ABSTRACT
Recent research reveals that the YEATS domains preferentially recognize crotonylated lysines on histones. Here, we discuss the molecular mechanisms that enable this recognition and the biological significances of this interaction. The dynamics of histone crotonylation and its potential roles in the regulation of gene expression will also be discussed.
KEYWORDS: aromatic-π stacking, histone crotonylation, reader, transcription, YEATS domain
Introduction
Post-translational modifications (PTMs) on histones are important epigenetic regulatory mechanisms operating in diverse biological processes, such as gene expression, development, DNA repair, and chromatin dynamics. Archetypical histone PTM lysine acetylation (Kac) has been proposed to function by neutralizing the positive charge on the lysine side chain, thereby weakening the electrostatic interaction between histone and DNA and creating a permissive chromatin environment for transcription.1 Kac also serves as docking sites for various protein complexes in transcriptional regulation.2 Acyl moieties other than the acetyl group can also modify histone lysines in vivo. Thanks to the recent progress of proteomic technology, a growing number of novel lysine acylations have been identified, including propionylation (Kpr), butyrylation (Kbu), crotonylation (Kcr), 2-hydroxyisobutyrylation (Khib), and 2-hydroxybutyrylation (Kbhb).3-5 Accumulating evidence indicates that these new histone acyl modifications may have differentiated functions from histone Kac, and that competition among these different acylations could be a critical epigenetic regulatory mechanism.6 Importantly, since the bio-generation of various lysine acylations is closely linked to the metabolism and the energy status of the cell, these acyl marks provide a direct link between extra-/intra-cellular environments and transcriptional responses.5,7,8
Histone PTMs often have dedicated writers, erasers, and readers. It was observed that some histone acetyltransferases (HATs) and deacetylases (HDACs) are co-opted to install and remove other acyl species, although with relatively lower enzymatic activities.7,9 In contrast, readers recognizing these acyl marks have remained largely unknown.
Recently, the YEATS (Yaf9, ENL, AF9, Taf14, and Sas5) family proteins AF9, Taf14, and YEATS2 were reported as effective readers of histone Kcr, indispensable for Kcr-mediated active transcription.10-13 YEATS family proteins are usually implicated in chromatin remodeling and transcriptional regulation.14,15 While bromodomain, the prototypical reader of Kac, exhibits minimal binding to Kcr, the YEATS domain is the first reader module preferring Kcr to Kac. These two families of acyllysine readers might exert differential regulatory functions by engaging their own cognate targets.8
The expanding repertoire of histone lysine acylation
Up to now, about nine types of short-chain acylations on histone lysines besides acetylation have been reported, including formylation, propionylation, butyrylation, crotonylation, malonylation, succinylation, glutarylation, 2-hydroxyisobutyrylation, and 2-hydroxybutyrylation, and the list is still growing.5,16,17 Nowadays, the establishment, removal, and interpretation of these acyl marks, as well as their biological function, have been extensively studied.18 We will focus on Kcr in the remaining part of this commentary.
Histone Kcr functions in various biological processes
Histone Kcr is an active mark conserved from yeast to human.3 Kcr is found on all four core histones and is enriched at promoters or enhancers.3, 7 Like Kac, Kcr neutralizes the positive charge on histone lysines, de-compacts the chromatin structure, and facilitates transcription.19 Structurally Kcr is four-carbon in length and planar in shape, distinct from Kac and other lysine acyl modifications. Interestingly, in mouse testis, Kcr marks sex chromosome-linked escape genes responsible for spermatogenesis. In a globally repressive transcriptional environment in post-meiotic cells, most genes remain inactive, whereas Kcr facilitates a small group of sex chromosome-linked genes to escape this transcriptional repression.3,20 This anti-repression effect of Kcr might originate from the fact that Kcr is not a good substrate for HDACs, which effect repression of gene transcription by removing histone Kac.9 In this regard, Kcr may represent a “long-acting” form of Kac, which is conceivably beneficial to enable a robust and productive transcription. It is worth investigating whether this Kcr-mediated anti-repression mechanism operates in other biological processes.
The level of histone Kcr can be modulated by the fluctuation of cellular metabolic intermediates, such as crotonyl-CoA over acetyl-CoA ratio.7 A recent research found that histone Kcr increases and modulates gene expression during acute kidney injury (AKI), providing a protective effect against inflammation and mitochondrial stress during AKI.21 Further studies are needed to investigate the function of histone Kcr in other cellular processes, including virus infection (e.g., Borna disease virus),22 cell reprogramming and development, and human diseases.
An unique aromatic-π stacking mechanism for crotonyllysine recognition by YEATS domain
Previously, we found that the YEATS domain utilizes an aromatic “sandwich” cage to encapsulate Kac.23 We reasoned that the YEATS might accommodate the longer and more rigid Kcr better than Kac due to an end-open feature of the aromatic “sandwich” cage. To our expectation, Kcr enhances its binding to different YEATS domains by 2–5-fold as compared to Kac.10-13 By contrast, most bromodomains do not bind Kcr.10,24 These three YEATS domains adopt a highly similar structure while engaging Kcr. The extended side chain of Kcr fits snugly into the end-open narrow pocket of the YEATS domain (Figs. 1A and B). In contrast, the Kac-binding pockets of bromodomains are end-blocked, which can hardly tolerate Kcr. The second bromodomain of human TAF1 is the only one that displays detectable yet compromised binding to histone Kcr peptide with a binding KD of ∼110 μM for H4K5crK8cr peptide (KD ≈ 50 μM for H4K5acK8ac).10,24 Thus, the YEATS domains represent the first family of Kcr-preferential readers.
Figure 1.
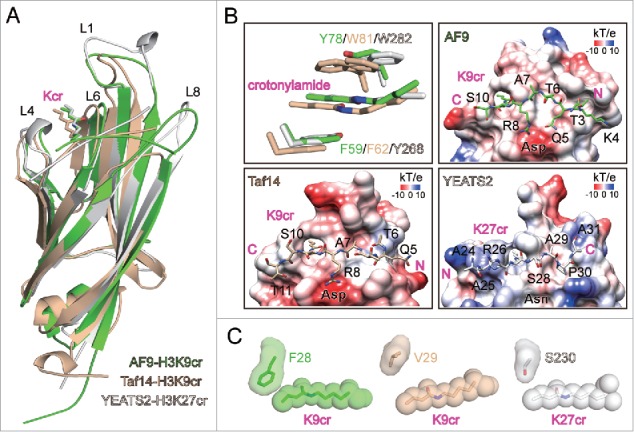
Molecular details of crotonyllysine recognition by YEATS domains. (A) Superimposition of AF9-H3K9cr, Taf14-H3K9cr, and YEATS2-H3K27cr complex structures. YEATS proteins and H3 peptides are depicted as cartoon, with sidechains of crotonyllysine shown as sticks. The color code is given in the bottom-right corner, and the same code is applied throughout the entire figure. (B) Aromatic-π-aromatic stacking and electrostatic potential surfaces ranging from −10 to +10 (kT/e) of AF9, Taf14, and YEATS2 YEATS domains. Key aromatic residues, crotonyllysine sidechains, and H3 peptides are shown as sticks. Oxygen atoms: red; Nitrogen atoms: blue. The W81 side chain of Taf14 is shown in dual conformation. (C) Relative positions of space-filling crotonyllysine and adjacent loop 1 residue in AF9, Taf14, and YEATS2 YEATS domain structures.
The planar crotonylamide group is sandwiched by two aromatic residues conserved among different YEATS domains (F59 and Y78 for AF9; F62 and W81 for Taf14; Y268 and W282 for YEATS2), which enables “aromatic-π-aromatic” stacking (also called “π–π–π” stacking) optimal for Kcr-specific recognition (Fig. 1B). Collectively, the unique aromatic-π stacking, the CH–π hydrogen bonding 25 as well as the hydrophobic interactions between the side chain of Kcr and the pocket residues contribute substantially to a preferential recognition of Kcr by the YEATS domains (Fig. 1B).
Site-selectivity of AF9, Taf14, and YEATS2 YEATS domains
Peptide array and calorimetric titration studies revealed that AF9 YEATS recognizes histone crotonylation at H3 K9, K18, and K27 with highest affinity for H3K9cr,10 and similar binding preference was observed for Taf14.12 By contrast, the YEATS domain of YEATS2 displays a selectivity only toward H3K27cr.11 Histone H3 K9, K18, and K27 share a common motif of “A(−2)R(−1)KS(+1).” Structural studies of AF9 and Taf14 YEATS domains bound to H3K9cr peptide revealed an acidic Asp residue that forms charge-stabilized hydrogen bonding interactions with H3R8 (Fig. 1B), stressing an “R(−1)K” recognition signature common for H3 K9, K18, and K27.23
Interestingly, YEATS2 has a neutral residue Asn instead of an Asp, which disfavors the recognition of “R(−1).” Correspondingly, co-crystal structural studies revealed that the H3K27cr peptide binds to the YEATS domain of YEATS2 in an opposite orientation as compared to that of AF9 and Taf14 (Fig. 1B).10-12 In this arrangement, H3 segment “K4-Q5-T6-A7-R8” that is N-terminal to H3K9 contributes to binding through extensive interactions with the surface formed by loops L6 and L8 of the YEATS domains of AF9 and Taf14. Whereas H3 segment “S28-A29-P30-A31” that is C-terminal to K27cr is nicely docked into and registered in the “L6-L8” surface of the YEATS2 YEATS domain (Figs. 1A and B). The involvement of a signature motif such as P30 at the “+3” position for recognition is unique to H3 K27 but not K9 and K18, thus explains the observed H3K27 site-selectivity. The histone peptide binding surface of YEATS2 is less negative (Fig. 1B), which partly accounts for the weak binding affinity between H3K27cr peptide and YEATS2 (Fig. 1B and refs.10-12). Of note, the primary sequences of loop L8 vary among different YEATS domains, providing one major determinant for histone Kcr site-selectivity (Fig. 1A and ref.23). In support of its importance, hot-spot mutations within loop L8 of the ENL YEATS have been found in Wilms tumor.26
Sensing the tip of acyllysine by the YEATS reader pocket
Besides Kcr, the YEATS domains can interact with several other types of short-chain lysine acylations, such as Kpr, Kbu, Khib.10-12 The YEATS domains of AF9 and YEATS2 displayed similar preference for histone Kpr and Kbu over Kac.10,11 Kpr and Kbu do not contain a double bond, thereby aromatic-π interaction could not contribute to the tip recognition of both acyllysines. In these cases, CH–π interaction and hydrophobic contacts likely dominates favorable recognition of Kpr and Kbu.25
In terms of acyl marks bulkier than Kcr, AF9 does not bind branched Khib or acidic succinylation at H3 K9.10 In contrast, YEATS2 binds H3K27hib well and 2-fold stronger than H3K27ac.11 A smaller side chain residue S230 within loop L1 of YEATS2 renders a more open pocket around the tip of the acyl group compared to AF9 (F28 for AF9 and ENL, H43 for GAS41 and Yaf9, V29 for Taf14, and L30 for Sas5). Such a feature likely accounts for the recognition of the bulky and branched Khib by YEATS2 (Figs. 1A and C and refs.11,23).
Regulation and possible roles of Kcr and YEATS proteins in vivo
The preferential readout of Kcr by the YEATS domains is implicated in diverse cellular processes (Fig. 2). We found that Kcr-AF9 YEATS positively regulates gene expression in inflammatory response.10
Figure 2.
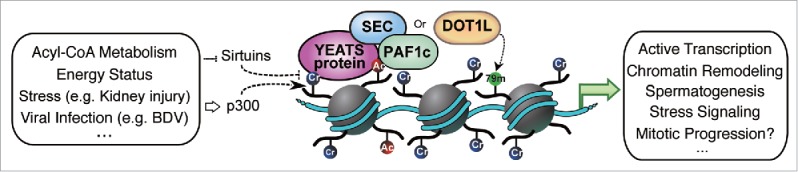
Histone crotonylation and YEATS proteins are involved in diverse biological processes.
Currently, four YEATS domain proteins are identified in human: AF9, ENL, Gas41, and YEATS2.14 AF9 or ENL is associated with several nuclear proteins/complexes, including super elongation complex (SEC), the histone H3K79-specific methyltransferase DOT1L, and the polymerase II-associated factor (PAF) complex. These complexes can facilitate pause-release and elongation in transcription. AF9 regulates HOX gene expression in both normal embryonic development and cancer cells via recruitment of the above complexes.23,27 Gas41 is a common subunit of the SRCAP remodeling and Tip60 HAT complexes, and is oncogenic. Meanwhile, GAS41 can interact with several transcription factors such as Myc.28 YEATS2 is an integral member of the ADA Two A-Containing (ATAC) HAT complex, which is involved in nucleosome remodeling, transcription regulation, stress signaling, and mitotic progression.29-31 YEATS2 also interacts with the TATA-binding protein (TBP) to modulate the basal transcription machinery.30 Yeast Taf14, Yaf9, and Sas5 are also associated with several distinct chromatin remodelers, transcription factors, or HAT complexes.14
Concluding remarks
Histone Kcr is a novel histone PTM closely related to active transcription. Its unique features have attracted extensive research efforts in the field of chromatin biology. YEATS domains recognize histone Kcr by a unique mechanism of aromatic-π stacking, and link Kcr to transcription, nucleosome remodeling, and other important cellular functions. It remains largely unknown how levels of Histone Kcr is modulated by extracellular signals and how Kcr–YEATS interactions activate downstream biological pathways. It is also tantalizing to investigate whether any other kind of reader of Kcr and newly identified acyllysine marks exists. If so, what are their recognizing mechanism and their functional outcome.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We sincerely thank the laboratories of Drs. C. David Allis, Xiaobing Shi and Yingming Zhao for their concerted collaborations on the YEATS projects.
Funding
This research was supported by grants from the Ministry of Science and Technology of the People's Republic of China (2016YFA0500700), the National Natural Science Foundation of China (91519304), and the Tsinghua University Initiative Scientific Research Program to H.L. Y.L. was supported by a Tsinghua Advanced Innovation fellowship from the Beijing Advanced Innovation Center for Structural Biology at Tsinghua University.
References
- [1].Allfrey VG, Faulkner R, Mirsky AE. Acetylation and methylation of histones and their possible role in the regulation of Rna synthesis. Proc Natl Acad Sci U S A 1964; 51:786-794; PMID:14172992; http://dx.doi.org/ 10.1073/pnas.51.5.786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dhalluin C, Carlson JE, Zeng L, He C, Aggarwal AK, Zhou MM. Structure and ligand of a histone acetyltransferase bromodomain. Nature 1999; 399:491-496; PMID:10365964; http://dx.doi.org/ 10.1038/20974 [DOI] [PubMed] [Google Scholar]
- [3].Tan M, Luo H, Lee S, Jin F, Yang JS, Montellier E, Buchou T, Cheng Z, Rousseaux S, Rajagopal N et al.. Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell 2011; 146:1016-1028; PMID:21925322; http://dx.doi.org/ 10.1016/j.cell.2011.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dai L, Peng C, Montellier E, Lu Z, Chen Y, Ishii H, Debernardi A, Buchou T, Rousseaux S, Jin F, et al.. Lysine 2-hydroxyisobutyrylation is a widely distributed active histone mark. Nat Chem Biol 2014; 10:365-370; PMID:24681537; http://dx.doi.org/ 10.1038/nchembio.1497 [DOI] [PubMed] [Google Scholar]
- [5].Xie Z, Zhang D, Chung D, Tang Z, Huang H, Dai L, Qi S, Li J, Colak G, Chen Y, et al.. Metabolic Regulation of Gene Expression by Histone Lysine β-Hydroxybutyrylation. Mol Cell 2016; 62:194-206; PMID:27105115; http://dx.doi.org/ 10.1016/j.molcel.2016.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Baumann K. Post-translational modifications: Crotonylation versus acetylation. Nat Rev Mol Cell Biol 2015; 16:265; PMID:25907603; http://dx.doi.org/ 10.1038/nrm3992 [DOI] [PubMed] [Google Scholar]
- [7].Sabari BR, Tang Z, Huang H, Yong-Gonzalez V, Molina H, Kong HE, Dai L, Shimada M, Cross JR, Zhao Y, et al.. Intracellular crotonyl-CoA stimulates transcription through p300-catalyzed histone crotonylation. Mol Cell 2015; 58:203-215; PMID:25818647; http://dx.doi.org/ 10.1016/j.molcel.2015.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dutta A, Abmayr SM, Workman JL. Diverse activities of histone acylations connect metabolism to chromatin function. Mol Cell 2016; 63:547-552; PMID:27540855; http://dx.doi.org/ 10.1016/j.molcel.2016.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bao X, Wang Y, Li X, Li XM, Liu Z, Yang T, Wong CF, Zhang J, Hao Q, Li XD. Identification of ‘erasers’ for lysine crotonylated histone marks using a chemical proteomics approach. Elife 2014; 3; PMID:25369635; http://dx.doi.org/ 10.7554/eLife.02999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Li Y, Sabari BR, Panchenko T, Wen H, Zhao D, Guan H, Wan L, Huang H, Tang Z, Zhao Y, et al.. Molecular coupling of histone crotonylation and active transcription by AF9 YEATS Domain. Mol Cell 2016; 62:181-193; PMID:27105114; http://dx.doi.org/ 10.1016/j.molcel.2016.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhao D, Guan H, Zhao S, Mi W, Wen H, Li Y, Zhao Y, Allis CD, Shi X, Li H. YEATS2 is a selective histone crotonylation reader. Cell Res 2016; 26:629-632; PMID:27103431; http://dx.doi.org/ 10.1038/cr.2016.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Andrews FH, Shinsky SA, Shanle EK, Bridgers JB, Gest A, Tsun IK, Krajewski K, Shi X, Strahl BD, Kutateladze TG. The Taf14 YEATS domain is a reader of histone crotonylation. Nat Chem Biol 2016; 12:396-398; PMID:27089029; http://dx.doi.org/ 10.1038/nchembio.2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhang Q, Zeng L, Zhao C, Ju Y, Konuma T, Zhou MM. Structural insights into Histone Crotonyl-Lysine recognition by the AF9 YEATS Domain. Structure 2016; 24(9):1606-1612; PMID:27545619; http://dx.doi.org/ 10.1016/j.str.2016.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Schulze JM, Wang AY, Kobor MS. YEATS domain proteins: a diverse family with many links to chromatin modification and transcription. Biochem Cell Biol 2009; 87:65-75; PMID:19234524; http://dx.doi.org/ 10.1139/O08-111 [DOI] [PubMed] [Google Scholar]
- [15].Schulze JM, Wang AY, Kobor MS. Reading chromatin: insights from yeast into YEATS domain structure and function. Epigenetics 2010; 5:573-577; PMID:20657183; http://dx.doi.org/ 10.4161/epi.5.7.12856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Xu YM, Du JY, Lau AT. Posttranslational modifications of human histone H3: an update. Proteomics 2014; 14:2047-2060; PMID:25044606; http://dx.doi.org/ 10.1002/pmic.201300435 [DOI] [PubMed] [Google Scholar]
- [17].Rousseaux S, Khochbin S. Histone acylation beyond acetylation: Terra incognita in chromatin biology. Cell J 2015; 17:1-6; PMID:25870829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Goudarzi A, Zhang D, Huang H, Barral S, Kwon OK, Qi S, Tang Z, Buchou T, Vitte AL, He T, et al.. Dynamic competing histone H4 K5K8 acetylation and butyrylation are hallmarks of highly active gene promoters. Mol Cell 2016; 62:169-180; PMID:27105113; http://dx.doi.org/ 10.1016/j.molcel.2016.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Suzuki Y, Horikoshi N, Kato D, Kurumizaka H. Crystal structure of the nucleosome containing histone H3 with crotonylated lysine 122. Biochem Biophys Res Commun 2016; 469:483-489; PMID:26694698; http://dx.doi.org/ 10.1016/j.bbrc.2015.12.041 [DOI] [PubMed] [Google Scholar]
- [20].Montellier E, Rousseaux S, Zhao Y, Khochbin S. Histone crotonylation specifically marks the haploid male germ cell gene expression program: post-meiotic male-specific gene expression. Bioessays 2012; 34:187-193; PMID:22170506; http://dx.doi.org/ 10.1002/bies.201100141 [DOI] [PubMed] [Google Scholar]
- [21].Ruiz-Andres O, Sanchez-Nino MD, Cannata-Ortiz P, Ruiz-Ortega M, Egido J, Ortiz A, Sanz AB. Histone lysine crotonylation during acute kidney injury in mice. Dis Model Mech 2016; 9(6):633-645; PMID:27125278; http://dx.doi.org/ 10.1242/dmm.024455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liu XHR, Zhang L, Wang X, Zhang LJ, Xie P. Borna disease virus alters histone lysine acetylation and crotonylation of human oligodendroglia cells. The 10th Biennial Conference of the Chinese Neuroscience Society Beijing, China, 2013. [Google Scholar]
- [23].Li Y, Wen H, Xi Y, Tanaka K, Wang H, Peng D, Ren Y, Jin Q, Dent SY, Li W, et al.. AF9 YEATS domain links histone acetylation to DOT1L-mediated H3K79 methylation. Cell 2014; 159:558-571; PMID:25417107; http://dx.doi.org/ 10.1016/j.cell.2014.09.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Flynn EM, Huang OW, Poy F, Oppikofer M, Bellon SF, Tang Y, Cochran AG. A subset of human bromodomains recognizes butyryllysine and crotonyllysine histone peptide modifications. Structure 2015; 23:1801-1814; PMID:26365797; http://dx.doi.org/ 10.1016/j.str.2015.08.004 [DOI] [PubMed] [Google Scholar]
- [25].Nishio M, Hirota M, Umezawa Y. The CH-[pi] interaction: evidence, nature, and consequences. New York: Wiley; 1998. [Google Scholar]
- [26].Perlman EJ, Gadd S, Arold ST, Radhakrishnan A, Gerhard DS, Jennings L, Huff V, Guidry Auvil JM, Davidsen TM, Dome JS, et al.. MLLT1 YEATS domain mutations in clinically distinctive Favourable Histology Wilms tumours. Nat Commun 2015; 6:10013; PMID:26635203; http://dx.doi.org/ 10.1038/ncomms10013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Collins EC, Appert A, Ariza-McNaughton L, Pannell R, Yamada Y, Rabbitts TH. Mouse Af9 is a controller of embryo patterning, like Mll, whose human homologue fuses with Af9 after chromosomal translocation in leukemia. Mol Cell Biol 2002; 22:7313-7324; PMID:12242306; http://dx.doi.org/ 10.1128/MCB.22.20.7313-7324.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Heisel S, Habel NC, Schuetz N, Ruggieri A, Meese E. The YEATS family member GAS41 interacts with the general transcription factor TFIIF. BMC Mol Biol 2010; 11:53; PMID:20618999; http://dx.doi.org/ 10.1186/1471-2199-11-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Suganuma T, Gutierrez JL, Li B, Florens L, Swanson SK, Washburn MP, Abmayr SM, Workman JL. ATAC is a double histone acetyltransferase complex that stimulates nucleosome sliding. Nat Struct Mol Biol 2008; 15:364-372; PMID:18327268; http://dx.doi.org/ 10.1038/nsmb.1397 [DOI] [PubMed] [Google Scholar]
- [30].Wang YL, Faiola F, Xu M, Pan S, Martinez E. Human ATAC Is a GCN5/PCAF-containing acetylase complex with a novel NC2-like histone fold module that interacts with the TATA-binding protein. J Biol Chem 2008; 283:33808-3380815; PMID:18838386; http://dx.doi.org/ 10.1074/jbc.M806936200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Orpinell M, Fournier M, Riss A, Nagy Z, Krebs AR, Frontini M, Tora L. The ATAC acetyl transferase complex controls mitotic progression by targeting non-histone substrates. EMBO J 2010; 29:2381-2394; PMID:20562830; http://dx.doi.org/ 10.1038/emboj.2010.125 [DOI] [PMC free article] [PubMed] [Google Scholar]


