Abstract
The Drosophila glucoside xylosyltransferase Shams xylosylates Notch and inhibits Notch signaling in specific contexts including wing vein development. However, the molecular mechanisms underlying context-specificity of the shams phenotype is not known. Considering the role of Delta-Notch signaling in wing vein formation, we hypothesized that Shams might affect Delta-mediated Notch signaling in Drosophila. Using genetic interaction studies, we find that altering the gene dosage of Delta affects the wing vein and head bristle phenotypes caused by loss of Shams or by mutations in the Notch xylosylation sites. Clonal analysis suggests that loss of shams promotes Delta-mediated Notch activation. Further, Notch trans-activation by ectopically overexpressed Delta shows a dramatic increase upon loss of shams. In agreement with the above in vivo observations, cell aggregation and ligand-receptor binding assays show that shams knock-down in Notch-expressing cells enhances the binding between Notch and trans-Delta without affecting the binding between Notch and trans-Serrate and cell surface levels of Notch. Loss of Shams does not impair the cis-inhibition of Notch by ectopic overexpression of ligands in vivo or the interaction of Notch and cis-ligands in S2 cells. Nevertheless, removing one copy of endogenous ligands mimics the effects of loss shams on Notch trans-activation by ectopic Delta. This favors the notion that trans-activation of Notch by Delta overcomes the cis-inhibition of Notch by endogenous ligands upon loss of shams. Taken together, our data suggest that xylosylation selectively impedes the binding of Notch with trans-Delta without affecting its binding with cis-ligands and thereby assists in determining the balance of Notch receptor’s response to cis-ligands vs. trans-Delta during Drosophila development.
Author summary
One of the key mechanisms used by neighboring cells to communicate with each other in animals is signaling through an evolutionarily conserved receptor family called Notch. Binding of Notch to ligands of the Delta/DLL and Serrate/JAG families from neighboring cells leads to activation of the signaling pathway and is called trans-activation. In contrast, interaction of Notch with ligands expressed in the same cell has an inhibitory effect on signaling and is known as cis-inhibition. The balance between trans- and cis- interactions ensures optimum Notch signaling in some contexts during animal development. We have used the model organism Drosophila (fruit fly) to decipher the mechanism through which a carbohydrate residue, xylose, regulates Notch signaling in specific contexts. We provide evidence that addition of xylose residues to the Notch receptor decreases its interaction with trans-Delta ligand without affecting its interaction with cis-ligands. Thereby, xylose tunes the Notch pathway by modulating the balance between Notch trans-activation by Delta and Notch cis-inhibition by same-cell ligands. Misregulation of Notch signaling causes a number of human diseases including cancer and developmental disorders. Therefore, understanding the role of xylosylation in Notch signaling can potentially establish a new framework for therapeutic targeting of this pathway.
Introduction
Notch signaling is a juxtacrine signaling pathway broadly used during animal development and tissue homeostasis [1]. Both Notch and its ligands are type I transmembrane proteins containing multiple epidermal growth factor-like (EGF) repeats, which are involved in Notch-ligand interactions and are the sites of several O-linked sugar modifications [2,3]. Interaction of the Delta/Serrate/Lag-2 (DSL) ligands on the surface of the signal-sending cell and the Notch receptor on the signal-receiving cell initiates Notch trans-activation and results in the induction of Notch downstream targets [4,5]. In contrast, binding of Notch and ligands expressed in the same cell can result in cis-inhibition of the pathway [6–10]. The balance between the cis and trans interactions of Notch with ligands is thought to determine whether each cell assumes a signal-sending or a signal-receiving role with regard to a given ligand [11,12].
The Notch ligands Delta and Serrate function redundantly in several contexts during fly development [13]. However, there are developmental processes in which Delta and Serrate show non-redundant roles [14–17]. For example, although Serrate plays a minor, fully redundant role during wing vein formation, Delta is the ligand primarily involved in wing vein development [13,15,18]. In this context, both trans-activatory and cis-inhibitory interactions between Delta and Notch are important for demarcation of the boundary between vein and intervein tissues [15,18]. Specifically, high levels of Delta in the central provein cells are thought to cis-inhibit Notch and allow the cells to assume the wing vein fate. Delta from central provein cells also trans-activates Notch in neighboring cells to prevent the adoption of vein fate and to establish the wing-intervein boundary. The haploinsufficient wing vein phenotypes exhibited by Notch+/–and Delta+/–animals and their mutual suppression in Notch+/–; Delta+/–double-heterozygous flies [19] further indicate that both cis-inhibition and trans-activation of Notch by Delta are required for proper wing vein formation, and that altering the relative expression levels of these two proteins can tip the balance between cis- and trans-interactions between them. However, it remains to be determined whether other mechanisms exist to regulate this balance.
Sugar modifications of the Notch receptors are known to affect Notch pathway activation at various steps, including folding, trafficking, ligand binding and potentially cleavage [20–22]. For instance, addition of N-acetylglucosamine (GlcNAc) to O-fucose residues on Notch EGF repeats by Fringe proteins differentially regulates the response of Notch to its ligands by regulating Notch-ligand interactions [23–26]. It is noteworthy that Fringe proteins promote the binding of Notch receptors to Delta ligands both in cis and in trans configurations [11] and as such, are not likely to be involved in regulating the balance between these opposing activities of Delta ligands. Another type of Notch sugar modification is the addition of O-glucose onto Notch EGF repeats by the protein O-glucosyltransferase 1 (Poglut1). In Drosophila, Poglut1 is encoded by rumi [27] and promotes Notch activation [27–30]. O-glucosylated EGF repeats also serve as a substrate for the addition of xylose by glucoside xylosyltransferase (GXYLT) enzymes [31,32]. Functional studies of the fly GXYLT, Shams, indicate that addition of xylose onto a specific subset of Notch EGF repeats (EGF16-20) negatively regulates Notch signaling in specific contexts, i.e. wing veins and head bristles [32]. Loss of xylose from Notch results in increased cell surface expression of Notch in the pupal wing but not in third instar larval wing discs [32], suggesting that additional mechanisms underlie the context-specificity of the shams loss-of-function phenotype.
Here, we provide evidence that Notch xylosylation by Shams decreases Delta-mediated trans-activation of Notch by reducing the binding of Notch to trans-Delta without affecting the binding of Notch to cis-ligands. The effect of loss of Shams on trans-activation of Notch by overexpressed Delta can be mimicked by decreasing the level of endogenous cis-ligands, suggesting that upon loss of Notch xylosylation, Delta trans-activation overcomes Notch cis-inhibition by ligands. Altogether, our observations indicate that Shams regulates the balance between trans-activation and cis-inhibition of Notch by Delta to ensure optimal Notch activation in several contexts during Drosophila development.
Results
Increased gene dosage of Delta enhances the wing vein loss upon lack of Notch xylosylation
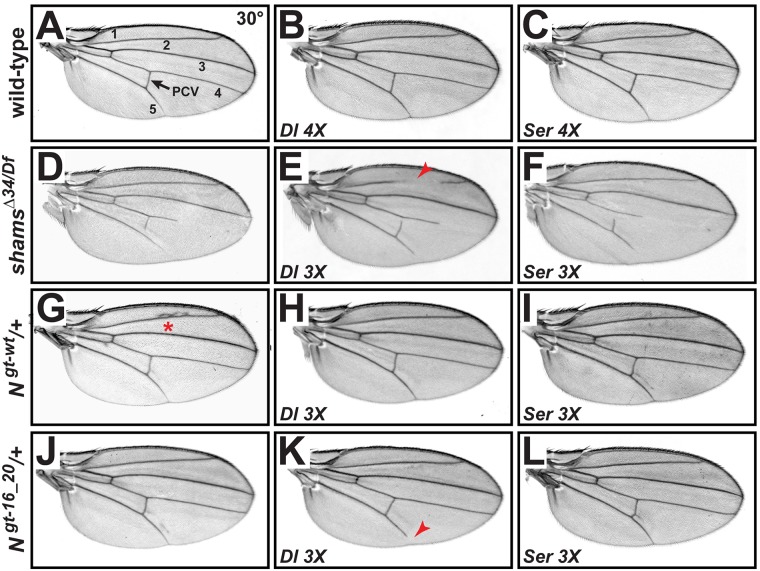
To assess the role of Delta in the wing vein loss phenotype observed in shams mutants, we performed gene-dosage experiments using Delta genomic rescue transgenes [11]. Providing two additional genomic copies (4X) of Delta in a wild-type background does not generate any adult wing phenotypes at 30°C (Fig 1A and 1B) or at room temperature [11]. The absence of phenotype is likely due to a simultaneous increase in the level of cis-inhibition and trans-activation of Notch upon increasing ligand levels. As previously reported, loss of shams results in a temperature-sensitive loss of distal part of adult wing veins L4 and L5 and a partial loss of the posterior cross-vein (Fig 1D) [32]. In a shamsΔ34/Df null background, providing one additional copy of Delta results in a fully penetrant, partial loss of wing vein L2 in addition to L4, L5 and posterior cross vein (Fig 1E), suggesting that shams mutants are sensitive to increased Delta levels compared to control animals. We performed similar genetic interaction experiments in flies harboring wild-type Notchgt-wt or xylosylation-deficient Notchgt-16_20 genomic transgenes [28]. Increasing the gene dosage of Delta does not result in wing vein loss in N+/+; Ngt-wt/+ animals, which have three copies of the wild-type Notch (Fig 1G and 1H). However, providing an additional copy of Delta in N+/+; Ngt-16_20/+ animals results in a partially penetrant loss of the distal wing vein L5 (Fig 1J and 1K), which resembles the shams mutant phenotype at 25°C [32]. Together, these data indicate that Notch signaling in shams mutants is sensitive to Delta levels and support the hypothesis that lack of Notch xylosylation affects Delta-mediated signaling. We also examined the effects of a Serrate transgene in similar experiments. Providing two additional copies of Serrate does not generate any wing vein loss in a wild-type background (Fig 1C) [11]. Moreover, increasing Serrate gene dosage does not enhance the wing vein loss phenotype in a shamsΔ34/Df null background (Fig 1F). Finally, N+/+; Ngt-wt/+ and N+/+; Ngt-16_20/+ animals do not exhibit wing vein loss upon addition of an extra copy of Serrate (Fig 1I and 1L). These results indicate that in the context of wing vein formation, lack of xylosylation does not render Notch sensitive to Serrate levels.
Fig 1. Shams inhibits Notch activation in response to increased levels of Delta but not Serrate.
All wings are from females raised at 30°C. (A) Adult wing of a wild-type fly. Wing veins are numbered. The arrow marks the posterior cross-vein (PCV). (B,C) Adding two additional genomic copies of Delta (Dlgt-wt) (B) or Serrate (Sergt-wt) (C) does not affect adult wings. (D) shamsΔ34/Df mutants partially lose L4 and L5 wing veins. (E) Dlgt-wt/+ shamsΔ34/Df animals have a partial loss of L2 vein (red arrow), which is not observed in shamsΔ34/Df animals. (F) Sergt-wt/+ shamsΔ34/Df wings are indistinguishable from shamsΔ34/Df wings (compare to D). (G) Providing an additional genomic copy of Notch (Ngt-wt) induces extra wing vein material at the L2 (red asterisk) and occasionally at the L5 wing vein indicative of Confluens phenotype. (H) Ngt-wt/+; Dlgt-wt/+ animals exhibit no wing vein defects (compare to G). (I) Ngt-wt/+; Sergt-wt/+animals do not show extra vein around L2 but occasionally show extra wing vein near L5. (J) Providing an additional genomic copy of Notch lacking the functional xylosylation sites (Ngt-16_20) induces extra wing vein material near the L5 wing vein. (K) Ngt-16_20/+; Dlgt-wt/+animals exhibit a partial loss of L5 (71% penetrant, n = 17). (L) Ngt-16_20/+; Sergt-wt/+ wings are indistinguishable from Ngt-16_20 wings (n = 15) (compare to J).
Removing one copy of Delta suppresses the loss of wing vein and head bristles in shams mutants
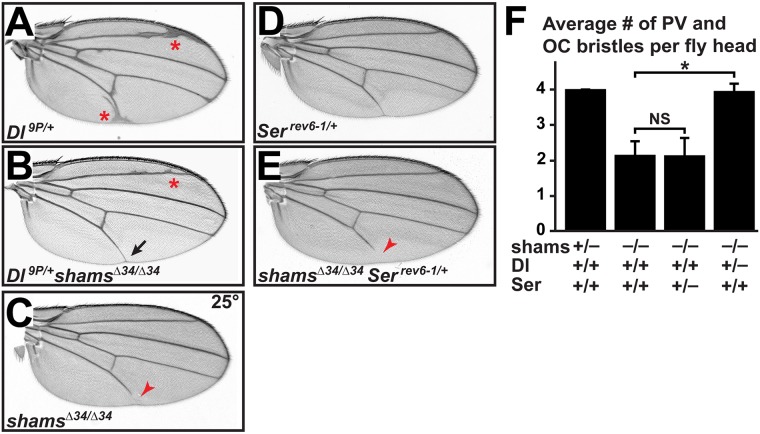
Genetic interaction experiments were performed to examine the effect of decreasing Delta levels on the shams mutant phenotypes. Loss of one copy of Delta in Delta9P/+ (Dl9P/+) animals results is extra wing vein material (Fig 2A) [33]. When one copy of Delta is removed in a shamsΔ34/Δ34 background, the shams mutant wing vein loss is completely suppressed, and the extra wing vein phenotype of Dl9P/+ is partially suppressed (Fig 2B and 2C). We have previously reported that loss of shams also results in the loss of post-vertical (PV) and ocellar (OC) bristles in the adult head [32]. Genetic interaction studies indicate that removing one copy of Delta in shams mutants rescues the loss of head bristles (Fig 2F) similar to the wing vein loss phenotype. Together, these observations support the notion that the shams loss-of-function phenotypes are due to increased Delta-mediated signaling. We also examined the effect of decreasing Serrate levels on the above-mentioned phenotypes (loss of wing vein and head bristles). Removing one copy of Serrate does not affect the loss of wing vein and head bristles in shams mutants (Fig 2D–2F). These observations indicate that altered Serrate-mediated signaling is not likely to contribute to shams loss-of-function phenotypes. Surprisingly, removing one copy of Serrate in shamsΔ34/Δ34 mutant animals results in wing margin loss in some animals (S1A Fig; 21% penetrant, n = 73), which resembles the loss-of-function mutants of Notch and Serrate. Adding one copy of a Serrate genomic rescue transgene [11] fully rescues the wing margin phenotype without affecting the wing vein loss caused by the loss of shams (S1B Fig), indicating that the observed wing margin loss is indeed due to decreased Serrate levels in a shams mutant background. This indicates that in the wing margin, shams might play a redundant role which is revealed when one copy of Serrate is removed. Thus, a role for Shams in Serrate-mediated Notch signaling remains a distinct possibility in the context of wing margin formation.
Fig 2. Removing one copy of Delta suppresses the loss of wing vein and head bristles in shams mutants.
(A-F) All animals raised at 25°C. (A) Adult wing from a Dl9P/+ animal. Note the wing vein thickening and extra wing vein material (red asterisks). (B) Dl9P/+ shamsΔ34/Δ34 adult wings show a suppression of the vein thickening and extra wing veins present in Dl9P/+ mutants (compare to A) and the wing vein loss (black arrow) present in shamsΔ34/ Δ34 mutants (red arrowhead in C). (D) Serrev6-1/+ adult wings do not exhibit any defects. (E) shamsΔ34/Δ34 Serrev6-1/+animals exhibit wing vein loss similar to shamsΔ34/Δ34 mutants (compare to C). (F) Shown is the average number of post-vertical (PV) and ocellar (OC) bristles in adult heads of the indicated genotypes. shamsΔ34/+ animals have a total of 4 PV and OC bristles, similar to wild-type animals. Removing one copy of Delta but not Serrate restores the head bristles in shams mutant animals. Error bars are standard error of mean. *P<0.001, NS: not significant.
Loss of shams enhances Delta-mediated Notch activation in Drosophila wing tissue
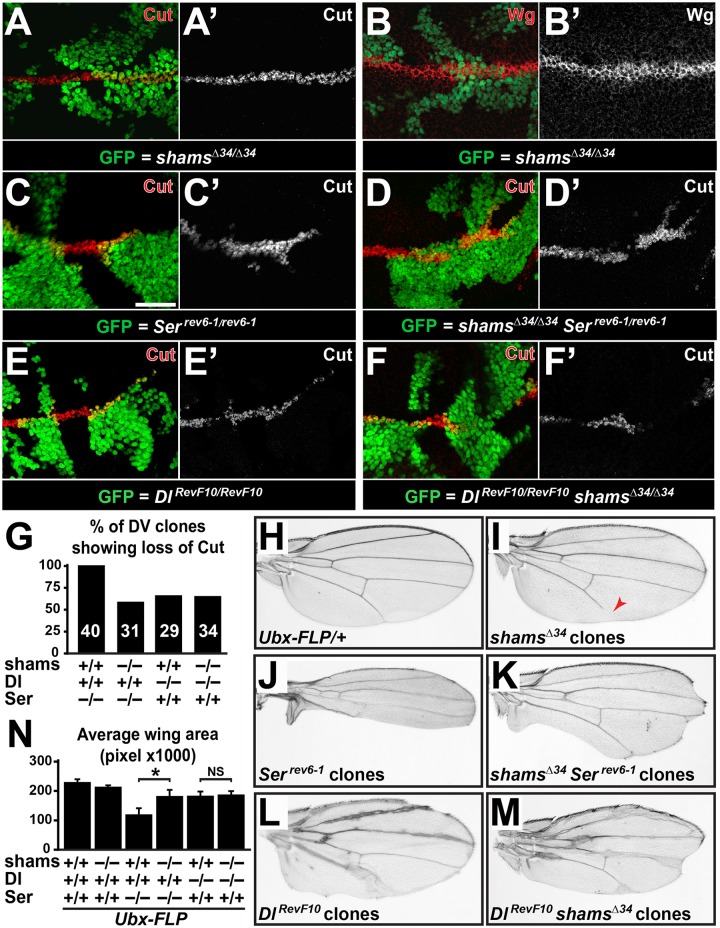
To examine the effects of loss of shams on the Notch activity in the wing imaginal disc, we performed clonal analysis in the 3rd instar larval wing discs by using the MARCM system [34]. We expressed Flippase with an Ultrabithorax enhancer (Ubx-FLP), which induces FRT-mediated recombination in the wing imaginal discs with a high efficiency [27,35]. We first examined the effect of loss of shams on Notch activity by staining for Notch targets Cut and Wingless (Wg) in shamsΔ34 mutants clones. These mutant clones do not exhibit clear abnormalities in the expression of Cut and Wg when compared to adjacent wild-type or heterozygous cells (Fig 3A–3B’). However, slight broadening observed in Cut and Wg expression in shamsΔ34 mutants clones supports a mild increase in Notch activation (Fig 3A–3B’). To further assess the effect of loss of shams on ligand-mediated Notch activity, we examined Cut expression in single-mutant clones for ligands (Serrev6-1 and DlRevF10) and double-mutant clones for ligands and shams (shamsΔ34 Serrev6-1 and DlRevF10 shamsΔ34). To analyze the effects of loss of shams on each ligand without interference from the other ligand, we focused on mutant clones which cross the dorsal-ventral (DV) boundary. Serrev6-1 clones show a highly penetrant loss of Cut, except for the cells that directly abut the wild-type tissue (Fig 3C, 3C’ and 3G; n = 40). This observation indicates that when cells around the DV boundary lack Serrate, they fail to activate the Notch target Cut despite the presence of Delta in the clones. In contrast, Cut-positive cells non-adjacent to the clone boundary are present in 42% of shamsΔ34 Serrev6-1 double-mutant clones which cross the DV boundary (Fig 3D, 3D’ and 3G; n = 31). Since Delta is the only ligand remaining in these clones, these observations are in agreement with the conclusion that loss of Shams promotes Delta-mediated Notch activation. Next, we performed a similar analysis on Delta single-mutant and Delta shams double-mutant clones, where Serrate is the only remaining ligand. DlRevF10 single-mutant clones crossing the boundary showed a ~65% penetrant loss of Cut expression (Fig 3E, 3E’ and 3G; n = 29). Similarly, DlRevF10 shamsΔ34 double-mutant clones which cross the DV boundary exhibited a ~64% penetrant loss of Cut expression (Fig 3F, 3F’ and 3G; n = 34). The comparable loss of Cut expression in DlRevF10 and DlRevF10 shamsΔ34 clones suggests that loss of shams does not alter the activation of Notch by Serrate, which is the only remaining ligand inside these clones.
Fig 3. Simultaneous loss of shams partially rescues Notch signaling in Serrate clones spanning the DV boundary, where Delta is the only ligand.
(A-N) All animals raised at 25°C. (A-F’) Third instar wing imaginal discs with mutant cells marked by the presence of GFP. Scale bar in (C) is 25 μm and applies to A-F’. Dorsal is up, anterior to the left. (A-B’) shamsΔ34 mutant clones do not seem to show altered Cut (A-A’) and Wingless (Wg) (B,B’) expression domains at the developing wing margin except a slight broadening in clone area compared to wild-type. (C,C’) Serrev6-1 mutant clones lack Cut expression (red) except for cells directly adjacent to wild-type cells (number of clones n = 40). (D,D’) shamsΔ34 Serrev6-1 double-mutant clones often express Cut in cells at the clonal boundary and within the mutant clones (number of clones n = 31). (E-F’) Comparable lack of Cut expression in DlRevF10 mutant clones (E,E’; number of clones n = 29) and DlRevF10 shamsΔ34 double mutant clones (F,F’; number of clone n = 34). (G) Graph represents % of mutant clones crossing the DV boundary which show loss of Cut expression in indicated genotypes. Number of clones scored for each genotype is shown. (H-M) Adult wings harboring the indicated single or double-mutant clones generated by the Ubx-FLP transgene. Clones are not marked. (N) Graph represents average wing area (n = 13 for each genotype) in the indicated genotypes. Error bars are standard error of mean. *P<0.05, NS: not significant.
In addition to wing disc stainings, we examined the effects of shamsΔ34, Serrev6-1 and DlRevF10 single-mutant and shamsΔ34 Serrev6-1 and DlRevF10 shamsΔ34 double-mutant clones on the adult wing size. Although the clones in these adult wings are not marked, inspection of 3rd instar wing imaginal discs indicates that Ubx-FLP induces sizeable clones in 100% of the discs. Adult wings harboring shamsΔ34 clones do not exhibit any wing margin phenotypes, although some animals show wing vein loss (Fig 3I). Adult wings harboring clones of the Serrev6-1 allele show severe wing scalloping (Fig 3J), in agreement with previous reports on this allele and other Serrate null alleles [36–38]. However, the degree of scalloping in adult wings harboring shamsΔ34 Serrev6-1 double-mutant clones is significantly less than that of the wings harboring Ser rev6-1 clones (Fig 3K). Indeed, the average area of the wings harboring Serrate clones was significantly less than that of the wings harboring Serrate shams double-mutant clones (Fig 3N; P<0.05; n = 13 for each genotype). In contrast, the average area of wings harboring DlRevF10 clones was not significantly different from the wings harboring DlRevF10 shamsΔ34 double mutant clones (Fig 3L–3N; P>0.05; n = 13 for each genotype). These results are in agreement with the data obtained from clonal analysis in larval wing discs and further support the notion that loss of shams partially suppresses the phenotypes caused by the loss of Serrate, likely by enhancing the Delta-mediated Notch activation in the developing wing.
Loss of Shams augments trans-activation of Notch by ectopic Delta
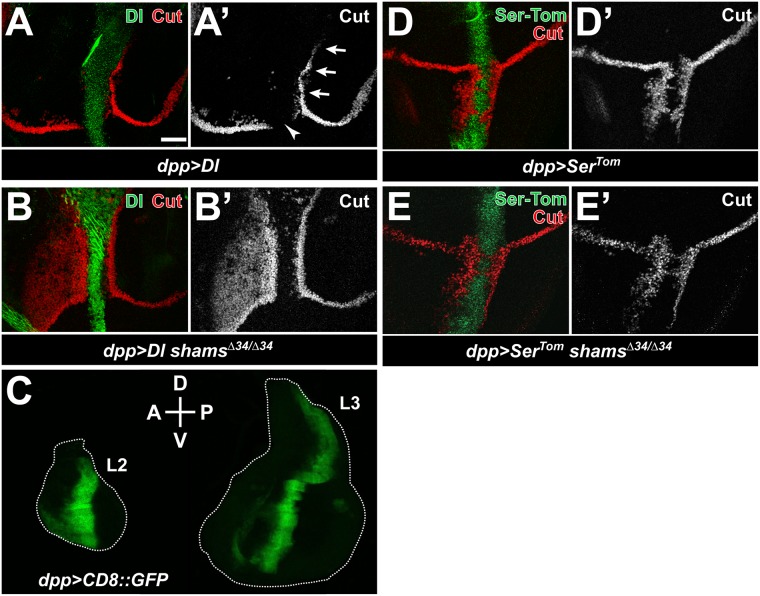
In some cell types, the decision whether Notch signaling is activated or inhibited is determined based on the relative levels of Notch trans-activation and cis-inhibition by its ligands [6–8]. Given the complex interplay between trans and cis functions of ligands and potential feedback mechanisms, we used the GAL4-UAS system [39] to overexpress Delta or Serrate along the anterior-posterior boundary of the developing 3rd instar wing imaginal discs and assessed the effects of loss of shams on the cis and trans effects of each ligand on Notch. When raised at 25°C, animals overexpressing Delta within the Dpp expression domain (dpp>Dl) exhibit two phenotypes in wing imaginal discs: loss of the Cut-positive cells and disruption of the endogenous wing margin within the Dpp expression domain due to cis-inhibition (Fig 4A and 4A’; arrowhead), and ectopic Cut-positive cells that flank the Dpp expression domain in the dorsal-posterior quadrant due to trans-activation (Fig 4A and 4A’; arrows). In a shamsΔ34/Δ34 mutant background, there is no obvious effect on cis-inhibition within the Delta-expressing stripe (Fig 4B and 4B’). However, we observe broad ectopic activation of Cut in a significant portion of the dorsal-anterior quadrant (Fig 4B and 4B’). These observations support the notion that loss of Shams promotes Delta-mediated trans-activation of Notch without affecting cis-inhibition. Of note, in late 2nd instar wing discs, the dpp-GAL4 driver shows a much broader anterior expression domain compared to 3rd instar wing discs (Fig 4C), providing a likely explanation for the wide Cut expression domain observed in the dorsal-anterior quadrant of dpp>Dl shamsΔ34/Δ34 3rd instar discs.
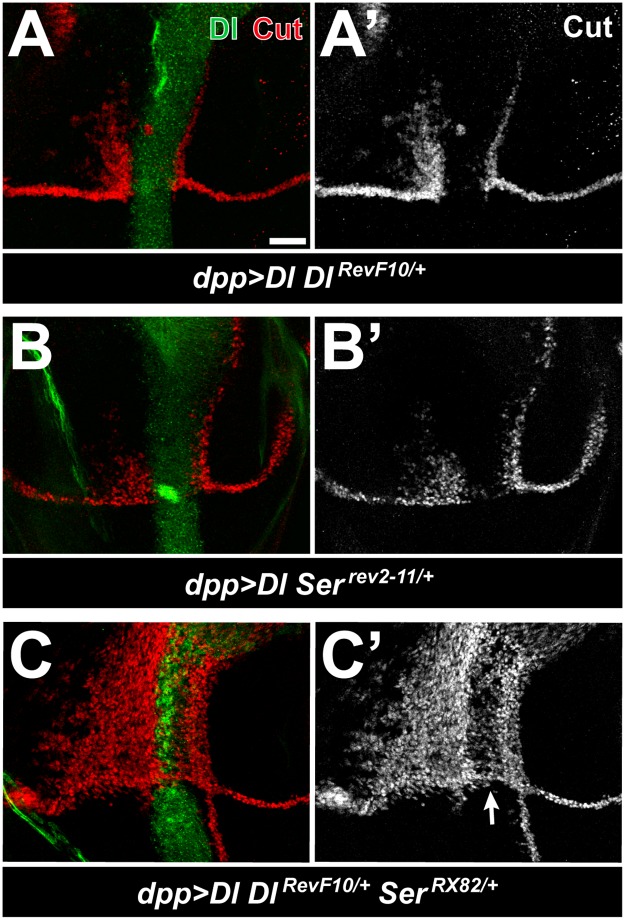
Fig 4. Loss of Shams augments trans-activation of Notch by ectopic Delta.
(A-D’) Third instar wing imaginal discs raised at 25°C. Staining with anti-Delta antibody and Tomato fluorescence from the SerrateTom hybrid protein are shown in green; Cut staining is shown in red. Anterior is to the left, and dorsal is up. Scale bar in (A) is 25 μm and applies to all panels except C. (A-A’). Expression of Delta using dpp-GAL4 (dpp>Dl) in a wild-type background. Arrows and the arrowhead indicate trans-activation and cis-inhibition, respectively. (B,B’) In a shamsΔ34/Δ34 background, dpp>Dl induces broad activation of Cut anterior to the Dpp domain. (C) Wing imaginal discs from dpp>CD8::GFP second (L2) and third (L3) instar larvae are shown. Note the anterior broadening of the CD8::GFP signal in L2 compared to L3 disc. (D-D’) Expression of SerrateTom using dpp-GAL4 (dpp>SerTom) in a wild-type background. (E-E’) Loss of shams does not alter Cut expression induced by dpp>SerTom.
To determine the effects of loss of Notch xylosylation on Serrate-mediated trans-activation and cis-inhibition of Notch, we performed similar overexpression experiments using a Tomato-tagged Serrate transgene (UAS-SerTom) [40]. As expected, overexpression of SerTom results in cis-inhibition of Notch at the endogenous wing margin region and trans-activation of Notch outside of the Dpp expression domain in the ventral compartment (Fig 4D and 4D’). In contrast to dpp>Dl, no changes in Cut activation in or outside of the Dpp expression domain was observed between dpp>SerTom and dpp>SerTom shamsΔ34/Δ34 animals (Fig 4D–4E’). These data indicate that in the developing wing disc, loss of Shams does not affect the trans-activation and cis-inhibition of Notch mediated by overexpressed Serrate.
Notch trans-activation by dpp>Dl overcomes its cis-inhibition by endogenous ligands in shams mutants
Given the dramatic increase in the ability of dpp>Dl to trans-activate Notch upon loss of Shams, we decided to further explore the mechanism that prevents dpp>Dl from activating Notch in a shams+/+ background. In the sensory bristle lineage, decreasing the endogenous levels of Delta enhances the trans-activation of Notch by overexpressed Delta [9]. Accordingly, we examined the effects of removing one copy of endogenous Delta and/or Serrate on the expression of Cut in dpp>Dl larvae. In a DlRevF10 or Serrev2-11 heterozygous background, dpp>Dl induces moderate levels of Notch trans-activation in the dorsal-anterior quadrant (Fig 5A–5B’, compare to Fig 4A and 4A’). Moreover, in a DlRevF10/+ SerRX82/+ double-heterozygous background, dpp>Dl results in massive induction of Cut in the dorsal-anterior quadrant, broadening of the Cut expression domain in the dorsal-posterior quadrant, and appearance of Cut-expressing cells in the ventral-posterior compartment (Fig 5C and 5C’). In addition, Delta-mediated cis-inhibition is significantly decreased, because many cells in the Dpp expression domain express Cut and the endogenous wing margin is restored (Fig 5C and 5C’; arrow). These observations indicate that cis-inhibition of Notch by endogenous ligands potently limits the ability of overexpressed Delta to trans-activate Notch outside of the Dpp domain and cooperates with overexpressed Delta to cis-inhibit Notch inside of the expression domain, in agreement with the previous report by Jacobsen et al [9]. Therefore, loss of shams and decreasing endogenous ligands both result in a similar increase in the ability of dpp>Dl to trans-activate Notch in 3rd instar wing discs, suggesting that in shams mutants, Delta mediated trans-activation of Notch is able to overcome the cis-inhibitory effect of endogenous ligands.
Fig 5. Reducing the level of endogenous ligands increases the trans activity of overexpressed Delta.
(A-C’) Third instar larval wing discs raised at 25°C. Delta is visualized in green and Cut in red. Anterior is to the left, and dorsal is up. Scale bar = 25 μm. (A-B’) In a DlRevF10/+ (A,A’) or Serrev2-11/+ (B,B’) heterozygous background, dpp>Dl induces Cut activation anterior to the Dpp domain (compare to Fig 4A). (C,C’) dpp>Dl Dl+/–Ser+/–animals induce broad expression of Cut anterior to and within the Dpp domain and show some expansion of the ectopic Cut posterior to the Dpp domain. Cut expression in the wing margin is restored (arrow in C’).
Cell-based aggregation and binding assays indicate increased binding between trans-Delta and Notch upon shams knockdown
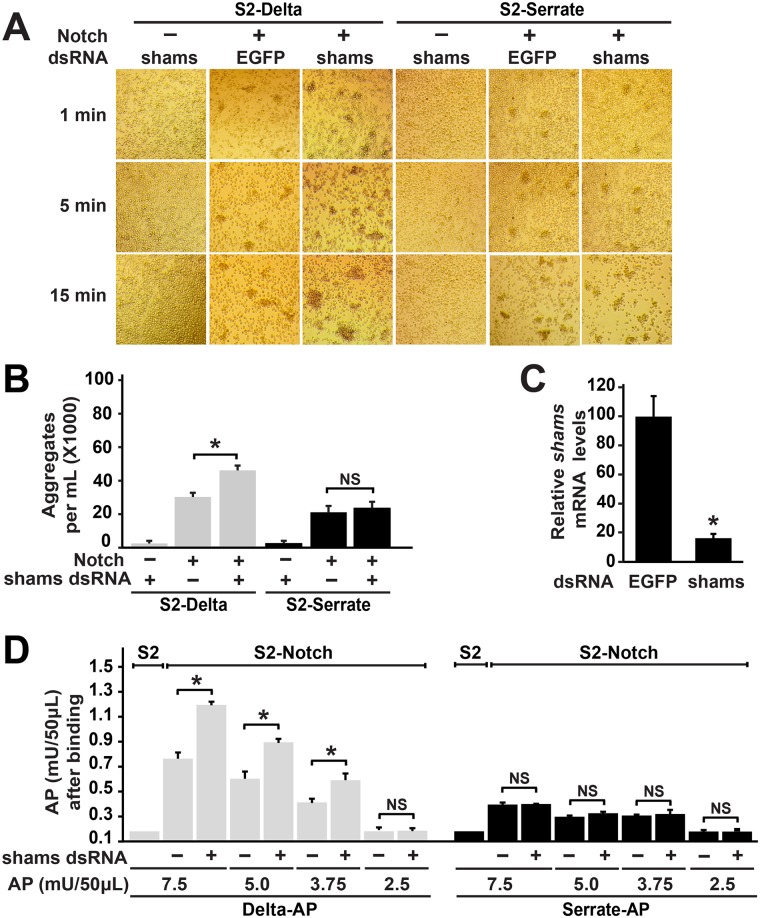
To determine whether the effects of Shams on Delta-mediated signaling can be explained at the level of Notch-ligand binding, we performed S2 co-culture assays and assessed the effects of shams knock-down (KD) on the rate of aggregation formation and size of the aggregates formed between S2-Notch (S2-N) and S2-Delta (S2-Dl) or S2-SerrateTom (S2-SerTom) cells, which are indications for Notch-ligand binding [40,41]. For control experiments, we used plain S2 cells, which do not express Notch and Delta [41], and EGFP dsRNA. When co-cultured with plain S2 cells treated with shams dsRNA, S2-Dl cells form small aggregates which do not increase with time (Fig 6A). Co-culture of S2-Dl and control S2-N cells for one minute resulted in the formation of small aggregates, which increased in number and size as time elapsed (Fig 6A). Co-culture of S2-Dl with Shams KD S2-N cells formed much larger cell aggregates at 5 and 15 minutes (Fig 6A).Quantification of cell aggregates containing more than 6 cells at 5 minutes of co-culture showed that the number of aggregates was significantly higher when S2-N cells were incubated with shams dsRNA (Fig 6B; P<0.01). qRT-PCR experiments indicate that shams dsRNA decreases shams mRNA levels greater than 80% in S2 cells (Fig 6C). These observations suggest that Shams decreases the binding between Notch and Delta in trans. Of note, co-culture experiments between S2-SerTom and S2-N cells showed that the size and the number of the S2-SerTom/S2-N aggregates did not change when shams levels were decreased (Fig 6A and 6B). These observations suggest that Shams KD does not affect the binding between Notch and trans-Serrate.
Fig 6. Cell-based aggregation and binding assays indicate increased binding between trans-Delta and Notch upon shams knockdown.
(A) Representative images of co-culture assays between the indicated cell types at 1, 5 and 15 minutes are shown. (A) Shams KD in S2-N cells enhances their aggregation with S2-Dl cells but not with S2-Ser cells. (B) Quantification of number of cell aggregates greater than 6 cells after 5 minutes of co-culture. Error bars indicate standard error. Shams KD in S2-N cells increases the number of aggregates with S2-Dl cells but not with S2-Ser cells. *P<0.01, NS: not significant. (C) Relative shams mRNA levels are measured by qRT-PCR in S2 cells treated with either the control EGFP dsRNA or shams dsRNA. *P<0.01. (D) Graph shows the average AP (Delta-AP or Serrate-AP) units bound to S2 cells (control) and S2-N cells (treated with EGFP or shams dsRNA). Error bars indicate standard error. Shams KD in S2-N cells increases binding with Delta-AP but not with Serrate-AP. *P<0.01, NS: not significant.
To more directly assess the effects of Shams KD on Notch-ligand binding, we used a quantitative receptor-ligand binding assay [23,42]. We incubated control and Shams KD S2-N cells with various concentrations of alkaline phosphatase (AP)-tagged ligand extracellular domains (Delta-AP or Serrate-AP) and asked whether Shams KD alters the binding of each ligand to S2-N cells in terms of the AP activity. Binding of each ligand-AP to plain S2 cells, which do not express Notch [41], was used as control. In agreement with the aggregation assays, treating S2-N cells with shams dsRNA significantly increased the binding of Delta-AP to these cells compared to EGFP dsRNA treated S2-N cells (Fig 6D). However, the amount of Serrate-AP bound to Shams KD and control S2-N cells was comparable at all four Serrate-AP concentrations tested (Fig 6D). These observations indicate that Shams negatively regulates Notch-Delta interaction but does not affect Notch-Serrate interaction.
Binding of Notch with trans-ligands can be affected by cell surface levels of Notch. Recent work has shown that combined loss of O-fucose and xylose residues from Notch alters its trafficking in 3rd instar wing discs, but loss of xylose by itself does not [43]. We have previously reported that loss of Shams or mutations that prevent the addition of xylose-glucose-O glycans to Notch EGF16-20 did not affect cell-surface expression of Notch in the 3rd instar wing discs, but enhanced the cell surface levels of Notch in the developing pupal wing disc [32]. Accordingly, we examined whether shams KD in S2 cells affects the cell surface expression of Notch. Detergent-free immunofluorescent staining of control and shams KD S2-N cells with an antibody against the Notch extracellular domain did not show any significant changes in the surface levels of Notch when shams is decreased (S2A and S2B Fig; n = 40 cells for each groups). Thus, the increased aggregation in Shams KD S2-N/S2-Dl co-cultures is not caused by an increase in Notch surface expression.
Although Serrate does not contain any predicted O-glucosylation sites, one of the Delta EGF repeats harbors the consensus O-glucosylation motif [44]. However, adding shams dsRNA to S2-Dl cells did not alter the size or the number of aggregates formed when cultured with S2-N cells (S3A and S3B Fig). We conclude that shams KD in Delta-expressing cells does not affect Notch-Delta trans-binding and that Shams functions in Notch-expressing cells. We also examined the effect of shams KD on surface distribution of Delta in S2-Dl cells using cell surface immunostaining with an antibody against the extracellular domain of Delta. Shams dsRNA treated S2-Dl cells did not show any significant change in surface level of Delta as compared to EGFP dsRNA treated S2-Dl cells (S3C and S3D Fig; n = 40 cells per group). This observation indicates that shams KD does not affect the surface level of Delta in S2 cells. We also examined whether loss of shams affects surface expression of Delta in vivo. Detergent-free staining of 3rd instar wing imaginal discs did not show any significant difference in surface expression of Delta between shamsΔ34 mutant clones and the neighboring wild-type and heterozygous cells (S3E Fig; n = 12 clones), indicating that Shams does not regulate the surface levels of Delta in 3rd instar wing imaginal discs.
Shams knockdown does not alter the inhibitory effect of cis-ligands on cell aggregation
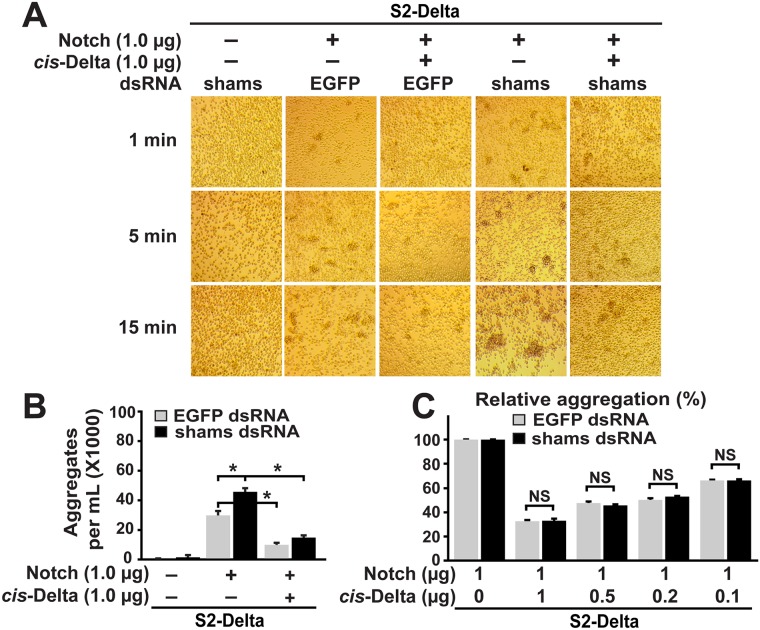
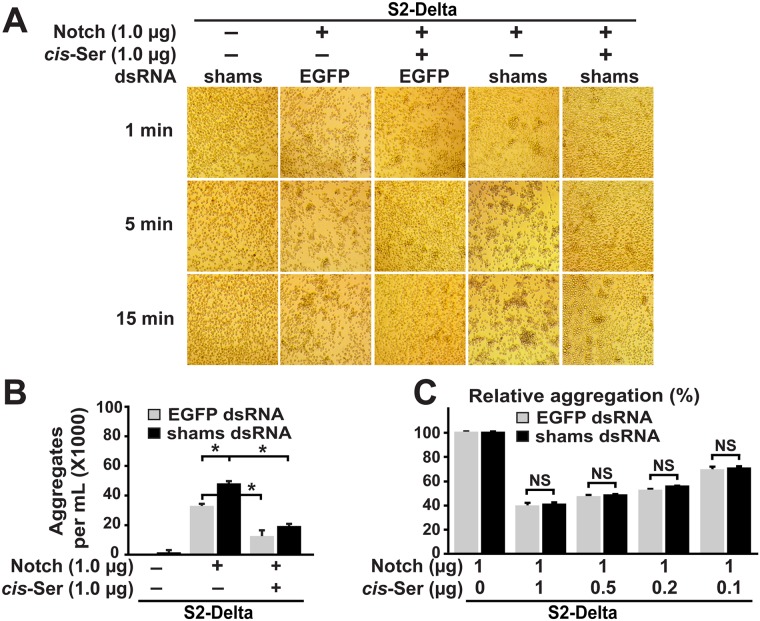
To determine whether Shams modulates binding between Notch and cis-ligands, we asked whether shams KD affects the ability of ligands co-expressed with Notch to decrease the aggregation between the Notch-expressing cells and S2-Dl cells. To this end, we performed aggregation assays between S2-Dl cells and S2 cells transiently transfected with a Notch expression vector as control (S2-Ntransient) or equal amounts of Notch and cis-ligand expression vectors (S2-N&Dltransient or S2-N&Sertransient). The relative aggregation between S2-Dl and S2-Ntransient cells in the presence and absence of cis-ligands was used as an indication for the degree of cis-inhibitory effect of each ligand. For example, if the number of aggregates between S2-Dl and S2-N&Sertransient cells is 25% of the number of aggregates between S2-Dl and S2-Ntransient cells (i.e. relative aggregation 25%), we would conclude that cis-Serrate was able to block the interaction between Notch and trans-Delta by 75%. Co-culture of S2-Dl and control S2-Ntransient cells formed small aggregates, which grew in size and number with time, similar to the co-culture between S2-Dl and stable S2-N cells (Figs 7A and 8A, compare to Fig 6A). The aggregation was dramatically decreased when S2-Dl cells were co-cultured with S2-N&Dltransient or S2-N&Sertransient cells (Figs 7A, 7B, 8A and 8B). This is most likely due to cis-inhibition of Notch by ligands expressed in the same cell, as shown previously in Drosophila and mammalian cell-culture assays [11,40]. Co-culture of S2-Dl cells and shams KD S2-Ntransient cells formed aggregates more quickly and resulted in larger aggregates, recapitulating the results seen with stable S2-N cells (Figs 7A and 8A). Addition of cis-ligands to these cells (shams KD S2-N&Dltransient or S2-N&Sertransient) also decreased their aggregation with S2-Dl cells (Figs 7A, 7B, 8A and 8B). Importantly, quantification of aggregates after five minutes of co-culture showed that the magnitude of this cis-inhibition was comparable to the cis-inhibition observed in co-culture between S2-Dl and control S2-N&Dltransient or S2-N&Sertransient cells, which were incubated with EGFP dsRNA instead of shams dsRNA (Figs 7C and 8C). These observations suggest that shams KD does not diminish the ability of cis-ligands to oppose the binding between Notch and trans-Delta.
Fig 7. Shams knockdown does not alter the inhibitory effect of cis-Delta on cell aggregation mediated by Notch and trans-Delta.
(A) Co-expression of Delta and Notch in a 1:1 ratio in S2 cells significantly decreases their ability to aggregate with S2-Dl cells. Shams KD does not affect the ability of Notch to respond to cis-Delta. (B) Quantification of number of cell aggregates greater than 6 cells after 5 minutes of co-culture. Error bars indicate standard error. *P<0.01. (C) Relative aggregation between S2-Dl cells and S2 cells co-transfected with indicated ratios of Notch and cis-Delta expression constructs. Number of cell aggregates greater than 6 cells after 5 minutes of co-culture were quantified for each combination and shown as a percentage of the number of aggregates between S2-Delta and S2-Ntransient cells in the absence of cis-Delta. Error bars indicate standard error. NS: not significant.
Fig 8. Shams knockdown does not alter the inhibitory effect of cis-Serrate on cell aggregation mediated by Notch and trans-Delta.
(A) Co-expression of Serrate and Notch in a 1:1 ratio in S2 cells significantly decreases their ability to aggregate with S2-Dl cells. Shams KD does not affect the ability of Notch to respond to cis-Serrate. (B) Quantification of number of cell aggregates greater than 6 cells after 5 minutes of co-culture. Error bars indicate standard error. *P<0.01. (C) Relative aggregation between S2-Dl cells and S2 cells co-transfected with indicated ratio of Notch and cis-Serrate expression constructs. Number of cell aggregates greater than 6 cells after 5 minutes of co-culture were quantified for each combination and shown as a percentage of the number of aggregates between S2-Delta and S2-Ntransient cells in the absence of cis-Serrate. Error bars indicate standard error. NS: not significant.
One caveat of this experiment is that the relative level of cis-ligands expressed in these cells might be too high and therefore be able to inhibit trans-Delta/Notch binding irrespective of shams levels. To address this concern, we performed three additional sets of aggregation assays for each cis-ligand and successively decreased the ratio of cis-ligand expression plasmid to the Notch expression plasmid used in each set (0.5:1, 0.2:1, 0.1:1). To ensure similar baseline levels of trans-Dl/Notch binding, we used the same amount of Notch expression plasmid in all experiments and only changed the amount of cis-ligand expression plasmids. Aggregation between S2-Dl and S2-Ntransient cells was once again inhibited by expression of cis-ligands (both cis-Delta and cis-Serrate) even at lowest levels. Quantitation of the number of cell aggregates greater than six cells at five minutes of co-culture showed that the magnitude of inhibition by cis-ligands was concentration-dependent: the lower the ratio of cis-ligand to Notch expression plasmid, the lower the ability of cis-ligands to decrease the number of aggregates between S2-Dl and S2-Ntransient cells (Figs 7C and 8C). These data indicate that the level of cis-ligands used in our assays are not saturating. Under each cis-ligand/Notch ratio, the cis-inhibitory effect of each ligand on S2-Dl/S2-Ntransient aggregation was almost identical in cells treated with EGFP dsRNA and shams dsRNA (Figs 7C and 8C). These observations indicate that shams KD does not decrease the ability of cis-ligands to block the interaction between trans-Delta and Notch, and suggest that Shams does not affect the binding between Notch and cis-ligands. Taken together, these cell culture data support the conclusion that loss of Shams specifically enhances the binding of Notch with trans-Delta without affecting its binding with cis-ligands.
Discussion
Modifications of the extracellular domain of Notch receptor by glycosylation influence its activity in different contexts [20–22]. Xylosylation of the Notch receptor by the glycosyltransferase enzyme Shams has been reported to negatively regulate Notch signaling in Drosophila [32]. Loss of Notch xylosylation is associated with increased Notch expression at the cell surface in pupal wing disc but not in the larval wing imaginal discs [32]. However, the mechanisms underlying the tissue-specific phenotypes of shams and the effects of xylosylation on other steps of Notch signaling such as ligand binding and cis-inhibition are not known. In the present study, we provide several lines of evidence suggesting that the wing vein loss phenotype observed upon loss of Notch xylosylation results from a specific increase in Delta-mediated Notch activation. First, adding one copy of Delta enhances wing vein loss phenotype in shams mutant animals. Second, a Notch transgene with mutations in functional xylosylation sites results in wing vein loss when combined with an extra copy of Delta. Third, removing one copy of Delta suppresses the wing vein phenotype in shams mutant animals. Last, Shams KD in S2-N cells enhances their binding with AP-tagged Delta and their aggregation with S2-Dl cells without affecting cell surface levels of Notch. Since functional xylose residues reside in EGF16-20 of the Notch receptor [32], our work identifies the glycosylation of this domain as a novel mechanism for the modulation of Delta-mediated Notch activation in Drosophila. Although Notch EGF11-12 are required for binding of Notch to both ligands [2], a mutation in Notch EGF8 affects Notch-ligand binding and signaling in a ligand-selective manner [42]. Together with this report [42], our data suggest that distinct EGF repeats other than EGF11-12 are involved in preferential or exclusive modulation of the response of Notch to its ligands. Moreover, the differential effects of decreasing Notch xylosylation on the binding and response of Notch to trans-Delta versus cis-ligands suggest different domains or structural conformations of Notch might be involved in binding to trans- versus cis-ligands.
The increased Delta-mediated signaling upon loss of shams can theoretically be due to increased trans-activation, decreased cis-inhibition, or both. If the primary mechanism for activation of Notch signaling and loss of wing vein in shams mutants were decreased cis-inhibition by Delta, removing one copy of Delta should have enhanced the wing vein loss phenotype in shams mutants. On the contrary, the shams wing vein loss was suppressed upon Delta heterozygosity. Moreover, loss of shams dramatically enhances Notch trans-activation but does not affect Notch cis-inhibition by Delta in dpp>Dl animals, and shams KD promotes S2 cell aggregation mediated by Notch and trans-Delta without decreasing the inhibitory effect of cis-Delta on Notch in these assays. Therefore, although it is still possible that Shams plays a minor role in the interaction of Notch with cis-ligands in certain contexts, our data strongly suggest that the primary mechanism for the wing vein loss in shams mutants is an increase in Notch trans-activation by Delta, not a decrease in Notch cis-inhibition by Delta.
In line with a previous report in the bristle lineage [9], we find that removing one copy of Delta and/or Serrate in dpp>Dl animals results in ectopic activation of Notch in the dorsal-anterior quadrant, indicating that cis-inhibition by endogenous ligands normally opposes the trans-activation of Notch by ectopic Delta in this region. These observations are in agreement with quantitative analyses indicating that the balance between the activity of trans- and cis-ligands determines whether a given cell assumes a signal-receiving state or not [11,12]. A similar increase in Delta-mediated trans-activation of Notch is seen in dpp>Dl animals upon loss of Shams despite the presence of endogenous ligands. Accordingly, we propose that Shams functions to regulate the balance between trans-activation of Notch by Delta and cis-inhibition of Notch by ligands, and that in the absence of shams, trans-activation of Notch by Delta overcomes the cis-inhibitory effects of ligands. Our cell aggregation assays suggest that Shams mediates this role by impeding the ability of Notch to bind Delta in trans, without altering the binding of Notch to cis-ligands. As shown in Fig 1, increasing the gene dosage of Delta by itself or combined with an additional copy of wild-type Notch does not result in wing vein loss, likely because the balance between the Notch and Delta levels and also the balance between the trans and cis activities of Delta are preserved. Based on our model, loss of xylose residues on Notch due to loss of Shams or mutations in biologically-relevant Shams target sites on Notch tips the balance between trans- and cis- activities of Delta in favor of trans-Delta, as evidenced by the net gain of Notch signaling and loss of wing vein in animals with three copies of Delta and simultaneous loss of Notch xylosylation.
Both Shams and Fringe regulate Notch signaling by adding carbohydrate residues to O-linked monosaccharides on Notch EGF repeats and generating disaccharides: xylose-glucose-O in the case of Shams, and GlcNAc-fucose-O in the case of Fringe [23,25,32,45]. Moreover, as shown here for Shams and previously for Fringe [23,46], both proteins regulate Notch-ligand interactions. However, the effects of these enzymes on the binding and response of Notch to Delta versus Serrate and to trans-Delta versus cis-Delta seem to be distinct from each other. Fringe promotes Delta-mediated trans-activation and simultaneously decreases Serrate-mediated trans-activation of Notch [26]. Moreover, it has recently been shown that Fringe proteins affect the trans and cis interaction of Notch with each ligand in the same direction, i.e., they promote Notch-Delta interactions both in cis and in trans, and inhibit Notch-Serrate interaction both in cis and in trans [11]. In contrast, our data indicate that Shams decreases the binding of Notch to and its activation by trans-Delta without affecting its interactions with cis-ligands. Further, our aggregation assays and most of our in vivo observations indicate that binding of Notch to and its activation by trans-Serrate is not significantly affected by Shams, although the appearance of a low-penetrance wing margin loss phenotype in shams–/–Serrate+/–animals suggests that Shams might play a redundant role in Serrate-induced Notch signaling in some contexts. The functional differences between Shams and Fringe likely explain their distinct mutant phenotypes in the wing, i.e. loss of wing vein in the case of shams and wing vein thickening and loss of wing margin in the case of fringe mutants [47]. The distinct roles of Fringe and Shams in regulating Notch signaling along with the differentially distributed EGF repeats with Shams (xylose) versus Fringe (GlcNAc) elongation across the Notch extracellular domain [32,48] suggest that the combined function of the sugar modifications mediated by these enzymes ensures optimal level of Notch pathway activity in several contexts during fly development.
Materials and methods
Drosophila strains and genetics
The following strains were used in this study: y w, y w; D/TM6, Tb1, w; nocSco/CyO, w; nocSco/CyO; TM3, Sb1/TM6, Tb1, dpp-GAL4, Df(3R)BSC494/TM6C, Sb1, Dl9P/TM6, Tb1, DlRevF10 SerRX82/TM6, Tb1, UAS-CD8::GFP (Bloomington Drosophila Stock Center), shamsΔ34/TM6, Tb1 [32], PBac{Ngt-wt}VK22, PBac{Ngt-16_20}VK22, [28], P{Dlgt-wt}attP2, PBac{Dlgt-wt}VK37, PBac{Sergt-wt}VK37 [11], y w Ubx-FLP tub-GAL4 UAS-GFPnls-6X-Myc; FRT82B y+ tub-GAL80/TM6, Ubx [27], w; UAS-Dl (Gary Struhl), DlRevF10/TM6, Tb1 [49], Serrev6-1/TM6, Tb1 [50], Serrev2-11/TM6, Tb1 [50], SerRX106 [51], UAS-Serwt-Tomato (UAS-SerTom) [40], FRT82B shamsΔ34 Serrev6-1/TM6, Tb1, Dl9P shamsΔ34/TM6, Tb1, FRT82B shamsΔ34 DlRevF10/TM6, Tb1, FRT82B DlRevF10/TM6, Tb1, dpp-GAL4 shamsΔ34/TM6, Tb1, UAS-Dl; shamsΔ34/TM6b,Tb1, UAS-SerTom/CyO; shamsΔ34/TM6b, Tb1, y w Ubx-FLP/FM7; FRT82B Sb63 y+/TM6, Ubx (this study).
Drosophila genetics
All crosses were performed on standard media. All crosses were incubated at 25°C except for adult wings listed at 30°C, which were incubated at 25°C until late larval stage and shifted to 30°C during pupal stages. To generate MARCM clones [52] in 3rd instar wing discs, y w Ubx-FLP tub-GAL4 UAS-GFPnls-6X-Myc; FRT82B y+ tub-GAL80/TM6, Ubx females were crossed to FRT82B mutant/TM6, Tb1 males, wherein mutant stands for shamsΔ34, Serrev6-1, DeltaRevF10, DeltaRevF10 shamsΔ34, or shamsΔ34 Serrev6-1. Crosses were kept at 25°C and mosaic Tb+ 3rd instar larvae were selected for dissection. To generate clones in adult wings, FRT82B shamsΔ34/TM6, Tb1, FRT82B Serrev6-1/TM6, Tb1, FRT82B shamsΔ34 Serrev6-1/TM6, Tb1, FRT82B DeltaRevF10/TM6, Tb1, and FRT82B DeltaRevF10 shamsΔ34/TM6, Tb1 males were crossed to y w Ubx-FLP/FM7; FRT82B Sb63 y+/TM6, Ubx females and raised at 25°C. Sb63, Tb+ flies were selected for scoring the wings. All of the scored flies had regions of Sb+ microchaete on the thorax, confirming the generation of mutant clones in wing imaginal discs. dpp>Dl shamsΔ34/Δ34 animals were generated by crossing animals harboring a dpp-GAL4 shamsΔ34 recombinant chromosome to UAS-Dl; shamsΔ34/TM6b,Tb1 animals. Tb+ 3rd instar larvae were selected for dissection. To generate dpp>SerTom shamsΔ34/Δ34 animals, dpp-GAL4 shamsΔ34 animals were crossed to UAS-SerTom/CyO; shamsΔ34/TM6b, Tb1 animals. Tb+ 3rd instar larvae expressing Tomato were selected for dissection. To examine the expression pattern of cDNAs driven by dpp-GAL4 in larval wing imaginal disc, dpp-GAL4/TM6, Tb1 males were crossed to UAS-CD8::GFP females. Late 2nd and late 3rd instar Tb+ larvae were selected and dissected.
Dissections, staining, image acquisition and processing
Dissection and staining were performed by using standard methods. For surface staining, S2 cells were incubated with antibodies against the Notch extracellular domain (NECD) or the Delta extracellular domain (Dl-ECD) in the absence of detergent. A similar detergent-free protocol was used for Delta surface staining of wing imaginal discs. Antibodies used were mouse α-Cut (2B10) 1:500, mouse anti-Wg (4D4) 1:100, mouse anti-NECD (C458.2H) 1:100 (all from DSHB), guinea pig anti-Dl-ECD 1:3000 (Gift from M. Muskavitch) [15], goat α-mouse-Cy3 1:500, donkey α-mouse-Cy5 1:500 and donkey α-Guinea Pig-Cy3 1:500 (Jackson ImmunoResearch Laboratories). Adult wings were imaged using Zeiss Axioscope-A1 and Nikon Ci-L upright microscopes. Wing areas were measured in term of square pixels using ImageJ 1.47. Confocal images were scanned using a Leica TCS-SP5 microscope and processed with Amira5.2.2. Images were processed with Adobe Photoshop CS5; Figures were assembled in Adobe Illustrator CS5.
S2 culture, ligand binding and cell aggregation assays
S2 cells (Invitrogen) were cultured in Schneider's Drosophila Medium (Lonza) supplemented with 10% fetal bovine serum and penicillin-streptomycin (100 U/mL). For S2-N and S2-Dl stable cell lines (DGRC, Bloomington, USA), 200 nM methotrexate (Sigma-Aldrich) was added. S2-SerTom stable cells [40] were cultured in M3 medium (Gibco) supplemented with 10% fetal bovine serum and 100 μg/mL of hygromycin B. For knock-down, 7.5 μg of either EGFP dsRNA (control) or shams dsRNA was added to the culture medium of S2, S2-N or S2-Dl cells, and the cells were cultured for 24 hours at 25°C prior to induction with CuS04 (0.7 mM).
Ligand-receptor binding assays were performed as described previously [42] with minor modifications. In brief, S2 cells (2 x 106 cells/well) were transiently transfected with the constructs expressing the extracellular domain of Delta or Serrate fused to alkaline phosphatase (6.0 μg of either pMT-Delta-AP or pMT-Serrate-AP per well). After 24 hours, the transfected cells were induced by adding 0.7mM CuSO4 to the media. After three days of induction, conditioned media were collected and the amount of AP was determined by quantifying AP activity in each medium by following the manufacturer’s instructions (Phospha-Light System, Applied Biosystems) and using FLUOstar OPTIMA (BMG Labtech). In parallel, plain S2 or S2-N cells (2 x 106 cells/well) were induced by 0.7mM CuSO4 after 24 hours of treatment with EGFP dsRNA or shams dsRNA. After three days of induction, cells (S2 or S2-N cells) were collected and incubated with conditioned media (containing defined concentrations of Delta-AP or Serrate-AP) for 90 minutes on rotary shaker. After three times washing and cell lysis, the endogenous AP activity was heat inactivated (60°C for 10 min). The amount of AP-tagged ligand bound to S2-N cells was assayed as per the manufacturer’s instructions (Phospha-Light System, Applied Biosystems) and using FLUOstar OPTIMA (BMG Labtech). Binding of each ligand (Delta-AP or Serrate-AP) with plain S2 cells (which lack endogenous Notch protein [41]) was used as control. Experiments were performed in triplicate and repeated three times.
Cells were incubated with CuSO4 for 1–2 days and then used in aggregation assays. For each aggregation assay, 2.5 x 105 of the dsRNA-treated cells (S2, S2-N, S2-Dl) were mixed with 5 x 105 S2-Dl, S2-SerTom or S2-N cells (induced with 0.7 mM CuSO4 for 3 hours prior to co-culture) in a total volume of 200 μl medium in a 24-well plate.
For co-culture assays expressing cis-ligands, after 24 hours of treatment with either EGFP or shams dsRNA, S2 cells were transiently transfected with 2 μg total of either pBluescript (control), pBluescript and pMT-Notch (S2-Ntransient), pMT-Notch and pMT-Delta (S2-N&Dltransient), or pMT-Notch and pMT-Serrate (S2-N&Sertransient) using 3 μL of FuGENE HD (Promega). One μg of pMT-Notch was used in all assays. The remaining 1 μg was either pBluescript alone, pMT-Delta alone, pMT-Serrate alone, or a mixture of 0.5 μg, 0.2 μg or 0.1 μg of pMT-Delta or pMT-Serrate and pBluescript (Figs 7C and 8C). For aggregation assays, 2.5 x 105 dsRNA treated cells were mixed with 5 x 105 S2-Dl cells (induced for 3 hours prior to co-culture with CuSO4) in a total volume of 200μL in a 24-well plate. Co-cultured cells were gently shaken at 150 rpm to allow aggregation. Images of aggregate formation were taken at reported time points. The number of cell aggregates was quantified using a hemocytometer after 5 minutes of co-culture. Each assay was repeated at least three times. P-values were determined either by Student’s t-test or by One-Way ANOVA with Tukey’s multiple comparisons test.
Generation of dsRNA for S2 aggregation assays
EGFP dsRNA primers:
Forward primer-GAAATTAATACGACTCACTATAGGGGGTGAGCAAGGGCGAGGAG
Reverse primer-GAAATTAATACGACTCACTATAGGGGGTCTTTGCTCAGGGCGG
shams dsRNA Primers:
Forward primer-TTAATACGACTCACTATAGGGGAGATGCTGTATGTGGACACGGAT
Reverse Primer-TTAATACGACTCACTATAGGGGAGATCCGTGGATAACCTTAACGA
Template DNAs were generated by PCR amplification from pAct-EGFP plasmid DNA or y w genomic DNA. Purified PCR products were used as template for in vitro transcription reactions using the T7 MEGAscript kit (Ambion). Double-stranded RNA was purified with RNeasy Mini kit (Qiagen).
qRT-PCR assays
shams and Rp49 (RpL32) mRNA expression in S2 cells treated with either EGFP or shams dsRNA were assayed by qRT-PCR using TaqMan One-Step RT-PCR Master Mix and TaqMan primers/probe sets (Life Technologies Dm02144576_g1 and Dm02151827_g). Relative shams mRNA levels were compared using the 2–ΔΔCT method. P-values were determined by Student’s t-test.
Supporting information
(A-B) All animals raised at 25°C. (A) Wing margin loss is observed in 21% of Serrev6-1/+ shamsΔ34/Δ34 animals (n = 73). (B) Adding one copy of a Serrate genomic transgene rescues the wing margin loss in these animals (n = 31).
(TIF)
(A) Representative images showing cell surface Notch expression (red) in S2 cell (control) and S2-N cells (treated with EGFP or shams dsRNA). No difference in expression levels is apparent. Scale bar = 25 μm. (B) Graph shows the average fluorescence intensity in dsRNA (EGFP or shams) treated S2-N cells (n = 40 cells in each group). Error bars indicate standard error. NS: not significant.
(TIF)
(A) Cell aggregation assays were performed between S2-N cells with S2 cells treated with shams dsRNA (Shams KD), S2-Dl cells treated with a control dsRNA (EGFP) or S2-Dl cells treated with shams dsRNA. Representative images of each co-culture at 1, 5 and 15 minutes are shown. (B) Quantification of number of cell aggregates greater than 6 cells after 5 minutes of co-culture. Error bars indicate standard error. Note that Shams KD does not change the number of aggregates significantly (P>0.05). NS: not significant. (C) Representative images showing cell surface Delta expression (red) in S2 cells (control) and S2-Dl cells (treated with EGFP or shams dsRNA). No difference in expression levels is apparent. Scale bar = 25 μm. (D) Graph shows the average fluorescence intensity of surface Delta in dsRNA (EGFP or shams) treated S2-Dl cells (n = 40 cells for each group). Error bars indicate standard error. NS: not significant. (E) Detergent-free immunostaining for Delta extracellular domain (Dl-ECD) in third instar wing imaginal discs harboring shamsΔ34 MARCM clones (marked with GFP; n = 12). No difference in surface level of Delta between wild-type and mutant cells is apparent. Scale bar = 25 μm.
(TIF)
Acknowledgments
We thank Shinya Yamamoto and Robert Haltiwanger for discussions and advice on ligand-binding assays; The Bloomington Drosophila Stock Center, the Developmental Studies Hybridoma Bank, the Microscopy Core of the BCM IDDRC, the BCM Integrated Microscopy Core, Spyros Artavanis-Tsakonas, Robert Fleming, Marc Muskavitch, Ken Irvine and Gary Struhl for reagents.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
We acknowledge support from the Mizutani Foundation for Glycoscience (grant #110071 to HJN), the NIH/NIGMS (R01GM084135 to HJN), The Microscopy Core of the BCM IDDRC (1U54HD083092; the Eunice Kennedy Shriver NICHD), the Bloomington Drosophila Stock Center (NIH P40OD018537), and the BCM Integrated Microscopy Core, which is funded by the NIH (HD007495, DK56338, and CA125123), the Dan L. Duncan Cancer Center, and the John S. Dunn Gulf Coast Consortium for Chemical Genomics. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Artavanis-Tsakonas S, Muskavitch MA (2010) Notch: the past, the present, and the future. Curr Top Dev Biol 92: 1–29. 10.1016/S0070-2153(10)92001-2 [DOI] [PubMed] [Google Scholar]
- 2.Rebay I, Fleming RJ, Fehon RG, Cherbas L, Cherbas P, et al. (1991) Specific EGF repeats of Notch mediate interactions with Delta and Serrate: implications for Notch as a multifunctional receptor. Cell 67: 687–699. [DOI] [PubMed] [Google Scholar]
- 3.Moloney DJ, Shair LH, Lu FM, Xia J, Locke R, et al. (2000) Mammalian Notch1 is modified with two unusual forms of O-linked glycosylation found on epidermal growth factor-like modules. J Biol Chem 275: 9604–9611. [DOI] [PubMed] [Google Scholar]
- 4.Bray SJ (2006) Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 7: 678–689. 10.1038/nrm2009 [DOI] [PubMed] [Google Scholar]
- 5.Fortini ME (2009) Notch signaling: the core pathway and its posttranslational regulation. Dev Cell 16: 633–647. 10.1016/j.devcel.2009.03.010 [DOI] [PubMed] [Google Scholar]
- 6.de Celis JF, Bray S (1997) Feed-back mechanisms affecting Notch activation at the dorsoventral boundary in the Drosophila wing. Development 124: 3241–3251. [DOI] [PubMed] [Google Scholar]
- 7.Doherty D, Feger G, Younger-Shepherd S, Jan LY, Jan YN (1996) Delta is a ventral to dorsal signal complementary to Serrate, another Notch ligand, in Drosophila wing formation. Genes Dev 10: 421–434. [DOI] [PubMed] [Google Scholar]
- 8.Micchelli CA, Rulifson EJ, Blair SS (1997) The function and regulation of cut expression on the wing margin of Drosophila: Notch, Wingless and a dominant negative role for Delta and Serrate. Development 124: 1485–1495. [DOI] [PubMed] [Google Scholar]
- 9.Jacobsen TL, Brennan K, Arias AM, Muskavitch MA (1998) Cis-interactions between Delta and Notch modulate neurogenic signalling in Drosophila. Development 125: 4531–4540. [DOI] [PubMed] [Google Scholar]
- 10.del Alamo D, Rouault H, Schweisguth F (2011) Mechanism and significance of cis-inhibition in Notch signalling. Curr Biol 21: R40–47. 10.1016/j.cub.2010.10.034 [DOI] [PubMed] [Google Scholar]
- 11.LeBon L, Lee TV, Sprinzak D, Jafar-Nejad H, Elowitz MB (2014) Fringe proteins modulate Notch-ligand cis and trans interactions to specify signaling states. Elife 3: e02950 10.7554/eLife.02950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sprinzak D, Lakhanpal A, Lebon L, Santat LA, Fontes ME, et al. (2010) Cis-interactions between Notch and Delta generate mutually exclusive signalling states. Nature 465: 86–90. 10.1038/nature08959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng C, Younger-Shepherd S, Jan LY, Jan YN (1998) Delta and Serrate are redundant Notch ligands required for asymmetric cell divisions within the Drosophila sensory organ lineage. Genes Dev 12: 1086–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Celis JF, Tyler DM, de Celis J, Bray SJ (1998) Notch signalling mediates segmentation of the Drosophila leg. Development 125: 4617–4626. [DOI] [PubMed] [Google Scholar]
- 15.Huppert SS, Jacobsen TL, Muskavitch MA (1997) Feedback regulation is central to Delta-Notch signalling required for Drosophila wing vein morphogenesis. Development 124: 3283–3291. [DOI] [PubMed] [Google Scholar]
- 16.Lebestky T, Jung SH, Banerjee U (2003) A Serrate-expressing signaling center controls Drosophila hematopoiesis. Genes Dev 17: 348–353. 10.1101/gad.1052803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Troost T, Klein T (2012) Sequential Notch signalling at the boundary of fringe expressing and non-expressing cells. PLoS ONE 7: e49007 10.1371/journal.pone.0049007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Celis JF, Bray S, Garcia-Bellido A (1997) Notch signalling regulates veinlet expression and establishes boundaries between veins and interveins in the Drosophila wing. Development 124: 1919–1928. [DOI] [PubMed] [Google Scholar]
- 19.de Celis JF, Bray SJ (2000) The Abruptex domain of Notch regulates negative interactions between Notch, its ligands and Fringe. Development 127: 1291–1302. [DOI] [PubMed] [Google Scholar]
- 20.Haltom AR, Jafar-Nejad H (2015) The multiple roles of epidermal growth factor repeat O-glycans in animal development. Glycobiology 25: 1027–1042. 10.1093/glycob/cwv052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeuchi H, Haltiwanger RS (2014) Significance of glycosylation in Notch signaling. Biochem Biophys Res Commun 453: 235–242. 10.1016/j.bbrc.2014.05.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stanley P, Okajima T (2010) Roles of glycosylation in Notch signaling. Curr Top Dev Biol 92: 131–164. 10.1016/S0070-2153(10)92004-8 [DOI] [PubMed] [Google Scholar]
- 23.Bruckner K, Perez L, Clausen H, Cohen S (2000) Glycosyltransferase activity of Fringe modulates Notch-Delta interactions. Nature 406: 411–415. 10.1038/35019075 [DOI] [PubMed] [Google Scholar]
- 24.Hicks C, Johnston SH, diSibio G, Collazo A, Vogt TF, et al. (2000) Fringe differentially modulates Jagged1 and Delta1 signalling through Notch1 and Notch2. Nat Cell Biol 2: 515–520. 10.1038/35019553 [DOI] [PubMed] [Google Scholar]
- 25.Moloney DJ, Panin VM, Johnston SH, Chen J, Shao L, et al. (2000) Fringe is a glycosyltransferase that modifies Notch. Nature 406: 369–375. 10.1038/35019000 [DOI] [PubMed] [Google Scholar]
- 26.Panin VM, Papayannopoulos V, Wilson R, Irvine KD (1997) Fringe modulates Notch-ligand interactions. Nature 387: 908–912. 10.1038/43191 [DOI] [PubMed] [Google Scholar]
- 27.Acar M, Jafar-Nejad H, Takeuchi H, Rajan A, Ibrani D, et al. (2008) Rumi is a CAP10 domain glycosyltransferase that modifies Notch and is required for Notch signaling. Cell 132: 247–258. 10.1016/j.cell.2007.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leonardi J, Fernandez-Valdivia R, Li YD, Simcox AA, Jafar-Nejad H (2011) Multiple O-glucosylation sites on Notch function as a buffer against temperature-dependent loss of signaling. Development 138: 3569–3578. 10.1242/dev.068361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishio A, Sasamura T, Ayukawa T, Kuroda J, Ishikawa HO, et al. (2015) O-fucose monosaccharide of Drosophila Notch has a temperature-sensitive function and cooperates with O-glucose glycan in Notch transport and Notch signaling activation. J Biol Chem 290: 505–519. 10.1074/jbc.M114.616847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perdigoto CN, Schweisguth F, Bardin AJ (2011) Distinct levels of Notch activity for commitment and terminal differentiation of stem cells in the adult fly intestine. Development 138: 4585–4595. 10.1242/dev.065292 [DOI] [PubMed] [Google Scholar]
- 31.Sethi MK, Buettner FF, Krylov VB, Takeuchi H, Nifantiev NE, et al. (2010) Identification of glycosyltransferase 8 family members as xylosyltransferases acting on O-glucosylated notch epidermal growth factor repeats. J Biol Chem 285: 1582–1586. 10.1074/jbc.C109.065409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee TV, Sethi MK, Leonardi L, Rana NA, Buettner FF, et al. (2013) Negative regulation of Notch signaling by xylose. PLoS Genet 9: e1003547 10.1371/journal.pgen.1003547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vassin H, Campos-Ortega JA (1987) Genetic Analysis of Delta, a Neurogenic Gene of Drosophila melanogaster. Genetics 116: 433–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee T, Luo L (2001) Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci 24: 251–254. [DOI] [PubMed] [Google Scholar]
- 35.Emery G, Hutterer A, Berdnik D, Mayer B, Wirtz-Peitz F, et al. (2005) Asymmetric Rab 11 endosomes regulate delta recycling and specify cell fate in the Drosophila nervous system. Cell 122: 763–773. 10.1016/j.cell.2005.08.017 [DOI] [PubMed] [Google Scholar]
- 36.de Celis JF, Garcia-Bellido A, Bray SJ (1996) Activation and function of Notch at the dorsal-ventral boundary of the wing imaginal disc. Development 122: 359–369. [DOI] [PubMed] [Google Scholar]
- 37.Speicher SA, Thomas U, Hinz U, Knust E (1994) The Serrate locus of Drosophila and its role in morphogenesis of the wing imaginal discs: control of cell proliferation. Development 120: 535–544. [DOI] [PubMed] [Google Scholar]
- 38.Diaz-Benjumea FJ, Cohen SM (1995) Serrate signals through Notch to establish a Wingless-dependent organizer at the dorsal/ventral compartment boundary of the Drosophila wing. Development 121: 4215–4225. [DOI] [PubMed] [Google Scholar]
- 39.Brand AH, Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. [DOI] [PubMed] [Google Scholar]
- 40.Fleming RJ, Hori K, Sen A, Filloramo GV, Langer JM, et al. (2013) An extracellular region of Serrate is essential for ligand-induced cis-inhibition of Notch signaling. Development 140: 2039–2049. 10.1242/dev.087916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fehon RG, Kooh PJ, Rebay I, Regan CL, Xu T, et al. (1990) Molecular interactions between the protein products of the neurogenic loci Notch and Delta, two EGF-homologous genes in Drosophila. Cell 61: 523–534. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto S, Charng WL, Rana NA, Kakuda S, Jaiswal M, et al. (2012) A mutation in EGF repeat-8 of Notch discriminates between Serrate/Jagged and Delta family ligands. Science 338: 1229–1232. 10.1126/science.1228745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsumoto K, Ayukawa T, Ishio A, Sasamura T, Yamakawa T, et al. (2016) Dual Roles of O-Glucose Glycans Redundant with Monosaccharide O-Fucose on Notch in Notch Trafficking. J Biol Chem 291: 13743–13752. 10.1074/jbc.M115.710483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jafar-Nejad H, Leonardi J, Fernandez-Valdivia R (2010) Role of glycans and glycosyltransferases in the regulation of Notch signaling. Glycobiology 20: 931–949. 10.1093/glycob/cwq053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu A, Haines N, Dlugosz M, Rana NA, Takeuchi H, et al. (2007) In vitro reconstitution of the modulation of Drosophila Notch-ligand binding by Fringe. J Biol Chem 282: 35153–35162. 10.1074/jbc.M707040200 [DOI] [PubMed] [Google Scholar]
- 46.Okajima T, Xu A, Irvine KD (2003) Modulation of notch-ligand binding by protein O-fucosyltransferase 1 and fringe. J Biol Chem 278: 42340–42345. 10.1074/jbc.M308687200 [DOI] [PubMed] [Google Scholar]
- 47.Correia T, Papayannopoulos V, Panin V, Woronoff P, Jiang J, et al. (2003) Molecular genetic analysis of the glycosyltransferase Fringe in Drosophila. Proc Natl Acad Sci U S A 100: 6404–6409. 10.1073/pnas.1131007100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harvey BM, Rana NA, Moss H, Leonardi J, Jafar-Nejad H, et al. (2016) Mapping Sites of O-Glycosylation and Fringe Elongation on Drosophila Notch. J Biol Chem 291: 16348–16360. 10.1074/jbc.M116.732537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haenlin M, Kramatschek B, Campos-Ortega JA (1990) The pattern of transcription of the neurogenic gene Delta of Drosophila melanogaster. Development 110: 905–914. [DOI] [PubMed] [Google Scholar]
- 50.Fleming RJ, Scottgale TN, Diederich RJ, Artavanis-Tsakonas S (1990) The gene Serrate encodes a putative EGF-like transmembrane protein essential for proper ectodermal development in Drosophila melanogaster. Genes Dev 4: 2188–2201. [DOI] [PubMed] [Google Scholar]
- 51.Thomas U, Speicher SA, Knust E (1991) The Drosophila gene Serrate encodes an EGF-like transmembrane protein with a complex expression pattern in embryos and wing discs. Development 111: 749–761. [DOI] [PubMed] [Google Scholar]
- 52.Lee T, Winter C, Marticke SS, Lee A, Luo L (2000) Essential roles of Drosophila RhoA in the regulation of neuroblast proliferation and dendritic but not axonal morphogenesis. Neuron 25: 307–316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A-B) All animals raised at 25°C. (A) Wing margin loss is observed in 21% of Serrev6-1/+ shamsΔ34/Δ34 animals (n = 73). (B) Adding one copy of a Serrate genomic transgene rescues the wing margin loss in these animals (n = 31).
(TIF)
(A) Representative images showing cell surface Notch expression (red) in S2 cell (control) and S2-N cells (treated with EGFP or shams dsRNA). No difference in expression levels is apparent. Scale bar = 25 μm. (B) Graph shows the average fluorescence intensity in dsRNA (EGFP or shams) treated S2-N cells (n = 40 cells in each group). Error bars indicate standard error. NS: not significant.
(TIF)
(A) Cell aggregation assays were performed between S2-N cells with S2 cells treated with shams dsRNA (Shams KD), S2-Dl cells treated with a control dsRNA (EGFP) or S2-Dl cells treated with shams dsRNA. Representative images of each co-culture at 1, 5 and 15 minutes are shown. (B) Quantification of number of cell aggregates greater than 6 cells after 5 minutes of co-culture. Error bars indicate standard error. Note that Shams KD does not change the number of aggregates significantly (P>0.05). NS: not significant. (C) Representative images showing cell surface Delta expression (red) in S2 cells (control) and S2-Dl cells (treated with EGFP or shams dsRNA). No difference in expression levels is apparent. Scale bar = 25 μm. (D) Graph shows the average fluorescence intensity of surface Delta in dsRNA (EGFP or shams) treated S2-Dl cells (n = 40 cells for each group). Error bars indicate standard error. NS: not significant. (E) Detergent-free immunostaining for Delta extracellular domain (Dl-ECD) in third instar wing imaginal discs harboring shamsΔ34 MARCM clones (marked with GFP; n = 12). No difference in surface level of Delta between wild-type and mutant cells is apparent. Scale bar = 25 μm.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.