1G), never induced C15 up-regulation (S6C Fig), confirming that LAE-RFP repression by Bowl stabilization within the tarsal cells occurs independently of C15 TF activity.
Lastly, though the distal selector gene Dll is required directly or indirectly for expression of bowl, C15 and rn [5, 12, 28–30], none was upregulated in mosaic leg discs misexpressing Dll activator (S6D and S6E Fig and Fig 6C) and none affected in turn Dll expression in clonal loss- or gain-of-function (see S5A Fig for rn as well as S7A and S7C Fig for bowl and C15, respectively). While as expected (see Fig 1F) LAE-RFP was up-regulated in bowl mutant clones (S7A Fig), its re-activation occurred only in Dll-expressing cells, either distally or proximally (compare white and yellow arrows, respectively). The observation that LAE-RFP could only be de-repressed in Dll+ cells is consistent with a critical activating role of the Dll TF in bab2 expression throughout limb development.
In conclusion, these results indicate that rn is also a repressed target of Bowl and C15, drawing new connections within the gene regulatory network governing distal leg development.
Discussion
The bric-à-brac2 expression pattern in the developing leg is dynamic [17–19]. EGFR and Notch pathways have been proposed to regulate bab2 expression in a timely fashion to restrict precisely its expression domain in the distal leg [8, 16]. We previously showed that the Distal-less HD and Rotund ZF transcription factors are central to activating bab2 in the developing distal leg, by binding directly a single tissue-specific enhancer, the LAE [19]. Here, we identified two transcriptional repressors, the C15 HD and the Bowl ZF proteins, that likewise act though LAE by repressing directly bab2 in pretarsal (Bowl and C15) as well as in proximal ts1 and distal ts5 cells (Bowl), to delimit precisely its final tarsal expression domain within the Dll-expressing cells. Moreover, we found that C15 does not require regular partnership with Aristaless for bab2 repression. Instead, C15 interacts functionally with Distal-less, engaging a direct molecular partnership through their DNA-binding homeodomain and competing each other for neighboring binding sites within LAE. Altogether our data allow us to propose an updated model for leg-specific bab2 regulation, taking into account repressive cell-signaling inputs from EGFR and Notch pathways.
The Lines/Bowl pathway shapes bab2 expression pattern in the developing tarsus
Repressive bowl activity has been previously proposed to mediate graded bab2 extinction in response to N signaling, in distal ts5 and in proximal ts1 cells [13, 16]. As for other tissues, bowl activity in the leg disc is down-regulated by lines activity [13, 15, 31], presumably through a direct physical association between the Bowl and Lines proteins [15]. In the present study, we provided several lines of evidence that the Lines/Bowl pathway regulates bab2 expression in developing distal leg cells through direct interaction of the Bowl ZF domain with conserved Odd family binding motifs within CR2 [21, 22, 32]. First, both bab2 expression and LAE-RFP reporter activity are cell-autonomously de-repressed in bowl mutant clones. Second, ectopic nuclear stabilization of Bowl TF (through lines down-regulation) is sufficient for cell-autonomous bab2 (LAE-RFP) down-regulation in developing tarsal cells. Third, recombinant Bowl ZF interacts directly with conserved LAE motifs, in a sequence-specific manner. Lastly, these binding sites are relevant in vivo, since ectopically stabilized Bowl protein is unable to extinguish a bab2 reporter gene mutant for the two biochemically-defined binding sites, confirming their critical role in mediating LAE enhancer repression.
As predicted from a bacterial one-hybrid assay [21], Bowl and Odd proteins interact in vitro with highly similar DNA-binding motifs [22] (this study). It is worth noting that bab2 is the first direct target gene identified so far for the Bowl TF. Although the near-palindromic CR2 cognate site is critical, a second one situated approximately 100 bp away is required specifically for full repression of the LAE enhancer in proximal leg tissues (Fig 2C). How the two Bowl ZF-binding sites cooperate in proximal ts1 cells remains to be deciphered. Interestingly, cooperation between distant cognate sites has been suggested for transcriptional targets of the related Odd TF during embryogenesis [22].
C15 acts independently of Al to mediate repressive EGFR signaling input on bab2 expression
Initially expressed within a broad, distal circular domain at the early L3 stage, bab2 is extinguished in the developing pretarsus in response to EGFR signaling activation at early-mid L3 stage [5]. Among known EGFR signaling targets [3], we provided several lines of evidence that the C15 transcription factor is directly repressing bab2 and do so independently of its regular partner Al. First, C15 protein behaves as a strong LAE interactor in yeast cells. Second, C15 homeodomain interacts strongly in vitro with dedicated binding sites within the critical CR1 LAE sequence, without synergistic effect of Al (contrary to Bar enhancer sequences). Third, both bab2 expression and LAE-RFP reporter activity are de-repressed distally in leg discs deficient for C15 activity. Fourth, ectopic expression of the C15 homeoprotein is sufficient to extinguish cell-autonomously bab2 (and LAE reporter activity) in tarsal cells, even in Al deficient cells. Altogether our data suggest that Bar regulation by the C15-Al heterodimer does not reflect a rule. Characterization of other direct target genes, among candidates such as rn (this work) and Dl [8], will indicate to what extent C15 TF is acting independently of Al.
C15 and Dll interact physically and compete for LAE binding
Within the essential CR1 sequence, C15 interacts strongly with an A/T-rich motif (S6) adjacent to the critical TAAT-rich Dll homeoprotein interacting DBS (Fig 4), which is not required for efficient C15 binding to its neighboring site S6. This proximity has prompted us to examine whether C15 and Dll are able to bind simultaneously to CR1 sequences encompassing both sites. Surprisingly, in EMSA experiments, simultaneous addition of both homeoproteins never revealed C15-Dll heterodimeric binding. We showed that C15 is able to compete with Dll upon binding to CR1 sequences, in a dose-sensitive manner (Fig 6A). Moreover, we found that C15, but not Al, interacts physically with Dll through their homeodomain (Fig 6B), providing a molecular mechanism for their antagonistic DNA binding to LAE sequences. Thus, C15 engages intermolecular interactions with both Al and Dll, through their related DNA-binding domain but with opposite outcomes: cooperative binding to specific DNA sequence (C15-Al) versus competition for LAE binding (C15-Dll). Further studies will determine relevant mechanism(s) of bab2 repression by C15 among at least three non-exclusive possibilities: (1) Dll-independent C15 repressive activity through direct binding on its own cognate sites within LAE (presumably through interaction with co-repressors, see below); (2) direct C15 binding to the S6 CR1 sequence sterically hindering Dll binding to its own adjacent cognate site (DBS); and (3) heterodimerization with Dll, inhibiting its direct interaction with LAE or changing its global DNA binding specificity, independently of C15 DNA binding per se. Whatever the mechanism(s) involved, our data suggest that C15 and Dll compete functionally in vivo in differentially regulating LAE activity, i.e. repression versus activation (Fig 6C). Given that both C15 and Dll (as well as Al) are co-expressed in pretarsal cells, we speculate that the relative amounts of C15 and Dll homeoproteins are critical for full repression in the distal leg. Interestingly, mammalian homologs of Dll form heterodimers with repressive Msx-type homeoproteins, through their respective HDs, with mutually antagonistic interactions in vitro and in-vivo [33], indicating an evolutionarily-conserved propensity of Dll-type HDs in assembling heterodimeric complexes with repressive homeoproteins.
We thus propose that at early-mid L3 stage, before C15 activation, Dll expressed throughout the distal territory allows bab2 induction as a distal circular domain, by binding the DBS within LAE. At the onset of C15 expression in response to EGFR signaling, increasing amount of C15 protein competes with Dll activator in pretarsal cells, thus resolving bab2 expression pattern as a single ring of cells at the mid L3 stage.
As stated above, in addition to interacting and/or competing with activating Dll on binding CR1 sequences, C15 might repress LAE activity on its own. Consistently, C15 harbors an eh1-type repressor domain that can interact with the Groucho (Gro) co-repressor [34]. Additionally, we showed that Bowl must function together with C15 to fully repress LAE-activity (Fig 7C). It is noteworthy that Bowl also harbors an eh1 repressive motif [35]. As for C15 (above), Bowl ZF protein is therefore predicted to interact with the Gro co-repressor and both repressive transcription factors may extinguish bab2 through the same molecular mechanisms, delimiting the tarsal expression domain in a timely manner. Future work should determine whether the C15 transcription factor functions together with the Gro protein to extinguish bab2 in the developing pretarsal primordium.
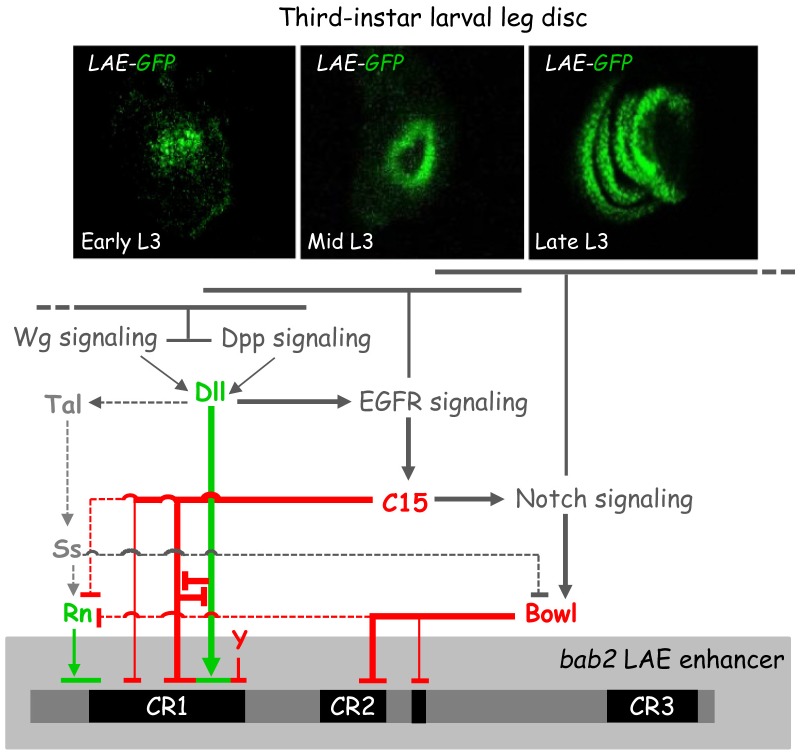
A model for molecular integration of multiple cell signaling inputs by the LAE CRM
Taken together with our previous work [19], we propose an updated model for LAE regulatory activity in the developing distal leg (Fig 9). The tissue-specificity of the LAE enhancer is ensured by its CR1-2 region, while its 3’-end moiety, containing CR3, is mainly required for transcriptional enhancer strength. Situated downstream of the Wg and Dpp signaling inputs, the Distal-less homeodomain TF is required to induce and maintain overall bab2 expression within the developing tarsus, from L3 to adult stages, by acting through CR1 [19]. Given that Dll is expressed early on during larval development, well before bab2 expression onset as a circular distal domain, another transcriptional activator functioning at early-mid L3 stage remains to be identified. This factor may operate through activating CR1 sequences overlapping the C15 binding sites (S4B Fig).
Fig 9. An updated model for limb-specific bab2 regulation: The LAE enhancer integrates multiple cell-signaling pathways.
See main text for details.
From early-mid to mid-late L3 stages, in response to Tal signaling induced by EGFR together with Dll inputs and via spineless-mediated activation [10, 29], Rn is activated in the ts1-3 primordia and up-regulates LAE activity in the proximalmost bab2-expressing cells. Given the dynamic activity of Bowl protein during tarsal growth, de Celis and Bray [16] anticipated that a transiently-expressed bab2 activator must counteract repressive Bowl TF in developing tarsal cells. Our previous work indicates that the Rn ZF protein could be this factor [19]. The Rn transcriptional activator is indeed expressed at the right time and in the proper cells, counteracting directly ongoing repressive Bowl on LAE enhancer activity. Interestingly, dynamic bowl activity is under the negative control of the transiently-accumulated Spineless (Ss) bHLH-PAS TF [16], which itself is positively required for rn activation [10]. Lastly, Bowl activity represses rn expression (this work), suggesting that Ss may in fact induce rn by counteracting Bowl repression.
Concomitant to the onset of Rn activity in proximal tarsal cells, and in response to EGFR signaling at early-mid L3 stage, C15 is activated and alone (i.e. without Al) extinguishes bab2 in the pretarsal primordium, presumably in a dose-dependent manner (see above), through direct competitive interactions with Dll (this work). At the same time, C15 cell-autonomously down-regulates Dl in the developing pretarsal cells, allowing bowl up-regulation in response to activated N signaling at the tarsus/pretarsus boundary [8]. Thus, C15 is required non-cell-autonomously for bowl expression in developing ts5 [8]. There, the Bowl TF in turn represses LAE enhancer activity (through its CR2 cognate site) to reshape the distalmost bab2-expressing ring during mid to late L3 stages. Lastly, at the late L3 stage and beyond up to the adult stage, graded bab2 expression is observed in each tarsal segment [17]. To complete our model regarding the molecular inputs controlling LAE enhancer activity, the previously-identified CR1-binding inter-ring repressive activity [19] (Factor Y in Fig 9) and the bab2 activator acting during the early L3 stage (X) remain to be characterized.
Finally, this work also shed light to new epistatic relationships between bab2 regulators within the leg genetic cascade. In addition to bab2, we showed that C15 and bowl regulate also rotund. Nevertheless, direct interactions with rn enhancer sequences remain to be established. It would be interesting to determine whether C15 (i) functions jointly with Bowl and/or (ii) antagonizes Dll activity in repressing other target genes.
Materials and methods
Fly stocks, culture and genetic manipulations
Drosophila lines were grown on standard yeast extract-sucrose medium. The vasa-PhiC31 ZH2A attP stock (kindly provided by F. Karch) was used to generate the mutant LAE-GFP reporter lines, as previously described [19]. LAE-RFP constructs were generated by insertions on both the ZH2A (X chromosome) and ZH86Fb (third chromosome) attP landing platforms, and they display identical expression patterns [19]. UAS-C15, UAS-Dll and UAS-Rn stocks were obtained from T. Kojima, S. Cohen and the Bloomington stock Center (#7403), respectively. C152/TM6B, Tb1 stock was kindly provided by G. Campbell. Mutant mitotic clones for null alleles of bowl, C15 and rotund were generated with the following genotypes: y w LAEwt-RFP; DllGal4EM2012 UAS-Flp / +; Ub-GFP FRT40A/bowl1 FRT40A (i.e., bowl mutant clones are GFP negative; Figs 1F, 7C and 8B and S7A Fig), y w LAEwt-RFP; DllGal4EM2012 UAS-Flp / +; FRT82B/FRT82B Ub-GFP C152 (i.e., C15 mutant clones harbor two Ub-GFP copies; Fig 7A and 7B) or y w LAEwt-GFP (or LAEm3-GFP); DllGal4EM2012 UAS-Flp / +; Ub-RFP FRT82B / FRT82B C152 and y w LAEwt-GFP; DllGal4EM2012 UAS-Flp / +; Ub-RFP FRT82B/FRT82B rn16 (i.e., C15 or rn mutant clones are marked by the absence of RFP fluorescence; Fig 7D and S5A–S5C and S7C Figs). UAS-dsRNA stocks used to obtain RNAi against lines (#40939) or al (#26747) were obtained from the Bloomington stock Center. “Flip-out” mitotic clones over-expressing dsRNA against lines were generated by 40 mn heat shocks at 38°C, in early second- to early-mid L3 larvae of genotypes: y w LAEwt-RFP hsFlp; UAS-dsRNAlines / Pact>y+>Gal4 UAS-GFP (i.e., FO clones express GFP in Fig 1G) and y w LAEwt-GFP (or LAEm3-GFP) hsFlp; UAS-dsRNAlines / Pact>y+>Gal4 UAS-RFP (i.e., Lines-deficient FO clones express RFP in Figs 2F, 2G and 8C and S6B, S6C and S7B Figs). FO clones overexpressing C15, Rn or Dll were generated by 40 mn heat shocks at 38°C, in mid second to early-mid L3 larvae of genotypes: y w LAEwt-RFP hsFlp; UAS-C15 / Pact>y+>Gal4 UAS-GFP; (+/-UAS-dsRNAal / +) (i.e., FO clones express GFP; Figs 3E, 5C, 5D, 8D and 8E and S6A and S7D Figs), y w LAEwt-RFP hsFlp; UAS-Rn / Pact>y+>Gal4 UAS-GFP (i.e., FO clones express GFP; Fig 6D and S5D and S5E Fig), and y w LAEwt-RFP hsFlp; Pact>y+>Gal4 UAS-GFP / +; UAS Dll / + (i.e., FO clones express GFP: Fig 6C and S6D and S6E Fig), respectively.
Reporter construction and mutagenesis
LAE fragments were cloned into pBP-S3aG vector as previously described [19]. pLAE-GFP or -RFP site-directed mutagenesis was performed by PCR, using the overlap extension method [36]. All plasmid constructs used in this work were sequence verified. Mutated LAE reporters were all inserted on the same ZH2A attP landing platform.
Yeast one hybrid assay
The Y1H screen was performed as described in [23]. LAE and mini-LAE DNA baits were amplified by PCR and cloned into pENTRY-5’ using standard restriction-ligation techniques. The sequence-verified LAE baits were sub-cloned into the Y1H-compatible pMW2 (HIS3) vector by Gateway LR cloning. LAE destination clones were integrated in Y1H-YM4271 yeast strain using lithium acetate-polyethylene glycol transformation followed by selection on a synthetic complete (SC) (-His) medium plate.
Immuno-histochemistry and microscopy
Leg discs were dissected from wandering L3 larvae. Indirect immuno-fluorescence was carried out as previously described [19] using a LEICA TCS SP5 or SP8 confocal microscope. Rat anti-Bab2 [18], rabbit anti-Bowl [16], rabbit anti-C15 [9] and rat anti-Al [8] antibodies, kindly provided by F. Laski, S. Bray, T. Kojima and G. Campbell, respectively, were used at 1/2000, 1/1000, 1/200 and 1/1000, respectively. Guinea pig antibody raised against Roe (an isoform encoded by the rotund/roe locus, see below) was prepared by Eurogentec, from a full-length GST-Roe fusion (a kind gift from D. del Alamo) [37] extracted from E. coli and enriched by affinity chromatography. Note that (i) anti-Roe cross-reacts with Rn, because both proteins share a large common C-terminal region (with the ZF domain) and (ii) Roe protein isoform is not expressed in the leg [27]. Anti-Roe/Rn sera from terminal bleeds was used for immunocytology without purification at a 1/500 dilution.
Recombinant proteins
The Open Reading Frame (ORF) encompassing the C15 HD-encoding sequence (codons 192 to 292) was amplified by PCR, using as a template a full length (FL) cDNA insert (IP08859), obtained from the Drosophila Genomic Resource Center (DGRC), and inserted as an EcoRI/XhoI fragment into pGex4T, to yield the pGexC15HD construct. Similarly, Dll-encoding sequences (full-length ORF or codons 120–188) were amplified by PCR, using as a template a full ORF cDNA insert [23], and inserted as EcoRI/XhoI fragments into pGex4T, to yield the pGexDllFL and pGexDllHD constructs. Non-fused GST (produced from empty pGex4T), GST-C15HD and both GST-Dll proteins were all expressed in BL21 [DE3] cells (Novagen), from which soluble forms were purified on glutathione-Sepharose beads (GE Healthcare) and then isolated through elution with free glutathione. Purified proteins were used in EMSA, as described in [26]. Given that FL Bowl protein appeared toxic when expressed in E. coli cells, ZF domain encoding bowl ORF (codons 220 to 382) was amplified by PCR, using as template a FL cDNA clone (a kind gift from Sarah Bray), and inserted as an EcoRI/XhoI fragment into pGex4T, to yield the pGexBowlZF construct. The GST-BowlZF fusion was expressed in BL21 [DE3] cells, from which soluble forms were purified by glutathione-Sepharose affinity. For control EMSA and pull-down experiments, transformation with empty pGex4T was also performed, to produce and then purify soluble non-fused GST.
Electrophoretic mobility-shift assay
EMSA experiments with Bowl and HD proteins were performed as described in [22] and [26], respectively, using purified GST fusion proteins (above) and/or in vitro translated Al HD (see below). Protein-DNA complexes were separated by electrophoresis on 6% native polyacrylamide gels and revealed by PhosphoImager detection. DNA Probes (see Figs 2A and 4B for sequences) were made from annealed synthetic oligonucleotides and 5’end-labeled with [32P]dCTP using Klenow DNA polymerase, as previously described [19]. However, a single labeled dCTP (instead of 8 for the LAE-derived probes) was incorporated into the Odd-binding probe, as described in [22]. The BarEnh probe is described in [26].
GST-pull down assay
Pull down was down as previously described [38], in duplicate. 35S protein probes (C15HD, DllHD and AlHD) were obtained from coupled in vitro transcription/translation (TNT with rabbit reticulocyte extracts; Promega Inc.) from DNA matrices generated by PCR, using 5’ oligonucleotides comprising each a T7 RNA polymerase promoter and a translation initiating ATG within an optimal Kozak context (sequences of oligonucleotides are available upon request). Radiolabeled C15 and Dll HDs (amino acids 192–292 and 120–188, respectively) were produced from full-length cDNA clones (described above). In vitro translated Al HD (amino acids 86–156) was produced from a full-length cDNA clone (RE68460), obtained from the Drosophila Genomic Resource Center (DGRC).
LAE sequence identification and alignment
bric-à-brac locus sequences were recovered from dipteran genomic sequences and aligned as previously described [19].
Supporting information
(A-B) Late L3 leg discs (distalmost confocal views) expressing either the LAEwt-GFP (A) or LAEΔ2-GFP (B) reporter. Merged GFP fluorescence (green) and Bowl immunostaining (magenta) are shown. Boxed areas are magnified for the merged markers in (A’) and (B’), as well as GFP expression in isolation in (A”) and (B”), respectively. While LAEwt-GFP fluorescence abuts the Bowl+ domain at the ts5-pt boundary, its mutant derivative ΔCR2 is specifically up-regulated in many ts5 cells and remained repressed in the raw of bowl-expressing pretarsal cells (magenta arrows). (C-D) Late L3 leg discs (distalmost confocal views) expressing the LAEm3-RFP reporter. Merged GFP fluorescence (green) and C15 (C) or Bowl (D) immunostaining (magenta) are shown. Boxed areas are magnified for the merged markers in (C’) and (D’), as well as GFP expression in isolation in (C”) and (D”), respectively. Note that C15-expressing pretarsal (pt) cells abut GFP-expressing ts4-5 cells [white bracket in (C”)], indicating that Bowl-insensitive LAEm3-GFP is still repressed in the developing pretarsus, including in a row of Bowl-expressing cells [white bracket in panel (D”)].
(TIF)
(A-B) CR2 (A) and 3’-neighboring region of CR2 (B) sequences of 27 dipterans are aligned. Drosophilidae, C. capitata, G. morsitans and M. domestica LAE-like sequences were identified though BLAST analyses using the Trace archive nucleotide blast server at NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi) and were aligned with the D. melanogaster LAE sequence using MAFFT (http://mafft.cbrc.jp/alignment/server/index.html). Homology shading was performed using BoxShade (http://www.ch.embnet.org/software/BOX_form.html). Bowl BS positions are indicated by blue dashed boxes. The near-palindromic site within CR2 is indicated by an orange dashed box.
(TIF)
(A-B) Late L3 late discs from wild-type (A) or C152 homozygous mutant (B) expressing LAE-RFP. Merged RFP fluorescence (red) and Bowl immunostaining (magenta) are shown, as well as the latter in isolation, in (A’) and (B’). It is noteworthy that bowl expression at the ts5/pretarsal (pt) boundary [yellow arrows in (A) and in (A’)] is no longer detected in the C15 loss-of-function developing distal leg, while remaining unaffected at the tibial (ti)/ts1 boundary [white arrows in (B) and (B’)].
(TIF)
(A) C15 homeodomain binds distinct A/T-rich motifs within the LAE CR1 sequence. EMSA experiment with retarded complexes obtained from purified GST-C15HD and unfused GST as a negative control. A non-specific retarded complex is indicated by asterisks. See Fig 4B for probed CR1 sequences. (B) The C15 repressor binding sites within CR1 are critical for LAE activation in vivo. GFP (green) and RFP (red) fluorescence are shown for late L3 leg discs expressing LAEwt-GFP together with wild-type (upper panels) or mutated (lower panels) LAE-RFP constructs. The F2LAES3+4+6m construct mutated for both C15 binding sites within CR1 (identical to the CR1S3+4+6m DNA probe depicted in B; lane 10) does not expressed RFP within the leg discs, suggesting that the S3-4 and S6 motifs encompass activating sequences.
(TIF)
Mosaic late L3 leg discs expressing LAE-GFP (A-C) or LAE–RFP (D-E) and harboring either rotund null clones (detected by the loss of RFP) (A-C) or FO clones (specifically expressing GFP) overexpressing the Rn protein (D-E). Merged RFP fluorescence (red), GFP fluorescence (green) and Dll (A, D), Bowl (B, E) or C15 (C) immunostaining (magenta) are shown, as well as the latter and GFP or RFP markers in isolation, in (A’-E’) and (A”-E”), respectively. Mitotic clones are circled with white dashed lines. In striking contrast to LAE-RFP (specifically affected in ts1-2 rings, white arrows), Dll, bowl and C15 expression remained unchanged in rn null clones. Similarly, misexpressed Rn TF never detectably affected Dll, bowl nor C15 (see Fig 6D) expression, while LAE-RFP was slightly up-regulated in ts3-5 FO clones (white arrow).
(TIF)
Mosaic late L3 leg discs expressing LAE-RFP and harboring FO clones (specifically expressing GFP) ectopically-expressing C15 (A), Bowl (B-C) or Dll (D-E). Merged markers and Bowl, C15 or Rn immunostaining (magenta) are shown, as well as LAE-RFP fluorescence in isolation. FO mitotic clones are circled with white dashed lines and some are indicated with white or yellow arrows. Lateral confocal views are shown in (A) and (B) with distalmost pretarsal cells on the left and right side, respectively. Note that ectopically-expressed C15 protein down-regulated bowl expression at the pt-ts5 boundary, while the converse was not observed (yellow vs white arrows). As expected LAE-RFP was autonomously extinguished in Lines-depleted tarsal FO cells (C”), but C15 protein was never detected there (C’) (yellow arrows), thus discarding the possibility that C15 misexpression contributes to LAE-RFP repression by ectopically-stabilized nuclear Bowl. Lastly, whereas rn remained unaffected in all Dll-misexpressing FO clones (E’, yellow arrow), a faint cell-autonomous LAE-RFP up-regulation was observed (albeit not in all cells) in some proximalmost clones (E”, yellow arrow), indicating that misexpressed Dll is sufficient to activate the LAE in proximal cells, as shown in C15-expressing distal cells (Fig 6C).
(TIF)
Mosaic late L3 leg discs expressing LAE-RFP (A, B, D) or LAE-GFP (C) and harboring loss- or gain-of-function clones for bowl (A, B) or C15 (C-D). Merged RFP fluorescence (red), GFP fluorescence (green) and Dll immunostaining (magenta) are shown, as well as the Dll and RFP or GFP markers in isolation, in (A’-D’) and (A”-D”), respectively. Mitotic clones are circled with white dashed lines. In striking contrast to bab2 reporters, Dll expression was never detectably affected in any mosaic discs (white arrows). As expected, LAE reporter activity was down-regulated in FO clones misexpressing Bowl and C15 proteins. LAE-RFP up-regulation was observed in all bowl mutant cells, provided that they are located within the Dll-expressing cells (compare A’ and A”, white vs yellow arrows).
(TIF)
Acknowledgments
We thank S. Bray, G. Campbell, T. Kojima, D. del Alamo, F. Laski, J.L. Couderc, S. Cohen and the Bloomington Stock Center for fly stocks and reagents. We are grateful to K. Hens, C. Faucher, C. Immarigeon and M. de Taffin de Tilques for help during the course of this work, to E. Guillou for comments on the manuscript, and to Caroline Monod for her careful proofreading of the manuscript. We thank J. Favier for technical assistance, particularly in managing the transgenic platform, and V. Nicolas for fly facility. Lastly, we acknowledge the Toulouse RIO Imaging platform.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from the French governmental agency for research (CNRS), Paul Sabatier University (UPS) and the "Association pour la Recherche sur le Cancer" foundation, as well as internal support provided by the EPFL School of Life Sciences (to BD). AB was supported by the CNRS, the Midi Pyrenees Region and ARC. LHMV was supported by the Mexican CONACYT. MHB was supported by the French MENRT. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript."
References
- 1.Zeller R, López-Ríos J, Zuniga A. Vertebrate limb bud development: moving towards integrative analysis of organogenesis. Nat Rev Genet. 2009;10(12):845–58. 10.1038/nrg2681 [DOI] [PubMed] [Google Scholar]
- 2.Estella C, Voutev R, Mann RS. A dynamic network of morphogens and transcription factors patterns the fly leg. Curr Top Dev Biol. 2012;98:173–98. 10.1016/B978-0-12-386499-4.00007-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kojima T. The mechanism of Drosophila leg development along the proximodistal axis. Dev Growth Differ. 2004;46(2):115–29. 10.1111/j.1440-169X.2004.00735.x [DOI] [PubMed] [Google Scholar]
- 4.Suzanne M. Molecular and cellular mechanisms involved in leg joint morphogenesis. Semin Cell Dev Biol. 2016. [DOI] [PubMed] [Google Scholar]
- 5.Galindo MI, Bishop SA, Greig S, Couso JP. Leg patterning driven by proximal-distal interactions and EGFR signaling. Science. 2002;297(5579):256–9. 10.1126/science.1072311 [DOI] [PubMed] [Google Scholar]
- 6.Kojima T, Sato M, Saigo K. Formation and specification of distal leg segments in Drosophila by dual Bar homeobox genes, BarH1 and BarH2. Development. 2000;127(4):769–78. [DOI] [PubMed] [Google Scholar]
- 7.Campbell G. Distalization of the Drosophila leg by graded EGF-receptor activity. Nature. 2002;418(6899):781–5. 10.1038/nature00971 [DOI] [PubMed] [Google Scholar]
- 8.Campbell G. Regulation of gene expression in the distal region of the Drosophila leg by the Hox11 homolog, C15. Dev Biol. 2005;278(2):607–18. 10.1016/j.ydbio.2004.12.009 [DOI] [PubMed] [Google Scholar]
- 9.Kojima T, Tsuji T, Saigo K. A concerted action of a paired-type homeobox gene, aristaless, and a homolog of Hox11/tlx homeobox gene, clawless, is essential for the distal tip development of the Drosophila leg. Dev Biol. 2005;279(2):434–45. 10.1016/j.ydbio.2004.12.005 [DOI] [PubMed] [Google Scholar]
- 10.Natori K, Tajiri R, Furukawa S, Kojima T. Progressive tarsal patterning in the Drosophila by temporally dynamic regulation of transcription factor genes. Dev Biol. 2012;361(2):450–62. 10.1016/j.ydbio.2011.10.031 [DOI] [PubMed] [Google Scholar]
- 11.Hao I, Green RB, Dunaevsky O, Lengyel JA, Rauskolb C. The odd-skipped family of zinc finger genes promotes Drosophila leg segmentation. Dev Biol. 2003;263(2):282–95. [DOI] [PubMed] [Google Scholar]
- 12.Rauskolb C. The establishment of segmentation in the Drosophila leg. Development. 2001;128(22):4511–21. [DOI] [PubMed] [Google Scholar]
- 13.Greenberg L, Hatini V. Essential roles for lines in mediating leg and antennal proximodistal patterning and generating a stable Notch signaling interface at segment borders. Dev Biol. 2009;330(1):93–104. 10.1016/j.ydbio.2009.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green RB, Hatini V, Johansen KA, Liu XJ, Lengyel JA. Drumstick is a zinc finger protein that antagonizes Lines to control patterning and morphogenesis of the Drosophila hindgut. Development. 2002;129(15):3645–56. [DOI] [PubMed] [Google Scholar]
- 15.Hatini V, Green RB, Lengyel JA, Bray SJ, Dinardo S. The Drumstick/Lines/Bowl regulatory pathway links antagonistic Hedgehog and Wingless signaling inputs to epidermal cell differentiation. Genes Dev. 2005;19(6):709–18. 10.1101/gad.1268005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Celis Ibeas JM, Bray SJ. Bowl is required downstream of Notch for elaboration of distal limb patterning. Development. 2003;130(24):5943–52. 10.1242/dev.00833 [DOI] [PubMed] [Google Scholar]
- 17.Godt D, Couderc JL, Cramton SE, Laski FA. Pattern formation in the limbs of Drosophila: bric a brac is expressed in both a gradient and a wave-like pattern and is required for specification and proper segmentation of the tarsus. Development. 1993;119(3):799–812. [DOI] [PubMed] [Google Scholar]
- 18.Couderc JL, Godt D, Zollman S, Chen J, Li M, Tiong S, et al. The bric à brac locus consists of two paralogous genes encoding BTB/POZ domain proteins and acts as a homeotic and morphogenetic regulator of imaginal development in Drosophila. Development. 2002;129(10):2419–33. [DOI] [PubMed] [Google Scholar]
- 19.Baanannou A, Mojica-Vazquez LH, Darras G, Couderc JL, Cribbs DL, Boube M, et al. Drosophila distal-less and Rotund bind a single enhancer ensuring reliable and robust bric-a-brac2 expression in distinct limb morphogenetic fields. PLoS Genet. 2013;9(6):e1003581 10.1371/journal.pgen.1003581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu J, Dong PD, Panganiban G. Limb type-specific regulation of bric a brac contributes to morphological diversity. Development. 2002;129(3):695–704. [DOI] [PubMed] [Google Scholar]
- 21.Enuameh MS, Asriyan Y, Richards A, Christensen RG, Hall VL, Kazemian M, et al. Global analysis of Drosophila Cys2-His2 zinc finger proteins reveals a multitude of novel recognition motifs and binding determinants. Genome Res. 2013;23(6):928–40. 10.1101/gr.151472.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng X, Brodsky MH, Wolfe SA. A bacterial one-hybrid system for determining the DNA-binding specificity of transcription factors. Nat Biotechnol. 2005;23(8):988–94. 10.1038/nbt1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hens K, Feuz JD, Isakova A, Iagovitina A, Massouras A, Bryois J, et al. Automated protein-DNA interaction screening of Drosophila regulatory elements. Nat Methods. 2011;8(12):1065–70. 10.1038/nmeth.1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noyes MB. Analysis of specific protein-DNA interactions by bacterial one-hybrid assay. Methods Mol Biol. 2012;786:79–95. 10.1007/978-1-61779-292-2_5 [DOI] [PubMed] [Google Scholar]
- 25.Noyes MB, Christensen RG, Wakabayashi A, Stormo GD, Brodsky MH, Wolfe SA. Analysis of homeodomain specificities allows the family-wide prediction of preferred recognition sites. Cell. 2008;133(7):1277–89. 10.1016/j.cell.2008.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyazono K, Zhi Y, Takamura Y, Nagata K, Saigo K, Kojima T, et al. Cooperative DNA-binding and sequence-recognition mechanism of aristaless and clawless. EMBO J. 2010;29(9):1613–23. 10.1038/emboj.2010.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.St Pierre SE, Galindo MI, Couso JP, Thor S. Control of Drosophila imaginal disc development by rotund and roughened eye: differentially expressed transcripts of the same gene encoding functionally distinct zinc finger proteins. Development. 2002;129(5):1273–81. [DOI] [PubMed] [Google Scholar]
- 28.Galindo MI, Pueyo JI, Fouix S, Bishop SA, Couso JP. Peptides encoded by short ORFs control development and define a new eukaryotic gene family. PLoS Biol. 2007;5(5):e106 10.1371/journal.pbio.0050106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pueyo JI, Couso JP. The 11-aminoacid long Tarsal-less peptides trigger a cell signal in Drosophila leg development. Dev Biol. 2008;324(2):192–201. 10.1016/j.ydbio.2008.08.025 [DOI] [PubMed] [Google Scholar]
- 30.Campbell G, Tomlinson A. The roles of the homeobox genes aristaless and Distal-less in patterning the legs and wings of Drosophila. Development. 1998;125(22):4483–93. [DOI] [PubMed] [Google Scholar]
- 31.Del Signore SJ, Hayashi T, Hatini V. odd-skipped genes and lines organize the notum anterior-posterior axis using autonomous and non-autonomous mechanisms. Mech Dev. 2012;129(5–8):147–61. 10.1016/j.mod.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nitta KR, Jolma A, Yin Y, Morgunova E, Kivioja T, Akhtar J, et al. Conservation of transcription factor binding specificities across 600 million years of bilateria evolution. Elife. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H, Hu G, Wang H, Sciavolino P, Iler N, Shen MM, et al. Heterodimerization of Msx and Dlx homeoproteins results in functional antagonism. Mol Cell Biol. 1997;17(5):2920–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wehn A, Campbell G. Genetic interactions among scribbler, Atrophin and groucho in Drosophila uncover links in transcriptional repression. Genetics. 2006;173(2):849–61. 10.1534/genetics.105.055012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldstein RE, Cook O, Dinur T, Pisanté A, Karandikar UC, Bidwai A, et al. An eh1-like motif in odd-skipped mediates recruitment of Groucho and repression in vivo. Mol Cell Biol. 2005;25(24):10711–20. 10.1128/MCB.25.24.10711-10720.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77(1):51–9. [DOI] [PubMed] [Google Scholar]
- 37.del Alamo D, Mlodzik M. Self-modulation of Notch signaling during ommatidial development via the Roughened eye transcriptional repressor. Development. 2008;135(17):2895–904. Epub 008 Jul 24. 10.1242/dev.022194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benassayag C, Plaza S, Callaerts P, Clements J, Romeo Y, Gehring WJ, et al. Evidence for a direct functional antagonism of the selector genes proboscipedia and eyeless in Drosophila head development. Development. 2003;130(3):575–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A-B) Late L3 leg discs (distalmost confocal views) expressing either the LAEwt-GFP (A) or LAEΔ2-GFP (B) reporter. Merged GFP fluorescence (green) and Bowl immunostaining (magenta) are shown. Boxed areas are magnified for the merged markers in (A’) and (B’), as well as GFP expression in isolation in (A”) and (B”), respectively. While LAEwt-GFP fluorescence abuts the Bowl+ domain at the ts5-pt boundary, its mutant derivative ΔCR2 is specifically up-regulated in many ts5 cells and remained repressed in the raw of bowl-expressing pretarsal cells (magenta arrows). (C-D) Late L3 leg discs (distalmost confocal views) expressing the LAEm3-RFP reporter. Merged GFP fluorescence (green) and C15 (C) or Bowl (D) immunostaining (magenta) are shown. Boxed areas are magnified for the merged markers in (C’) and (D’), as well as GFP expression in isolation in (C”) and (D”), respectively. Note that C15-expressing pretarsal (pt) cells abut GFP-expressing ts4-5 cells [white bracket in (C”)], indicating that Bowl-insensitive LAEm3-GFP is still repressed in the developing pretarsus, including in a row of Bowl-expressing cells [white bracket in panel (D”)].
(TIF)
(A-B) CR2 (A) and 3’-neighboring region of CR2 (B) sequences of 27 dipterans are aligned. Drosophilidae, C. capitata, G. morsitans and M. domestica LAE-like sequences were identified though BLAST analyses using the Trace archive nucleotide blast server at NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi) and were aligned with the D. melanogaster LAE sequence using MAFFT (http://mafft.cbrc.jp/alignment/server/index.html). Homology shading was performed using BoxShade (http://www.ch.embnet.org/software/BOX_form.html). Bowl BS positions are indicated by blue dashed boxes. The near-palindromic site within CR2 is indicated by an orange dashed box.
(TIF)
(A-B) Late L3 late discs from wild-type (A) or C152 homozygous mutant (B) expressing LAE-RFP. Merged RFP fluorescence (red) and Bowl immunostaining (magenta) are shown, as well as the latter in isolation, in (A’) and (B’). It is noteworthy that bowl expression at the ts5/pretarsal (pt) boundary [yellow arrows in (A) and in (A’)] is no longer detected in the C15 loss-of-function developing distal leg, while remaining unaffected at the tibial (ti)/ts1 boundary [white arrows in (B) and (B’)].
(TIF)
(A) C15 homeodomain binds distinct A/T-rich motifs within the LAE CR1 sequence. EMSA experiment with retarded complexes obtained from purified GST-C15HD and unfused GST as a negative control. A non-specific retarded complex is indicated by asterisks. See Fig 4B for probed CR1 sequences. (B) The C15 repressor binding sites within CR1 are critical for LAE activation in vivo. GFP (green) and RFP (red) fluorescence are shown for late L3 leg discs expressing LAEwt-GFP together with wild-type (upper panels) or mutated (lower panels) LAE-RFP constructs. The F2LAES3+4+6m construct mutated for both C15 binding sites within CR1 (identical to the CR1S3+4+6m DNA probe depicted in B; lane 10) does not expressed RFP within the leg discs, suggesting that the S3-4 and S6 motifs encompass activating sequences.
(TIF)
Mosaic late L3 leg discs expressing LAE-GFP (A-C) or LAE–RFP (D-E) and harboring either rotund null clones (detected by the loss of RFP) (A-C) or FO clones (specifically expressing GFP) overexpressing the Rn protein (D-E). Merged RFP fluorescence (red), GFP fluorescence (green) and Dll (A, D), Bowl (B, E) or C15 (C) immunostaining (magenta) are shown, as well as the latter and GFP or RFP markers in isolation, in (A’-E’) and (A”-E”), respectively. Mitotic clones are circled with white dashed lines. In striking contrast to LAE-RFP (specifically affected in ts1-2 rings, white arrows), Dll, bowl and C15 expression remained unchanged in rn null clones. Similarly, misexpressed Rn TF never detectably affected Dll, bowl nor C15 (see Fig 6D) expression, while LAE-RFP was slightly up-regulated in ts3-5 FO clones (white arrow).
(TIF)
Mosaic late L3 leg discs expressing LAE-RFP and harboring FO clones (specifically expressing GFP) ectopically-expressing C15 (A), Bowl (B-C) or Dll (D-E). Merged markers and Bowl, C15 or Rn immunostaining (magenta) are shown, as well as LAE-RFP fluorescence in isolation. FO mitotic clones are circled with white dashed lines and some are indicated with white or yellow arrows. Lateral confocal views are shown in (A) and (B) with distalmost pretarsal cells on the left and right side, respectively. Note that ectopically-expressed C15 protein down-regulated bowl expression at the pt-ts5 boundary, while the converse was not observed (yellow vs white arrows). As expected LAE-RFP was autonomously extinguished in Lines-depleted tarsal FO cells (C”), but C15 protein was never detected there (C’) (yellow arrows), thus discarding the possibility that C15 misexpression contributes to LAE-RFP repression by ectopically-stabilized nuclear Bowl. Lastly, whereas rn remained unaffected in all Dll-misexpressing FO clones (E’, yellow arrow), a faint cell-autonomous LAE-RFP up-regulation was observed (albeit not in all cells) in some proximalmost clones (E”, yellow arrow), indicating that misexpressed Dll is sufficient to activate the LAE in proximal cells, as shown in C15-expressing distal cells (Fig 6C).
(TIF)
Mosaic late L3 leg discs expressing LAE-RFP (A, B, D) or LAE-GFP (C) and harboring loss- or gain-of-function clones for bowl (A, B) or C15 (C-D). Merged RFP fluorescence (red), GFP fluorescence (green) and Dll immunostaining (magenta) are shown, as well as the Dll and RFP or GFP markers in isolation, in (A’-D’) and (A”-D”), respectively. Mitotic clones are circled with white dashed lines. In striking contrast to bab2 reporters, Dll expression was never detectably affected in any mosaic discs (white arrows). As expected, LAE reporter activity was down-regulated in FO clones misexpressing Bowl and C15 proteins. LAE-RFP up-regulation was observed in all bowl mutant cells, provided that they are located within the Dll-expressing cells (compare A’ and A”, white vs yellow arrows).
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.