Abstract
Background
The degree of pain caused by the implantation of cardiac electronic devices (CEDs) and the type of anesthesia or perioperative pain management used with the procedure have been insufficiently studied. The aim of this study was to analyze perioperative pain management, as well as intensity and location of pain among patients undergoing implantation of CED, and to compare the practice with published guidelines.
Patients and methods
This was a combined retrospective and prospective study conducted at the tertiary hospital, University Hospital Split, Croatia. The sample included 372 patients who underwent CED implantation. Perioperative pain management was analyzed retrospectively in 321 patients who underwent CED implantation during 2014. In a prospective study, intensity and location of pain before, during, and after the procedure were measured by using a numerical rating scale (NRS) ranging from 0 to 10 in 51 patients at the same institution from November 2014 to August 2015.
Results
A quarter of patients received analgesia or sedation before surgery. All the patients received local lidocaine anesthesia. After surgery, 31% of patients received pain medication or sedation. The highest pain intensity was observed during CED implantation with the highest NRS pain score being 8. Some patients reported severe pain (NRS >5) also at 1, 3, 6, 8, and 24 hours after surgery. The most common pain locations were surgical site, shoulder, and chest. Adherence to guidelines for acute perioperative pain management was insufficient.
Conclusion
Patients may experience severe pain during and after CED implantation. Perioperative pain management was suboptimal, and higher doses of sedation and intensive analgesia are required. Guidelines for acute perioperative pain management and anesthesia during CED implantation should be developed.
Keywords: cardiac electronic devices, perioperative pain management, postoperative pain, analgesics, pain intensity, guidelines
Introduction
The use of implantable cardiac electronic devices (CEDs), including pacemakers, implantable cardioverter-defibrillators, and cardiac resynchronized therapy (CRT), is increasing worldwide for the treatment of cardiac arrhythmias.1–3
Pain-free life is nowadays considered an essential human right.4 However, despite the growing consumption of analgesics worldwide,5–9 the prevalence of postoperative pain in various surgical disciplines is worrying.10–12 It is known that poorly treated acute perioperative pain may lead to chronic postsurgical pain (CPSP) and that the prevalence of CPSP is also disturbingly high. For example, an Italian study showed that the incidence of CPSP at 6 months was 45.2% for mild pain, 15.9% for moderate pain, and 2.7% for severe pain, whereas the incidence of CPSP at 12 months was 35.9%, 11.8%, and 2.5% for mild, moderate, and severe pain, respectively.11 Multiple factors may be associated with postoperative pain,13 and perioperative pain management is not necessarily conducted according to the recommended international guidelines.14
No data have been published to provide evidence about the degree of pain caused by implantation of CEDs, the type of anesthesia used during the procedure, or perioperative pain management. The aim of this study was to explore the patterns of anesthesia and perioperative pain management during CED implantation from patient records and to study pain intensity and location experienced by patients perioperatively in a prospective study.
Patients and methods
Design and setting
This study included both retrospective and prospective data collection. Data on anesthesia and analgesia were collected retrospectively for all patients (N=321) who underwent surgical cardiac device implantation at the University Hospital Split, Croatia, from January to December 2014. Data on pain intensity and location during CED implantation were collected prospectively from a convenience sample of 51 patients treated between November 2014 and August 2015.
Ethics
The study was approved by the ethics committee of The University Hospital Split. Patient consent was not required to access their medical records for the retrospective study because all data analyzed in the retrospective study were collected as part of routine diagnosis and treatment. Patients were recruited to the prospective study after receiving detailed information about the study and providing informed consent.
Data collection
Types of anesthesia, analgesia, and perioperative pain management were analyzed during and after cardiac device therapy and compared to the American Society of Anesthesiologists (ASA) guidelines for perioperative pain management.15
The data collected in the retrospective part of the study included age, sex, diagnosis, type and dosage of premedication, type of cardiac device, type and dosage of anesthesia, and type and dosage of analgesia for each day if it was administered on more than 1 day (on each postoperative day, whether the patient received pain medicine, type of analgesia, and dose were noted).
In the prospective part of the study, pain intensity was measured before, during, and after implantation (at 1, 3, 6, and 8 hours after device implantation, and 24 hours after implantation if the patient was still in the hospital). Pain intensity was measured by using a numerical rating scale (NRS) ranging from 0 to 10, with 0 indicating no pain and 10 indicating the worst pain imaginable. Patients were also asked about the location of their pain. Data about pain intensity and location were recorded by a nurse on a special data collection form, based on the patients’ self-report.
Statistics
Descriptive statistics were performed by using Microsoft Excel (Microsoft Inc., Redmond, WA, USA). Frequencies and percentages were calculated for the analyzed categorical variables. For continuous variables, mean and standard deviation were calculated.
Results
Retrospective study
A total of 321 patients, who underwent CED implantation during 2014 at the University Hospital, Split, were included in the retrospective study. They comprised 201 male (63%) and 120 female (37%) patients with an average age of 76 (range: 24–103) years. The three most common indications for surgery were atrioventricular block, atrial fibrillation, and sinus node dysfunction.
Patients included in the retrospective study received several types of devices: pacemaker (96%), cardioverter-defibrillator (2%), CRT (1%), and “loop recorder” (0.6%). Local anesthesia with 5–10 mg lidocaine was applied during CED implantation as the only type of anesthesia in all patients. None of the patients received general anesthesia.
Of the 321 patients, 242 (75%) did not receive any drug before the procedure. Among the 25% of patients who received premedication, the most commonly used drugs were a combination of tramadol and acetaminophen (28%), tramadol (25%), diazepam (20%), acetaminophen, and ibuprofen (8%), whereas a few patients received methylphenobarbital (2%), zolpidem (2%), diclofenac (1%), fentanyl (1%), indomethacin (1%), or morphine (1%).
The most common doses of premedication were 37.5 mg/325 mg of a combination of tramadol and acetaminophen (29%), 5 mg of diazepam (17%), 50 mg of tramadol (16%), and 500 mg of acetaminophen (12%). The analysis of postoperative analgesia showed that 223/321 (69%) patients did not receive any analgesia after the operation. Among the 31% of patients who did receive analgesia or sedation after the operation, the most commonly used drugs were a combination of tramadol/acetaminophen (26%), tramadol (23%), and diazepam (18%) (Table 1).
Table 1.
Analgesia received after cardiac electronic device implantation in 321 patients
| Postoperative analgesia | N (%) |
|---|---|
| Tramadol/acetaminophen | 31 (26) |
| Tramadol | 28 (23) |
| Diazepam | 22 (18) |
| Acetaminophen | 21 (17) |
| Ibuprofen | 11 (9) |
| Methylphenobarbital | 2 (2) |
| Diclofenac | 1 (1) |
| Fentanyl | 1 (1) |
| Indomethacin | 1 (1) |
| Metamizole | 1 (1) |
The most common doses for postoperative analgesic drugs were 37.5 mg/325 mg of a combination of tramadol/acetaminophen (28%), 500 mg of acetaminophen (18%), and 5 mg of diazepam (17%). Patients who received analgesics most commonly received them within 3 days of the surgery (ranging from 1 to 9 days postimplantation). They received analgesics most commonly once (19%) or twice (16%) a day.
Prospective study
A total of 51 patients who underwent CED implantation from November 2014 to August 2015 were included in the prospective study. The highest recorded intensity of pain on the NRS was 8 during the procedure, 7 at 1 h postoperatively, and 6 at the remaining four postoperative measurements. The average postoperative pain at all time points was <4 (Figure 1). The most common locations of pain were the site of operation (44%), shoulder (5%), and chest (2%) (Table 2).
Figure 1.
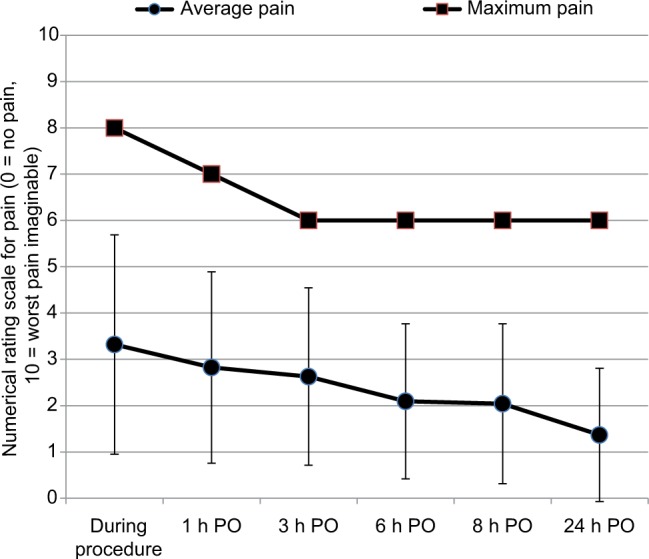
Maximum and average pain intensity during and after cardiac electronic device implantation (PO, postoperatively). Average pain presented as mean and standard deviation.
Table 2.
Location of the most intense pain in 51 patients undergoing cardiac electronic device implantation
| Site | N (%) |
|---|---|
| Procedural site | 137 (44) |
| Chest | 7 (2) |
| Shoulder | 17 (5) |
| Arm | 2 (0.6) |
| Neck | 3 (0.9) |
| No answer | 85 (27) |
Comparison with guidelines for acute perioperative pain management
Postoperative pain management during CED implantation at the study center was compared to the ASA guidelines for perioperative pain management.15 It was found that the study center did not adhere to the guidelines. Health workers involved with the postoperative care of patients were not trained by the anesthesiologists. Patients’ medical notes did not contain information about previous pain intensity for any of the patients. Furthermore, the notes did not contain any information on any side effects of the drugs. Multimodal pain therapy was not used, and acetaminophen was rarely used as postoperative analgesia. Patients were not placed in separate rooms for postoperative recovery because CED implantation is considered a minor procedure in the day hospital, and there was no intensive patient follow-up.
Discussion
Our retrospective analysis indicated that patients undergoing CED implantation at the study center received inadequate perioperative pain management. It was found that 75% of patients did not receive any kind of premedication. All procedures were conducted under local anesthesia with lidocaine. Only 25% of patients received any analgesics or sedative drugs after the procedure. Furthermore, the prospective analysis showed that patients experienced severe pain during the procedure, with an intensity of up to 8, indicating that anesthesia and analgesia were inadequate.
Guidelines for treating perioperative pain were published by the ASA in 2012.15 The aim of the guidelines was to increase the efficacy and safety of acute perioperative pain management, to reduce the risk of side effects, to preserve patients’ functions, and to increase patients’ quality of life during the perioperative period.15 The guidelines emphasize the importance of preventing the development of side effects and chronic pain due to inappropriate analgesia. They also emphasize education of the hospital staff by anesthesiologists. This training should include education about pain assessment and on pharmacological and nonpharmacological treatments for pain. According to the guidelines, anesthesiologists should regularly evaluate pain intensity, and the efficiency and side effects of therapy, and document these. Preoperative assessment of patients should include taking a detailed patient history, especially about previous pain, physical examination, and planning pain control considering the procedure that the patient is undergoing.15
A number of studies on the quality of postoperative pain management have been conducted in different countries to improve postoperative care and implement the guidelines. For example, a study conducted by Apfelbaum et al16 in the USA showed that 80% of patients experienced pain after the procedure. Among these, 86% had moderate, severe, or extremely severe pain which in many cases occurred after being discharged from hospital.16
Maier et al17 analyzed pain treatment in German hospitals during a 3-year period by interviewing patients undergoing surgical and nonsurgical procedures about their intensity of pain and efficiency of the treatment of pain. They found that 55% of patients from the surgical group and 57% from the nonsurgical group were not satisfied with their pain treatment. Furthermore, 39% of nonsurgical and 15% of surgical patients did not receive any analgesia even though they had pain. Pain therapy was considered inadequate for 46% of nonsurgical and 30% of surgical patients. The authors concluded that pain after surgical or nonsurgical procedures is still very common and its treatment is inadequate.17
A similar study conducted by Weiran et al18 in China indicated that postoperative pain was relieved within 3 days for 83% of patients. However, 20% of interviewed patients were not satisfied with their pain treatment, whereas 52% did not receive any analgesia even though 91% of patients reported pain. The authors concluded that, even though effective methods of pain treatment are available, there is a need for additional education of hospital staff, patients, and their families about pain treatment and also a need for better communication with patients in the postoperative period.18
The studies mentioned thus far did not relate specifically to CED implantation but to various other surgical procedures. However, Anderson et al19 recently presented a summary of their research conducted in London, UK, about analgesia after CED implantation at the 15th World Congress on Pain. These findings indicated that acetaminophen was given during the procedure only to patients who were under general anesthesia and that the same patients received opioids in 43% of cases. Morphine was given to 83% of patients who underwent local anesthesia. They found that only 48% of patients received analgesia after discharge despite high pain intensity (>6) but a year later, after educating the hospital staff, that number increased to 53%. They concluded that pain after CED implantation is underrated and that hospital staff did not administer analgesia consistently. Even though educational programs helped to increase administration of analgesics after discharge, development of guidelines for pain treatment in cardiac device implantation was proposed.19
In this study, none of the patients received morphine after the procedure, which might indicate a lack of very severe pain among patients or a reluctance of physicians to administer morphine. In contrast to the study by Anderson et al19 in which 29% of patients underwent CED implantation under general anesthesia, all patients in the present study received local lidocaine anesthesia.
It was found that, at the study center in 2014, 75% of patients did not receive any analgesic or sedative premedication, and 69% of patients did not receive any pain medication after the operation. As indicated by the high pain intensities reported during the procedure by patients in the prospective study, perioperative pain management was inadequate. It is possible that some patients received analgesics not for postoperative pain, but for painful comorbidities, considering the advanced age of the population. It is also possible that the patients are self-medicating and sharing analgesics between themselves to alleviate pain. It is known that sharing of even prescription analgesics is a common behavior that is viewed positively by patients and even some physicians.20,21
Comparing the practice at the study center to the ASA guidelines for perioperative pain management indicated lack of compliance with those guidelines. In particular, it was noted that there was no policy for acute pain management at the hospital. Very few studies of acute pain management procedures have been conducted in tertiary hospitals in Croatia. A study conducted in another department of the same hospital indicated inadequate perioperative pain management in patients undergoing complex ophthalmic procedures.14 Another study of chronic pain treatment in the same hospital showed long waiting times for referral to a tertiary pain clinic and for procedures and referrals to specialists.22 Understaffed pain clinics in Croatia and a narrow focus of work may explain their limited involvement in acute pain management.23 ASA guidelines recommend multimodal pain management, but this was not observed in the present study or the previous studies on acute perioperative pain management,14,24 and the treatment of chronic pain25 at the same hospital.
Despite the ASA guidelines for acute perioperative pain treatment,15 it was found that anesthesiologists do not educate hospital staff on pain treatment. There is no routine evaluation of pain intensity, therapy efficiency, or side effects. Patients’ medical records did not contain information about previous pain intensity or any side effects of the therapy. Even though CED implantation is a minor procedure, in most cases conducted under local anesthesia, and not requiring a long stay in the hospital, there is still a possibility of pain during the procedure and in the early postoperative period. Local guidelines for acute pain management should be developed, taking into account the complexity of the procedure and specific conditions of the setting.
These prospective data showed that patients experienced the most pain during the procedure, with some patients reporting severe pain (NRS score >5) in all postoperative measurements. Patients’ pain reports during the procedure showed high variability, but pain is a highly subjective sensation; hence, this variability can be expected. Considering that almost every patient felt some postoperative pain, there is room for improving perioperative pain management related to CED implantation. Education of the hospital staff, in line with the ASA guidelines for perioperative pain management,15 could be the first step to remedy this situation.
Strengths of this study include its originality, since any previous studies on this subject could not be found. Furthermore, findings of this study are significant because this study identified the problem that no patients undergoing CED implantation had adequate pain management and pointed out the way to solve it by educating the staff and the introduction of acute pain management guidelines to alleviate suffering of patients and enable pain-free CED implantation procedures.
Limitations
Limitations of the study are its cross-sectional nature and relatively small number of patients involved in the prospective part because it was conducted in a single center; thus, it may not be representative of other centers, and there were some missing data on pain location. Further observational studies should explore pain intensity associated with CED implantation in terms of risk factors for pain development and factors related to the procedure (size of the device, extent of the surgical trauma, and duration of the procedure) and patient condition. Further studies on this subject should also include randomized controlled trials to assess the efficacy and safety of various analgesics and tranquilizers in the CED implantation setting as no such studies could be found in the medical literature.
Conclusion
All patients undergoing CED implantation at the study center received local anesthesia, and perioperative pain management was inadequate. Education of staff and introduction of acute pain management guidelines should be the next step to alleviate suffering of patients and enable pain-free CED implantation procedures.
Acknowledgments
The authors are grateful to all patients who participated in the study and to Dr Antonia Jelicic Kadic for critical reading of the manuscript. Many thanks to Prof Elizabeth Wager, of Sideview, Princes Risborough, for language editing; Sideview specializes in medical and academic publications.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Herce B, Nazeyrollas P, Lesaffre F, et al. Risk factors for infection of implantable cardiac devices: data from a registry of 2496 patients. Europace. 2013;15(1):66–70. doi: 10.1093/europace/eus284. [DOI] [PubMed] [Google Scholar]
- 2.Hill PE. Complications of permanent transvenous cardiac pacing: a 14-year review of all transvenous pacemakers inserted at one community hospital. Pacing Clin Electrophysiol. 1987;10(3 Pt 1):564–570. doi: 10.1111/j.1540-8159.1987.tb04521.x. [DOI] [PubMed] [Google Scholar]
- 3.Gadler F, Valzania C, Linde C. Current use of implantable electrical devices in Sweden: data from the Swedish pacemaker and implantable cardioverter-defibrillator registry. Europace. 2015;17(1):69–77. doi: 10.1093/europace/euu233. [DOI] [PubMed] [Google Scholar]
- 4.Lohman D, Schleifer R, Amon JJ. Access to pain treatment as a human right. BMC Med. 2010;8:8. doi: 10.1186/1741-7015-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krnic D, Anic-Matic A, Dosenovic S, Zezelic S, Draganic P, Puljak L. National consumption of opioid and nonopioid analgesics in Croatia: 2007–2013. Ther Clin Risk Manag. 2015;11:1305–1314. doi: 10.2147/TCRM.S86226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamunen K, Laitinen-Parkkonen P, Paakkari P, et al. What do different databases tell about the use of opioids in seven European countries in 2002? Eur J Pain. 2008;12(6):705–715. doi: 10.1016/j.ejpain.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 7.De Conno F, Ripamonti C, Brunelli C. Opioid purchases and expenditure in nine western European countries: “are we killing off morphine?”. Palliat Med. 2005;19(3):179–184. doi: 10.1191/0269216305pm1002oa. [DOI] [PubMed] [Google Scholar]
- 8.Lasinkas M. Trends in the Consumption of Analgesic Drugs in Lithuania iz 2005–2007 [master’s thesis] Kauno Medicinos Universitetas; [Accessed December 3, 2008]. Available from: http://vddb.library.lt/obj/LT-eLABa-0001:E.02~2008~D_20080616_100523-46119. [Google Scholar]
- 9.Gudin JA. The changing landscape of opioid prescribing: long-acting and extended-release opioid class-wide Risk Evaluation and Mitigation Strategy. Ther Clin Risk Manag. 2012;8:209–217. doi: 10.2147/TCRM.S28764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Awan H, Durrani Z. Postoperative pain management in the surgical wards of a tertiary care hospital in Peshawar. J Pak Med Assoc. 2015;65(4):358–361. [PubMed] [Google Scholar]
- 11.Sansone P, Pace MC, Passavanti MB, Pota V, Colella U, Aurilio C. Epidemiology and incidence of acute and chronic post-surgical pain. Ann Ital Chir. 2015;86:285–292. [PubMed] [Google Scholar]
- 12.Joris JL, Georges MJ, Medjahed K, et al. Prevalence, characteristics and risk factors of chronic postsurgical pain after laparoscopic colorectal surgery: retrospective analysis. Eur J Anaesthesiol. 2015;32(10):712–717. doi: 10.1097/EJA.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 13.Lesin M, Domazet Bugarin J, Puljak L. Factors associated with postoperative pain and analgesic consumption in ophthalmic surgery: a systematic review. Surv Ophthalmol. 2015;60(3):196–203. doi: 10.1016/j.survophthal.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Lesin M, Sundov ZD, Jukic M, Puljak L. Postoperative pain in complex ophthalmic surgical procedures: comparing practice with guidelines. Pain Med. 2014;15(6):1036–1042. doi: 10.1111/pme.12433. [DOI] [PubMed] [Google Scholar]
- 15.Thompson M, Tiwari A, Fu R, Moe E, Buckley DI. A Framework to Facilitate the Use of Systematic Reviews and Meta-analyses in the Design of Primary Research Studies. Rockville, MD: Agency for Healthcare Research and Quality; 2012. [Accessed March 29, 2017]. (AHRQ Publication No. 12-EHC009-EF). [Prepared by the Oregon Evidence-based Practice Center under Contract HHSA 290-2007-10057-I] Available from: https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0076923/ [PubMed] [Google Scholar]
- 16.Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97(2):534–540. doi: 10.1213/01.ANE.0000068822.10113.9E. [DOI] [PubMed] [Google Scholar]
- 17.Maier C, Nestler N, Richter H, et al. The quality of pain management in German hospitals. Dtsch Arztebl Int. 2010;107(36):607–614. doi: 10.3238/arztebl.2010.0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiran L, Lei Z, Woo SM, et al. A study of patient experience and perception regarding postoperative pain management in Chinese hospitals. Patient Prefer Adherence. 2013;7:1157–1162. doi: 10.2147/PPA.S53235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson RH, Cox FJ, Jaggar SI. Analgesia provision for cardiac device implantation – an unrecognised need; 15th World Congress on Pain; October 6–11, 2014; Buenos Aires, Argentina. 2014. [Accessed March 29, 2017]. Available from: http://docplayer.net/44253580-15th-world-congress-on-pain.html. [Google Scholar]
- 20.Markotic F, Puljak L. Risks associated with borrowing and sharing of prescription analgesics among patients observed by pain management physicians in Croatia: a qualitative study. J Pain Res. 2016;9:1143–1151. doi: 10.2147/JPR.S118945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markotic F, Vrdoljak D, Puljiz M, Puljak L. Risk perception about medication sharing among patients: a focus group qualitative study on borrowing and lending of prescription analgesics. J Pain Res. 2017;10:365–374. doi: 10.2147/JPR.S123554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Triva P, Jukic M, Puljak L. Access to public healthcare services and waiting times for patients with chronic nonmalignant pain: feedback from a tertiary pain clinic. Acta Clin Croatica. 2013;52(1):79–85. [PubMed] [Google Scholar]
- 23.Fidahic M, Dogan K, Sapunar D, Puljak L. National survey of pain clinics in Croatia: organization and services. Acta Med Acad. 2015;44(1):18–30. doi: 10.5644/ama2006-124.123. [DOI] [PubMed] [Google Scholar]
- 24.Boric K, Boric M, Boric T, Puljak L. Analysis of perioperative pain management in vascular surgery indicates that practice does not adhere with guidelines: a retrospective cross-sectional study. J Pain Res. 2017;10:203–209. doi: 10.2147/JPR.S123894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jukic M, Kardum G, Sapunar D, Puljak L. Treatment of chronic musculoskeletal back pain in a tertiary care pain clinic. J Musculoskelet Pain. 2012;20(4):277–283. [Google Scholar]


